Abstract
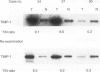
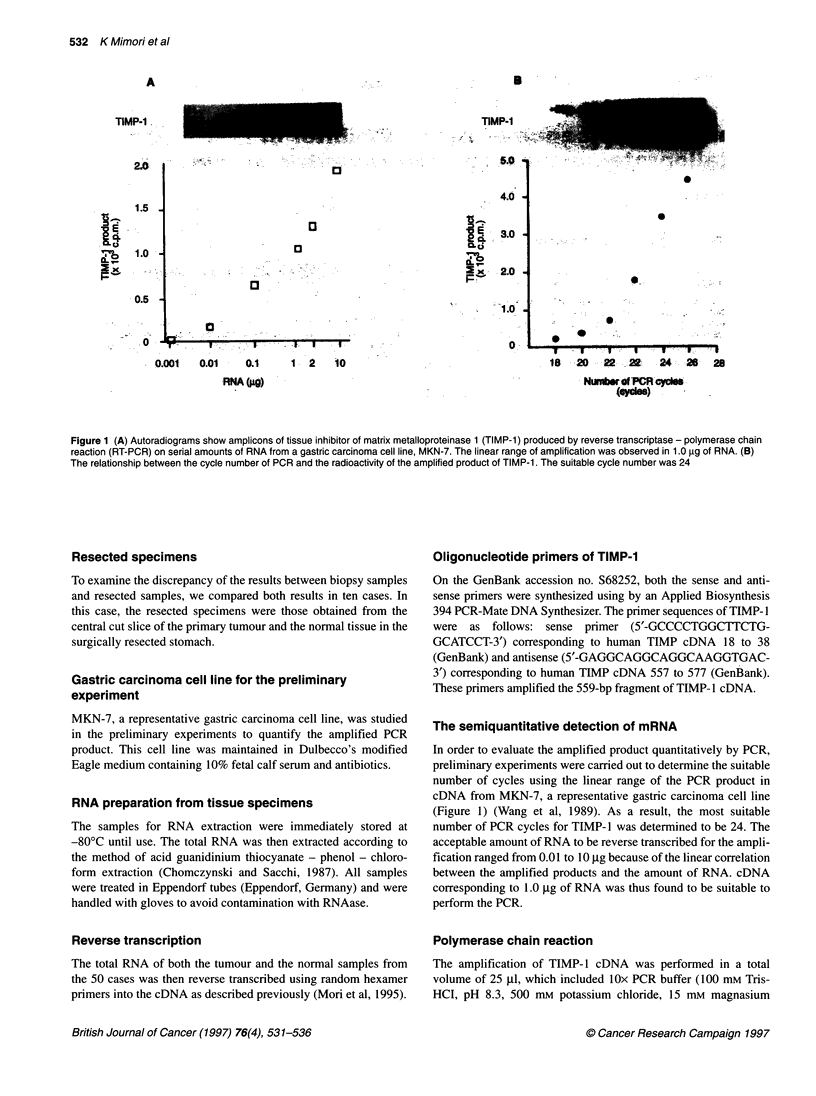
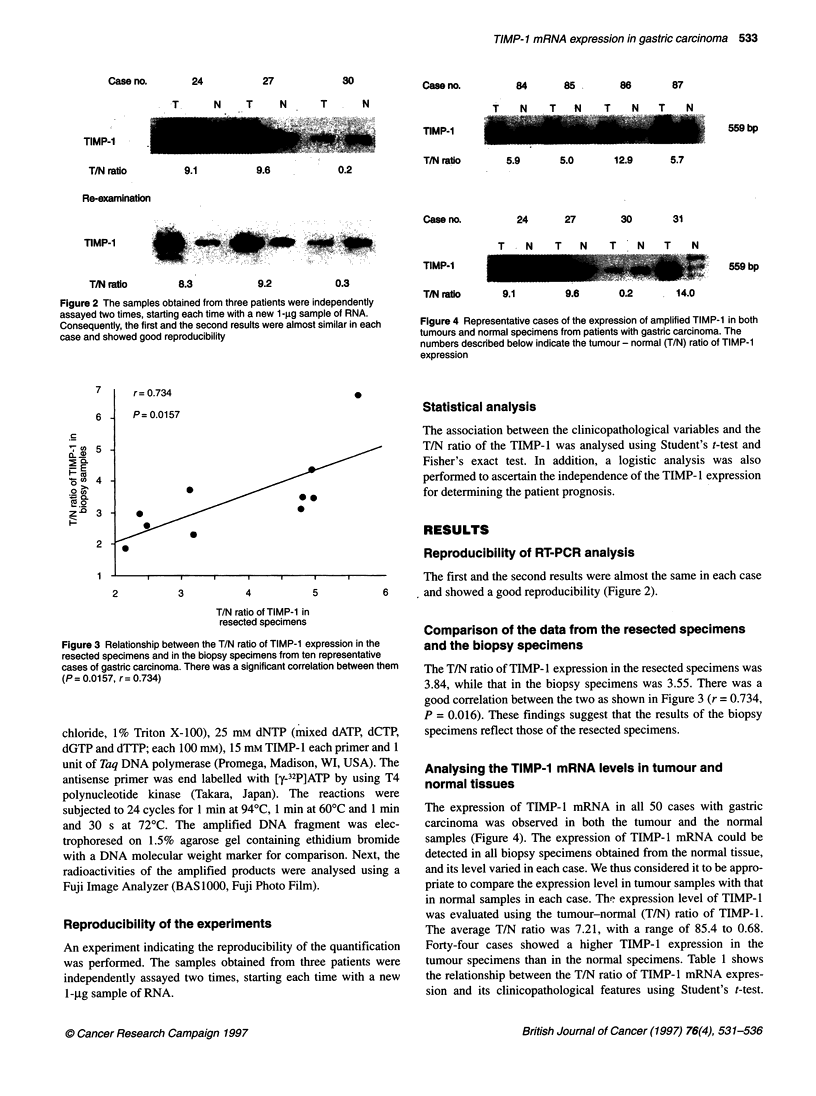
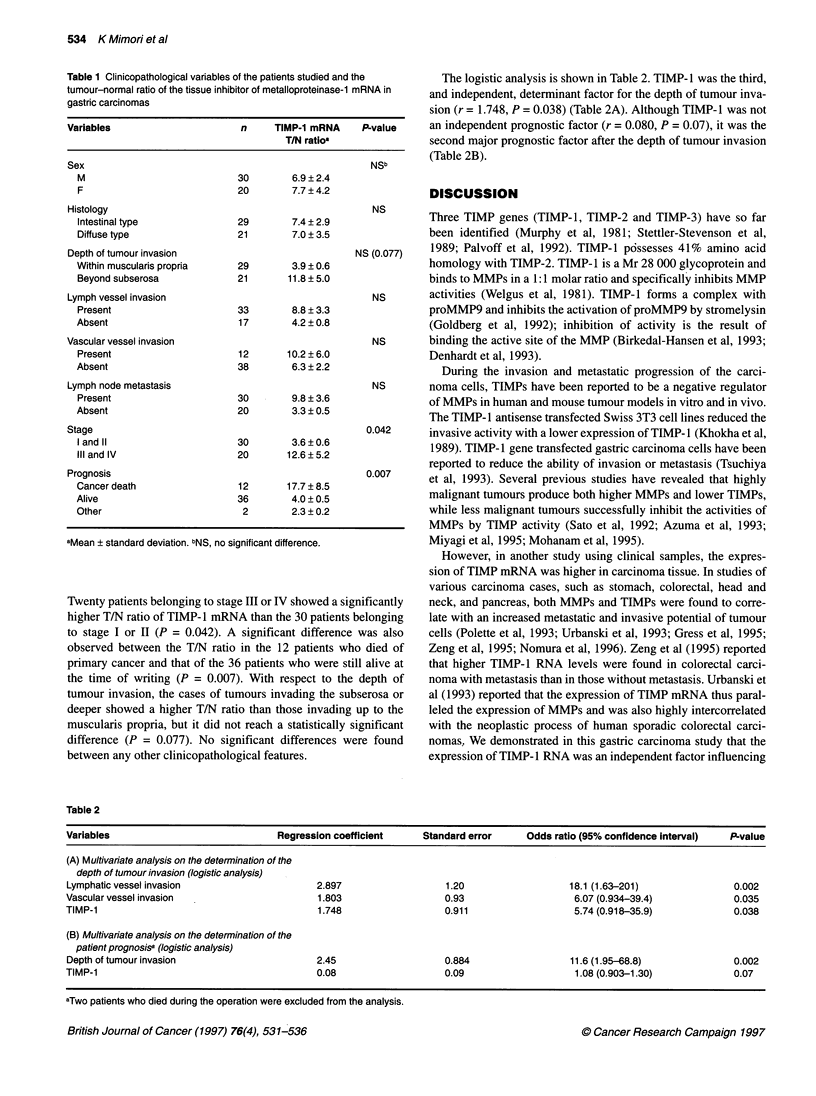
Tissue inhibitor of metalloproteinase (TIMP) has been reported to inhibit tumour invasion through an inactivation of matrix metalloproteinase (MMP) both in vitro and in vivo. Among the TIMP family, TIMP-1 possesses not only proteinase inhibitory activity but also a growth-promoting function. However, the significance of the expression of TIMP-1 in human gastric carcinoma tissue has yet to be clarified. In 50 examined cases of gastric carcinoma, 44 (88%) cases showed a higher expression of TIMP-1 mRNA in the biopsy samples from the tumour tissue (T) than in the biopsy samples from the corresponding normal tissue (N), as determined by semiquantitative reverse transcriptase-polymerase chain reaction (RT-PCR). In a multivariate analysis, the T/N ratio of TIMP-1 mRNA was found to be an independent factor influencing the depth of tumour invasion and was the second most important factor in determining the prognosis of patients. As RT-PCR assay can be performed on biopsy specimens obtained before surgery, an evaluation of the TIMP-1 expression in biopsy specimens by RT-PCR may thus provide useful preoperative information on tumour aggressiveness.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrez M. V., Meldrum C. J., Sim A. T., Aebersold R. H., Clark I. M., Cawston T. E., Burns G. F. A fibroblast elongation factor purified from colon carcinoma cells shares sequence identity with TIMP-1. Biochem Biophys Res Commun. 1995 Jan 17;206(2):590–600. doi: 10.1006/bbrc.1995.1084. [DOI] [PubMed] [Google Scholar]
- Avalos B. R., Kaufman S. E., Tomonaga M., Williams R. E., Golde D. W., Gasson J. C. K562 cells produce and respond to human erythroid-potentiating activity. Blood. 1988 Jun;71(6):1720–1725. [PubMed] [Google Scholar]
