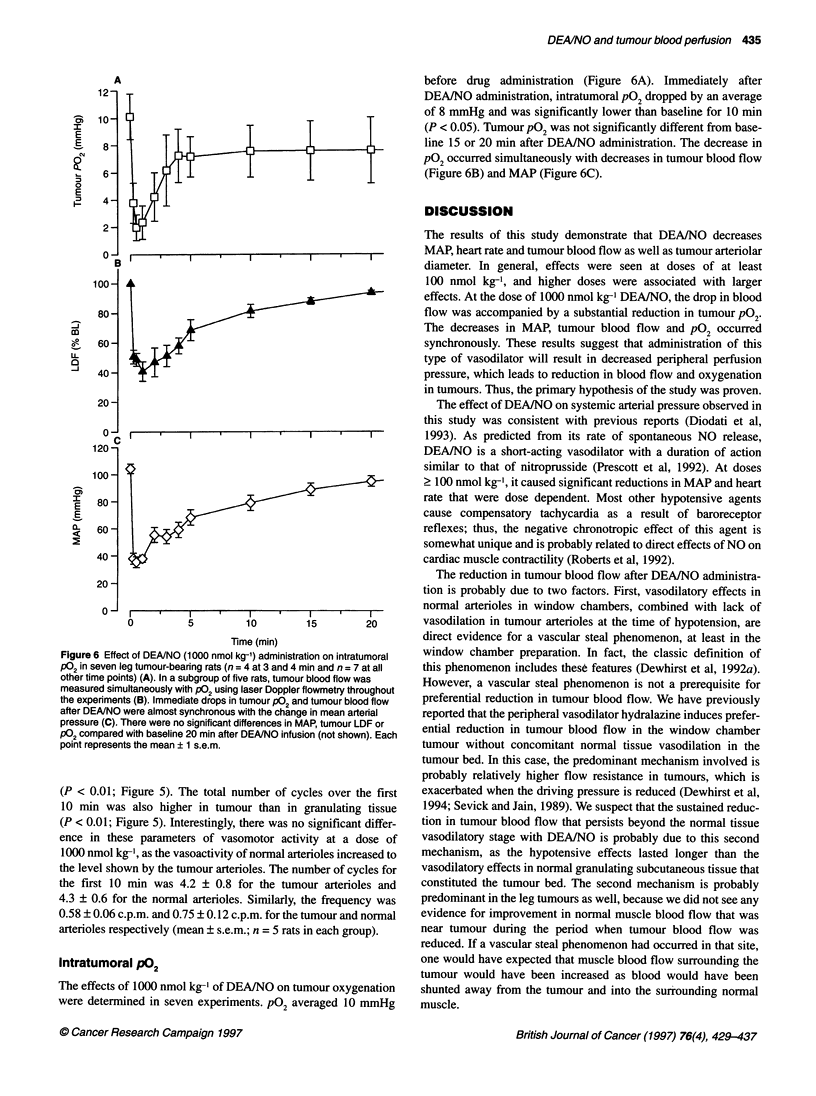
Abstract
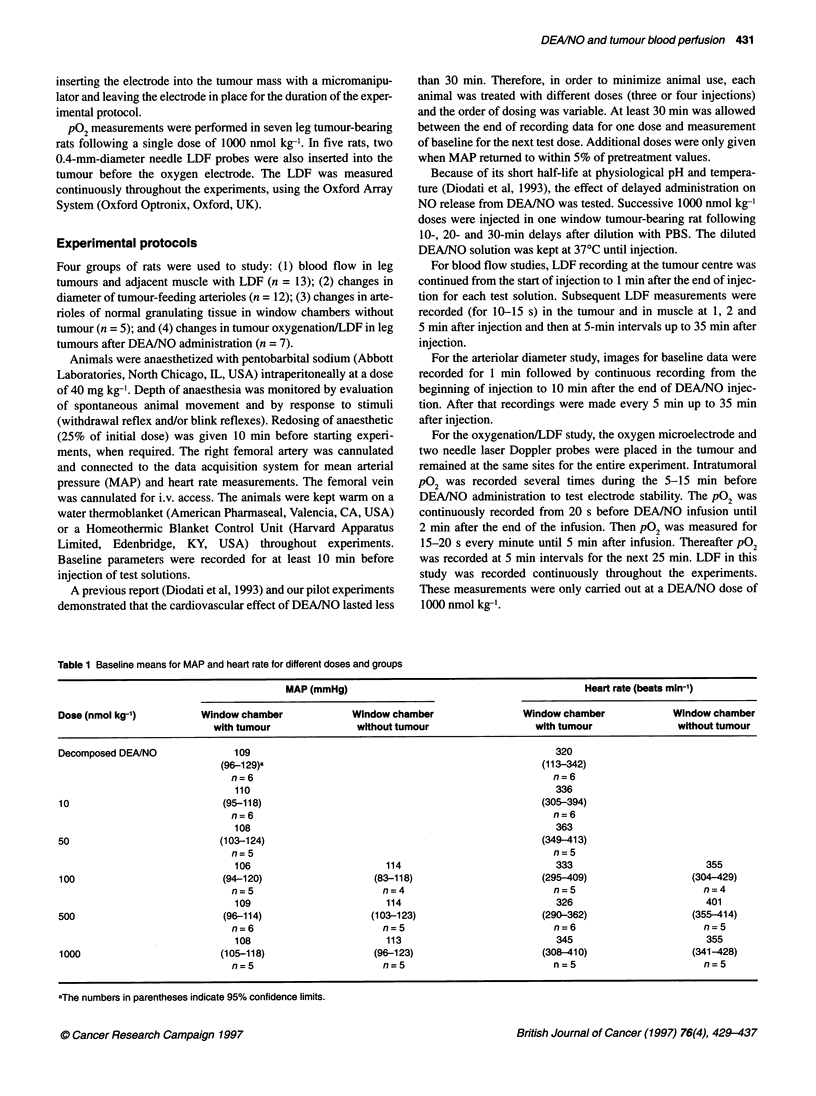
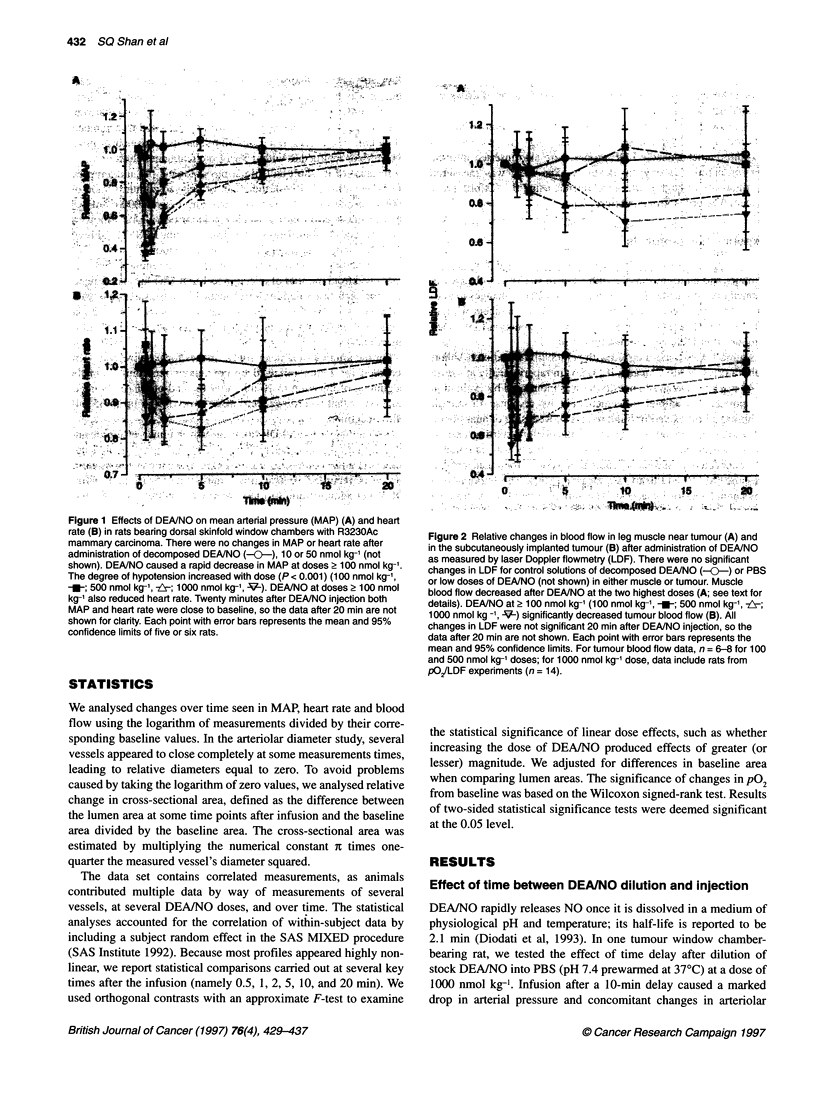
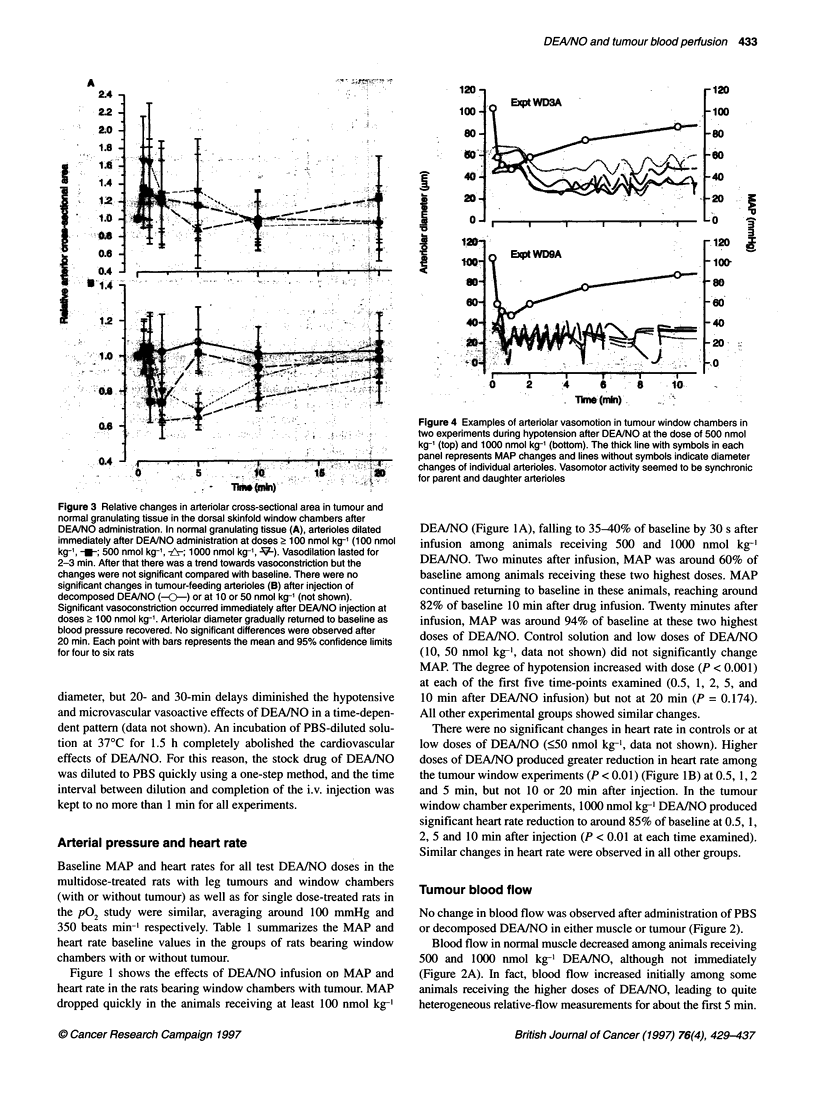
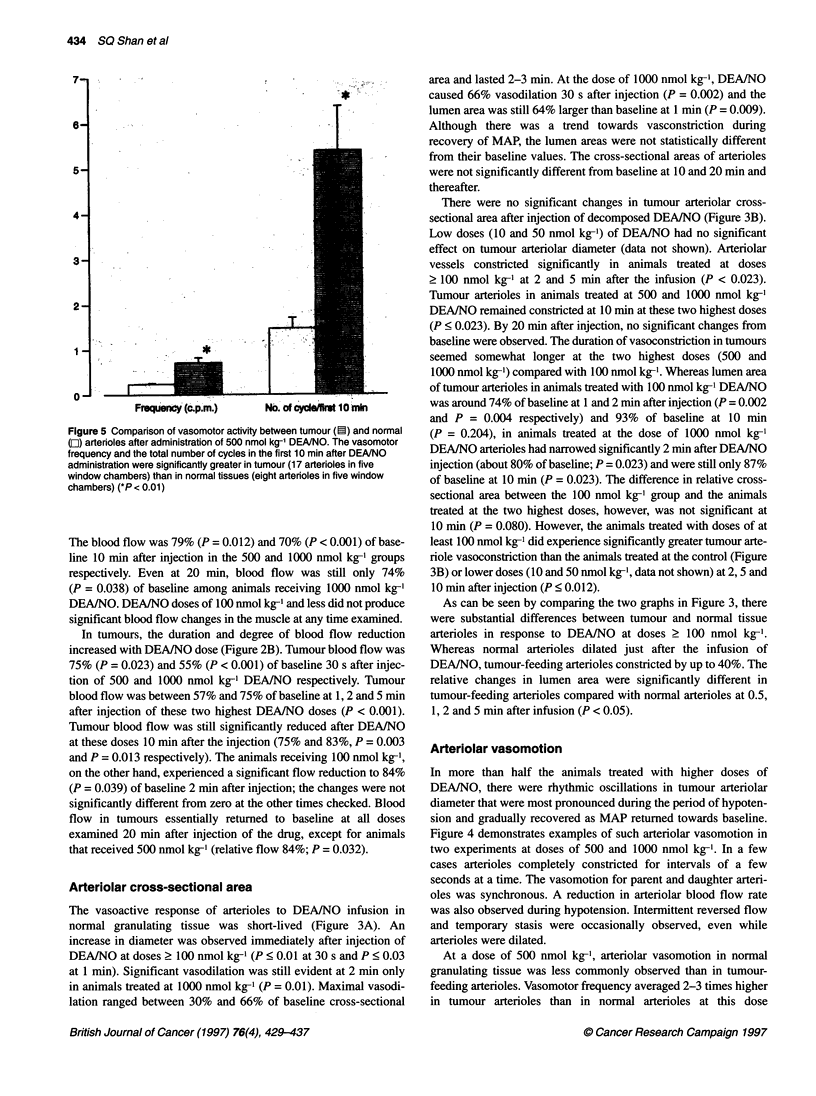
The effects of intravenous diethylamine/nitric oxide (DEA/NO), a short-acting nitric oxide (NO) donor, on systemic haemodynamics, muscle and tumour blood flow (MBF and TBF) and tumour oxygenation were examined in rats bearing subcutaneous R3230Ac carcinoma in the leg. The effects of DEA/NO on the diameters of tumour-feeding and normal arterioles were evaluated in window chambers with and without implanted tumours. DEA/NO reduced mean arterial pressure (MAP) when given at doses > or = 100 nmol kg(-1), with maximal suppression at 0.5-1 min followed by return to baseline within 20 min. DEA/NO did not affect MBF except at the highest doses (500 and 1000 nmol kg(-1)). In contrast, DEA/NO reduced TBF and constricted tumour arterioles at doses > or = 100 nmol kg(-1). Tumour arteriolar vasomotion occurred in more than half the animals during hypotension and with a significantly higher frequency than in normal granulating tissue at a dose of 500 nmol kg(-1). Normal arterioles rapidly and significantly vasodilated for about 3 min and then returned to baseline. The reductions in TBF and MAP were accompanied by synchronous reduction in tumour pO2. Our findings suggest that DEA/NO decreases TBF in two ways. In the window chamber model, vascular steal occurs as normal arterioles adjacent to tumour dilate more than tumour