Abstract
The C syndrome is characterized by trigonocephaly and associated anomalies, such as unusual facies, psychomotor retardation, redundant skin, joint and limb abnormalities, and visceral anomalies. In an individual with the C syndrome who harbors a balanced chromosomal translocation, t(3;18)(q13.13;q12.1), we discovered that the TACTILE gene for CD96, a member of the immunoglobulin superfamily, was disrupted at the 3q13.3 breakpoint. In mutation analysis of nine karyotypically normal patients given diagnoses of the C or C-like syndrome, we identified a missense mutation (839C→T, T280M) in exon 6 of the CD96 gene in one patient with the C-like syndrome. The missense mutation was not found among 420 unaffected Japanese individuals. Cells with mutated CD96 protein (T280M) lost adhesion and growth activities in vitro. These findings indicate that CD96 mutations may cause a form of the C syndrome by interfering with cell adhesion and growth.
The C (Opitz trigonocephaly) syndrome (MIM %211750) is a malformation syndrome of unknown cause, and its mode of inheritance has been suggested to be autosomal recessive. The syndrome comprises trigonocephaly and associated anomalies, such as unusual facies, wide alveolar ridges, multiple buccal frenula, limb defects, visceral anomalies, redundant skin, psychomotor retardation, and hypotonia.1,2
Recently, Bohring et al.3,4 suggested the delineation or existence of a severe form of the C syndrome (the C-like syndrome, or Bohring-Opitz syndrome [MIM 605039]). More recently, Osaki et al.5 reported on a newborn infant who had many clinical features similar to those of the C-like syndrome but did not have exophthalmoses, which has been regarded as a hallmark of the C-like syndrome. They suggested that the manifestations in this patient are a further indication of overlap between the C-like syndrome and the C syndrome. Thus, it is controversial whether there is (1) a gradient of spectrum in the C syndrome, from the mild form (C syndrome) to the severe form (C-like syndrome), or (2) genetic heterogeneity among the patients with the C syndrome.
In addition, various chromosomal abnormalities, especially those that include chromosome 3, have been reported in patients originally described as having the C syndrome.2 These include 3p monosomy,6 distal 3p trisomy,7 3q trisomy,8 distal 3q trisomy with deletion of distal 3p,9 and inversion in chromosome 3.10 Although these cases might be removed from the C syndrome because they involve chromosome abnormalities, it is possible that there could be putative genes (or multiple loci) related to trigonocephaly and, even further, to pathogenesis of the C syndrome in chromosome 3.2,10
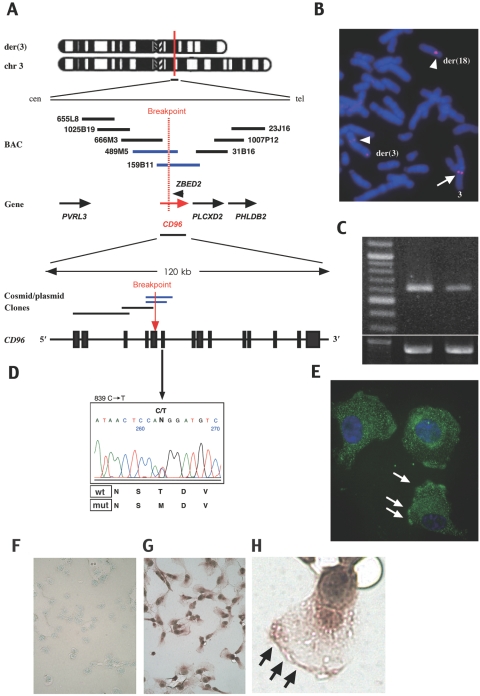
We encountered a boy with the C syndrome and a de novo balanced translocation, 46,XY,t(3;18)(q13.13;q12.1).11 By construction of a BAC/cosmid contig covering the breakpoints, we found the CD96 (TACTILE) gene (GenBank accession number NM_198196) encoding a member of the immunoglobulin superfamily12 at the 3q13.13 breakpoint (fig. 1A). The CD96 gene consists of 15 exons and spans ∼120 kb in the genome. Precise structural analysis around the breakpoint showed that the gene was disrupted by the translocation in exon 5, probably leading to premature termination or loss of expression of CD96 protein. There is no gene or poly-A signal in a 500-kb region telomeric to the breakpoint of chromosome 18, according to the Ensembl Genome Browser Web site. FISH analysis with use of a BAC clone, RP11-158B11, demonstrated split signals on the two derivative chromosomes 3 and 18 (fig. 1B). Semiquantitative RT-PCR analysis showed that CD96 expression in B cells of the patient was reduced to 45.8% of the normal level (fig. 1C). Although one of the zinc-finger genes, ZEBD2 (GenBank accession number NM_024508.3) exists near the breakpoint, in intron 6 of CD96 it has the opposite direction (fig. 1A), and its expression was not reduced in the patient (data not shown). At the other breakpoint, 18q12.1, we could not find any genes or ESTs, according to the Genome Browser Web site (data not shown). We surveyed in this patient copy-number changes for the whole genome by the use of Human Mapping 50K Array Xba240 (Affymetrix). No pathogenic deletions or duplications were detected (data not shown).
