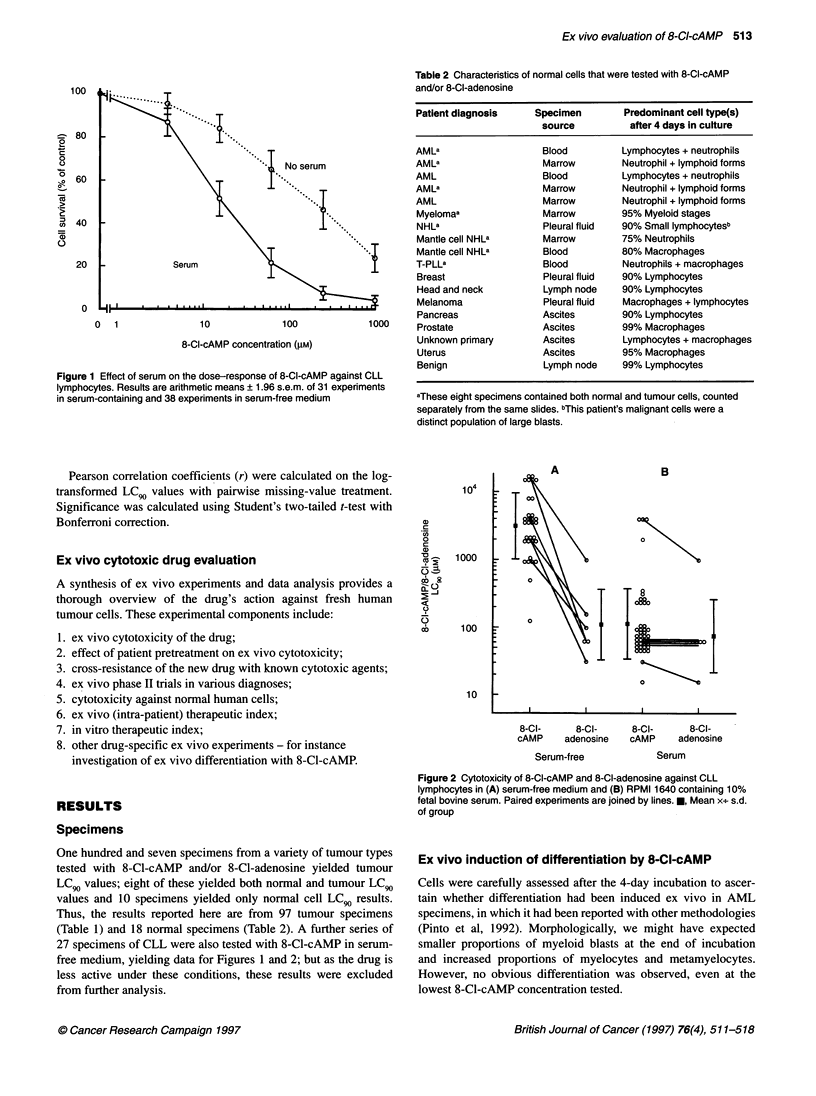
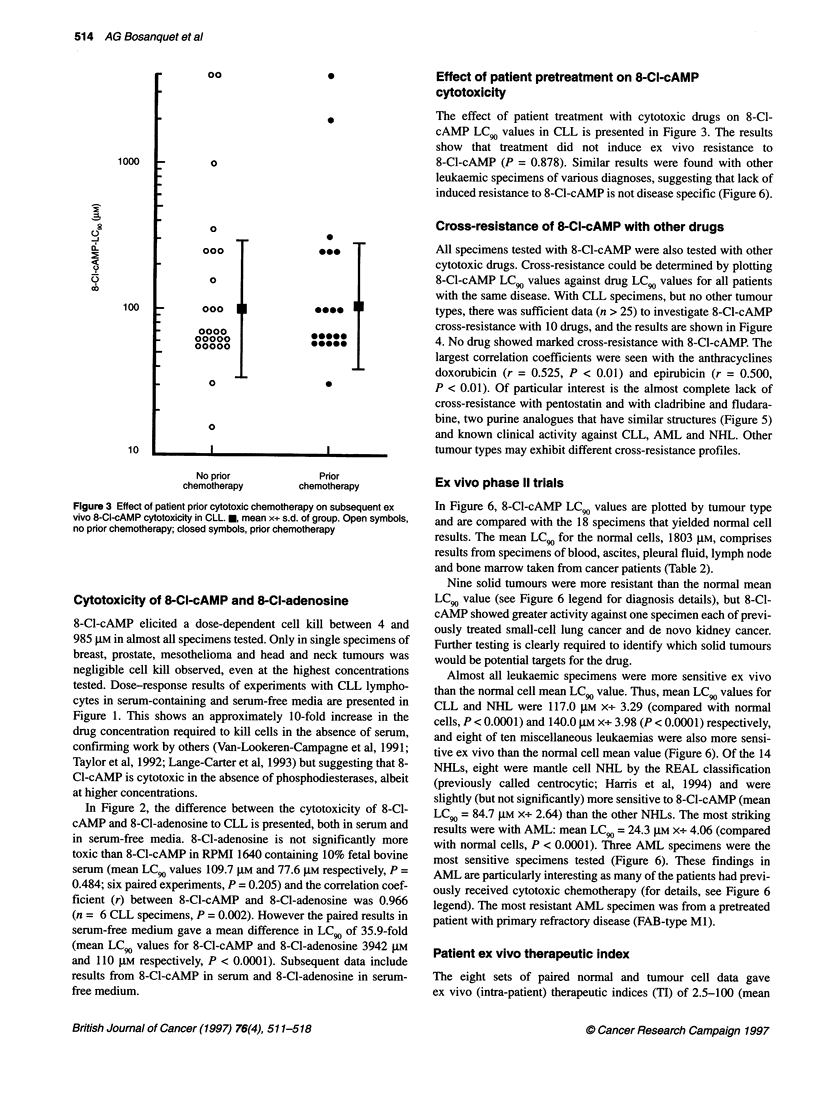
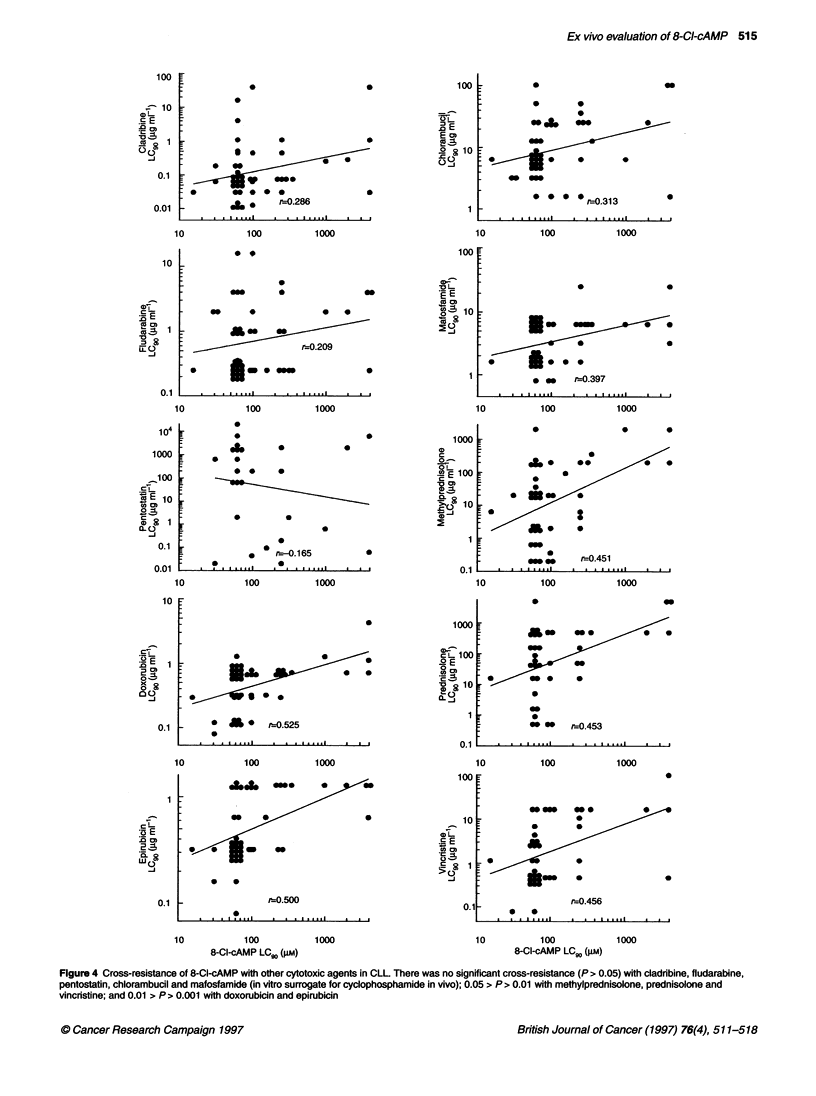
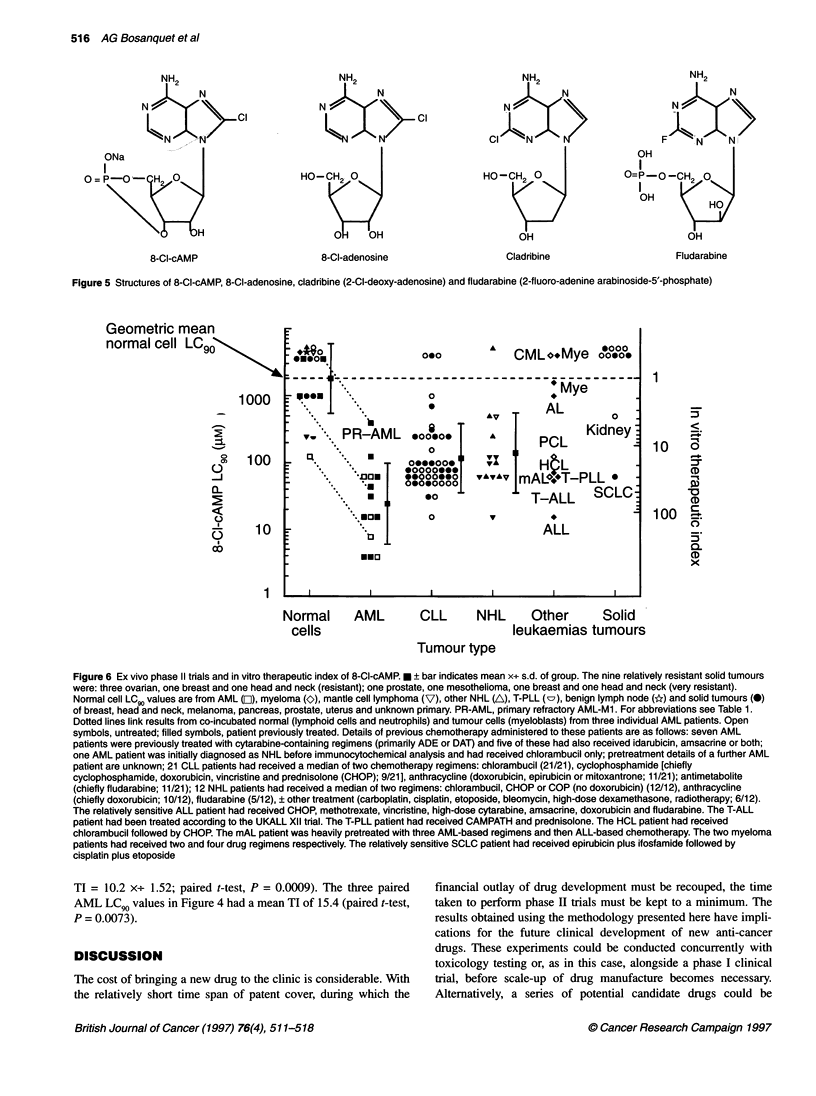
Abstract
There is a pressing need to reduce the time and cost of developing new cytotoxic agents and to accurately identify clinically active agents at an early stage. In this study, the differential staining cytotoxicity (DiSC) assay was used to assess the efficacy of the novel antitumour cAMP analogue, 8-chloro-cAMP (8-Cl-cAMP) (and its metabolite 8-Cl-adenosine) against 107 fresh specimens of human neoplastic and normal cells. Diagnoses included chronic and acute leukaemias, myeloma, non-Hodgkin's lymphoma (NHL) and miscellaneous solid tumours. The aim was to identify targets for subsequent phase I, II and III trials. 8-Cl-cAMP was tested at 4-985 microM, along with standard chemotherapeutic drugs. 8-Cl-cAMP and its metabolite caused no morphologically observable cell differentiation but induced dose-dependent cytotoxicity. Compared with untreated patients, previously treated chronic lymphocytic leukaemia (CLL) patients showed no increase in ex vivo resistance to 8-Cl-cAMP (P = 0.878); minimal cross-resistance with other cytotoxic drugs was detected. Compared with normal cells (mean LC90 = 1803 microM), 8-Cl-cAMP showed significant ex vivo activity against CLL (117.0 microM; P < 0.0001) and NHL (140.0 microM; P < 0.0001), of which eight were mantle cell NHL (84.7 microM), and greatest activity against cells from patients with acute myeloid leukaemia (AML; mean LC90 = 24.3 microM; in vitro therapeutic index 74-fold, P < 0.0001). Solid tumour specimens were comparatively resistant to 8-Cl-cAMP. The results highlight the clinical potential of 8-Cl-cAMP, point to several new phase I, II and III trial possibilities and provide a rationale for the inclusion of ex vivo cytotoxic drug evaluation in the drug development process.