Abstract
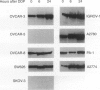
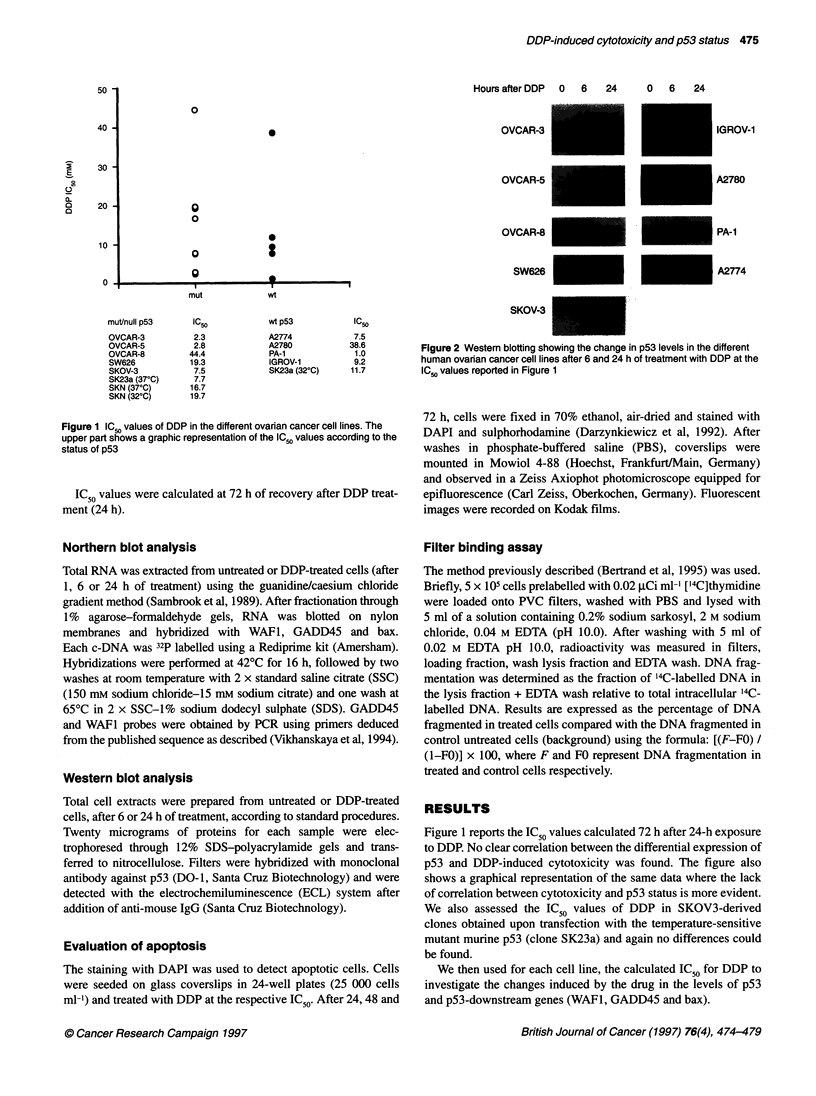
Nine human ovarian cancer cell lines that express wild-type (wt) or mutated (mut) p53 were used to evaluate the cytotoxicity induced by cisplatin (DDP). The concentrations inhibiting the growth by 50% (IC50) were calculated for each cell line, and no differences were found between cells expressing wt p53 and mut p53. Using, for each cell line, the DDP IC50, we found that these concentrations were able to induce an increase in p53 levels in all four wt-p53-expressing cell lines and in one out of five mut-p53-expressing cell lines. WAF1 and GADD45 mRNAs were also increased by DDP treatment, independently of the presence of a wt p53. Bax levels were only marginally affected by DDP, and this was observed in both wt-p53- and mut-p53-expressing cells. DDP-induced apoptosis was evident 72 h after treatment, and the percentage of cells undergoing apoptosis was slightly higher for wt-p53-expressing cells. However, at doses near the IC50, the percentage of apoptotic cells was less than 20% in all the cell lines investigated. We conclude that the presence of wt p53 is not a determinant for the cytotoxicity induced by DDP in human ovarian cancer cell lines.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akashi M., Hachiya M., Osawa Y., Spirin K., Suzuki G., Koeffler H. P. Irradiation induces WAF1 expression through a p53-independent pathway in KG-1 cells. J Biol Chem. 1995 Aug 11;270(32):19181–19187. doi: 10.1074/jbc.270.32.19181. [DOI] [PubMed] [Google Scholar]
- Brown R., Clugston C., Burns P., Edlin A., Vasey P., Vojtesek B., Kaye S. B. Increased accumulation of p53 protein in cisplatin-resistant ovarian cell lines. Int J Cancer. 1993 Oct 21;55(4):678–684. doi: 10.1002/ijc.2910550428. [DOI] [PubMed] [Google Scholar]
- Cannistra S. A. Cancer of the ovary. N Engl J Med. 1993 Nov 18;329(21):1550–1559. doi: 10.1056/NEJM199311183292108. [DOI] [PubMed] [Google Scholar]
- Damia G., Imperatori L., Stefanini M., D'Incalci M. Sensitivity of CHO mutant cell lines with specific defects in nucleotide excision repair to different anti-cancer agents. Int J Cancer. 1996 Jun 11;66(6):779–783. doi: 10.1002/(SICI)1097-0215(19960611)66:6<779::AID-IJC12>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Bruno S., Del Bino G., Gorczyca W., Hotz M. A., Lassota P., Traganos F. Features of apoptotic cells measured by flow cytometry. Cytometry. 1992;13(8):795–808. doi: 10.1002/cyto.990130802. [DOI] [PubMed] [Google Scholar]