- Azuma M., Tamatani T., Fukui K., Yoshida H., Kamogashira T., Ogino K., Suzuki T., Sato M. Role of plasminogen activators, metalloproteinases and the tissue inhibitor of metalloproteinase-1 in the metastatic process of human salivary-gland adenocarcinoma cells. Int J Cancer. 1993 Jun 19;54(4):669–676. doi: 10.1002/ijc.2910540424. [DOI] [PubMed] [Google Scholar]
- Belloc C., Lu H., Soria C., Fridman R., Legrand Y., Menashi S. The effect of platelets on invasiveness and protease production of human mammary tumor cells. Int J Cancer. 1995 Jan 27;60(3):413–417. doi: 10.1002/ijc.2910600324. [DOI] [PubMed] [Google Scholar]
- Birkedal-Hansen H., Moore W. G., Bodden M. K., Windsor L. J., Birkedal-Hansen B., DeCarlo A., Engler J. A. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4(2):197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- Campbell E. J., Cury J. D., Shapiro S. D., Goldberg G. I., Welgus H. G. Neutral proteinases of human mononuclear phagocytes. Cellular differentiation markedly alters cell phenotype for serine proteinases, metalloproteinases, and tissue inhibitor of metalloproteinases. J Immunol. 1991 Feb 15;146(4):1286–1293. [PubMed] [Google Scholar]
- Chesler L., Golde D. W., Bersch N., Johnson M. D. Metalloproteinase inhibition and erythroid potentiation are independent activities of tissue inhibitor of metalloproteinases-1. Blood. 1995 Dec 15;86(12):4506–4515. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T., Feng B., Edwards D. R., Cocuzzi E. T., Malyankar U. M. Tissue inhibitor of metalloproteinases (TIMP, aka EPA): structure, control of expression and biological functions. Pharmacol Ther. 1993 Sep;59(3):329–341. doi: 10.1016/0163-7258(93)90074-n. [DOI] [PubMed] [Google Scholar]
- Docherty A. J., Lyons A., Smith B. J., Wright E. M., Stephens P. E., Harris T. J., Murphy G., Reynolds J. J. Sequence of human tissue inhibitor of metalloproteinases and its identity to erythroid-potentiating activity. Nature. 1985 Nov 7;318(6041):66–69. doi: 10.1038/318066a0. [DOI] [PubMed] [Google Scholar]
