Abstract
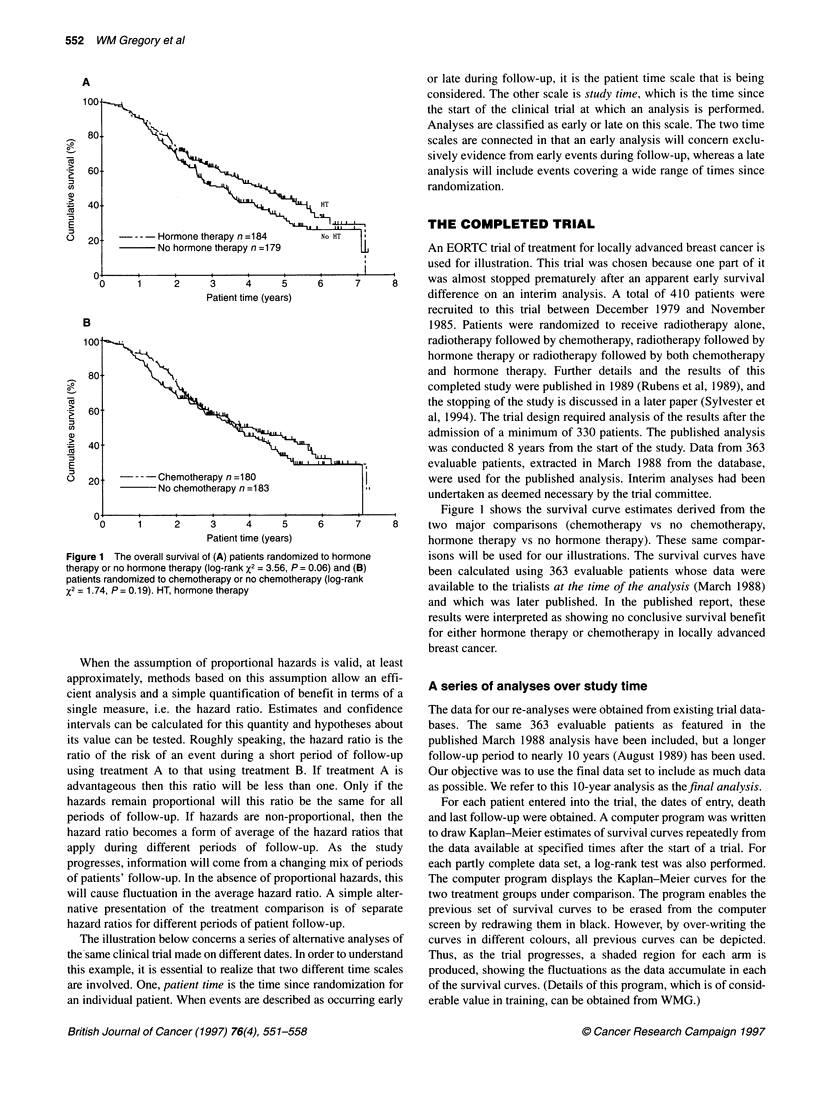
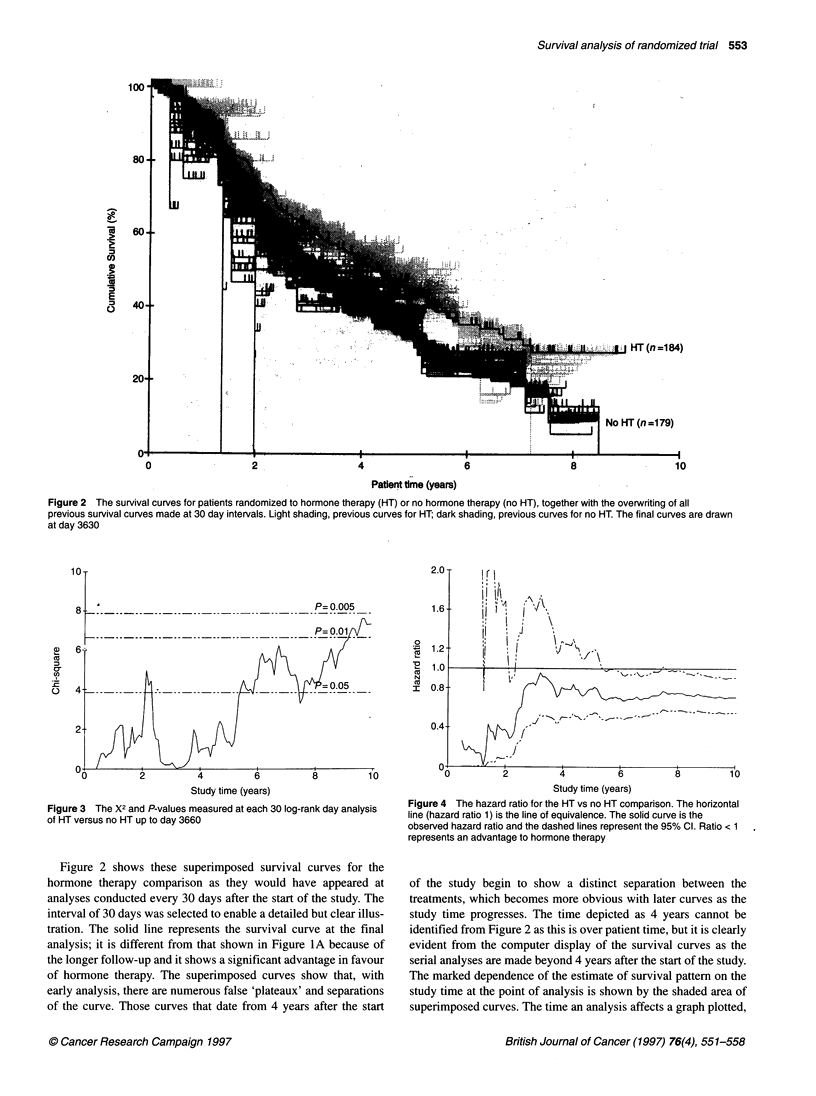
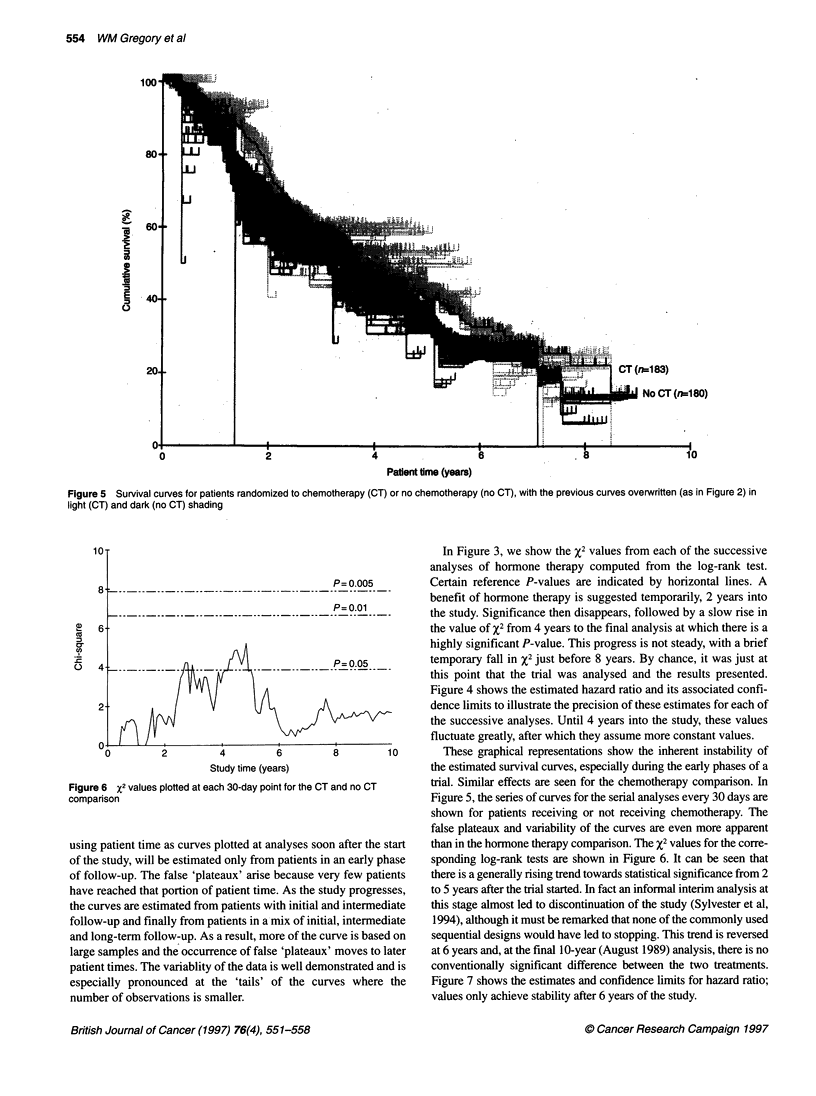
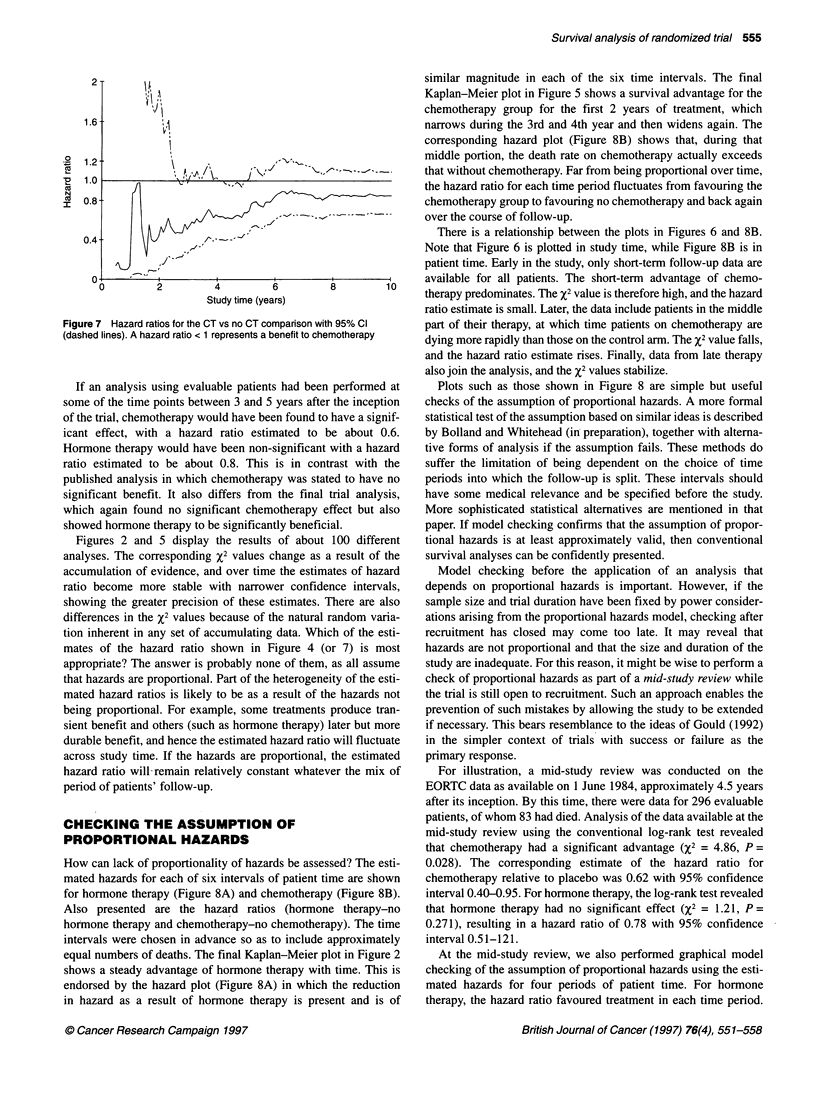
Data from a completed randomized trial in breast cancer are used to demonstrate and quantify the variation in estimated survival curves and log-rank statistics at different times throughout a trial. False 'plateaux' are common, as are wide fluctuations in chi2 values obtained from the log-rank test when there are few events. We show how analyses conducted at different times can demonstrate different effects. Long follow-up is often necessary to allow correct interpretation of results. We discuss the assumption of proportional hazards and the consequences of making that assumption inappropriately. We show how checking whether hazards are proportional can help in avoiding erroneous conclusions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fayers P. M., Cook P. A., Machin D., Donaldson N., Whitehead J., Ritchie A., Oliver R. T., Yuen P. On the development of the Medical Research