Abstract
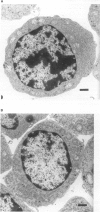
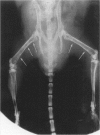
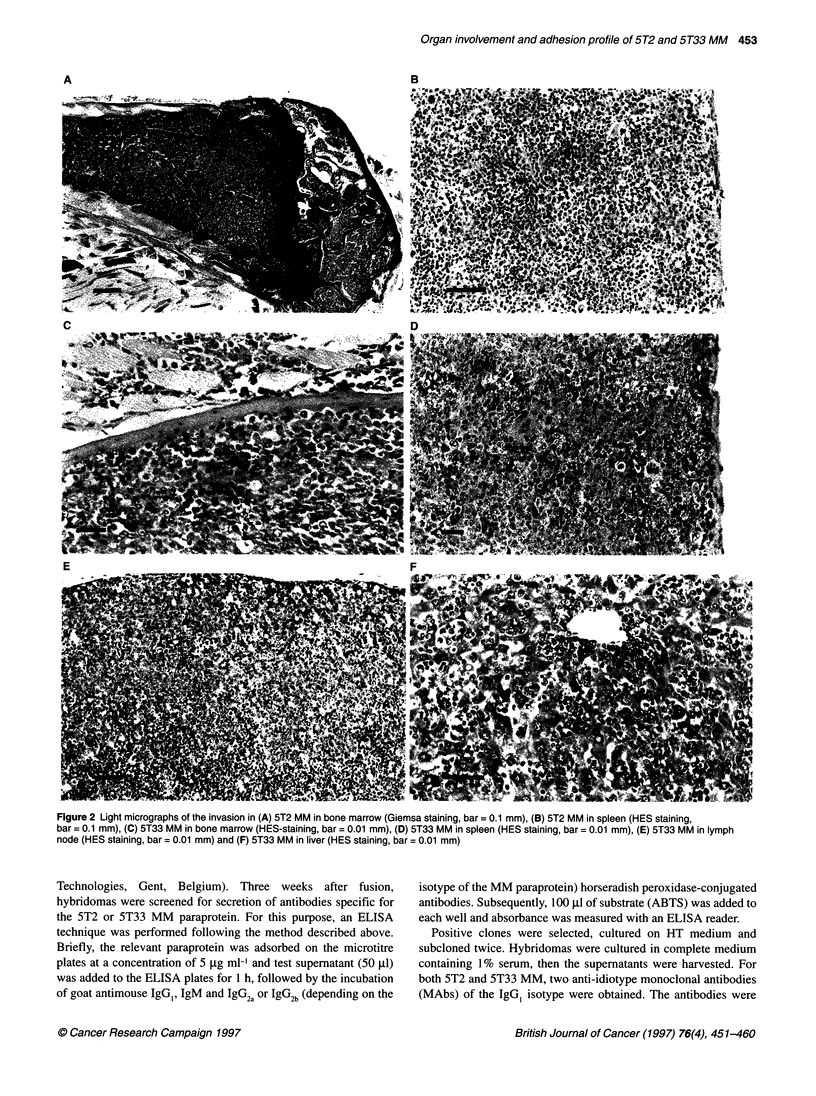
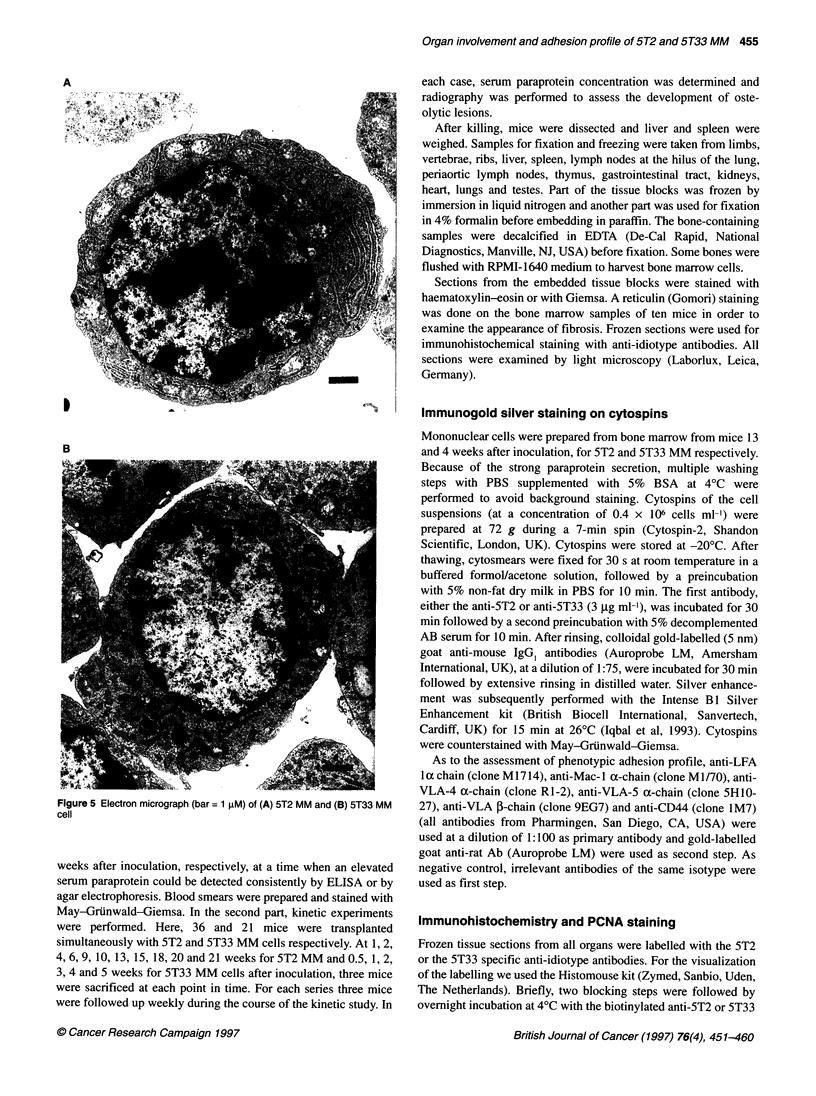
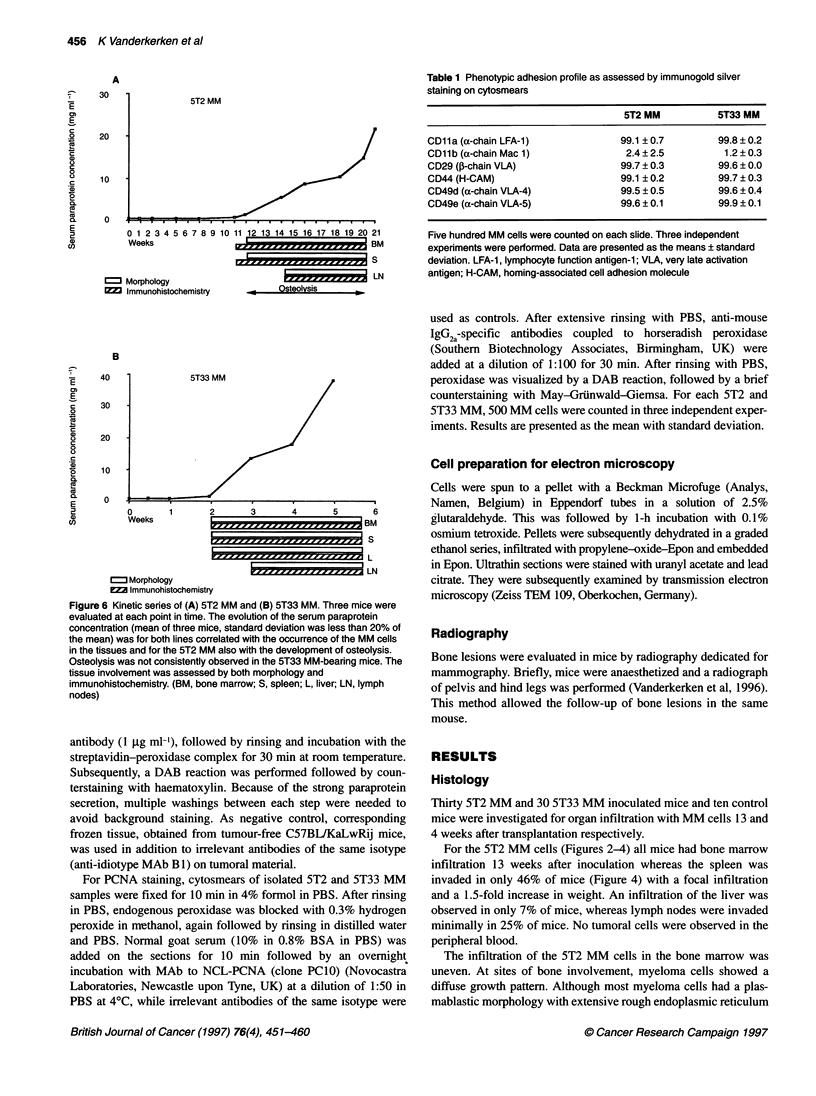
The aim of this study was to evaluate the tissue infiltration and phenotypic adhesion profile of 5T2 multiple myeloma (MM) and 5T33 MM cells and to correlate it with that observed in human disease. For each line, 30 mice were intravenously inoculated with myeloma cells and at a clear-cut demonstrable serum paraprotein concentration; mice were sacrificed and a number of organs removed. The haematoxylin-eosin stainings on paraffin sections were complemented with immunohistochemistry using monoclonal antibodies developed against the specific MM idiotype. When analysed over time, 5T2 MM cells could be observed in bone marrow samples from week 9 after transfer of the cells. For the 5T33 MM, a simultaneous infiltration was observed in bone marrow, spleen and liver 2 weeks after inoculation. Osteolytic lesions consistently developed in the 5T2 MM, but this was not consistent for 5T33 MM. PCNA staining showed a higher proliferative index for the 5T33 MM cells. The expression of adhesion molecules was analysed by immunohistochemistry on cytosmears: both 5T2 MM and 5T33 MM cells were LFA-1, CD44, VLA-4 and VLA-5 positive. We conclude that both lines have a phenotypic adhesion profile analogous to that of human MM cells. As the 5T2 MM cells are less aggressive than the 5T33 MM cells, their organ distribution is more restricted to the bone marrow and osteolytic lesions are consistently present, the former cell line induces myeloma development similar to the human disease.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahsmann E. J., Lokhorst H. M., Dekker A. W., Bloem A. C. Lymphocyte function-associated antigen-1 expression on plasma cells correlates with tumor growth in multiple myeloma. Blood. 1992 Apr 15;79(8):2068–2075. [PubMed] [Google Scholar]