Figure 1. .
CD96 mutations in the C or C-like syndrome and its expression. A, BAC and cosmid/plasmid map spanning the 3q13.3 breakpoint of a patient with a translocation, t(3;18)(q13.13;q12.1). The red vertical line and dashed red line indicate the breakpoint. Blue bars for BACs or plasmids indicate clones covering the breakpoint (detected by FISH analysis). A red horizontal arrow indicates the CD96 gene. A red vertical arrow indicates the breakpoint in the CD96 gene. B, FISH analysis of the patient harboring the translocation. FISH signals for BAC RP11-159B11 are separated into two chromosomes, der(3) and der(18), whose signals are indicated by arrowheads. An arrow indicates signals on a normal chromosome 3. C, RT-PCR for CD96 expression in the B cells of an unaffected individual and the patient with C syndrome. The left, middle, and right lanes depict a 100-bp ladder, an unaffected control, and the patient with C syndrome with translocation, respectively. The lower panel indicates expression of GAPDH as the control. D, Direct DNA-sequence analysis of CD96 demonstrates a point mutation in a patient. E–H, Immunocytochemical analysis for CD96 localization in HT1080 cells with use of rabbit anti-CD96 antibody and FITC-conjugated (E) or HRP-conjugated (G–H) goat anti-rabbit IgG. E, Counterstained with 4′,6-diamidino-2-phenylindole (original magnification 400×). F–H, Counterstained with methyl green. F, Negative control. Original magnification was 100× (F and G) or 400× (H). White arrows (E) and black arrows (H) indicate regions adherent to the plastic dish.
We examined nine karyotypically normal Japanese patients who were given clinical diagnoses of the C or C-like syndrome. The syndromes were diagnosed by the presence of trigonocephaly and associated combinations of major clinical findings that are observed in >70% of reported patients with the C or C-like syndromes—that is, upslanting palpebral fissures, epicanthial folds, strabismus, depressed nasal root, anomalous and posteriorly angulated ears, capillary hemangioma, redundant skin, and joint contractures (table 1).2 Two of the patients were reported as having C-like syndrome,5,13 and the information about seven others was unpublished. First, we examined these patients for deletions or duplications by FISH analysis, using RP11-159B11 as a probe. However, no deletions were detected in any of them (data not shown). We then performed direct sequencing analysis of the candidate genes, CD96 and ZEBD2. Primer pairs and PCR conditions for amplification of the candidate genes are listed in table 2. In one patient who was given a diagnosis of C-like syndrome,5 we identified a de novo missense mutation (c.839C→T) in exon 6 of CD96 (fig. 1D). The c.839C→T substitution predicts a threonine-to-methionine change (T280M) at nucleotide position 839, close to the third immunoglobulin-like domain. The threonine residue was conserved in some species—that is, chimpanzee, monkey, dog, opossum, and armadillo. The missense mutation was not found among 420 unaffected Japanese individuals.
Table 1. .