- Goldberg G. I., Strongin A., Collier I. E., Genrich L. T., Marmer B. L. Interaction of 92-kDa type IV collagenase with the tissue inhibitor of metalloproteinases prevents dimerization, complex formation with interstitial collagenase, and activation of the proenzyme with stromelysin. J Biol Chem. 1992 Mar 5;267(7):4583–4591. [PubMed] [Google Scholar]
- Gress T. M., Müller-Pillasch F., Lerch M. M., Friess H., Büchler M., Adler G. Expression and in-situ localization of genes coding for extracellular matrix proteins and extracellular matrix degrading proteases in pancreatic cancer. Int J Cancer. 1995 Aug 9;62(4):407–413. doi: 10.1002/ijc.2910620409. [DOI] [PubMed] [Google Scholar]
- Horikoshi T., Danenberg K. D., Stadlbauer T. H., Volkenandt M., Shea L. C., Aigner K., Gustavsson B., Leichman L., Frösing R., Ray M. Quantitation of thymidylate synthase, dihydrofolate reductase, and DT-diaphorase gene expression in human tumors using the polymerase chain reaction. Cancer Res. 1992 Jan 1;52(1):108–116. [PubMed] [Google Scholar]
- Khokha R., Waterhouse P., Yagel S., Lala P. K., Overall C. M., Norton G., Denhardt D. T. Antisense RNA-induced reduction in murine TIMP levels confers oncogenicity on Swiss 3T3 cells. Science. 1989 Feb 17;243(4893):947–950. doi: 10.1126/science.2465572. [DOI] [PubMed] [Google Scholar]
- Lotz M., Guerne P. A. Interleukin-6 induces the synthesis of tissue inhibitor of metalloproteinases-1/erythroid potentiating activity (TIMP-1/EPA). J Biol Chem. 1991 Feb 5;266(4):2017–2020. [PubMed] [Google Scholar]
- Miyagi E., Yasumitsu H., Hirahara F., Minaguchi H., Koshikawa N., Miyazaki K., Umeda M. Characterization of matrix-degrading proteinases and their inhibitors secreted by human gynecological carcinoma cells. Jpn J Cancer Res. 1995 Jun;86(6):568–576. doi: 10.1111/j.1349-7006.1995.tb02436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanam S., Wang S. W., Rayford A., Yamamoto M., Sawaya R., Nakajima M., Liotta L. A., Nicolson G. L., Stetler-Stevenson W. G., Rao J. S. Expression of tissue inhibitors of metalloproteinases: negative regulators of human glioblastoma invasion in vivo. Clin Exp Metastasis. 1995 Jan;13(1):57–62. doi: 10.1007/BF00144019. [DOI] [PubMed] [Google Scholar]
