Abstract
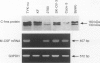
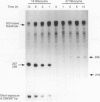
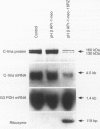
Co-expression of macrophage colony-stimulating factor (M-CSF) and its receptor (c-fms) is often found in ovarian epithelial carcinoma, suggesting the existence of autocrine regulation of cell growth by M-CSF. To block this autocrine loop, we have developed hammerhead ribozymes against c-fms mRNA. As target sites of the ribozyme, we chose the GUC sequence in codon 18 and codon 27 of c-fms mRNA. Two kinds of ribozymes were able to cleave an artificial c-fms RNA substrate in a cell-free system, although the ribozyme against codon 18 was much more efficient than that against codon 27. We next constructed an expression vector carrying a ribozyme sequence that targeted the GUC sequence in codon 18 of c-fms mRNA. It was introduced into TYK-nu cells that expressed M-CSF and its receptor. Its transfectant showed a reduced growth potential. The expression levels of c-fms protein and mRNA in the transfectant were clearly decreased with the expression of ribozyme RNA compared with that of an untransfected control or a transfectant with the vector without the ribozyme sequence. These results suggest that the ribozyme against GUC in codon 18 of c-fms mRNA is a promising tool for blocking the autocrine loop of M-CSF in ovarian epithelial carcinoma.
Full text
PDF

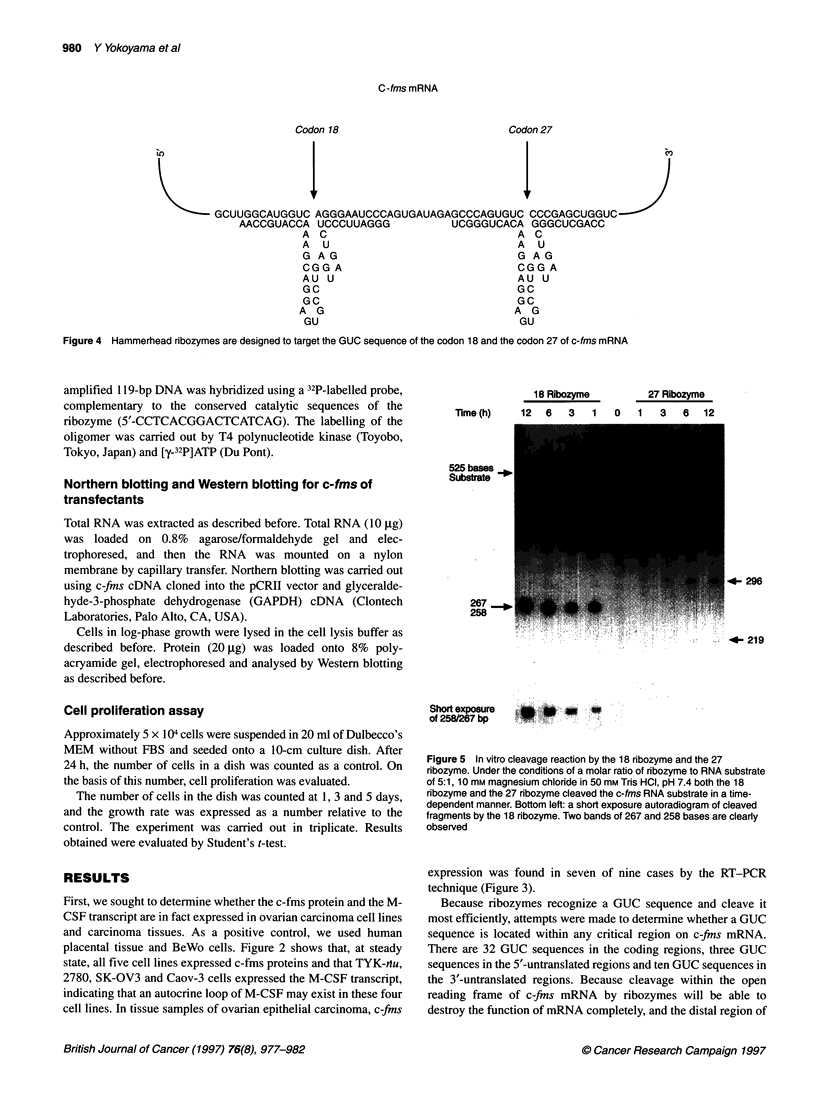
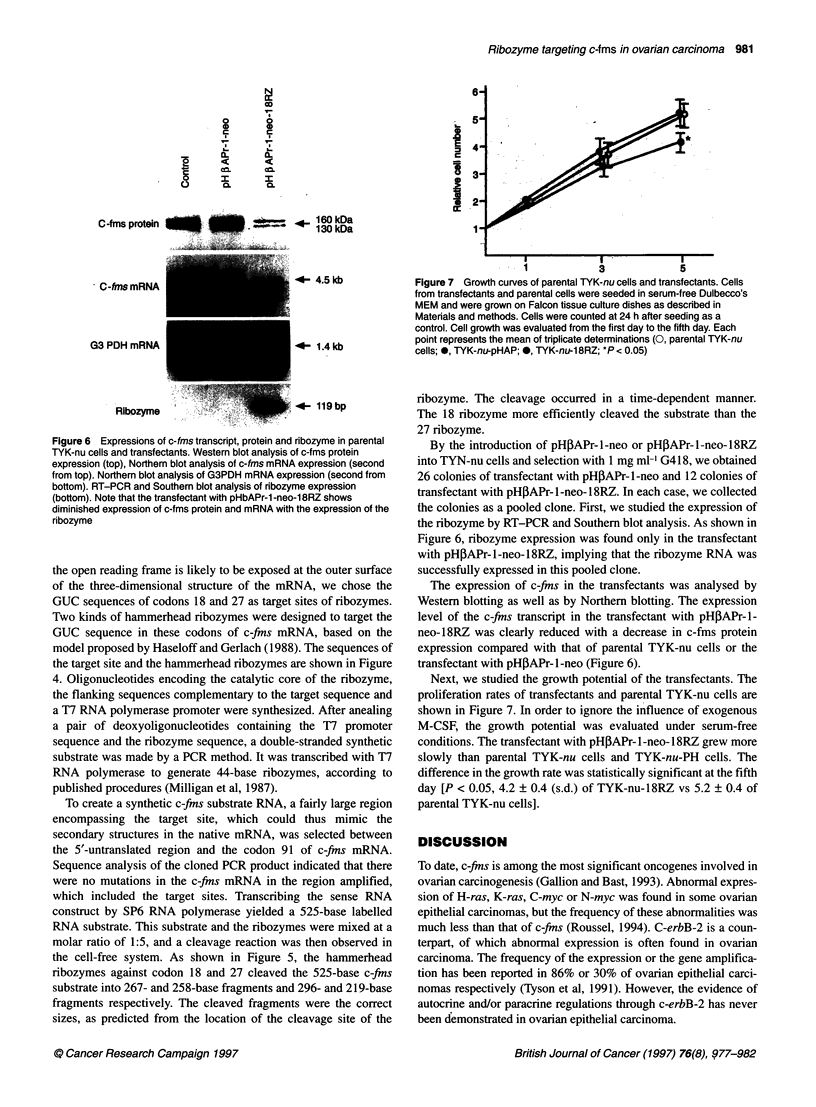

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adcock I. M., Brown C. R., Kwon O., Barnes P. J. Oxidative stress induces NF kappa B DNA binding and inducible NOS mRNA in human epithelial cells. Biochem Biophys Res Commun. 1994 Mar 30;199(3):1518–1524. doi: 10.1006/bbrc.1994.1403. [DOI] [PubMed] [Google Scholar]
