Abstract
Background
Mechanisms of neurovascular coupling—the relationship between neuronal chemoelectrical activity and compensatory metabolic and hemodynamic changes—appear to be preserved across species from rats to humans despite differences in scale. However, previous work suggests that the highly cellular dense mouse somatosensory cortex has different functional hemodynamic changes compared to other species.
Methods
We developed novel hardware and software for 2-dimensional optical spectroscopy (2DOS). Optical changes at four simultaneously recorded wavelengths were measured in both rat and mouse primary somatosensory cortex (S1) evoked by forepaw stimulation to create four spectral maps. The spectral maps were converted to maps of deoxy-, oxy-, and total-hemoglobin (HbR, HbO, and HbT) concentration changes using the modified Beer-Lambert law and phantom HbR and HbO absorption spectra.
Results
Functional hemodynamics were different in mouse versus rat neocortex. On average, hemodynamics were as expected in rat primary somatosensory cortex (S1): the fractional change in the log of HbT concentration increased monophasically 2 s after stimulus, whereas HbO changes mirrored HbR changes, with HbO showing a small initial dip at 0.5 s followed by a large increase 3.0 s post stimulus. In contrast, mouse S1 showed a novel type of stimulus-evoked hemodynamic response, with prolonged, concurrent, monophasic increases in HbR and HbT and a parallel decrease in HbO that all peaked 3.5–4.5 s post stimulus onset. For rats, at any given time point the average size and shape of HbO and HbR forepaw maps were the same, whereas surface veins distorted the shape of the HbT map. For mice, HbO, HbR, and HbT forepaw maps were generally the same size and shape at any post-stimulus time point.
Conclusions
2DOS using image splitting optics is feasible across species for brain mapping and quantifying the map topography of cortical hemodynamics. These results suggest that during physiologic stimulation, different species and/or cortical architecture may give rise to different hemodynamic changes during neurovascular coupling.
Introduction
Brain function is derived from the dynamic spatiotemporal interactions between neurons, astrocytes, and surrounding erythrocytes and vasculature, known as “neurovascular coupling.” Neurovascular coupling mechanisms appear to be grossly preserved across mammalian species from rats to humans (Cannestra et al., 1996; Grinvald et al., 1986; Malonek et al., 1997) despite differences in scale. However, capillary bed density may place a limit on various hemodynamic homeostatic mechanisms (Harrison et al., 2002b), such as functional hyperemia. Compared to other species (Changizi, 2001, 2005; Harrison et al., 2002a), mouse cortex is one of the most neuronally dense (Pasternak and Woolsey, 1975), and appears to have less dependence on functional hyperemia than rat (Ayata et al., 2004; Prakash, 1999; Prakash and Frostig, 1997; Prakash et al., 2005). A decreased dependence on functional hyperemia may serve an adaptive role to preserve brain homeostatic conditions in different brain architectures within and across species. However, the details of such differences remain largely speculative.
Optical imaging techniques used for brain mapping of intrinsic signals (as opposed to extrinsic signals from applied dyes) detect changes predominantly in light scattering and from hemoglobin absorption (Frostig et al., 1990; Grinvald et al., 1986; Obrig and Villringer, 2003; Toga and Mazziotta, 2002). Moreover, historical, technologic, and theoretical considerations further separate intrinsic signal optical imaging techniques into two classes: visible-spectrum and near-infrared. Visible-spectrum techniques can detect changes on the order of tens of micrometers to a maximum depth of about 1 mm below the pial surface (Ratzlaff and Grinvald, 1991), and can only be performed on visible cortex, such as during neurosurgery in humans (Haglund et al., 1992), or through the intact, transparent skull of mice (Prakash et al., 2000). Near-infrared techniques can detect changes on the order of centimeters to a maximum depth of about 2 cm below the pial surface, and can be performed non-invasively (Boas et al., 2001; Elwell et al., 1993; Fuster et al., 2005; Obrig and Villringer, 2003).
Various versions and names of these basic methodologies exist, such as optical imaging of intrinsic signals (OIS), optical imaging spectroscopy, and near infrared spectroscopy (Obrig and Villringer, 2003). However, each method has a trade-off either in spatial, temporal, or spectral resolutions. A newly developed multi-wavelength, visible-spectrum, 2-dimensional optical spectroscopy (2DOS) method using a filter-wheel and charge coupled device (CCD) camera has minimized some of the trade-offs, but still has limited spectral and temporal resolutions (Berwick et al., 2005; Dunn et al., 2003; Sheth et al., 2005).
We developed a different 2DOS method that uses a commercially available image splitter, in tandem with a microscope and CCD camera. Compared to 2DOS using a filter-wheel (Berwick et al., 2005; Dunn et al., 2003; Sheth et al., 2005), this new method was not limited in temporal resolution by a filter wheel, and had better spatial resolution (due to a new camera), and had comparable spectral resolution.
Here we used this new 2DOS method to explore how hemodynamic responses during neurovascular coupling differed in mouse versus rat S1 cortex.
Methods
Six adult male Sprague Dawley rats (704 ± 131 g (mean ± SD)) and six adult male C57Bl/6 mice (26 ± 4 g) were used for this study. The rodents were prepared for imaging using previously described methodology (Blood et al., 1995; Cannestra et al., 1996; Prakash et al., 2000) in accordance with the institutional Animal Research Committee guidelines.
Surgical Preparation
Anesthesia was induced with gaseous halothane (4–5%) and maintained (1–2%) at a depth such that the rodent had no response to toe pinch. For imaging preparation, all rodents were placed in a species-appropriate stereotactic frame (David Kopf Instruments, Tujunga, CA). A midline scalp incision was made to expose the bone over the parietal cortex of one or both hemispheres. For rats, the bone over the hemisphere to be imaged was thinned with a dental drill, and silicone oil was applied to increase the translucency. For mice no thinning was necessary as the bone is already transparent (Prakash and Frostig, 1997; Prakash et al., 2000). After surgery, the animal, while still in the stereotactic frame, was transferred to the imaging stage. Halothane was discontinued and replaced with enflurane (1.5–2.5%). During imaging, anesthesia was adjusted so that the animal maintained a corneal blink reflex but no response to toe pinch. Core body temperature was monitored with a rectal temperature probe and maintained with a heating pad.
Stimulation trials
Sterile stainless steel needle electrodes were inserted into the plantar surface of forepaw between the fourth and fifth digits. Return electrodes were inserted into the plantar surface of forepaw between the first and second digits. Imaging sessions consisted of 16–32 trials of forepaw stimulation. One stimulation trial consisted of 25 s of recording with a 6 s inter-trial interval. Electrical stimulus (1 s, 1.0 mA, 5 Hz, 1 ms current pulse) was delivered 5 s after the start of each trial. Stimulus and trial parameters were controlled using Labview software with a National Instruments controller board (National Instruments) and a Master-8 (A.M.P.I. Co.) controller.
