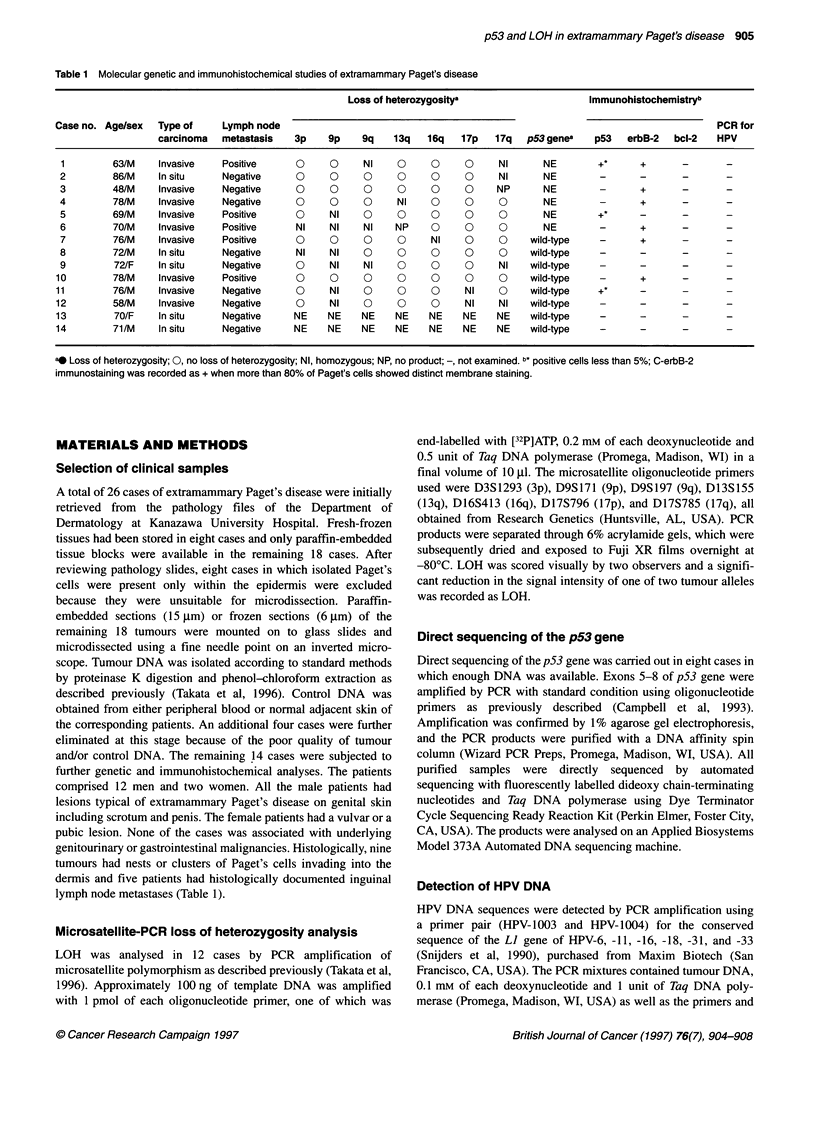
Abstract
Extramammary Paget's disease is a particular form of skin cancer of unknown histogenesis. To look for the genetic defects underlying the pathogenesis of this tumour, we have examined loss of heterozygosity (LOH), p53 and human papillomavirus (HPV) status, and the expression of c-erbB-2 and bcl-2 proteins in 14 cases. Unexpectedly, no LOH was detected at several loci commonly lost in other human cancers (namely 3p, 9p, 9q, 13q, 16q, 17p, and 17q) in 12 tumours examined. Altered p53 protein expression was entirely or mostly negative in all 14 cases. Direct sequencing of exons 5-8 of the p53 gene in eight cases revealed no mutation. Polymerase chain reaction amplification of the L1 gene of human papillomavirus (HPV) did not detect the virus that could inactivate p53 and retinoblastoma tumour-suppressor gene products. As expected, c-erbB-2 proto-oncogene protein was overexpressed in six cases. The expression of bcl-2 was negative in all cases. The results presented in this study suggest that molecular events underlying extramammary Paget's disease differ from those of other common epithelial malignancies and that tumour-suppressor genes located in chromosome regions not examined in this study may be important.