arterioles during the initial period of hypotension. In leg tumours, the predominant mechanism is attributable to reduced perfusion pressure induced by lowered MAP, which decreases flow to the tumour, probably because of relatively higher flow resistance. The vasoconstriction and vasomotion in tumour arterioles during DEA/NO-induced hypotension may reflect differences in regulatory metabolism of NO between neoplastic and normal arterioles. Thus, intravenous injection of a short-acting NO donor, DEA/NO, decreases MAP and heart rate, leading to subsequent decreases in tumour blood flow and oxygenation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acker J. C., Dewhirst M. W., Honoré G. M., Samulski T. V., Tucker J. A., Oleson J. R. Blood perfusion measurements in human tumours: evaluation of laser Doppler methods. Int J Hyperthermia. 1990 Mar-Apr;6(2):287–304. doi: 10.3109/02656739009141139. [DOI] [PubMed] [Google Scholar]
- Andrade S. P., Hart I. R., Piper P. J. Inhibitors of nitric oxide synthase selectively reduce flow in tumor-associated neovasculature. Br J Pharmacol. 1992 Dec;107(4):1092–1095. doi: 10.1111/j.1476-5381.1992.tb13412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertuglia S., Colantuoni A., Coppini G., Intaglietta M. Hypoxia- or hyperoxia-induced changes in arteriolar vasomotion in skeletal muscle microcirculation. Am J Physiol. 1991 Feb;260(2 Pt 2):H362–H372. doi: 10.1152/ajpheart.1991.260.2.H362. [DOI] [PubMed] [Google Scholar]