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borsellino N., Crescimanno M., Leonardi V., D'Alessandro N. Effects of 8-chloro-cyclic adenosine monophosphate on the growth and sensitivity to doxorubicin of multidrug-resistant tumour cell lines. Pharmacol Res. 1994 Jul;30(1):81–90. doi: 10.1016/1043-6618(94)80090-1. [DOI] [PubMed] [Google Scholar]
- Bosanquet A. G., Bell P. B. Enhanced ex vivo drug sensitivity testing of chronic lymphocytic leukaemia using refined DiSC assay methodology. Leuk Res. 1996 Feb;20(2):143–153. doi: 10.1016/0145-2126(95)00127-1. [DOI] [PubMed] [Google Scholar]
- Bosanquet A. G., Bell P. B. Novel ex vivo analysis of nonclassical, pleiotropic drug resistance and collateral sensitivity induced by therapy provides a rationale for treatment strategies in chronic lymphocytic leukemia. Blood. 1996 Mar 1;87(5):1962–1971. [PubMed] [Google Scholar]
- Bosanquet A. G. Correlations between therapeutic response of leukaemias and in-vitro drug-sensitivity assay. Lancet. 1991 Mar 23;337(8743):711–714. doi: 10.1016/0140-6736(91)90287-y. [DOI] [PubMed] [Google Scholar]
- Bosanquet A. G., McCann S. R., Crotty G. M., Mills M. J., Catovsky D. Methylprednisolone in advanced chronic lymphocytic leukaemia: rationale for, and effectiveness of treatment suggested by DiSC assay. Acta Haematol. 1995;93(2-4):73–79. doi: 10.1159/000204115. [DOI] [PubMed] [Google Scholar]
- Buraczewska I., Szumiel I., Zagórski S., Afanasjev G. G. Effects of 8-chloroadenosine-3',5'-monophosphate in combination with irradiation in L5178Y mouse lymphoblasts. Acta Oncol. 1994;33(6):671–675. doi: 10.3109/02841869409121781. [DOI] [PubMed] [Google Scholar]