Image Acquisition
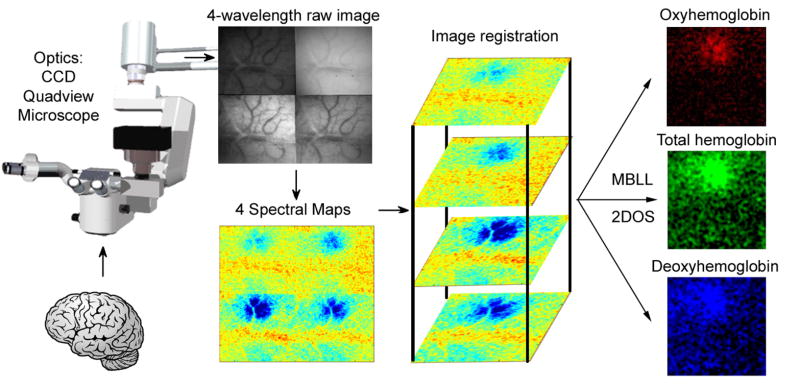
The parietal bone was epi-illuminated with white light from a voltage-regulated halogen light source (Cuda I-150) via fiber optic illumination guides, and images were collected with a cooled charge-coupled device (Photonmax 512-B, Princeton Instruments, Trenton, NJ) mounted over the imaging region on a custom built microscope with a Quadview (Optical Insights) attached. The Quadview split the light into four sub-images which were filtered at 560±5 nm, 570±5 nm, 577±5 nm, and 610±5 nm (Corion, Holliston, MA). Images were acquired at 0.62–1.00X magnification (2 frames/s, 400 ms exposure time, 512 × 512 pixel array (256 × 256 for sub-images)) using Winview software and stored on a desktop computer (Figure 1).
Figure 1. Schematic of hardware and software for 2D Optical Spectroscopy (2DOS).
The Quadview optics splits the image of the brain into 4 separate simultaneously acquired spectral images. Trial averaging of sensory stimulation yields 4 separate spectral maps. After image registration of the spectral maps, the modified Beer-Lambert law (MBLL) is applied to create HbO, HbR, and HbT maps.
Sub-image Registration
2DOS with a Quadview requires multi-temporal and multi-modal image registration (Zitova and Flusser, 2003). Sub-images were registered using a method similar to automated image registration (Woods et al., 1998). We used normalized cross-correlation of intensity as a similarity measure and Newton type maximization to cross-correlate the sub-images across frames. Image contrast is also wavelength dependent, hence the need for multi-modal analysis. To reduce the effect of this contrast inconsistency, gray-level transformations were applied before registration so that sub-images had the same contrast. Non-uniformity of reflected light also introduced different shading patterns. For example, areas near the center of images are brighter and those near the edges are darker. Additionally, different shading patterns also were wavelength-dependent. To reduce the effect of shading patterns, pixels areas near the edges were masked and eliminated from calculation of cross-correlation values
2-D Optical Spectroscopy
Theoretical considerations
The Beer-Lambert law describes the attenuation of light transmitted through a medium in the presence of absorbers, which is proportional to the concentration and absorption coefficient of the absorbers and the path length traveled by the photons (see equation below). In the visible wavelength range used here and for most OIS studies, HbR and HbO are the most important absorbers. Because there is also attenuation of incident light due to scattering (from large molecules and lipids), the path length is effectively increased. Calculation of this path length adjustment previously has been done via Monte Carlo simulation (Mayhew et al., 2000; Mayhew et al., 1999) or by using a phantom solution (Sheth et al., 2004b). With a phantom solution, the two contributors to light attenuation—absorption and scattering—can be held constant, allowing calculation of the effective path length adjustment.
Execution
Trials were averaged and absorbance at each registered, pixel, frame, and wavelength was computed as follows: , where is the intensity during the baseline frames and Iλ is the intensity during the particular frame. The reflectance spectra was then analyzed using the modified differential Beer Lambert law equation: . The wavelength dependent absorption coefficients αλ were calculated empirically using an in vitro phantom model (Sheth et al., 2004b) to determine changes in HbO and HbR concentrations (Δ[HbO] , Δ[HbR] ). Such changes were calculated from the absorbance data after applying a gaussian filter with a half-width of five pixels. The Δ[HbT] was calculated by summing Δ[HbO] and Δ[HbR] values. The Δ[HbO], Δ[HbR], and Δ[HbT] values were used to generate their respective maps (Figure 1.).
Quantitative Analysis
The location and values for the maximum and minimum of each frame for HbO, HbR and HbT were determined, as well as the median value. Similar to previous analysis (Prakash et al., 2000), the area contained within multiple isocontours (95%, 80%, 65%, and 50% between the maximum and median) were calculated. The location of the maximum was also used as the center-point to divide the image into eight even sectors and areas contained within each sector and isocontour were calculated. This analysis was repeated for each post-stimulus frame. Statistical comparisons were performed using analysis of variance (ANOVA) with post-hoc Tukey’s test (p< 0.05).
Results
Representative comparisons of the spatiotemporal evolution of the S1 forepaw maps in rat versus mouse can be found in the online supplemental material section.
Both rat and mouse showed evoked monophasic increases in HbT (functional hyperemia). The major differences between species were: 1) rats showed biphasic evoked changes in HbO and HbR, whereas mice showed monophasic changes. 2) Functional hyperemia in mice was associated with increased HbR, whereas in rats functional hyperemia was associated with decreased HbR (Figures 2–4).
Figure 2. Comparison of rat and mouse hemodynamic changes during neurovascular coupling.

Oxy-, deoxy-, and total-hemoglobin (HbO, HbR, and HbT) S1 forepaw maps in rat (left) and mouse (right) at the times of maximal change reveal that functional hyperemia (increased HbT) in rats is associated with decreased HbR, whereas functional hyperemia in mice is associated with increased HbR. Also note that in rats, the HbT map (green patch) is derived largely from surface veins and arteries, whereas the HbR map (black patch) and HbO (red patch) are similar and derived from the parenchyma and capillary bed. In mice all three maps show similar topologies. See also movie available online in supplemental materials for more details.
Figure 4. Time course of hemodynamic changes of neurovascular coupling in mouse.
Stimulus was given at time 0. Unlike rat: 1) changes appeared monophasic, and 2) there was a prolonged increase in HbR and HbT, and a parallel decrease in HbO.