- Arceci R. J., Shanahan F., Stanley E. R., Pollard J. W. Temporal expression and location of colony-stimulating factor 1 (CSF-1) and its receptor in the female reproductive tract are consistent with CSF-1-regulated placental development. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8818–8822. doi: 10.1073/pnas.86.22.8818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baiocchi G., Kavanagh J. J., Talpaz M., Wharton J. T., Gutterman J. U., Kurzrock R. Expression of the macrophage colony-stimulating factor and its receptor in gynecologic malignancies. Cancer. 1991 Feb 15;67(4):990–996. doi: 10.1002/1097-0142(19910215)67:4<990::aid-cncr2820670422>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Bast R. C., Jr, Boyer C. M., Jacobs I., Xu F. J., Wu S., Wiener J., Kohler M., Berchuck A. Cell growth regulation in epithelial ovarian cancer. Cancer. 1993 Feb 15;71(4 Suppl):1597–1601. doi: 10.1002/cncr.2820710426. [DOI] [PubMed] [Google Scholar]
- Cech T. R., Bass B. L. Biological catalysis by RNA. Annu Rev Biochem. 1986;55:599–629. doi: 10.1146/annurev.bi.55.070186.003123. [DOI] [PubMed] [Google Scholar]
- Croxtall J. D., Pollard J. W., Carey F., Forder R. A., White J. O. Colony stimulating factor-1 stimulates Ishikawa cell proliferation and lipocortin II synthesis. J Steroid Biochem Mol Biol. 1992 Apr;42(2):121–129. doi: 10.1016/0960-0760(92)90020-j. [DOI] [PubMed] [Google Scholar]
- Dorai T., Kobayashi H., Holland J. F., Ohnuma T. Modulation of platelet-derived growth factor-beta mRNA expression and cell growth in a human mesothelioma cell line by a hammerhead ribozyme. Mol Pharmacol. 1994 Sep;46(3):437–444. [PubMed] [Google Scholar]
- Filderman A. E., Bruckner A., Kacinski B. M., Deng N., Remold H. G. Macrophage colony-stimulating factor (CSF-1) enhances invasiveness in CSF-1 receptor-positive carcinoma cell lines. Cancer Res. 1992 Jul 1;52(13):3661–3666. [PubMed] [Google Scholar]
- Gallion H. H., Bast R. C., Jr National Cancer Institute Conference on Investigational Strategies for Detection and Intervention in Early Ovarian Cancer. Cancer Res. 1993 Aug 15;53(16):3839–3842. [PubMed] [Google Scholar]
- Gunning P., Leavitt J., Muscat G., Ng S. Y., Kedes L. A human beta-actin expression vector system directs high-level accumulation of antisense transcripts. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4831–4835. doi: 10.1073/pnas.84.14.4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseloff J., Gerlach W. L. Simple RNA enzymes with new and highly specific endoribonuclease activities. Nature. 1988 Aug 18;334(6183):585–591. doi: 10.1038/334585a0. [DOI] [PubMed] [Google Scholar]