- Mori M., Barnard G. F., Staniunas R. J., Jessup J. M., Steele G. D., Jr, Chen L. B. Prothymosin-alpha mRNA expression correlates with that of c-myc in human colon cancer. Oncogene. 1993 Oct;8(10):2821–2826. [PubMed] [Google Scholar]
- Mori M., Mimori K., Inoue H., Barnard G. F., Tsuji K., Nanbara S., Ueo H., Akiyoshi T. Detection of cancer micrometastases in lymph nodes by reverse transcriptase-polymerase chain reaction. Cancer Res. 1995 Aug 1;55(15):3417–3420. [PubMed] [Google Scholar]
- Mori M., Mimori K., Ueo H., Karimine N., Barnard G. F., Sugimachi K., Akiyoshi T. Molecular detection of circulating solid carcinoma cells in the peripheral blood: the concept of early systemic disease. Int J Cancer. 1996 Dec 11;68(6):739–743. doi: 10.1002/(SICI)1097-0215(19961211)68:6<739::AID-IJC8>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Murphy G., Cawston T. E., Reynolds J. J. An inhibitor of collagenase from human amniotic fluid. Purification, characterization and action on metalloproteinases. Biochem J. 1981 Apr 1;195(1):167–170. doi: 10.1042/bj1950167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura H., Fujimoto N., Seiki M., Mai M., Okada Y. Enhanced production of matrix metalloproteinases and activation of matrix metalloproteinase 2 (gelatinase A) in human gastric carcinomas. Int J Cancer. 1996 Feb 20;69(1):9–16. doi: 10.1002/(SICI)1097-0215(19960220)69:1<9::AID-IJC3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Pavloff N., Staskus P. W., Kishnani N. S., Hawkes S. P. A new inhibitor of metalloproteinases from chicken: ChIMP-3. A third member of the TIMP family. J Biol Chem. 1992 Aug 25;267(24):17321–17326. [PubMed] [Google Scholar]