Full text
PDF
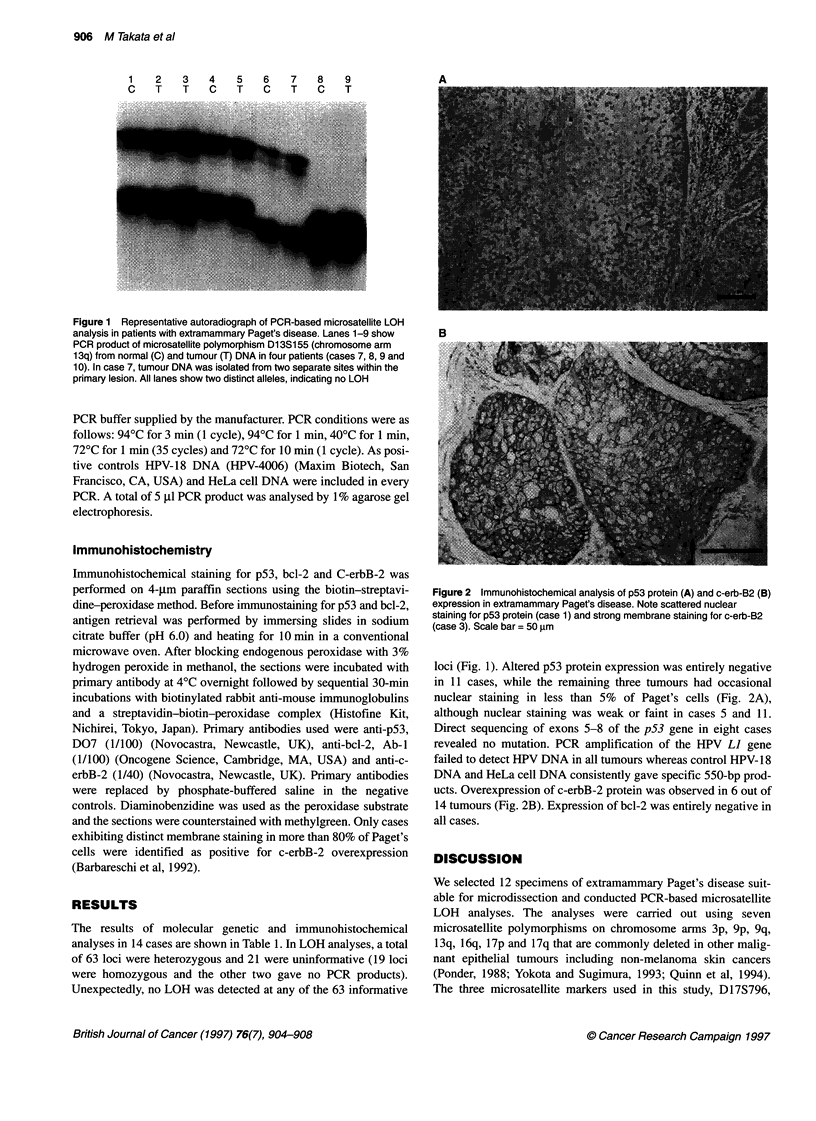


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbareschi M., Leonardi E., Mauri F. A., Serio G., Dalla Palma P. p53 and c-erbB-2 protein expression in breast carcinomas. An immunohistochemical study including correlations with receptor status, proliferation markers, and clinical stage in human breast cancer. Am J Clin Pathol. 1992 Oct;98(4):408–418. doi: 10.1093/ajcp/98.4.408. [DOI] [PubMed] [Google Scholar]
- Busby-Earle R. M., Steel C. M., Bird C. C. Cervical carcinoma: low frequency of allele loss at loci implicated in other common malignancies. Br J Cancer. 1993 Jan;67(1):71–75. doi: 10.1038/bjc.1993.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell C., Quinn A. G., Ro Y. S., Angus B., Rees J. L. p53 mutations are common and early events that precede tumor invasion in squamous cell neoplasia of the skin. J Invest Dermatol. 1993 Jun;100(6):746–748. doi: 10.1111/1523-1747.ep12475717. [DOI] [PubMed] [Google Scholar]
- Campo M. S., Moar M. H., Sartirana M. L., Kennedy I. M., Jarrett W. F. The presence of bovine papillomavirus type 4 DNA is not required for the progression to, or the maintenance of, the malignant state in cancers of the alimentary canal in cattle. EMBO J. 1985 Jul;4(7):1819–1825. doi: 10.1002/j.1460-2075.1985.tb03856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey G., Lopez M. E., Ramos J. C., Plummer S. J., Arboleda M. J., Shaughnessy M., Karlan B., Slamon D. J. DNA sequence analysis of exons 2 through 11 and immunohistochemical staining are required to detect all known p53 alterations in human malignancies. Oncogene. 1996 Nov 7;13(9):1971–1981. [PubMed] [Google Scholar]
- Demopoulos R. I. Fine structure of the extramammary Paget's cell. Cancer. 1971 May;27(5):1202–1210. doi: 10.1002/1097-0142(197105)27:5<1202::aid-cncr2820270527>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Devilee P., Cornelisse C. J. Somatic genetic changes in human breast cancer. Biochim Biophys Acta. 1994 Dec 30;1198(2-3):113–130. doi: 10.1016/0304-419x(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Dyson N., Howley P. M., Münger K., Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989 Feb 17;243(4893):934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- Greenblatt M. S., Bennett W. P., Hollstein M., Harris C. C. Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res. 1994 Sep 15;54(18):4855–4878. [PubMed] [Google Scholar]