- Carmichael J. Cancer chemotherapy: identifying novel anticancer drugs. BMJ. 1994 May 14;308(6939):1288–1290. doi: 10.1136/bmj.308.6939.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho-Chung Y. S., Clair T. The regulatory subunit of cAMP-dependent protein kinase as a target for chemotherapy of cancer and other cellular dysfunctional-related diseases. Pharmacol Ther. 1993 Nov;60(2):265–288. doi: 10.1016/0163-7258(93)90010-b. [DOI] [PubMed] [Google Scholar]
- Cummings J., Leonard R. C., Miller W. R. Sensitive determination of 8-chloroadenosine 3',5'-monophosphate and 8-chloroadenosine in plasma by high-performance liquid chromatography. J Chromatogr B Biomed Appl. 1994 Aug 5;658(1):183–188. doi: 10.1016/0378-4347(94)00200-2. [DOI] [PubMed] [Google Scholar]
- Gazdar A. F., Steinberg S. M., Russell E. K., Linnoila R. I., Oie H. K., Ghosh B. C., Cotelingam J. D., Johnson B. E., Minna J. D., Ihde D. C. Correlation of in vitro drug-sensitivity testing results with response to chemotherapy and survival in extensive-stage small cell lung cancer: a prospective clinical trial. J Natl Cancer Inst. 1990 Jan 17;82(2):117–124. doi: 10.1093/jnci/82.2.117. [DOI] [PubMed] [Google Scholar]
- Glazer R. I., Rohlff C. Transcriptional regulation of multidrug resistance in breast cancer. Breast Cancer Res Treat. 1994;31(2-3):263–271. doi: 10.1007/BF00666159. [DOI] [PubMed] [Google Scholar]
- Hanauske A. R., Wüster K. C., Lehmer A., Rotter M., Schneider P., Kaeser-Fröhlich A., Rastetter J., Depenbrock H. Activity of NK 611, a new epipodophyllotoxin derivative, against colony forming units from freshly explanted human tumours in vitro. Eur J Cancer. 1995 Sep;31A(10):1677–1681. doi: 10.1016/0959-8049(95)00245-e. [DOI] [PubMed] [Google Scholar]
- Harland S. J., Newell D. R., Siddik Z. H., Chadwick R., Calvert A. H., Harrap K. R. Pharmacokinetics of cis-diammine-1,1-cyclobutane dicarboxylate platinum(II) in patients with normal and impaired renal function. Cancer Res. 1984 Apr;44(4):1693–1697. [PubMed] [Google Scholar]