Temporal Analysis
Rat
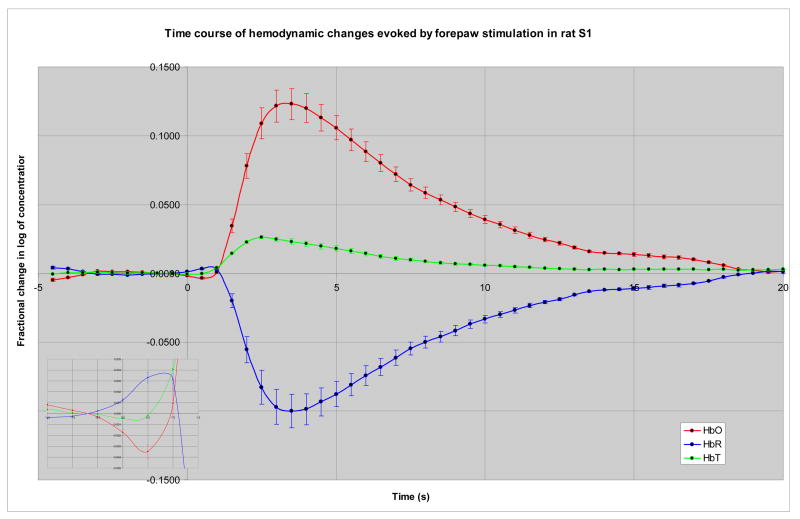
On average, in rat S1 cortex the time course of the maximal fractional change in the log of HbT concentration increased monophasically to 26.2×10−3 ± 1.1×10−3 peaking 2.0–3.0 s after stimulus; HbR changes were biphasic, with a small but significant increase to 4.3×10−3 ± 1.1×10−3 at 0.5–1.0 s, followed by a larger significant decrease to −99.8×10− 3 ± 12.2×10−3 peaking 3.0–3.5 s post-stimulus; HbO changes mirrored HbR changes with a small initial dip to −4.7×10−3 ± 1.3×10−3 at 0.5 s followed by a large increase to 123.0×10−v3 ± 11.2×10−3 at 3.0–3.5 s post stimulus. All changes returned to baseline by 23.5–25.0 s post-stimulus (Figure 3). These results were generally consistent with previous reports of the temporal dynamics of neurovascular coupling (Cannestra et al., 1998; Devor et al., 2003; Grinvald et al., 1986; Hess et al., 2000; Liu et al., 2005; Martindale et al., 2003; Mayhew et al., 1999; Sheth et al., 2004b; Ugurbil et al., 1999; Vanzetta et al., 2005)
Figure 3. Time course of hemodynamic changes during neurovascular coupling in rat.
Stimulus was given at time 0. Within 0.5–1.0s there was an increase in HbR that that is counterbalanced by an equal decrease in HbO (inset on left). This initial phase was then followed by a prolonged second phase of increased HbO and HbT, with decreased HbR, which peaked 2.5–3.5s after stimulus.
Mouse
Unexpectedly, in contrast to rat and other reported species, the time course of sensory evoked hemodynamic changes in mice was different. In mouse S1 cortex the maximal fractional changes of the log of concentrations of HbR, HbT, and HbO were monophasic, with increases in HbR to 9.5×10−3 ± 3.6×10−3, HbT to 2.5×10−3 ± 0.6×10−3, and HbO to −7.1×10−3 ± 3.1×10−3. All changes peaked 3.5–4.5 s post stimulus onset. Changes generally returned to baseline by 19.0 s post-stimulus, but in some animals changes lasted beyond 20.0 s (Figure 4).
Spatial Analysis
Size
Rat
The average spatial extent of the forepaw map at the time of maximal response for HbR, HbO, and HbT are shown below on Table 1. The size and topology of the maps, on average, did not vary significantly over time as seen in the supplemental video.
Table 1.
Rat S1 forepaw representation size (mm2, peak frame(s), 160 trials)
| HbO | HbR | HbT | |
|---|---|---|---|
| Area at 95% height | 0.07±0.00 | 0.08±0.00 | 0.07±0.00 |
| Area at 80% height | 0.46±0.06 | 0.53±0.05 | 0.50±0.06 |
| Area at 65% height | 1.17±0.16 | 1.25±0.12 | 1.19±0.15 |
| Area at 50% height | 2.31±0.30 | 2.31±0.25 | 2.32±0.28 |
Mouse
The average spatial extent of the forepaw map at the time of maximal response for HbR, HbO, and HbT are shown below on Table 2. The size and shape of the maps, on average, did not vary significantly over time as seen on the supplemental videos. Although there was a trend for the HbT map to be larger than the HbR map, and HbR to be larger than HbO map, these trends were not statistically significant (see also Figures 2 and 5).
Table 2.
Mouse S1 forepaw representation size (mm2, peak frame(s), 160 trials)
| HbO | HbR | HbT | |
|---|---|---|---|
| Area at 95% height | 0.06±0.02 | 0.06±0.01 | 0.10±0.02 |
| Area at 80% height | 0.28±0.06 | 0.42±0.10 | 0.45±0.70 |
| Area at 65% height | 0.68±0.12 | 0.89±0.16 | 1.02±0.16 |
| Area at 50% height | 1.32±0.23 | 1.54±0.25 | 1.82±0.27 |
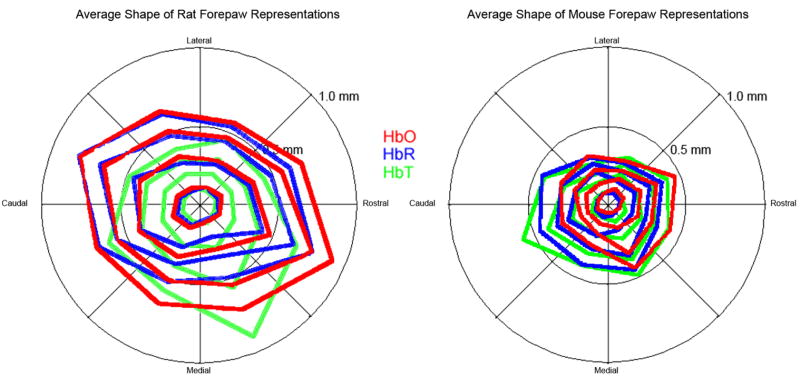
Figure 5. Shape of hemodynamic changes during neurovascular coupling in rat versus mouse.
In rat S1 cortex (left), HbO (red) and HbR (blue) maps of the forepaw had similar sizes and shapes, whereas HbT maps were on average more distorted due to signal from surface veins and arteries. In mouse S1 cortex (right) all three forepaw maps had similar topographic shapes.
Shape
Rat
HbR and HbO forepaw maps each had similar topographic shapes (Figure 5). This shape and orientation was similar to the anatomical forepaw representation in layer IV (Aangel et al., 1976; Dawson and Killackey, 1987; Pearson et al., 1996; Waters et al., 1995), but an order of magnitude larger. The average shape of the HbT map was more distorted due to apparent HbT changes within first and second order branches of surface veins and arteries.