- Gunn R. A., Gallager H. S. Vulvar Paget's disease: a topographic study. Cancer. 1980 Aug 1;46(3):590–594. doi: 10.1002/1097-0142(19800801)46:3<590::aid-cncr2820460327>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Hamm H., Vroom T. M., Czarnetzki B. M. Extramammary Paget's cells: further evidence of sweat gland derivation. J Am Acad Dermatol. 1986 Dec;15(6):1275–1281. doi: 10.1016/s0190-9622(86)70302-2. [DOI] [PubMed] [Google Scholar]
- Hart W. R., Millman J. B. Progression of intraepithelial Paget's disease of the vulva to invasive carcinoma. Cancer. 1977 Nov;40(5):2333–2337. doi: 10.1002/1097-0142(197711)40:5<2333::aid-cncr2820400549>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Horowitz J. M., Park S. H., Bogenmann E., Cheng J. C., Yandell D. W., Kaye F. J., Minna J. D., Dryja T. P., Weinberg R. A. Frequent inactivation of the retinoblastoma anti-oncogene is restricted to a subset of human tumor cells. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2775–2779. doi: 10.1073/pnas.87.7.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo R., Gatter K., Bartek J., Lane D., Harris A. L. Increased expression of mutant forms of p53 oncogene in primary lung cancer. Lancet. 1990 Mar 24;335(8691):675–679. doi: 10.1016/0140-6736(90)90801-b. [DOI] [PubMed] [Google Scholar]
- Jones R. E., Jr, Austin C., Ackerman A. B. Extramammary Paget's disease. A critical reexamination. Am J Dermatopathol. 1979 Summer;1(2):101–132. doi: 10.1097/00000372-197900120-00002. [DOI] [PubMed] [Google Scholar]
- Kamb A., Gruis N. A., Weaver-Feldhaus J., Liu Q., Harshman K., Tavtigian S. V., Stockert E., Day R. S., 3rd, Johnson B. E., Skolnick M. H. A cell cycle regulator potentially involved in genesis of many tumor types. Science. 1994 Apr 15;264(5157):436–440. doi: 10.1126/science.8153634. [DOI] [PubMed] [Google Scholar]
- Kanitakis J., Thivolet J., Claudy A. p53 protein expression in mammary and extramammary Paget's disease. Anticancer Res. 1993 Nov-Dec;13(6B):2429–2433. [PubMed] [Google Scholar]
- Kawatsu T., Miki Y. Triple extramammary Paget's disease. Arch Dermatol. 1971 Sep;104(3):316–319. [PubMed] [Google Scholar]
- Keatings L., Sinclair J., Wright C., Corbett I. P., Watchorn C., Hennessy C., Angus B., Lennard T., Horne C. H. c-erbB-2 oncoprotein expression in mammary and extramammary Paget's disease: an immunohistochemical study. Histopathology. 1990 Sep;17(3):243–247. doi: 10.1111/j.1365-2559.1990.tb00714.x. [DOI] [PubMed] [Google Scholar]
- Lee S. C., Roth L. M., Ehrlich C., Hall J. A. Extramammary Paget's disease of the vulva. A clinicopathologic study of 13 cases. Cancer. 1977 Jun;39(6):2540–2549. doi: 10.1002/1097-0142(197706)39:6<2540::aid-cncr2820390635>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Lu Q. L., Abel P., Foster C. S., Lalani E. N. bcl-2: role in epithelial differentiation and oncogenesis. Hum Pathol. 1996 Feb;27(2):102–110. doi: 10.1016/s0046-8177(96)90362-7. [DOI] [PubMed] [Google Scholar]