- Dewhirst M. W., Ong E. T., Klitzman B., Secomb T. W., Vinuya R. Z., Dodge R., Brizel D., Gross J. F. Perivascular oxygen tensions in a transplantable mammary tumor growing in a dorsal flap window chamber. Radiat Res. 1992 May;130(2):171–182. [PubMed] [Google Scholar]
- Dewhirst M. W., Tso C. Y., Oliver R., Gustafson C. S., Secomb T. W., Gross J. F. Morphologic and hemodynamic comparison of tumor and healing normal tissue microvasculature. Int J Radiat Oncol Biol Phys. 1989 Jul;17(1):91–99. doi: 10.1016/0360-3016(89)90375-1. [DOI] [PubMed] [Google Scholar]
- Dewhirst M. W., Vinuya R. Z., Ong E. T., Klitzman B., Rosner G., Secomb T. W., Gross J. F. Effects of bradykinin on the hemodynamics of tumor and granulating normal tissue microvasculature. Radiat Res. 1992 Jun;130(3):345–354. [PubMed] [Google Scholar]
- Diodati J. G., Quyyumi A. A., Keefer L. K. Complexes of nitric oxide with nucleophiles as agents for the controlled biological release of nitric oxide: hemodynamic effect in the rabbit. J Cardiovasc Pharmacol. 1993 Aug;22(2):287–292. doi: 10.1097/00005344-199308000-00018. [DOI] [PubMed] [Google Scholar]
- Doi K., Akaike T., Horie H., Noguchi Y., Fujii S., Beppu T., Ogawa M., Maeda H. Excessive production of nitric oxide in rat solid tumor and its implication in rapid tumor growth. Cancer. 1996 Apr 15;77(8 Suppl):1598–1604. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1598::AID-CNCR27>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- HOWARD-FLANDERS P. Effect of nitric oxide on the radiosensitivity of bacteria. Nature. 1957 Nov 30;180(4596):1191–1192. doi: 10.1038/1801191a0. [DOI] [PubMed] [Google Scholar]