Mouse
HbR, HbO, and HbT maps of the forepaw all had similar topographic shapes (Figure 5). Unlike rat, the shape of the HbT map was not significantly distorted compared to the HbR and HbO maps. The shape and orientation of the forepaw maps were similar to the anatomical forepaw representation in layer IV, but an order of magnitude larger (Oury et al., 2006; Vanderhaeghen et al., 2000; Woolsey and Van der Loos, 1970).
Comparative Analysis
In this study rats, on average, weighed 27 times more than mice. However, previous work has shown that average brain weight plateaus for Sprague-Dawley rats at 1415g (Donaldson, 1918) and for adult c57bl6 mouse brain at 500g (Williams, 2000), for an estimated brain weight ratio of 2.8.
The ratios of maximal fractional change for log of concentration HbO, HbR, and HbT of rat versus mouse were −17.4, −10.5, and 10.6 respectively. The sizes of the maps measured by 2DOS represent the horizontal surface area of sensory evoked metabolic and hyperemic changes. Table 3 depicts the comparison of such rat versus mouse map sizes.
Table 3.
Comparison of rat to mouse map sizes.
| HbO(rat/mouse) | HbR(rat/mouse) | HbT(rat/mouse) | average(rat/mouse) | |
|---|---|---|---|---|
| 95% | 1.17 | 1.33 | 0.70 | 1.07 |
| 80% | 1.64 | 1.26 | 1.11 | 1.34 |
| 65% | 1.72 | 1.40 | 1.17 | 1.43 |
| 50% | 1.75 | 1.50 | 1.27 | 1.51 |
| Average | 1.57 | 1.37 | 1.06 | 1.34 |
Summary
Rat S1 cortex showed expected, biphasic, mirror-image stimulus-evoked changes in HbR and HbO, with an initial dip in HbO at 0.5 s, followed by a larger increase at 2.5–3.5 s.
Mouse S1 cortex showed unexpected, monophasic, mirror-image stimulus-evoked changes in HbR and HbO, with a prolonged increase in HbR and small increase in HbT, and parallel decrease in HbO.
The amplitude of fractional changes in log of HbT, HbO, and HbR concentrations in rat cortex were 10–17 times greater than those in mouse cortex.
HbR and HbO maps in rats had similar topographical features (shape, size, location), but HbT maps were topographically distorted due to signal contributions from surface veins and arteries.
All three maps in mice had similar topographic features.
Map sizes did not generally change over post-stimulus time epochs for rats or mice, although HbT maps had greater variability.
On average, the area of rat compared to mouse maps were 1.34 times larger.
Discussion
2DOS-Validation and Resolution Considerations
Physiological
Here we showed that this new technique is sensitive for half-second temporal and micrometer spatial changes in hemodynamics, such as those in the mouse cortex, and the “initial dip” (Buxton et al., 2004) in rat cortex. Moreover, our findings in the rat—of biphasic changes in HbR and HbO, with a delayed monophasic increase in HbT—were also compatible with previous optical spectroscopy studies (Frostig et al., 1990; Jones et al., 2001; Malonek et al., 1997; Mayhew et al., 2000; Sheth et al., 2004a) suggesting that the 2DOS results are physiologically valid in rats. The unusual findings in mice are discussed below.
Spectral
While the optimal wavelengths and number of wavelengths to use has been examined for the technique of near-infrared spectroscopy, these parameters are not known for 2DOS methods in the visible spectrum (Boas et al., 2001; Obrig and Villringer, 2003; Okui and Okada, 2005; Sato et al., 2004). The wavelengths used in this study were comparable with previously reported 2D optical spectroscopy studies (Berwick et al., 2005; Dunn et al., 2003; Prakash et al., 2006; Sheth et al., 2005). Further studies are necessary to determine the best number of wavelengths, bandwidths, and particular wavelength combinations for optimal visible light 2DOS. Although we have found that 3 spectral maps are not usually sufficient to produce HbO, HbR, and HbT maps without significant noise (Prakash, Zumsteg, Biag, unpublished observations). Additionally, as the signal-to-noise ratio in mice at the maximal response is about ten, our results in mouse may demonstrate the lower end of sensitivity of the technique, especially for delineating the topographic features of the map at the area at half-height, where the signal-to-noise ratio approaches five.
Temporal
The temporal resolution for 2DOS is limited by a combination of the speed and noise of the CCD camera, and signal-to-noise considerations arising from both the 2DOS optics and the brain intrinsic signals. Comparable to most OIS studies, we chose a 2 Hz frame rate, which is adequate to observe to observe both oximetric and perfusion changes, although not the controversial signal from fast optical scatter changes in neuronal tissue (Steinbrink et al., 2005). Further studies are necessary to determine the upper limit of temporal resolution.
Spatial
Although the Quadview image splitter decreases the field of view by one-quarter, the spatial resolution of this 2DOS setup was comparable or better than previous generation OIS setups. Specifically, with the Quadview, the field of view of was 256×256 pixels, and with the adjustable microscope stage, the pixel size range was between 8×8 μm and 43×43 μm. For these experiments, we adjusted the field of view and magnification depending on the preparation of the individual animal, such that pixel dimensions were between 17×17 μm and 27×27 sμm.
Registration
General issues of registration are beyond the scope of this paper (Zitova and Flusser, 2003), however, 2DOS posed unique registration challenges. The Quadview image splitter theoretically should perfectly align sub-images into the four quadrants of the image. However, in our hands, due to small optical imperfections, each sub-image could not be perfectly aligned. Moreover, mechanical drifting or small movements of the preparation necessitated manual alignment before each experiment. Such alignment errors were generally small, ~1–2%, and could be translations, rotations, or distortions. Our sub-image registration algorithm generally improved the quality of the final maps. However, the registration algorithm was not always useful for low contrast sub-images, especially those from the 610 nm filter of mouse brain. As such, registration errors may degrade the spatial resolution of some 2DOS maps. Additionally, a Gaussian filter applied to the spectral data to reduce noise, also reduced the spatial resolution of the final 2DOS maps. Newer generation image-splitters may eliminate some of these limitations.
Comparison of Rat and Mouse Maps
Previously we showed that rat whisker maps are 1.6–2.0 times larger in area than mice (Prakash, 1999; Prakash and Frostig, 1997). Here, we found the rat forepaw maps to be 1.3–1.8 times larger than the mouse maps (when comparing the same measure, area at half-height). These findings suggest that the topographic features of the maps generated by this technique are reliable and valid, even in light of the technical considerations described above. Such differences in map size could reflect relative use-dependent differences in whisker versus forepaw in rats versus mice, alternatively, it could reflect differences in sophistication of cortical processing of the whisker versus forepaw information.
The scalars relating the size of functional maps to physiological parameters such brain mass, mean transit-time, capillary density, and neuronal density are largely unknown. Mice have one of the most neuronally dense brains (Changizi, 2001, 2005; Dawson, 2005; Harrison et al., 2002a; Pasternak and Woolsey, 1975). Neuronal density decreases as a power of 1/3 of brain volume (Changizi, 2005). The average weight of adult c57bl6 mouse brain in 500g (Williams, 2000) and the average adult Sprague-Dawley rat brain weighs 1415g (Donaldson, 1918). Hence mice should have ~1.4 times the neuronal density of rats.