- Alsina M., Boyce B. F., Mundy G. R., Roodman G. D. An in vivo model of human multiple myeloma bone disease. Stem Cells. 1995 Aug;13 (Suppl 2):48–50. [PubMed] [Google Scholar]
- Alsina M., Boyce B., Devlin R. D., Anderson J. L., Craig F., Mundy G. R., Roodman G. D. Development of an in vivo model of human multiple myeloma bone disease. Blood. 1996 Feb 15;87(4):1495–1501. [PubMed] [Google Scholar]
- Bartl R., Frisch B., Diem H., Mündel M., Nagel D., Lamerz R., Fateh-Moghadam A. Histologic, biochemical, and clinical parameters for monitoring multiple myeloma. Cancer. 1991 Nov 15;68(10):2241–2250. doi: 10.1002/1097-0142(19911115)68:10<2241::aid-cncr2820681024>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Bataille R., Chappard D., Marcelli C., Dessauw P., Sany J., Baldet P., Alexandre C. Mechanisms of bone destruction in multiple myeloma: the importance of an unbalanced process in determining the severity of lytic bone disease. J Clin Oncol. 1989 Dec;7(12):1909–1914. doi: 10.1200/JCO.1989.7.12.1909. [DOI] [PubMed] [Google Scholar]
- Brissinck J., Demanet C., Moser M., Leo O., Thielemans K. Treatment of mice bearing BCL1 lymphoma with bispecific antibodies. J Immunol. 1991 Dec 1;147(11):4019–4026. [PubMed] [Google Scholar]
- Butcher E. C., Picker L. J. Lymphocyte homing and homeostasis. Science. 1996 Apr 5;272(5258):60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- Degrassi A., Hilbert D. M., Rudikoff S., Anderson A. O., Potter M., Coon H. G. In vitro culture of primary plasmacytomas requires stromal cell feeder layers. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):2060–2064. doi: 10.1073/pnas.90.5.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faid L., Van Riet I., De Waele M., Facon T., Schots R., Lacor P., Van Camp B. Adhesive interactions between tumour cells and bone marrow stromal elements in human multiple myeloma. Eur J Haematol. 1996 Nov;57(5):349–358. doi: 10.1111/j.1600-0609.1996.tb01392.x. [DOI] [PubMed] [Google Scholar]