Comparison of Manifestations between Two of Our Patients and Frequencies of Major Findings in the C or C-like Syndromes[Note]
| Presence ina |
Frequency in |
|||
| Clinical Finding | Patient with Translocation | Patient with Mutation | C Syndrome2 | C-like Syndrome4 |
| Trigonocephaly | + | + | 23/23 | 13/13 |
| Upslanting palpebral fissures | + | + | 22/23 | 13/13 |
| Epicanthal folds | + | − | 20/22 | NM |
| Prominent eyes | + | − | NM | 13/13 |
| Strabismus | + | + | 16/22 | 8/8 |
| Depressed nasal bridge | + | + | 15/22 | 13/13 |
| Anomalous and posteriorly angulated ears | + | + | 18/21 | 12/13b |
| Wide alveolar ridges | + | − | 10/18 | 4/6 |
| High-arched palate | + | + | NM | NM |
| Capillary hemangioma | − | + | 9/17 | 13/13 |
| Redundant skin | + | − | 14/20 | NM |
| Joint contractures | − | + | 7/21 | 13/13 |
| Agenesis of the corpus callosum | + | + | NM | 7/10 |
| Failure to thrive | − | + | NM | 11/11 |
| Intrauterine growth retardation | − | + | NM | 12/13 |
| Seizures | − | − | 5/19 | 5/5 |
| Developmental retardation | ± | + | 18/19 | 9/9 |
| Congenital heart anomalies | − | − | 11/22 | 5/11 |
| Clinical diagnosis | C syndrome | C-like syndrome | … | … |
Note.— NM = not mentioned.
+ = present; − = absent. ± = borderline.
Low-set ears.
Table 2. .
Sequencing Primers and PCR Conditions for the CD96 and ZBED2 Genes
| Sequence(5′→3′) |
|||||
| Primer Name | Forward | Reverse | Tma (°C) |
MgCl2 (mM) |
Size (bp) |
| hCD96 ex1 | CAACTGCTCTGCGTGATATC | ACCCTTAGTAATGATTTGTCCT | 60 | 2.5 | 540 |
| hCD96 ex2 | CCTAAAGCAGCCAGGGAGAAA | ATGCTGAGCACCAAGCCTAAC | 58 | 1.25 | 657 |
| hCD96 ex3 | GAGGACAGATGAATCCCTATAC | ATAGACTCAGAGGCTTGCCTG | 60 | 1.8 | 424 |
| hCD96 ex4 | CAGACTTGCCAGTGCTGAGT | GGATGGACTAAGGTAGACTTC | 60 | 1.8 | 380 |
| hCD96 ex5 | GTAAATGAATCAGTGCTTGTCGA | GTATCCAGGGAAACAGACTCC | 62 | 2.5 | 429 |
| hCD96 ex6 | TCTGTATTCCCATGAAACTGTAG | TATGCAACCTGACACACCTTAC | 60 | 1.8 | 367 |
| hCD96 ex7 | CATCTCTATAGGAGATAGCCCA | ACACTCCACCCCCTTGGAAG | 58 | 1.25 | 472 |
| hCD96 ex8 | TTGATCATGCCATGCCTTGGC | TTTCACTGGAGTCCTACTTGTC | 58 | 1.25 | 446 |
| hCD96 ex9 | GCTGCCTAGTTTCCAGGCCA | ATGGGCAAGTTAATGTGACGTG | 58 | 1.25 | 485 |
| hCD96 ex10 | GGCTGTTCACTAAGATTCTTTCC | TAGTCACCGCAGAGTAACCCA | 58 | 1.25 | 343 |
| hCD96 ex11 | GCCAGCTAGTGTTCCTGCATA | GTCCATGGGTGTAGTCTCAGA | 60 | 1.8 | 386 |
| hCD96 ex12 | CAAGAATCCCTTCAACTCCCAC | TATATCTATCTGAGGCTGGCTTC | 62 | 1.8 | 355 |
| hCD96 ex13 | CAAATCTCAGGATCCCAGCCT | TTGACCCTGACAACACCTTATC | 62 | 1.25 | 499 |
| hCD96 ex14 | GCTTAGACATGCCCACCTCC | CAGCCTGACTAGGCCAATGC | 62 | 1.25 | 488 |
| hCD96 ex15 | TGTGACTAACAGGCACAGGGT | GGTTAAGCTTCAGGCGTTTGG | 58 | 1.25 | 467 |
| hCD96 ex15-2 | GAGAGCCAGAACTACCCAGC | CCACTCCCTACCCCCACTTT | 62 | 1.8 | 372 |
| hZBED2 15 | TGTGGTTCAAATAAGCTTTTGGC | … | 60 | 1.25 | 934 |
| hZBED2 23 | GTTTCGGCCAAGGGTCAGCA | … | … | … | … |
| hZBED2 35 | ACATGATGAGGCGGGAAGACGA | … | 60 | 1.25 | 657 |
| hZBED2 43 | AACAAAATGGAAGGGATGTACTG | … | … | … | … |
Annealing temperature.