- Debernardis D., Siré E. G., De Feudis P., Vikhanskaya F., Valenti M., Russo P., Parodi S., D'Incalci M., Broggini M. p53 status does not affect sensitivity of human ovarian cancer cell lines to paclitaxel. Cancer Res. 1997 Mar 1;57(5):870–874. [PubMed] [Google Scholar]
- Eastman A. Characterization of the adducts produced in DNA by cis-diamminedichloroplatinum(II) and cis-dichloro(ethylenediamine)platinum(II). Biochemistry. 1983 Aug 2;22(16):3927–3933. doi: 10.1021/bi00285a031. [DOI] [PubMed] [Google Scholar]
- Fan S., Smith M. L., Rivet D. J., 2nd, Duba D., Zhan Q., Kohn K. W., Fornace A. J., Jr, O'Connor P. M. Disruption of p53 function sensitizes breast cancer MCF-7 cells to cisplatin and pentoxifylline. Cancer Res. 1995 Apr 15;55(8):1649–1654. [PubMed] [Google Scholar]
- Gjerset R. A., Turla S. T., Sobol R. E., Scalise J. J., Mercola D., Collins H., Hopkins P. J. Use of wild-type p53 to achieve complete treatment sensitization of tumor cells expressing endogenous mutant p53. Mol Carcinog. 1995 Dec;14(4):275–285. doi: 10.1002/mc.2940140408. [DOI] [PubMed] [Google Scholar]
- Graniela Sirè E. A., Vikhanskaya F., Broggini M. Sensitivity and cellular response to different anticancer agents of a human ovarian cancer cell line expressing wild-type, mutated or no p53. Ann Oncol. 1995 Jul;6(6):589–593. doi: 10.1093/oxfordjournals.annonc.a059249. [DOI] [PubMed] [Google Scholar]
- Greenblatt M. S., Bennett W. P., Hollstein M., Harris C. C. Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res. 1994 Sep 15;54(18):4855–4878. [PubMed] [Google Scholar]
- Hoeijmakers J. H. Human nucleotide excision repair syndromes: molecular clues to unexpected intricacies. Eur J Cancer. 1994;30A(13):1912–1921. doi: 10.1016/0959-8049(94)00381-e. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers J. H. Nucleotide excision repair. II: From yeast to mammals. Trends Genet. 1993 Jun;9(6):211–217. doi: 10.1016/0168-9525(93)90121-w. [DOI] [PubMed] [Google Scholar]
- Kastan M. B., Onyekwere O., Sidransky D., Vogelstein B., Craig R. W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991 Dec 1;51(23 Pt 1):6304–6311. [PubMed] [Google Scholar]
- Lane D. P. Cancer. p53, guardian of the genome. Nature. 1992 Jul 2;358(6381):15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- Michieli P., Chedid M., Lin D., Pierce J. H., Mercer W. E., Givol D. Induction of WAF1/CIP1 by a p53-independent pathway. Cancer Res. 1994 Jul 1;54(13):3391–3395. [PubMed] [Google Scholar]
- Ozols R. F., Young R. C. Chemotherapy of ovarian cancer. Semin Oncol. 1991 Jun;18(3):222–232. [PubMed] [Google Scholar]