- Galand P., Degraef C. Cyclin/PCNA immunostaining as an alternative to tritiated thymidine pulse labelling for marking S phase cells in paraffin sections from animal and human tissues. Cell Tissue Kinet. 1989 Sep;22(5):383–392. doi: 10.1111/j.1365-2184.1989.tb00223.x. [DOI] [PubMed] [Google Scholar]
- Huang Y. W., Richardson J. A., Tong A. W., Zhang B. Q., Stone M. J., Vitetta E. S. Disseminated growth of a human multiple myeloma cell line in mice with severe combined immunodeficiency disease. Cancer Res. 1993 Mar 15;53(6):1392–1396. [PubMed] [Google Scholar]
- Kawakita N., Seki S., Sakaguchi H., Yanai A., Kuroki T., Mizoguchi Y., Kobayashi K., Monna T. Analysis of proliferating hepatocytes using a monoclonal antibody against proliferating cell nuclear antigen/cyclin in embedded tissues from various liver diseases fixed in formaldehyde. Am J Pathol. 1992 Feb;140(2):513–520. [PMC free article] [PubMed] [Google Scholar]
- Kawano M. M., Huang N., Harada H., Harada Y., Sakai A., Tanaka H., Iwato K., Kuramoto A. Identification of immature and mature myeloma cells in the bone marrow of human myelomas. Blood. 1993 Jul 15;82(2):564–570. [PubMed] [Google Scholar]
- Kawano M. M., Huang N., Tanaka H., Ishikawa H., Sakai A., Tanabe O., Nobuyoshi M., Kuramoto A. Homotypic cell aggregations of human myeloma cells with ICAM-1 and LFA-1 molecules. Br J Haematol. 1991 Dec;79(4):583–588. doi: 10.1111/j.1365-2141.1991.tb08085.x. [DOI] [PubMed] [Google Scholar]
- Kim I., Uchiyama H., Chauhan D., Anderson K. C. Cell surface expression and functional significance of adhesion molecules on human myeloma-derived cell lines. Br J Haematol. 1994 Jul;87(3):483–493. doi: 10.1111/j.1365-2141.1994.tb08302.x. [DOI] [PubMed] [Google Scholar]