Two patients had a homozygous 5-bp insertion (c.856-80insTTATG) in intron 6 of the CD96 gene. They showed an ∼40% reduction of CD96 expression in their B cells, compared with the normal control level (data not shown). However, this homozygous 5-bp insertion was found in 2 of 196 normal Japanese individuals examined. No copy-number variation around this region has been registered in the Database of Genomic Variants. Therefore, it is ambiguous whether the insertion is directly associated with the syndrome. There is also a possibility that small mutations in the promoter or enhancer region of CD96 or other mutations that affect CD96 expression, albeit undetected by our analyses, might reduce the gene expression in the patients. No mutation in ZEBD2 was found in any of the nine Japanese patients (data not shown).
We also examined 20 white patients for the CD96 gene, 18 of whom were given clinical diagnoses of the C syndrome and 2 of whom were given diagnoses of the C-like syndrome. However, the direct sequencing analysis could not detect any apparent mutations in any exons of the CD96 gene in these patients.
The patient having the missense mutation in CD96 had the following relatively severe clinical manifestations: trigonocephaly, ridging of the metopic suture with narrow forehead, a small hemangioma near the nose, thin upper lip, long philtrum, a high-arched palate with deep groove, low-set ears, a short neck, cryptorchidism, abnormality of the ventricular myocardium, mild optic-nerve atrophy, and hypoplasia of the corpus callosum, all of which led to the diagnosis of the C-like syndrome (table 1).5 The patient harboring the balanced translocation had less severe manifestations—that is, trigonocephaly, a prominent metopic ridge, upslanting palpebral fissures, epicanthal folds, thick and irregular alveolar ridges, thin upper lip, long philtrum, low-set ears, redundant nuchal skin, and agenesis of the corpus callosum (table 1).11 His phenotype satisfied the diagnosis of the C syndrome.
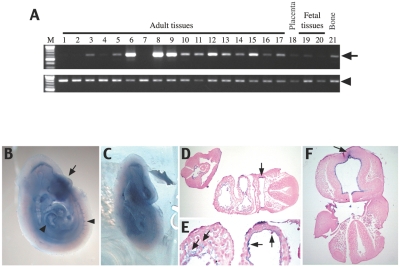
CD96 was identified as a human T-cell–activated antigen in long-term culture and is known to interact with the poliovirus receptor, CD155, to recognize targets for natural killer (NK) cells.14 To determine a possible role of CD96 in the C syndrome, we investigated its expression and function in humans and mice. CD96 was found to be localized in the cytoplasm and cell-adhesion sites of the cell surface when it was expressed in HT1080 cells (fig. 1E–1H). A CD96-CFP fusion protein gave the same result when it was transiently expressed in HT1080 (data not shown). These findings support the hypothesis that CD96 may act as a cell-adhesion molecule, as do some other proteins of the immunoglobulin superfamily, such as nectin.15 The human CD96 gene is strongly expressed in the adult lung, spleen, and thymus and is moderately expressed in the adult spinal cord, kidney, trachea, digestive tissues, prostate, placenta, bone, and fetal brain and liver (fig. 2A). In 10-d-postcoitum mouse (dpc) embryos, Cd96 is expressed in the forebrain and in a front part of the head tissues, cardiac jelly, endothelial cells, pharynx, and blood cells (fig. 2B–2F). These expression patterns are consistent with organs and tissues involved in the abnormalities of the C syndrome—that is, trigonocephaly, redundant nuchal skin, and cardiovascular abnormalities.
Figure 2. .