- MURRELL T. W., Jr, McMULLAN F. H. Extramammary Paget's disease. A report of two cases. Arch Dermatol. 1962 May;85:600–613. doi: 10.1001/archderm.1962.01590050030006. [DOI] [PubMed] [Google Scholar]
- Mazoujian G., Pinkus G. S., Haagensen D. E., Jr Extramammary Paget's disease--evidence for an apocrine origin. An immunoperoxidase study of gross cystic disease fluid protein-15, carcinoembryonic antigen, and keratin proteins. Am J Surg Pathol. 1984 Jan;8(1):43–50. [PubMed] [Google Scholar]
- Meissner K., Rivière A., Haupt G., Löning T. Study of neu-protein expression in mammary Paget's disease with and without underlying breast carcinoma and in extramammary Paget's disease. Am J Pathol. 1990 Dec;137(6):1305–1309. [PMC free article] [PubMed] [Google Scholar]
- Mori O., Hachisuka H., Nakano S., Sasai Y., Shiku H. Expression of ras p21 in mammary and extramammary Paget's disease. Arch Pathol Lab Med. 1990 Aug;114(8):858–861. [PubMed] [Google Scholar]
- Nakamura G., Shikata N., Shoji T., Hatano T., Hioki K., Tsubura A. Immunohistochemical study of mammary and extramammary Paget's disease. Anticancer Res. 1995 Mar-Apr;15(2):467–470. [PubMed] [Google Scholar]
- Nigro J. M., Baker S. J., Preisinger A. C., Jessup J. M., Hostetter R., Cleary K., Bigner S. H., Davidson N., Baylin S., Devilee P. Mutations in the p53 gene occur in diverse human tumour types. Nature. 1989 Dec 7;342(6250):705–708. doi: 10.1038/342705a0. [DOI] [PubMed] [Google Scholar]
- Nishi M., Yoshida H., Setoyama M., Tashiro M. Immunohistochemical study of c-erbB-2 oncoprotein expression in extramammary Paget's disease. Dermatology. 1994;188(2):100–102. doi: 10.1159/000247110. [DOI] [PubMed] [Google Scholar]
- Ordóez N. G., Awalt H., Mackay B. Mammary and extramammary Paget's disease. An immunocytochemical and ultrastructural study. Cancer. 1987 Mar 15;59(6):1173–1183. doi: 10.1002/1097-0142(19870315)59:6<1173::aid-cncr2820590624>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Ponder B. Cancer. Gene losses in human tumours. Nature. 1988 Sep 29;335(6189):400–402. doi: 10.1038/335400a0. [DOI] [PubMed] [Google Scholar]
- Quinn A. G., Sikkink S., Rees J. L. Basal cell carcinomas and squamous cell carcinomas of human skin show distinct patterns of chromosome loss. Cancer Res. 1994 Sep 1;54(17):4756–4759. [PubMed] [Google Scholar]
- Rehman I., Takata M., Wu Y. Y., Rees J. L. Genetic change in actinic keratoses. Oncogene. 1996 Jun 20;12(12):2483–2490. [PubMed] [Google Scholar]