- Lokhorst H. M., Lamme T., de Smet M., Klein S., de Weger R. A., van Oers R., Bloem A. C. Primary tumor cells of myeloma patients induce interleukin-6 secretion in long-term bone marrow cultures. Blood. 1994 Oct 1;84(7):2269–2277. [PubMed] [Google Scholar]
- Manning L. S., Berger J. D., O'Donoghue H. L., Sheridan G. N., Claringbold P. G., Turner J. H. A model of multiple myeloma: culture of 5T33 murine myeloma cells and evaluation of tumorigenicity in the C57BL/KaLwRij mouse. Br J Cancer. 1992 Dec;66(6):1088–1093. doi: 10.1038/bjc.1992.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okado T., Hawley R. G. Adhesion molecules involved in the binding of murine myeloma cells to bone marrow stromal elements. Int J Cancer. 1995 Dec 11;63(6):823–830. doi: 10.1002/ijc.2910630613. [DOI] [PubMed] [Google Scholar]
- Pellat-Deceunynck C., Barillé S., Puthier D., Rapp M. J., Harousseau J. L., Bataille R., Amiot M. Adhesion molecules on human myeloma cells: significant changes in expression related to malignancy, tumor spreading, and immortalization. Cancer Res. 1995 Aug 15;55(16):3647–3653. [PubMed] [Google Scholar]
- Pellat-Deceunynk C., Amiot M., Bataille R., Van Riet I., Van Camp B., Omede P., Boccadoro M. Human myeloma cell lines as a tool for studying the biology of multiple myeloma: a reappraisal 18 years after. Blood. 1995 Nov 15;86(10):4001–4002. [PubMed] [Google Scholar]
- Picker L. J. Control of lymphocyte homing. Curr Opin Immunol. 1994 Jun;6(3):394–406. doi: 10.1016/0952-7915(94)90118-x. [DOI] [PubMed] [Google Scholar]
- Radl J., Croese J. W., Zurcher C., Van den Enden-Vieveen M. H., de Leeuw A. M. Animal model of human disease. Multiple myeloma. Am J Pathol. 1988 Sep;132(3):593–597. [PMC free article] [PubMed] [Google Scholar]