- Kacinski B. M. CSF-1 and its receptor in ovarian, endometrial and breast cancer. Ann Med. 1995 Feb;27(1):79–85. doi: 10.3109/07853899509031941. [DOI] [PubMed] [Google Scholar]
- Kacinski B. M., Carter D., Mittal K., Yee L. D., Scata K. A., Donofrio L., Chambers S. K., Wang K. I., Yang-Feng T., Rohrschneider L. R. Ovarian adenocarcinomas express fms-complementary transcripts and fms antigen, often with coexpression of CSF-1. Am J Pathol. 1990 Jul;137(1):135–147. [PMC free article] [PubMed] [Google Scholar]
- Kacinski B. M., Scata K. A., Carter D., Yee L. D., Sapi E., King B. L., Chambers S. K., Jones M. A., Pirro M. H., Stanley E. R. FMS (CSF-1 receptor) and CSF-1 transcripts and protein are expressed by human breast carcinomas in vivo and in vitro. Oncogene. 1991 Jun;6(6):941–952. [PubMed] [Google Scholar]
- Kacinski B. M., Stanley E. R., Carter D., Chambers J. T., Chambers S. K., Kohorn E. I., Schwartz P. E. Circulating levels of CSF-1 (M-CSF) a lymphohematopoietic cytokine may be a useful marker of disease status in patients with malignant ovarian neoplasms. Int J Radiat Oncol Biol Phys. 1989 Jul;17(1):159–164. doi: 10.1016/0360-3016(89)90383-0. [DOI] [PubMed] [Google Scholar]
- Kawasaki E. S., Ladner M. B., Wang A. M., Van Arsdell J., Warren M. K., Coyne M. Y., Schweickart V. L., Lee M. T., Wilson K. J., Boosman A. Molecular cloning of a complementary DNA encoding human macrophage-specific colony-stimulating factor (CSF-1). Science. 1985 Oct 18;230(4723):291–296. doi: 10.1126/science.2996129. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y., Iwano I., Kato K. Effects of calmodulin antagonists on human ovarian cancer cell proliferation in vitro. Biochem Biophys Res Commun. 1984 Aug 30;123(1):385–392. doi: 10.1016/0006-291x(84)90425-x. [DOI] [PubMed] [Google Scholar]