To our knowledge, mouse brain capillary density (Verant et al., 2006; Wang et al., 1992) and mean transit time have not been directly compared to other species. However, mouse muscle capillary density is 1.86 times greater than rat (Schmidt-Nielsen and Pennycuik, 1961), and it is assumed that a similar scalar would apply to brain. However, if mouse brain has a disproportionately lower brain capillary density or slower mean transit time, then these could potentially cause a mismatch between oxygen consumption and functional hyperemia. On the other hand, mouse cortex is relatively resistant to ischemia (see below), and possible mechanisms to this resistance could be a disproportionately high brain capillary density and/or ability to alter mean transit time. Further study is required to resolve these issues.
Oxygen Consumption with Low Functional Hyperemia
We reported here that mouse brain has unique hemodynamic changes during neurovascular coupling. Specifically we found prolonged increases in HbR occurring after a brief stimulus, with only small increases in HbT. In contrast, rat brain (and other species) had a brief period of increase in HbR with a matching decrease in HbO. This was followed by increased HbT with concurrent decreased HbR and increased HbO. HbT increases reflect functional hyperemia, which occurs through hyperperfusion and/or hypervolemia. Although functional hyperperfusion has been reported in mice in response to sensory stimulation (Kazama et al., 2003; Niwa et al., 2000), it is not known if this functional hyperperfusion is less than, matches, or exceeds the functional increase in oxygen consumption (Shonat et al., 1997; Thomas et al., 2003). Previous reports suggest that evoked responses in mouse cortex may induce increased oxygen consumption with only a small increase in hyperemia. For example, hypersensitivity of murine blood vessels to extracellular potassium blunts functional hyperemia (Ayata et al., 2004). Additionally, this effect does not appear to be stimulus-, or anesthesia-dependent as very mild whisker stimulation under pentobarbital anesthesia also produces monophasic hemoglobin changes (Prakash, 1999; Prakash et al., 2005; Prakash et al., 2000), or hindpaw stimulation under alpha-chlorolose (Ahrens and Dubowitz, 2001). Similarly, mice under isoflurane (Mueggler et al., 2003) or awakening from anesthesia have monophasic positive blood oxygen level-dependent (BOLD) signals evoked by visual stimulus (Huang et al., 1996). A positive BOLD signal can arise from a decrease in HbR or an increase in blood volume with no change in oxygenation. One explanation for this apparent discrepancy may be that fMRI studies are more sensitive to detecting blood volume increases in large arteries and veins, which potentially from volume averaging could cancel out HbR changes in the intervening capillary beds. However, Huang et al. also reported significant regions that evoked negative monophasic BOLD signals (Huang et al., 1996), suggesting that HbR increases are indeed a major component of the evoked mouse hemodynamic response.
As mouse cortex is relatively resistant to ischemic infarction (Carmichael, 2003), such a dependence on oxygen consumption with only mild functional hyperemia may come with other counter-adaptations, such as a heavily collateralized vasculature, an unpredicted disproportionately higher capillary density, a better ability to adjust mean transit-time (see previous section), or neuronglial units better adapted for anaerobic metabolism. Given that acutely ischemic cortex in human brain may behave similarly to normal mouse cortex, i.e. with metabolic demands exceeding functional hyperemia, understanding such counter-adaptations may provide a potential insight into novel therapeutic targets or mechanisms.
Implications for Intraoperative Functional and Tumor Mapping
Intraoperative optical imaging and optical spectroscopy have been demonstrated to be a potentially useful neurosurgical tools for both brain mapping (Cannestra et al., 2000; Cannestra et al., 2001; Haglund et al., 1992; Lin et al., 2001; Nariai et al., 2005; Schwartz, 2005; Toga et al., 1995) and tumor margin demarcation (Cannestra et al., 2004; Toms et al., 2005). However, intraoperative OIS so far has only been demonstrated to be effective for arteriovenous malformation demarcation (Cannestra et al., 2004); while optical spectroscopy techniques may be able to distinguish cancerous tumors from healthy brain, they only can map tumor margins in one-dimension, point-by-point (Toms et al., 2005). Here we show the ability of 2DOS in rodents to provide high-resolution functional brain maps, even under conditions of low signal-to-noise. Both high-resolution 2D functional brain mapping and tumor margin demarcation may be possible with intraoperative 2DOS.
Supplementary Material
Supplemental Video. Peri-stimulus evolution of HbO (top row), HbR (middle row), and HbT (bottom row) forepaw maps in primary somatosensory cortex from a representative mouse (left 3 columns) and rat (right 3 columns). The first 5 seconds are baseline, then a 1 s, 5 Hz contralateral forepaw stimulus is delivered. Ignoring spurious noise, the shape (2nd and 5th columns) and size (3rd and 6th) columns of HbO and HbR maps did not significantly change up to ten seconds post-stimulus. The rat HbT was topographically different than the HbO and HbR maps as it emphasized venous and arterial changes.