Expression of the CD96 gene in fetal and adult tissues. A, Expression in human tissues. An arrow indicates CD96 cDNA. An arrowhead indicates GAPDH cDNA as control. Lane M, size marker (100-bp ladder); 1, brain (whole); 2, cerebellum; 3, spinal cord; 4, heart; 5, kidney; 6, lung; 7, skeletal muscle; 8, spleen; 9, thymus; 10, trachea; 11, stomach; 12, small intestine; 13, colon; 14, salivary gland; 15, prostate; 16, testis; 17, uterus; 18, placenta; 19, fetal brain; 20, fetal liver; and 21, bone. Lanes 1–17 and 21, adult tissues. Lanes 19 and 20, fetal tissues. B–F, Whole mount in situ hybridization with Cd96 antisense RNA in 10-dpc mouse embryo, showing high expression in developing forehead (arrow in B) and in heart and blood vessels (arrowheads in B). D–F, Horizontal sections of the embryo. Cd96 is expressed in the pharynx (arrow in D); in cardiac jelly, endocardial cells, and blood cells (arrow in E); and in forebrain tissues (arrow in F). All sections are counterstained with nuclear fast red.
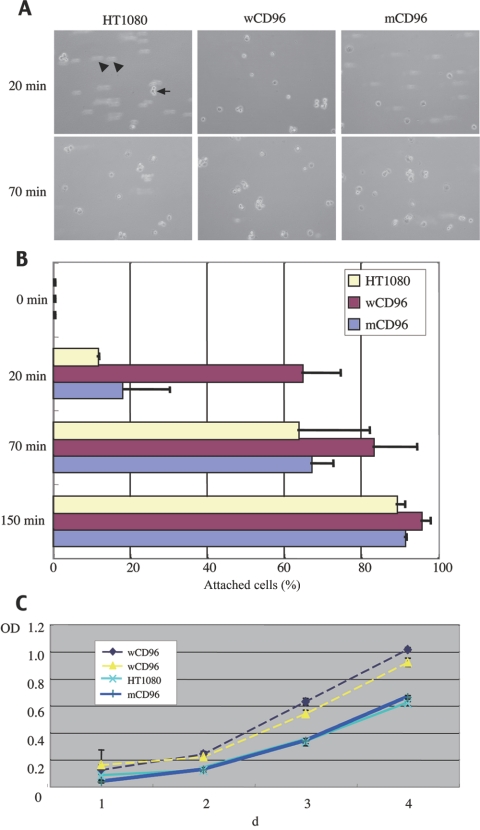
To analyze a potential role of CD96 in the morphological abnormalities of the C syndrome, we investigated the function of wild-type CD96 (wCD96) and mutated CD96 (mCD96 [c.839C→T]) in vitro. We constructed expression vectors for wCD96 and mCD96 using the strong CAG promoter,16 introduced each vector into HT1080 cells, and compared the characteristics of each transformant. A cell-adhesion assay with the HT1080 cell clones expressing wCD96 showed faster attachments on tissue-culture plates compared with mock clones, even under the condition of 10% serum-containing medium (fig. 3A and 3B), whereas those expressing mCD96 showed the same adherent activity as the mock cells (fig. 3A and 3B). The result suggests that CD96 protein is involved in cell-matrix adhesion in transfected HT1080 cells, but mCD96 protein loses the activity. A tetrazolium-based (MTS) assay on the transformants, performed to determine their effect on cell growth, showed 1.5 times more growth-promoting activity of wCD96 than was shown of mCD96 in HT1080 (fig. 3C). Many cell-adhesion molecules belonging to the immunoglobulin super family (IgCAMs) play important roles during embryogenesis or morphogenesis.17 For example, mutations in the gene for PVRL1/nectin-1, a member of IgCAM, are involved in the cause of cleft lip/palate–ectodermal dysplasia syndrome (MIM #225000).18,19
Figure 3. .
Functional characterization of wild-type and mutated CD96 proteins. A and B, Cell-adhesion assay for tissue-culture plates. A, Images captured after exposure with vibration for 20 min and 70 min. HT1080 indicates untransfected cells (control); wCD96 indicates highly expressed clone for wild-type CD96 in HT1080; and mCD96 indicates highly expressed clone for mutated CD96 in HT1080. The arrow indicates attached cell. Arrowheads indicate nonattached cells. B, Quantitated adhesion activity in each transformant. Attached cells and nonattached cells are counted in more than five different fields under a microscope. A total of at least 500 cells were counted for each experiment. Error bars are mean±SD. Adhesion activities are indicated by percentages of attached cells per total cell number at 0 min, 20 min, 70 min, and 150 min after spreading cells. C, Cell proliferation assessed by a tetrazolium-based (MTS) assay. The ordinates show the cell number expressed as arbitrary units. Two wCD96s are clones highly expressing wild-type CD96, HT1080 is an untransfected control clone, and mCD96 is a clone expressing mutated CD96. Error bars are mean±SD. Data shown are from three independent experiments, each performed in quadruplicate (n=12).