Council trial of alpha-interferon in metastatic renal carcinoma. Urological Working Party Renal Carcinoma Subgroup. Stat Med. 1994 Nov 15;13(21):2249–2260. doi: 10.1002/sim.4780132106. [DOI] [PubMed] [Google Scholar]
- Freedman L. S. Tables of the number of patients required in clinical trials using the logrank test. Stat Med. 1982 Apr-Jun;1(2):121–129. doi: 10.1002/sim.4780010204. [DOI] [PubMed] [Google Scholar]
- Gould A. L. Interim analyses for monitoring clinical trials that do not materially affect the type I error rate. Stat Med. 1992 Jan 15;11(1):55–66. doi: 10.1002/sim.4780110107. [DOI] [PubMed] [Google Scholar]
- Pepe M. S., Fleming T. R. Weighted Kaplan-Meier statistics: a class of distance tests for censored survival data. Biometrics. 1989 Jun;45(2):497–507. [PubMed] [Google Scholar]
- Peto R., Pike M. C., Armitage P., Breslow N. E., Cox D. R., Howard S. V., Mantel N., McPherson K., Peto J., Smith P. G. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977 Jan;35(1):1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubens R. D., Bartelink H., Engelsman E., Hayward J. L., Rotmensz N., Sylvester R., van der Schueren E., Papadiamantis J., Vassilaros S. D., Wildiers J. Locally advanced breast cancer: the contribution of cytotoxic and endocrine treatment to radiotherapy. An EORTC Breast Cancer Co-operative Group Trial (10792). Eur J Cancer Clin Oncol. 1989 Apr;25(4):667–678. doi: 10.1016/0277-5379(89)90203-4. [DOI] [PubMed] [Google Scholar]
- Souhami R. L. The clinical importance of early stopping of randomized trials in cancer treatments. Stat Med. 1994 Jul 15;13(13-14):1293–1295. doi: 10.1002/sim.4780131303. [DOI] [PubMed] [Google Scholar]
- Sylvester R., Bartelink H., Rubens R. A reversal of fortune: practical problems in the monitoring and interpretation of an EORTC breast cancer trial. Stat Med. 1994 Jul 15;13(13-14):1329–1335. doi: 10.1002/sim.4780131306. [DOI] [PubMed] [Google Scholar]
- Whitehead J. Interim analyses and stopping rules in cancer clinical trials. Br J Cancer. 1993 Dec;68(6):1179–1185. doi: 10.1038/bjc.1993.500. [DOI] [PMC free article] [PubMed] [Google Scholar]