Acknowledgments
This work was supported by NIH: MH52083 (A.T.) and the Adelson Program in Neural Repair and Rehabilitation (N.P.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aangel A, Berridge DA, Fox RE. The organization of the primary somatosensory and motor areas for the forepaw in the anaesthetized rat [proceedings] J Physiol (Lond) 1976;263:137P–138P. [PubMed] [Google Scholar]
- Ahrens ET, Dubowitz DJ. Peripheral somatosensory fMRI in mouse at 11.7 T. NMR Biomed. 2001;14:318–324. doi: 10.1002/nbm.709. [DOI] [PubMed] [Google Scholar]
- Ayata C, Shin HK, Salomone S, Ozdemir-Gursoy Y, Boas DA, Dunn AK, Moskowitz MA. Pronounced Hypoperfusion During Spreading Depression in Mouse Cortex. Journal of Cerebral Blood Flow & Metabolism. 2004;24:1172–1182. doi: 10.1097/01.WCB.0000137057.92786.F3. [DOI] [PubMed] [Google Scholar]
- Berwick J, Johnston D, Jones M, Martindale J, Redgrave P, McLoughlin N, Schiessl I, Mayhew JEW. Neurovascular coupling investigated with two-dimensional optical imaging spectroscopy in rat whisker barrel cortex. European Journal of Neuroscience. 2005;22:1655–1666. doi: 10.1111/j.1460-9568.2005.04347.x. [DOI] [PubMed] [Google Scholar]
- Blood AJ, Narayan SM, Toga AW. Stimulus parameters influence characteristics of optical intrinsic signal responses in somatosensory cortex. J Cereb Blood Flow Metab. 1995;15:1109–1121. doi: 10.1038/jcbfm.1995.138. [DOI] [PubMed] [Google Scholar]
- Boas DA, Gaudette T, Strangman G, Cheng X, Marota JJA, Mandeville JB. The Accuracy of Near Infrared Spectroscopy and Imaging during Focal Changes in Cerebral Hemodynamics. Neuroimage. 2001;13:76–90. doi: 10.1006/nimg.2000.0674. [DOI] [PubMed] [Google Scholar]
- Buxton RB, Uludag K, Dubowitz DJ, Liu TT. Modeling the hemodynamic response to brain activation. Neuroimage. 2004;23:S220–S233. doi: 10.1016/j.neuroimage.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Cannestra AF, Blood AJ, Black KL, Toga AW. The evolution of optical signals in human and rodent cortex. Neuroimage. 1996;3:202–208. doi: 10.1006/nimg.1996.0022. [DOI] [PubMed] [Google Scholar]
- Cannestra AF, Bookheimer SY, Pouratian N, O’Farrell A, Sicotte N, Martin NA, Becker D, Rubino G, Toga AW. Temporal and topographical characterization of language cortices using intraoperative optical intrinsic signals. Neuroimage. 2000;12:41–54. doi: 10.1006/nimg.2000.0597. [DOI] [PubMed] [Google Scholar]
- Cannestra AF, Pouratian N, Bookheimer SY, Martin NA, Beckerand DP, Toga AW. Temporal spatial differences observed by functional MRI and human intraoperative optical imaging. Cereb Cortex. 2001;11:773–782. doi: 10.1093/cercor/11.8.773. [DOI] [PubMed] [Google Scholar]
- Cannestra AF, Pouratian N, Forage J, Bookheimer SY, Martin NA, Toga AW. Functional magnetic resonance imaging and optical imaging for dominant-hemisphere perisylvian arteriovenous malformations. Neurosurgery. 2004;55:804–812. doi: 10.1227/01.neu.0000137654.27826.71. discussion 812–804. [DOI] [PubMed] [Google Scholar]
- Cannestra AF, Pouratian N, Shomer MH, Toga AW. Refractory periods observed by intrinsic signal and fluorescent dye imaging. J Neurophysiol. 1998;80:1522–1532. doi: 10.1152/jn.1998.80.3.1522. [DOI] [PubMed] [Google Scholar]
- Carmichael ST. Rodent models of focal stroke: size, mechanism, and purpose. NeuroRx. 2003;2005:396–409. doi: 10.1602/neurorx.2.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changizi MA. Principles underlying mammalian neocortical scaling. Biological Cybernetics. 2001;84:207–215. doi: 10.1007/s004220000205. [DOI] [PubMed] [Google Scholar]
- Changizi MA. Scaling the brain and its connections. In: Kaas JH, editor. Evolution of Nervous Systems. Elsevier; New York: 2005. [Google Scholar]
- Dawson DR, Killackey HP. The organization and mutability of the forepaw and hindpaw representations in the somatosensory cortex of the neonatal rat. Journal of Comparative Neurology. 1987;256:246–256. doi: 10.1002/cne.902560205. [DOI] [PubMed] [Google Scholar]
- Dawson TH. Modeling of vascular networks. J Exp Biol. 2005;208:1687–1694. doi: 10.1242/jeb.01622. [DOI] [PubMed] [Google Scholar]
- Devor A, Dunn AK, Andermann ML, Ulbert I, Boas DA, Dale AM. Coupling of total hemoglobin concentration, oxygenation, and neural activity in rat somatosensory cortex. Neuron. 2003;39:353–359. doi: 10.1016/s0896-6273(03)00403-3. [DOI] [PubMed] [Google Scholar]
- Donaldson HH. A Comparison of Growth Changes in the Nervous System of the Rat with Corresponding Changes in the Nervous System of Man. Proceedings of the National Academy of Sciences. 1918;4:280–283. doi: 10.1073/pnas.4.9.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AK, Devor A, Bolay H, Andermann ML, Moskowitz MA, Dale AM, Boas DA. Simultaneous imaging of total cerebral hemoglobin concentration, oxygenation, and blood flow during functional activation. Opt Lett. 2003;28:28–30. doi: 10.1364/ol.28.000028. [DOI] [PubMed] [Google Scholar]
- Elwell CE, Owen-Reece H, Cope M, Wyatt JS, Edwards AD, Delpy DT, Reynolds EO. Measurement of adult cerebral haemodynamics using near infrared spectroscopy. Acta Neurochir Suppl (Wien) 1993;59:74–80. doi: 10.1007/978-3-7091-9302-0_13. [DOI] [PubMed] [Google Scholar]
- Frostig RD, Lieke EE, Ts’o DY, Grinvald A. Cortical functional architecture and local coupling between neuronal activity and the microcirculation revealed by in vivo high-resolution optical imaging of intrinsic signals. Proc Natl Acad Sci U S A. 1990;87:6082–6086. doi: 10.1073/pnas.87.16.6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster J, Guiou M, Ardestani A, Cannestra A, Sheth S, Zhou YD, Toga A, Bodner M. Near-infrared spectroscopy (NIRS) in cognitive neuroscience of the primate brain. Neuroimage. 2005;26:215–220. doi: 10.1016/j.neuroimage.2005.01.055. [DOI] [PubMed] [Google Scholar]
- Grinvald A, Lieke E, Frostig RD, Gilbert CD, Wiesel TN. Functional architecture of cortex revealed by optical imaging of intrinsic signals. Nature. 1986;324:361–364. doi: 10.1038/324361a0. [DOI] [PubMed] [Google Scholar]
- Haglund MM, Ojemann GA, Hochman DW. Optical Imaging of Epileptiform and Functional Activity in Human Cerebral Cortex. Nature. 1992:358. doi: 10.1038/358668a0. [DOI] [PubMed] [Google Scholar]
- Harrison KH, Hof PR, Wang SSH. Scaling laws in the mammalian neocortex: Does form provide clues to function? Journal of Neurocytology. 2002a;31:289–298. doi: 10.1023/a:1024178127195. [DOI] [PubMed] [Google Scholar]