- Roth L. M., Lee S. C., Ehrlich C. E. Paget's disease of the vulva. A histogenetic study of five cases including ultrastructural observations and review of the literature. Am J Surg Pathol. 1977 Sep;1(3):193–206. doi: 10.1097/00000478-197709000-00001. [DOI] [PubMed] [Google Scholar]
- Scheffner M., Münger K., Byrne J. C., Howley P. M. The state of the p53 and retinoblastoma genes in human cervical carcinoma cell lines. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5523–5527. doi: 10.1073/pnas.88.13.5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijders P. J., van den Brule A. J., Schrijnemakers H. F., Snow G., Meijer C. J., Walboomers J. M. The use of general primers in the polymerase chain reaction permits the detection of a broad spectrum of human papillomavirus genotypes. J Gen Virol. 1990 Jan;71(Pt 1):173–181. doi: 10.1099/0022-1317-71-1-173. [DOI] [PubMed] [Google Scholar]
- Snow S. N., Desouky S., Lo J. S., Kurtycz D. Failure to detect human papillomavirus DNA in extramammary Paget's disease. Cancer. 1992 Jan 1;69(1):249–251. doi: 10.1002/1097-0142(19920101)69:1<249::aid-cncr2820690140>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- TOKER C. Some observations on Paget's disease of the nipple. Cancer. 1961 Jul-Aug;14:653–672. doi: 10.1002/1097-0142(199007/08)14:4<653::aid-cncr2820140402>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Takata M., Quinn A. G., Hashimoto K., Rees J. L. Low frequency of loss of heterozygosity at the nevoid basal cell carcinoma locus and other selected loci in appendageal tumors. J Invest Dermatol. 1996 May;106(5):1141–1144. doi: 10.1111/1523-1747.ep12340190. [DOI] [PubMed] [Google Scholar]
- Werness B. A., Levine A. J., Howley P. M. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990 Apr 6;248(4951):76–79. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]
- Wienecke R., Eckert F., Kaudewitz P., de Viragh P. A., Heidl G., Volkenandt M. p53 protein in benign and malignant sweat gland tumors. Am J Dermatopathol. 1994 Apr;16(2):126–129. doi: 10.1097/00000372-199404000-00003. [DOI] [PubMed] [Google Scholar]
- Wolber R. A., Dupuis B. A., Wick M. R. Expression of c-erbB-2 oncoprotein in mammary and extramammary Paget's disease. Am J Clin Pathol. 1991 Aug;96(2):243–247. doi: 10.1093/ajcp/96.2.243. [DOI] [PubMed] [Google Scholar]
- Yokota J., Sugimura T. Multiple steps in carcinogenesis involving alterations of multiple tumor suppressor genes. FASEB J. 1993 Jul;7(10):920–925. doi: 10.1096/fasebj.7.10.8344488. [DOI] [PubMed] [Google Scholar]