- Harris N. L., Jaffe E. S., Stein H., Banks P. M., Chan J. K., Cleary M. L., Delsol G., De Wolf-Peeters C., Falini B., Gatter K. C. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994 Sep 1;84(5):1361–1392. [PubMed] [Google Scholar]
- Lange-Carter C. A., Vuillequez J. J., Malkinson A. M. 8-Chloroadenosine mediates 8-chloro-cyclic AMP-induced down-regulation of cyclic AMP-dependent protein kinase in normal and neoplastic mouse lung epithelial cells by a cyclic AMP-independent mechanism. Cancer Res. 1993 Jan 15;53(2):393–400. [PubMed] [Google Scholar]
- Langeveld C. H., Jongenelen C. A., Heimans J. J., Stoof J. C. 8-Chloro-cyclic adenosine monophosphate, a novel cyclic AMP analog that inhibits human glioma cell growth in concentrations that do not induce differentiation. Exp Neurol. 1992 Aug;117(2):196–203. doi: 10.1016/0014-4886(92)90127-c. [DOI] [PubMed] [Google Scholar]
- Langeveld C. H., Jongenelen C. A., Heimans J. J., Stoof J. C. Growth inhibition of human glioma cells induced by 8-chloroadenosine, an active metabolite of 8-chloro cyclic adenosine 3':5'-monophosphate. Cancer Res. 1992 Jul 15;52(14):3994–3999. [PubMed] [Google Scholar]
- Larsson R., Fridborg H., Liliemark J., Csoka K., Kristensen J., de la Torre M., Nygren P. In vitro activity of 2-chlorodeoxyadenosine (CdA) in primary cultures of human haematological and solid tumours. Eur J Cancer. 1994;30A(7):1022–1026. doi: 10.1016/0959-8049(94)90136-8. [DOI] [PubMed] [Google Scholar]
- Martin K. J., Chen S. F., Clark G. M., Degen D., Wajima M., Von Hoff D. D., Kaddurah-Daouk R. Evaluation of creatine analogues as a new class of anticancer agents using freshly explanted human tumor cells. J Natl Cancer Inst. 1994 Apr 20;86(8):608–613. doi: 10.1093/jnci/86.8.608. [DOI] [PubMed] [Google Scholar]