- Harrison RV, Harel N, Panesar J, Mount RJ. Blood Capillary Distribution Correlates with Hemodynamic-based Functional Imaging in Cerebral Cortex. Cerebral Cortex. 2002b;12:225–233. doi: 10.1093/cercor/12.3.225. [DOI] [PubMed] [Google Scholar]
- Hess A, Stiller D, Kaulisch T, Heil P, Scheich H. New insights into the hemodynamic blood oxygenation level-dependent response through combination of functional magnetic resonance imaging and optical recording in gerbil barrel cortex. J Neurosci. 2000;20:3328–3338. doi: 10.1523/JNEUROSCI.20-09-03328.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Palyka I, Li H, Eisenstein EM, Volkow ND, Springer CS., Jr Magnetic resonance imaging (MRI) detection of the murine brain response to light: temporal differentiation and negative functional MRI changes. Proc Natl Acad Sci U S A. 1996;93:6037–6042. doi: 10.1073/pnas.93.12.6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M, Berwick J, Johnston D, Mayhew J. Concurrent optical imaging spectroscopy and laser-Doppler flowmetry: the relationship between blood flow, oxygenation, and volume in rodent barrel cortex. Neuroimage. 2001;13:1002–1015. doi: 10.1006/nimg.2001.0808. [DOI] [PubMed] [Google Scholar]
- Kazama K, Wang G, Frys K, Anrather J, Iadecola C. Angiotensin II attenuates functional hyperemia in the mouse somatosensory cortex. Am J Physiol Heart Circ Physiol. 2003 doi: 10.1152/ajpheart.00464.2003. [DOI] [PubMed] [Google Scholar]
- Lin WC, Toms SA, Johnson M, Jansen ED, Mahadevan-Jansen A. In Vivo Brain Tumor Demarcation Using Optical Spectroscopy. Photochemistry and Photobiology. 2001;73:396–402. doi: 10.1562/0031-8655(2001)073<0396:ivbtdu>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wang Z, Luo Q. Temporal clustering analysis of cerebral blood flow activation maps measured by laser speckle contrast imaging. Journal of Biomedical Optics. 2005;10:024019. doi: 10.1117/1.1891105. [DOI] [PubMed] [Google Scholar]
- Malonek D, Dirnagl U, Lindauer U, Yamada K, Kanno I, Grinvald A. Vascular imprints of neuronal activity: relationships between the dynamics of cortical blood flow, oxygenation, and volume changes following sensory stimulation. Proc Natl Acad Sci U S A. 1997;94:14826–14831. doi: 10.1073/pnas.94.26.14826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martindale J, Mayhew J, Berwick J, Jones M, Martin C, Johnston D, Redgrave P, Zheng Y. The hemodynamic impulse response to a single neural event. J Cereb Blood Flow Metab. 2003;23:546–555. doi: 10.1097/01.WCB.0000058871.46954.2B. [DOI] [PubMed] [Google Scholar]
- Mayhew J, Johnston D, Berwick J, Jones M, Coffey P, Zheng Y. Spectroscopic analysis of neural activity in brain: increased oxygen consumption following activation of barrel cortex. Neuroimage. 2000;12:664–675. doi: 10.1006/nimg.2000.0656. [DOI] [PubMed] [Google Scholar]
- Mayhew J, Zheng Y, Hou Y, Vuksanovic B, Berwick J, Askew S, Coffey P. Spectroscopic Analysis of Changes in Remitted Illumination: The Response to Increased Neural Activity in Brain. Neuroimage. 1999;10:304–326. doi: 10.1006/nimg.1999.0460. [DOI] [PubMed] [Google Scholar]
- Mueggler T, Baumann D, Rausch M, Staufenbiel M, Rudin M. Age-Dependent Impairment of Somatosensory Response in the Amyloid Precursor Protein 23 Transgenic Mouse Model of Alzheimer’s Disease. J Neurosci. 2003;23:8231–8236. doi: 10.1523/JNEUROSCI.23-23-08231.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nariai T, Sato K, Hirakawa K, Ohta Y, Tanaka Y, Ishiwata K, Ishii K, Kamino K, Ohno K. Imaging of somatotopic representation of sensory cortex with intrinsic optical signals as guides for brain tumor surgery. J Neurosurg. 2005;103:414–423. doi: 10.3171/jns.2005.103.3.0414. [DOI] [PubMed] [Google Scholar]
- Niwa K, Younkin L, Ebeling C, Turner SK, Westaway D, Younkin S, Ashe KH, Carlson GA, Iadecola C. Abeta 1-40-related reduction in functional hyperemia in mouse neocortex during somatosensory activation. Proc Natl Acad Sci U S A. 2000;97:9735–9740. doi: 10.1073/pnas.97.17.9735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrig H, Villringer A. Beyond the Visible--Imaging the Human Brain With Light. J Cereb Blood Flow Metab. 2003;23:1–18. doi: 10.1097/01.WCB.0000043472.45775.29. [DOI] [PubMed] [Google Scholar]
- Okui N, Okada E. Wavelength dependence of crosstalk in dual-wavelength measurement of oxy- and deoxy-hemoglobin. Journal of Biomedical Optics. 2005;10:011015–011018. doi: 10.1117/1.1846076. [DOI] [PubMed] [Google Scholar]
- Oury F, Murakami Y, Renaud JS, Pasqualetti M, Charnay P, Ren SY, Rijli FM. Hoxa2- and Rhombomere-Dependent Development of the Mouse Facial Somatosensory Map. Science. 2006;313:1408–1413. doi: 10.1126/science.1130042. [DOI] [PubMed] [Google Scholar]
- Pasternak JR, Woolsey TA. The number, size and spatial distribution of neurons in lamina IV of the mouse SmI neocortex. Journal of Comparative Neurology. 1975;160:291–306. doi: 10.1002/cne.901600303. [DOI] [PubMed] [Google Scholar]
- Pearson PP, Oladehin A, Li CX, Johnson EF, Weeden AM, Daniel CH, Waters RS. Relationship between representation of hindpaw and hindpaw barrel subfield (HBS) in layer IV of rat somatosensory cortex. Neuroreport. 1996;7:2317–2323. doi: 10.1097/00001756-199610020-00009. [DOI] [PubMed] [Google Scholar]
- Prakash N. Neurobiology and Behavior. University of California Irvine; Irvine: 1999. Rapid nerve growth factor-induced cortical plasticity mediated through the basal forebrain cholinergic system in adult rat in vivo; p. 166. [Google Scholar]
- Prakash N, Frostig RD. Characterization of the functional organization in mouse barrel cortex using intrinsic signal optical imaging through intact skull. Society for Neuroscience Abstracts. 1997;23:2343. [Google Scholar]
- Prakash N, Guiou MW, Sheth SA, Cury K, Theriot J, Toga AW. Hemodynamics in mouse neocortex evoked during somatosensory stimulation and during spreading depression. Society for Neuroscience; Washington DC: 2005. [Google Scholar]
- Prakash N, Theriot J, Mitsuyama S, Sheth SA, Sachdeva P, Ramachandra C, Toga AW. Spatiotemporal changes in oxy- and deoxy-hemoglobin evoked by forepaw and hindpaw stimulation in mouse somatosensory cortex visualized using full-field optical spectroscopy. Society for Neuroscience; Atlanta, Georgia: 2006. [Google Scholar]