The original report and other reports of affected sibs with the C syndrome suggested that the syndrome is inherited in an autosomal recessive fashion.1,2 Normal chromosomes in most patients, unaffected parents with multiaffected offsprings, the equal sex ratio of affected individuals, and consanguineous matings1,2,8 all support autosomal recessive inheritance. Meanwhile, many other patients have sporadic disease,2 and recurrence risk may be estimated to be 10%,8 which suggests the possibility of dominant inheritance or germline mosaicism.2,8,10 These findings imply that the C syndrome is genetically heterogeneous, and its inheritance mode is in debate. The CD96 aberrations found in our two patients were both in the heterozygous state without a copy-number variation in this region, which is consistent with an autosomal dominant condition. Since it is hard to assume that all reported sib cases would have originated in germline mosaicism in their respective parents, the CD96 deficiency identified in our patients cannot explain all patients with the C syndrome. However, since genetic heterogeneity is evident in the syndrome and many sporadic cases are known, our results suggest that a form of the C syndrome is caused by dysfunction of CD96. At least, the fact the mutations were found in the C and C-like syndromes may indicate that they are allelic.
A similar example is Cohen syndrome, where only ∼20% of patients were found to have mutations in a causative gene, COH1.20,21 The identification of a causative gene, CD96, may open a door to an understanding of the molecular pathology of the C syndrome.
Acknowledgments
We thank the patients and their families, for their participation in this study, and Dr. Takashi Muramatsu and Dr. Steven Howe, for their helpful advice and discussion. T.K. was supported by Grant-in-Aid for Scientific Research Category C number 17590289, and N.N. was supported by a Grant-in-Aid for Scientific Research on Priority Areas (Applied Genomics) from the Ministry of Education, Culture, Sports, Science and Technology of Japan and by SORST from the Japan Science and Technology Agency.
Web Resources
Accession numbers and URLs for data presented herein are as follows:
- Database of Genomic Variants, http://projects.tcag.ca/variation/
- Ensembl Genome Browser, http://www.ensembl.org/index.html
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for CD96 [accession number NM_198196] and ZBED2 [accession number NM_024508.3])
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.gov/Omim/ (for C syndrome, C-like syndrome, and cleft lip/palate–ectodermal dysplasia syndrome)
References
- 1.Opitz JM, Johnson RC, McCreadie SR, Smith DW (1969) The C syndrome of multiple congenital anomalies. Birth Defects 5:161–166 [Google Scholar]
- 2.Gorlin RJ, Cohen MM Jr, Hennekam RCM (2001) Syndromes of the head and neck, 4th ed. Oxford University Press, New York, pp 1145–1147 [Google Scholar]
- 3.Bohring A, Silengo M, Lerone M, Superneau DW, Spaich C, Braddock SR, Poss A, Opitz JM (1999) Severe end of Opitz trigonocephaly (C) syndrome or new syndrome? Am J Med Genet 85:438–446 [DOI] [PubMed] [Google Scholar]
- 4.Bohring A, Oudesluijs GG, Grange DK, Zampino G, Thierry P (2006) New cases of Bohring-Opitz syndrome, update, and critical review of the literature. Am J Med Genet A 140:1257–1263 [DOI] [PubMed] [Google Scholar]
- 5.Osaki M, Makita Y, Miura J, Abe N, Noguchi S, Miyamoto A (2006) A Japanese boy with apparent Bohring-Opitz or “C-like” syndrome. Am J Med Genet A 140:897–899 [DOI] [PubMed] [Google Scholar]