- Kommoss F., Wölfle J., Bauknecht T., Pfisterer J., Kiechle-Schwarz M., Pfleiderer A., Sauerbrei W., Kiehl R., Kacinski B. M. Co-expression of M-CSF transcripts and protein, FMS (M-CSF receptor) transcripts and protein, and steroid receptor content in adenocarcinomas of the ovary. J Pathol. 1994 Oct;174(2):111–119. doi: 10.1002/path.1711740207. [DOI] [PubMed] [Google Scholar]
- Milligan J. F., Groebe D. R., Witherell G. W., Uhlenbeck O. C. Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates. Nucleic Acids Res. 1987 Nov 11;15(21):8783–8798. doi: 10.1093/nar/15.21.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard J. W., Bartocci A., Arceci R., Orlofsky A., Ladner M. B., Stanley E. R. Apparent role of the macrophage growth factor, CSF-1, in placental development. Nature. 1987 Dec 3;330(6147):484–486. doi: 10.1038/330484a0. [DOI] [PubMed] [Google Scholar]
- Pollard J. W., Hunt J. S., Wiktor-Jedrzejczak W., Stanley E. R. A pregnancy defect in the osteopetrotic (op/op) mouse demonstrates the requirement for CSF-1 in female fertility. Dev Biol. 1991 Nov;148(1):273–283. doi: 10.1016/0012-1606(91)90336-2. [DOI] [PubMed] [Google Scholar]
- Rossi J. J., Cantin E. M., Sarver N., Chang P. F. The potential use of catalytic RNAs in therapy of HIV infection and other diseases. Pharmacol Ther. 1991;50(2):245–254. doi: 10.1016/0163-7258(91)90016-f. [DOI] [PubMed] [Google Scholar]
- Roussel M. F. Signal transduction by the macrophage-colony-stimulating factor receptor (CSF-1R). J Cell Sci Suppl. 1994;18:105–108. doi: 10.1242/jcs.1994.supplement_18.15. [DOI] [PubMed] [Google Scholar]
- Suzuki M., Ohwada M., Aida I., Tamada T., Hanamura T., Nagatomo M. Macrophage colony-stimulating factor as a tumor marker for epithelial ovarian cancer. Obstet Gynecol. 1993 Dec;82(6):946–950. [PubMed] [Google Scholar]
- Tyson F. L., Boyer C. M., Kaufman R., O'Briant K., Cram G., Crews J. R., Soper J. T., Daly L., Fowler W. C., Jr, Haskill J. S. Expression and amplification of the HER-2/neu (c-erbB-2) protooncogene in epithelial ovarian tumors and cell lines. Am J Obstet Gynecol. 1991 Sep;165(3):640–646. doi: 10.1016/0002-9378(91)90300-g. [DOI] [PubMed] [Google Scholar]