- Prakash N, Vanderhaeghen P, Cohen-Cory S, Frisen J, Flanagan JG, Frostig RD. Malformation of the functional organization of somatosensory cortex in adult ephrin-A5 knock-out mice revealed by in vivo functional imaging. J Neurosci. 2000;20:5841–5847. doi: 10.1523/JNEUROSCI.20-15-05841.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratzlaff EH, Grinvald A. A tandem-lens epifluorescence macroscope: hundred-fold brightness advantage for wide-field imaging. J Neurosci Methods. 1991;36:127–137. doi: 10.1016/0165-0270(91)90038-2. [DOI] [PubMed] [Google Scholar]
- Sato H, Kiguchi M, Kawaguchi F, Maki A. Practicality of wavelength selection to improve signal-to-noise ratio in near-infrared spectroscopy. Neuroimage. 2004;21:1554–1562. doi: 10.1016/j.neuroimage.2003.12.017. [DOI] [PubMed] [Google Scholar]
- Schmidt-Nielsen K, Pennycuik P. Capillary density in mammals in relation to body size and oxygen consumption. Am J Physiol. 1961;200:746–750. doi: 10.1152/ajplegacy.1961.200.4.746. [DOI] [PubMed] [Google Scholar]
- Schwartz TH. The Application of Optical Recording of Intrinsic Signals to Simultaneously Acquire Functional, Pathological and Localizing Information and Its Potential Role in Neurosurgery. Stereotact Funct Neurosurg. 2005;83:36–44. doi: 10.1159/000085025. [DOI] [PubMed] [Google Scholar]
- Sheth SA, Nemoto M, Guiou M, Walker M, Pouratian N, Hageman N, Toga AW. Columnar Specificity of Microvascular Oxygenation and Volume Responses: Implications for Functional Brain Mapping. J Neurosci. 2004a;24:634–641. doi: 10.1523/JNEUROSCI.4526-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth SA, Nemoto M, Guiou M, Walker M, Pouratian N, Toga AW. Linear and Nonlinear Relationships between Neuronal Activity, Oxygen Metabolism, and Hemodynamic Responses. Neuron. 2004b;42:347–355. doi: 10.1016/s0896-6273(04)00221-1. [DOI] [PubMed] [Google Scholar]
- Sheth SA, Nemoto M, Guiou MW, Walker MA, Toga AW. Spatiotemporal evolution of functional hemodynamic changes and their relationship to neuronal activity. Journal of Cerebral Blood Flow & Metabolism. 2005;25:830–841. doi: 10.1038/sj.jcbfm.9600091. [DOI] [PubMed] [Google Scholar]
- Shonat RD, Wachman ES, Niu W, Koretsky AP, Farkas DL. Near-simultaneous hemoglobin saturation and oxygen tension maps in mouse brain using an AOTF microscope. Biophys J. 1997;73:1223–1231. doi: 10.1016/S0006-3495(97)78155-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbrink J, Kempf FCD, Villringer A, Obrig H. The fast optical signal--Robust or elusive when non-invasively measured in the human adult? Neuroimage. 2005;26:996–1008. doi: 10.1016/j.neuroimage.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Thomas MP, Jackson SK, James PE. Variation in blood oxygenation and cerebral pO2 in a mouse model measured by EPR spectrometry. Adv Exp Med Biol. 2003;510:205–211. doi: 10.1007/978-1-4615-0205-0_34. [DOI] [PubMed] [Google Scholar]
- Toga AW, Cannestra AF, Black KL. The temporal/spatial evolution of optical signals in human cortex. Cereb Cortex. 1995;5:561–565. doi: 10.1093/cercor/5.6.561. [DOI] [PubMed] [Google Scholar]
- Toga AW, Mazziotta JC. Brain mapping : the methods. 2. Academic Press; Boston: 2002. [Google Scholar]
- Toms S, Lin WC, Weil R, Johnson M, Jansen E, Mahadevan-Jansen A. Intraoperative Optical Spectroscopy Identifies Infiltrating Glioma Margins with High Sensitivity. Neurosurgery. 2005;57:382–391. doi: 10.1227/01.neu.000176855.39826.2d. [DOI] [PubMed] [Google Scholar]
- Ugurbil K, Hu X, Chen W, Zhu XH, Kim SG, Georgopoulos A. Functional mapping in the human brain using high magnetic fields. Philos Trans R Soc Lond B Biol Sci. 1999;354:1195–1213. doi: 10.1098/rstb.1999.0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhaeghen P, Lu Q, Prakash N, Frisen J, Walsh CA, Frostig RD, Flanagan JG. A mapping label required for normal scale of body representation in the cortex. Nat Neurosci. 2000;3:358–365. doi: 10.1038/73929. [DOI] [PubMed] [Google Scholar]
- Vanzetta I, Hildesheim R, Grinvald A. Compartment-Resolved Imaging of Activity-Dependent Dynamics of Cortical Blood Volume and Oximetry. J Neurosci. 2005;25:2233–2244. doi: 10.1523/JNEUROSCI.3032-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verant P, Serduc R, Van Der Sanden B, Remy C, Vial J-C. A direct method for measuring mouse capillary cortical blood volume using multiphoton laser scanning microscopy. J Cereb Blood Flow Metab. 2006 doi: 10.1038/sj.jcbfm.9600415. [DOI] [PubMed] [Google Scholar]
- Wang DB, Blocher NC, Spence ME, Rovainen CM, Woolsey TA. Development and remodeling of cerebral blood vessels and their flow in postnatal mice observed with in vivo videomicroscopy. J Cereb Blood Flow Metab. 1992;12:935–946. doi: 10.1038/jcbfm.1992.130. [DOI] [PubMed] [Google Scholar]
- Waters RS, Li CX, McCandlish CA. Relationship between the organization of the forepaw barrel subfield and the representation of the forepaw in layer IV of rat somatosensory cortex. Exp Brain Research. 1995;103:183–197. doi: 10.1007/BF00231705. [DOI] [PubMed] [Google Scholar]
- Williams RW. Mapping Genes that Modulate Mouse Brain Development: A Quantitative Genetic Approach. Springer Verlag; New York: 2000. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. Automated image registration: I. General methods and intrasubject, intramodality validation. J Comput Assist Tomogr. 1998;22:139–152. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- Woolsey TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res. 1970;17:205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]
- Zitova B, Flusser J. Image registration methods: a survey. Image and Vision Computing. 2003;21:977–1000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Video. Peri-stimulus evolution of HbO (top row), HbR (middle row), and HbT (bottom row) forepaw maps in primary somatosensory cortex from a representative mouse (left 3 columns) and rat (right 3 columns). The first 5 seconds are baseline, then a 1 s, 5 Hz contralateral forepaw stimulus is delivered. Ignoring spurious noise, the shape (2nd and 5th columns) and size (3rd and 6th) columns of HbO and HbR maps did not significantly change up to ten seconds post-stimulus. The rat HbT was topographically different than the HbO and HbR maps as it emphasized venous and arterial changes.