- 6.Schwyzer U, Binkert F, Caflisch U, Baumgartner B, Schinzel A (1987) Terminal deletion of the short arm of chromosome 3, del(3pter-p25): a recognizable syndrome. Helv Paediatr Acta 42:309–311 [PubMed] [Google Scholar]
- 7.McGaughran J, Aftimos S, Oei P (2000) Trisomy of 3pter in a patient with apparent C (trigonocephaly) syndrome. Am J Med Genet 94:311–315 [DOI] [PubMed] [Google Scholar]
- 8.Sargent C, Burn J, Baraitser M, Pembrey ME (1985) Trigonocephaly and the Opitz C syndrome. J Med Genet 22:39–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Preus M, Vekemans M, Kaplan P (1986) Diagnosis of chromosome 3 duplication q23→qter, deletion p25→pter in a patient with the C (trigonocephaly) syndrome. Am J Med Genet 23:935–943 10.1002/ajmg.1320230409 [DOI] [PubMed] [Google Scholar]
- 10.Opitz JM, Putnam AR, Comstock JM, Chin S, Byrne JL, Kennedy A, Frikke MJ, Bernard C, Albrecht S, Der Kaloustian V, et al (2006) Mortality and pathological findings in C (Opitz trigonocephaly) syndrome. Fetal Pediatr Pathol 25:211–231 10.1080/15513810601015753 [DOI] [PubMed] [Google Scholar]
- 11.Chinen Y, Kaname T, Yanagi K, Naritomi K, Ohta T (2006) Opitz trigonocephaly C syndrome in a boy with a de novo balanced reciprocal translocation t(3;18)(q13.13;q12.1). Am J Med Genet A 140:1655–1657 [DOI] [PubMed] [Google Scholar]
- 12.Wang PL, O’Farrell S, Clayberger C, Krensky AM (1992) Identification and molecular cloning of TACTILE: a novel human T cell activation antigen that is a member of the Ig gene superfamily. J Immunol 148:2600–2608 [PubMed] [Google Scholar]
- 13.Nakane T, Kubota T, Fukushima Y, Hata Y, Ishii J, Komiyama A (2000) Opitz trigonocephaly (C)-like syndrome, or Bohring-Opitz syndrome: another example. Am J Med Genet 92:361–362 [DOI] [PubMed] [Google Scholar]
- 14.Fuchs A, Cella M, Giurisato E, Shaw AS, Colonna M (2004) CD96 (Tactile) promotes NK cell-target cell adhesion by interacting with the poliovius receptor (CD155). J Immunol 172:3994–3998 [DOI] [PubMed] [Google Scholar]
- 15.Sakisaka T, Takai Y (2004) Biology and pathology of nectins and nectin-like molecules. Curr Opin Cell Biol 16:513–521 10.1016/j.ceb.2004.07.007 [DOI] [PubMed] [Google Scholar]
- 16.Kaname T, Huxley C (2001) Simple and efficient vectors for retrofitting BACs and PACs with mammalian neoR and EGFP marker genes. Gene 266:147–153 10.1016/S0378-1119(01)00375-4 [DOI] [PubMed] [Google Scholar]
- 17.Krauss RS, Cole F, Gaio U, Takaesu G, Zhang W, Kang JS (2005) Close encounters: regulation of vertebrate skeletal myogenesis by cell-cell contact. J Cell Sci 118:2355–2362 10.1242/jcs.02397 [DOI] [PubMed] [Google Scholar]
- 18.Suzuki K, Hu D, Bustos T, Zlotogora J, Richieri-Costa A, Helms JA, Spritz RA (2000) Mutation of PVRL1, endoding a cell-cell adhesion molecule/herpesvirus receptor, in cleft lip/palate-ectodermal dysplasia. Nat Genet 25:427–430 10.1038/78119 [DOI] [PubMed] [Google Scholar]
- 19.Sozen MA, Suzuki K, Tolarova M, Bustos T, Fernandez Iglesias JE, Spritz RA (2001) Mutation of PVRL1 is associated with sporadic, non-syndromic cleft lip/palate in northern Venezuela. Nat Genet 29:141–142 10.1038/ng740 [DOI] [PubMed] [Google Scholar]
- 20.Kolehmainen J, Wilkinson R, Lehesjoki AE, Chandler K, Kivitie-Kallio S, Clayton-Smith J, Traskelin AL, Waris L, Saarinen A, Khan J, et al (2004) Delineation of Cohen syndrome following a large-scale genotype-phenotype screen. Am J Hum Genet 75:122–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mochida GH, Rajab A, Eyaid W, Lu A, Al-Nouri D, Kosaki K, Noruzinia M, Sarda P, Ishihara J, Bodell A, et al (2004) Broader geographical spectrum of Cohen syndrome due to COH1 mutations. J Med Genet 41:e87 10.1136/jmg.2003.014779 [DOI] [PMC free article] [PubMed] [Google Scholar]