- Nagourney R. A., Evans S. S., Messenger J. C., Su Y. Z., Weisenthal L. M. 2 chlorodeoxyadenosine activity and cross resistance patterns in primary cultures of human hematologic neoplasms. Br J Cancer. 1993 Jan;67(1):10–14. doi: 10.1038/bjc.1993.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio K., Morikage T., Kubota N., Ohmori T., Takeda Y., Fujiwara Y., Miki K., Abe K., Saijo N. Alteration of type II regulatory subunit of cAMP-dependent protein kinase in human cisplatin-resistant cells as a basis of collateral sensitivity to 8-chloro-cAMP. Jpn J Cancer Res. 1992 Jul;83(7):754–760. doi: 10.1111/j.1349-7006.1992.tb01976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North P. S., Davies S. L., Ciardiello F., Damiano V., Bianco C., Pepe S., Bianco A. R., Harris A. L., Hickson I. D., Tortora G. Overexpression of the RI alpha subunit of protein kinase A confers hypersensitivity to topoisomerase II inhibitors and 8-chloro-cyclic adenosine 3'5'-monophosphate in Chinese hamster ovary cells. Cancer Res. 1994 Aug 1;54(15):4123–4128. [PubMed] [Google Scholar]
- Pinto A., Aldinucci D., Gattei V., Zagonel V., Tortora G., Budillon A., Cho-Chung Y. S. Inhibition of the self-renewal capacity of blast progenitors from acute myeloblastic leukemia patients by site-selective 8-chloroadenosine 3',5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):8884–8888. doi: 10.1073/pnas.89.19.8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reece P. A., Bishop J. F., Olver I. N., Stafford I., Hillcoat B. L., Morstyn G. Pharmacokinetics of unchanged carboplatin (CBDCA) in patients with small cell lung carcinoma. Cancer Chemother Pharmacol. 1987;19(4):326–330. doi: 10.1007/BF00261482. [DOI] [PubMed] [Google Scholar]
- Rohlff C., Clair T., Cho-Chung Y. S. 8-Cl-cAMP induces truncation and down-regulation of the RI alpha subunit and up-regulation of the RII beta subunit of cAMP-dependent protein kinase leading to type II holoenzyme-dependent growth inhibition and differentiation of HL-60 leukemia cells. J Biol Chem. 1993 Mar 15;268(8):5774–5782. [PubMed] [Google Scholar]