- Kennovin G. D., Flitney F. W., Hirst D. G. "Upstream" modification of vasoconstrictor responses in rat epigastric artery supplying an implanted tumour. Adv Exp Med Biol. 1994;345:411–416. doi: 10.1007/978-1-4615-2468-7_54. [DOI] [PubMed] [Google Scholar]
- Kourembanas S., Marsden P. A., McQuillan L. P., Faller D. V. Hypoxia induces endothelin gene expression and secretion in cultured human endothelium. J Clin Invest. 1991 Sep;88(3):1054–1057. doi: 10.1172/JCI115367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebmann J., DeLuca A. M., Coffin D., Keefer L. K., Venzon D., Wink D. A., Mitchell J. B. In vivo radiation protection by nitric oxide modulation. Cancer Res. 1994 Jul 1;54(13):3365–3368. [PubMed] [Google Scholar]
- Linsenmeier R. A., Yancey C. M. Improved fabrication of double-barreled recessed cathode O2 microelectrodes. J Appl Physiol (1985) 1987 Dec;63(6):2554–2557. doi: 10.1152/jappl.1987.63.6.2554. [DOI] [PubMed] [Google Scholar]
- Meyer R. E., Shan S., DeAngelo J., Dodge R. K., Bonaventura J., Ong E. T., Dewhirst M. W. Nitric oxide synthase inhibition irreversibly decreases perfusion in the R3230Ac rat mammary adenocarcinoma. Br J Cancer. 1995 Jun;71(6):1169–1174. doi: 10.1038/bjc.1995.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J. B., Wink D. A., DeGraff W., Gamson J., Keefer L. K., Krishna M. C. Hypoxic mammalian cell radiosensitization by nitric oxide. Cancer Res. 1993 Dec 15;53(24):5845–5848. [PubMed] [Google Scholar]