- Radl J., Croese J. W., Zurcher C., van den Enden-Vieveen M. H., Brondijk R. J., Kazil M., Haaijman J. J., Reitsma P. H., Bijvoet O. L. Influence of treatment with APD-bisphosphonate on the bone lesions in the mouse 5T2 multiple myeloma. Cancer. 1985 Mar 1;55(5):1030–1040. doi: 10.1002/1097-0142(19850301)55:5<1030::aid-cncr2820550518>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Radl J., De Glopper E. D., Schuit H. R., Zurcher C. Idiopathic paraproteinemia. II. Transplantation of the paraprotein-producing clone from old to young C57BL/KaLwRij mice. J Immunol. 1979 Feb;122(2):609–613. [PubMed] [Google Scholar]
- Roldán E., García-Pardo A., Brieva J. A. VLA-4-fibronectin interaction is required for the terminal differentiation of human bone marrow cells capable of spontaneous and high rate immunoglobulin secretion. J Exp Med. 1992 Jun 1;175(6):1739–1747. doi: 10.1084/jem.175.6.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi H., Toyoshima T., Fukao K., Nakauchi H. Presence of hematopoietic stem cells in the adult liver. Nat Med. 1996 Feb;2(2):198–203. doi: 10.1038/nm0296-198. [DOI] [PubMed] [Google Scholar]
- Uchiyama H., Barut B. A., Chauhan D., Cannistra S. A., Anderson K. C. Characterization of adhesion molecules on human myeloma cell lines. Blood. 1992 Nov 1;80(9):2306–2314. [PubMed] [Google Scholar]
- Uchiyama H., Barut B. A., Mohrbacher A. F., Chauhan D., Anderson K. C. Adhesion of human myeloma-derived cell lines to bone marrow stromal cells stimulates interleukin-6 secretion. Blood. 1993 Dec 15;82(12):3712–3720. [PubMed] [Google Scholar]
- Van Riet I., Van Camp B. The involvement of adhesion molecules in the biology of multiple myeloma. Leuk Lymphoma. 1993 Apr;9(6):441–452. doi: 10.3109/10428199309145751. [DOI] [PubMed] [Google Scholar]
- Vanderkerken K., Goes E., De Raeve H., Radl J., Van Camp B. Follow-up of bone lesions in an experimental multiple myeloma mouse model: description of an in vivo technique using radiography dedicated for mammography. Br J Cancer. 1996 Jun;73(12):1463–1465. doi: 10.1038/bjc.1996.277. [DOI] [PMC free article] [PubMed] [Google Scholar]