- Perego P., Giarola M., Righetti S. C., Supino R., Caserini C., Delia D., Pierotti M. A., Miyashita T., Reed J. C., Zunino F. Association between cisplatin resistance and mutation of p53 gene and reduced bax expression in ovarian carcinoma cell systems. Cancer Res. 1996 Feb 1;56(3):556–562. [PubMed] [Google Scholar]
- Sakakura C., Sweeney E. A., Shirahama T., Igarashi Y., Hakomori S., Nakatani H., Tsujimoto H., Imanishi T., Ohgaki M., Ohyama T. Overexpression of bax sensitizes human breast cancer MCF-7 cells to radiation-induced apoptosis. Int J Cancer. 1996 Jul 3;67(1):101–105. doi: 10.1002/(SICI)1097-0215(19960703)67:1<101::AID-IJC17>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Vikhanskaya F., D'Incalci M., Broggini M. Decreased cytotoxic effects of doxorubicin in a human ovarian cancer-cell line expressing wild-type p53 and WAF1/CIP1 genes. Int J Cancer. 1995 May 4;61(3):397–401. doi: 10.1002/ijc.2910610320. [DOI] [PubMed] [Google Scholar]
- Vikhanskaya F., Erba E., D'Incalci M., Broggini M. Introduction of wild-type p53 in a human ovarian cancer cell line not expressing endogenous p53. Nucleic Acids Res. 1994 Mar 25;22(6):1012–1017. doi: 10.1093/nar/22.6.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagener C., Bargou R. C., Daniel P. T., Bommert K., Mapara M. Y., Royer H. D., Dörken B. Induction of the death-promoting gene bax-alpha sensitizes cultured breast-cancer cells to drug-induced apoptosis. Int J Cancer. 1996 Jul 3;67(1):138–141. doi: 10.1002/(SICI)1097-0215(19960703)67:1<138::AID-IJC22>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Wang X. W., Forrester K., Yeh H., Feitelson M. A., Gu J. R., Harris C. C. Hepatitis B virus X protein inhibits p53 sequence-specific DNA binding, transcriptional activity, and association with transcription factor ERCC3. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2230–2234. doi: 10.1073/pnas.91.6.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. W., Yeh H., Schaeffer L., Roy R., Moncollin V., Egly J. M., Wang Z., Freidberg E. C., Evans M. K., Taffe B. G. p53 modulation of TFIIH-associated nucleotide excision repair activity. Nat Genet. 1995 Jun;10(2):188–195. doi: 10.1038/ng0695-188. [DOI] [PubMed] [Google Scholar]
- Wosikowski K., Regis J. T., Robey R. W., Alvarez M., Buters J. T., Gudas J. M., Bates S. E. Normal p53 status and function despite the development of drug resistance in human breast cancer cells. Cell Growth Differ. 1995 Nov;6(11):1395–1403. [PubMed] [Google Scholar]
- Zeng Y. X., el-Deiry W. S. Regulation of p21WAF1/CIP1 expression by p53-independent pathways. Oncogene. 1996 Apr 4;12(7):1557–1564. [PubMed] [Google Scholar]