- Papenfuss H. D., Gross J. F., Intaglietta M., Treese F. A. A transparent access chamber for the rat dorsal skin fold. Microvasc Res. 1979 Nov;18(3):311–318. doi: 10.1016/0026-2862(79)90039-6. [DOI] [PubMed] [Google Scholar]
- Prescott D. M., Samulski T. V., Dewhirst M. W., Page R. L., Thrall D. E., Dodge R. K., Oleson J. R. Use of nitroprusside to increase tissue temperature during local hyperthermia in normal and tumor-bearing dogs. Int J Radiat Oncol Biol Phys. 1992;23(2):377–385. doi: 10.1016/0360-3016(92)90756-8. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., Vodovotz Y., Roche N. S., Sporn M. B., Nathan C. F. Role of nitric oxide in antagonistic effects of transforming growth factor-beta and interleukin-1 beta on the beating rate of cultured cardiac myocytes. Mol Endocrinol. 1992 Nov;6(11):1921–1930. doi: 10.1210/mend.6.11.1282674. [DOI] [PubMed] [Google Scholar]
- Schmidt J. A., Intaglietta M., Borgström P. Periodic hemodynamics in skeletal muscle during local arterial pressure reduction. J Appl Physiol (1985) 1992 Sep;73(3):1077–1083. doi: 10.1152/jappl.1992.73.3.1077. [DOI] [PubMed] [Google Scholar]
- Schneiderman G., Goldstick T. K. Oxygen electrode design criteria and performance characteristics: recessed cathode. J Appl Physiol Respir Environ Exerc Physiol. 1978 Jul;45(1):145–154. doi: 10.1152/jappl.1978.45.1.145. [DOI] [PubMed] [Google Scholar]
- Sevick E. M., Jain R. K. Geometric resistance to blood flow in solid tumors perfused ex vivo: effects of tumor size and perfusion pressure. Cancer Res. 1989 Jul 1;49(13):3506–3512. [PubMed] [Google Scholar]