- Rohlff C., Safa B., Rahman A., Cho-Chung Y. S., Klecker R. W., Glazer R. I. Reversal of resistance to adriamycin by 8-chloro-cyclic AMP in adriamycin-resistant HL-60 leukemia cells is associated with reduction of type I cyclic AMP-dependent protein kinase and cyclic AMP response element-binding protein DNA-binding activities. Mol Pharmacol. 1993 Mar;43(3):372–379. [PubMed] [Google Scholar]
- Tidefelt U., Sundman-Engberg B., Rhedin A. S., Paul C. In vitro drug testing in patients with acute leukemia with incubations mimicking in vivo intracellular drug concentrations. Eur J Haematol. 1989 Nov;43(5):374–384. doi: 10.1111/j.1600-0609.1989.tb00323.x. [DOI] [PubMed] [Google Scholar]
- Tortora G, Ciardiello F, Pepe S, Tagliaferri P, Ruggiero A, Bianco C, Guarrasi R, Miki K, Bianco AR. Phase I clinical study with 8-chloro-cAMP and evaluation of immunological effects in cancer patients. Clin Cancer Res. 1995 Apr;1(4):377–384. [PubMed] [Google Scholar]
- Van Lookeren Campagne M. M., Villalba Díaz F., Jastorff B., Kessin R. H. 8-Chloroadenosine 3',5'-monophosphate inhibits the growth of Chinese hamster ovary and Molt-4 cells through its adenosine metabolite. Cancer Res. 1991 Mar 15;51(6):1600–1605. [PubMed] [Google Scholar]
- Vandenberghe E. Mantle cell lymphoma. Blood Rev. 1994 Jun;8(2):79–87. doi: 10.1016/s0268-960x(05)80011-6. [DOI] [PubMed] [Google Scholar]
- Weisenthal L. M. Antineoplastic drug screening belongs in the laboratory, not in the clinic. J Natl Cancer Inst. 1992 Apr 1;84(7):466–469. doi: 10.1093/jnci/84.7.466. [DOI] [PubMed] [Google Scholar]
- Weisenthal L. M., Kern D. H. Prediction of drug resistance in cancer chemotherapy: the Kern and DiSC assays. Oncology (Williston Park) 1991 Sep;5(9):93-103; disc. 104, 111-4, 117-8. [PubMed] [Google Scholar]