- Polette M., Clavel C., Birembaut P., De Clerck Y. A. Localization by in situ hybridization of mRNAs encoding stromelysin 3 and tissue inhibitors of metallo-proteinases TIMP-1 and TIMP-2 in human head and neck carcinomas. Pathol Res Pract. 1993 Nov;189(9):1052–1057. doi: 10.1016/S0344-0338(11)80679-5. [DOI] [PubMed] [Google Scholar]
- Richards C. D., Agro A. Interaction between oncostatin M, interleukin 1 and prostaglandin E2 in induction of IL-6 expression in human fibroblasts. Cytokine. 1994 Jan;6(1):40–47. doi: 10.1016/1043-4666(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Sato H., Kida Y., Mai M., Endo Y., Sasaki T., Tanaka J., Seiki M. Expression of genes encoding type IV collagen-degrading metalloproteinases and tissue inhibitors of metalloproteinases in various human tumor cells. Oncogene. 1992 Jan;7(1):77–83. [PubMed] [Google Scholar]
- Stetler-Stevenson W. G., Krutzsch H. C., Liotta L. A. Tissue inhibitor of metalloproteinase (TIMP-2). A new member of the metalloproteinase inhibitor family. J Biol Chem. 1989 Oct 15;264(29):17374–17378. [PubMed] [Google Scholar]
- Tsuchiya Y., Sato H., Endo Y., Okada Y., Mai M., Sasaki T., Seiki M. Tissue inhibitor of metalloproteinase 1 is a negative regulator of the metastatic ability of a human gastric cancer cell line, KKLS, in the chick embryo. Cancer Res. 1993 Mar 15;53(6):1397–1402. [PubMed] [Google Scholar]
- Urbanski S. J., Edwards D. R., Hershfield N., Huchcroft S. A., Shaffer E., Sutherland L., Kossakowska A. E. Expression pattern of metalloproteinases and their inhibitors changes with the progression of human sporadic colorectal neoplasia. Diagn Mol Pathol. 1993 Jun;2(2):81–89. [PubMed] [Google Scholar]
- Welgus H. G., Jeffrey J. J., Eisen A. Z. The collagen substrate specificity of human skin fibroblast collagenase. J Biol Chem. 1981 Sep 25;256(18):9511–9515. [PubMed] [Google Scholar]
- Zeng Z. S., Cohen A. M., Zhang Z. F., Stetler-Stevenson W., Guillem J. G. Elevated tissue inhibitor of metalloproteinase 1 RNA in colorectal cancer stroma correlates with lymph node and distant metastases. Clin Cancer Res. 1995 Aug;1(8):899–906. [PubMed] [Google Scholar]