- Song C. W., Rhee J. G., Haumschild D. J. Continuous and non-invasive quantification of heat-induced changes in blood flow in the skin and RIF-1 tumour of mice by laser Doppler flowmetry. Int J Hyperthermia. 1987 Jan-Feb;3(1):71–77. doi: 10.3109/02656738709140374. [DOI] [PubMed] [Google Scholar]
- Vollmar B., Preissler G., Menger M. D. Hemorrhagic hypotension induces arteriolar vasomotion and intermittent capillary perfusion in rat pancreas. Am J Physiol. 1994 Nov;267(5 Pt 2):H1936–H1940. doi: 10.1152/ajpheart.1994.267.5.H1936. [DOI] [PubMed] [Google Scholar]
- Wood P. J., Sansom J. M., Butler S. A., Stratford I. J., Cole S. M., Szabo C., Thiemermann C., Adams G. E. Induction of hypoxia in experimental murine tumors by the nitric oxide synthase inhibitor, NG-nitro-L-arginine. Cancer Res. 1994 Dec 15;54(24):6458–6463. [PubMed] [Google Scholar]
- Wood P. J., Sansom J. M., Stratford I. J., Adams G. E., Szabo C., Thiemermann C., Vane J. R. Modification of metabolism of transplantable and spontaneous murine tumors by the nitric oxide synthase inhibitor, nitro-L-arginine. Int J Radiat Oncol Biol Phys. 1994 Jun 15;29(3):443–447. doi: 10.1016/0360-3016(94)90435-9. [DOI] [PubMed] [Google Scholar]
- Wood P. J., Stratford I. J., Adams G. E., Szabo C., Thiemermann C., Vane J. R. Modification of energy metabolism and radiation response of a murine tumour by changes in nitric oxide availability. Biochem Biophys Res Commun. 1993 Apr 30;192(2):505–510. doi: 10.1006/bbrc.1993.1444. [DOI] [PubMed] [Google Scholar]


