Abstract
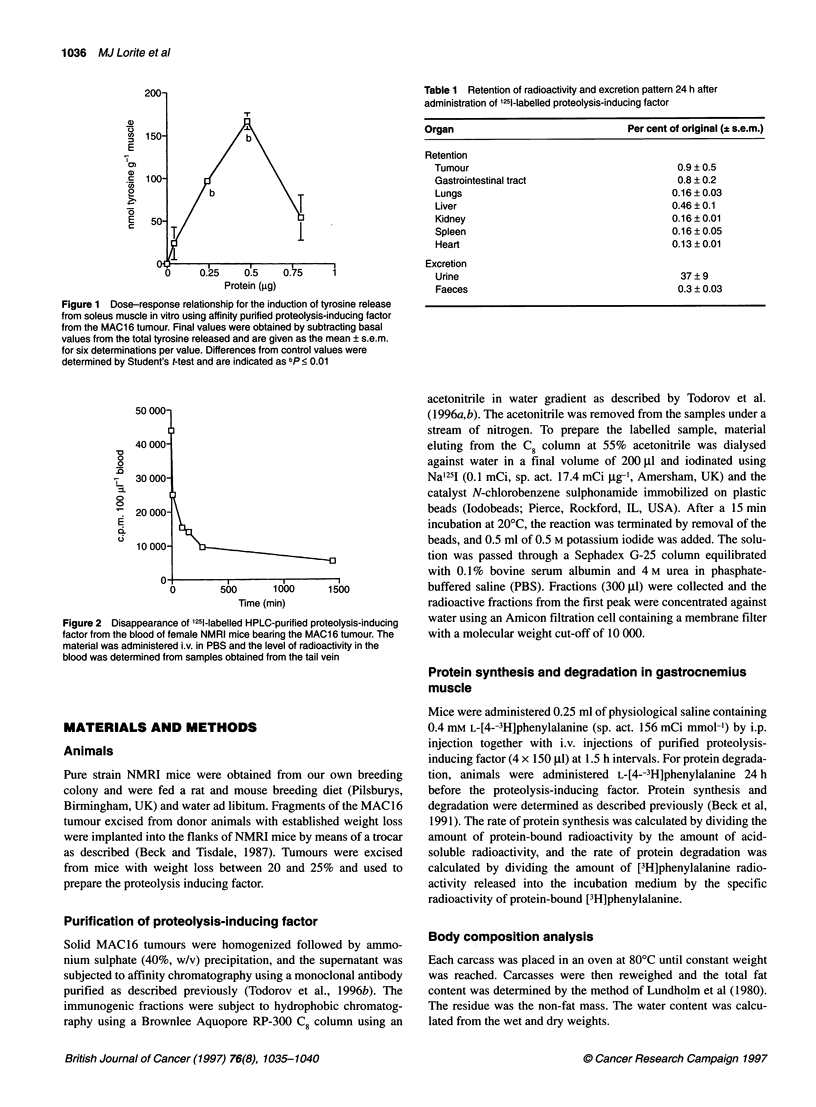
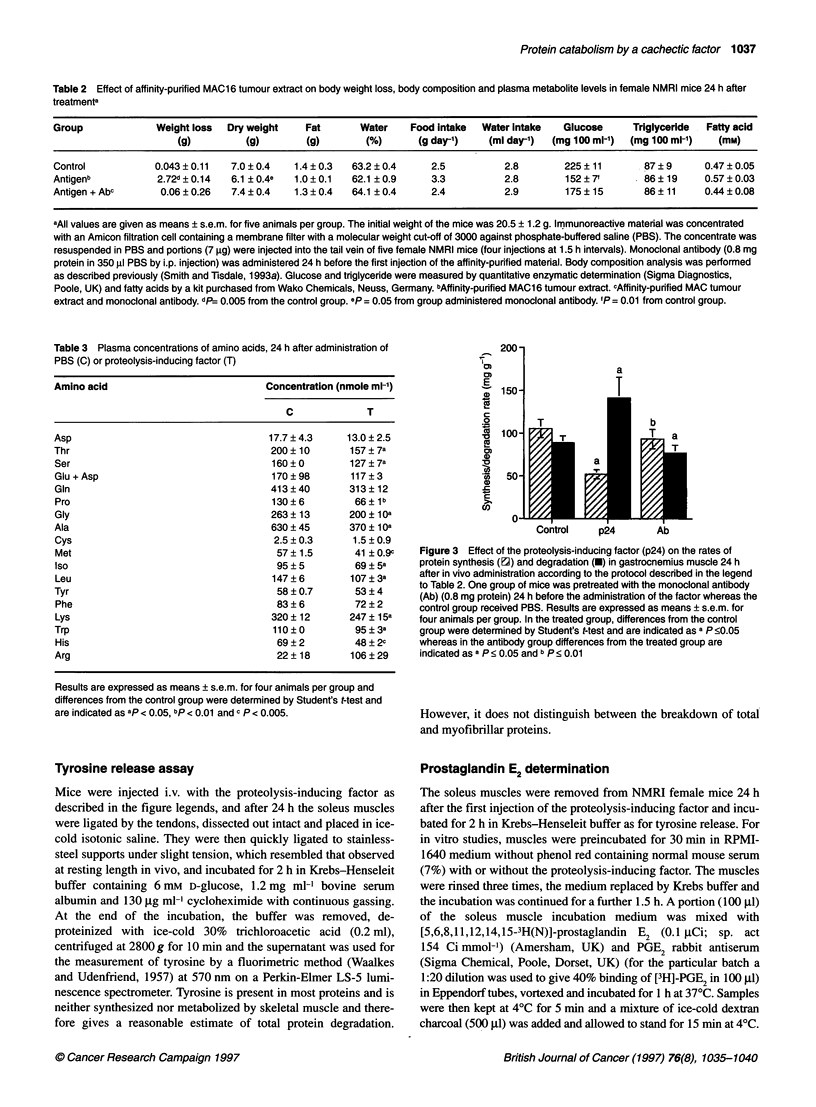
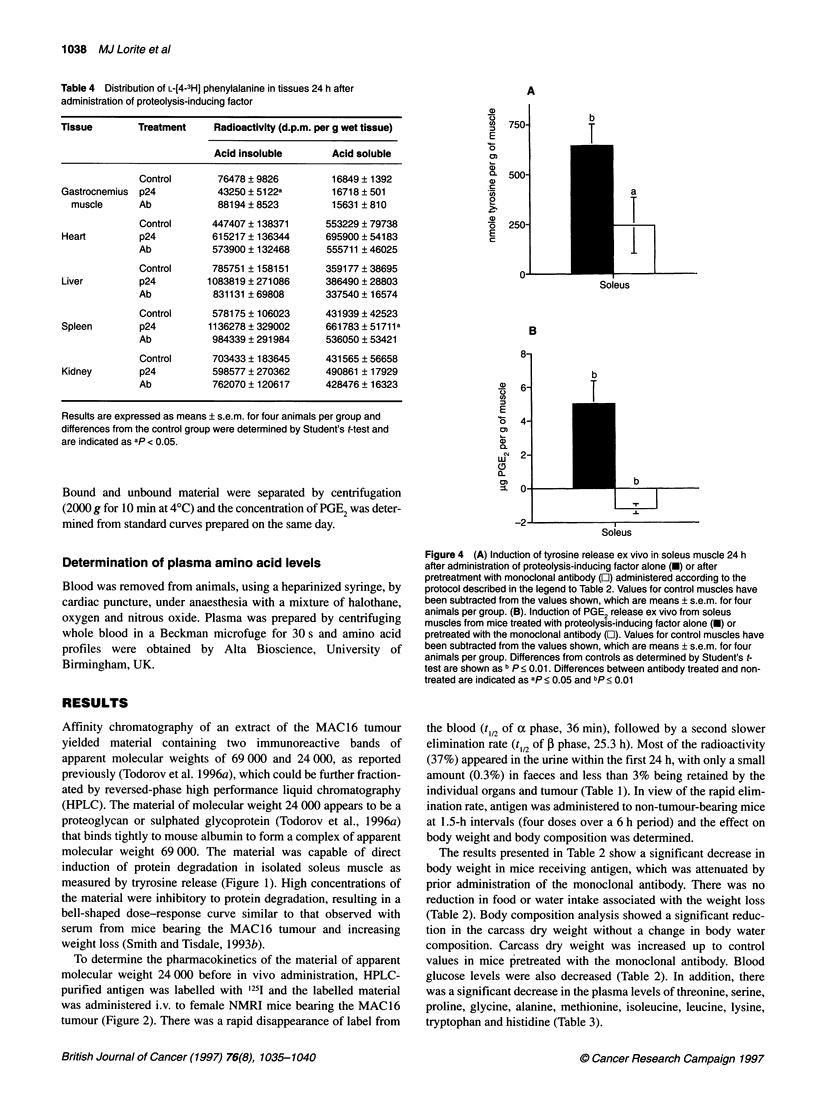
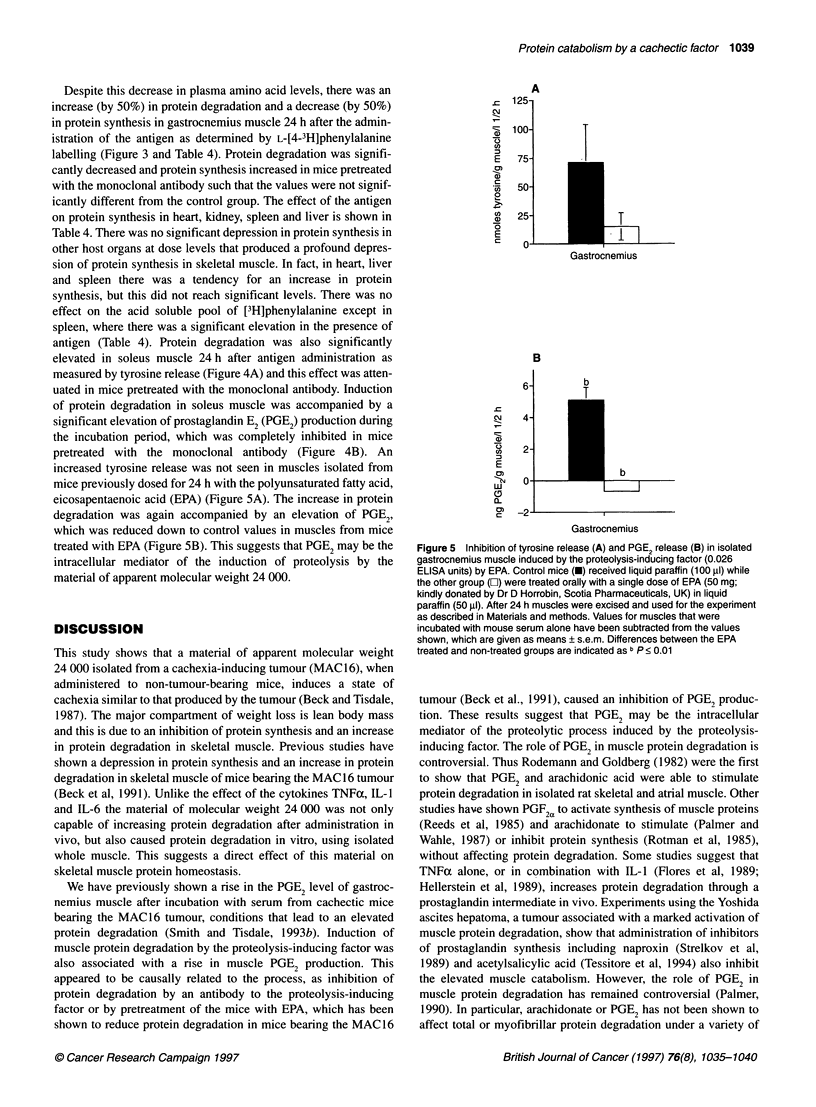
An antigen of apparent molecular weight of 24,000, reactive with a murine monoclonal antibody, has been isolated from a cachexia-inducing tumour (MAC 16) and has been shown to initiate muscle protein degradation in vitro using isolated soleus muscle. Administration of this material to female NMRI mice (20 g) produced a pronounced depression in body weight (2.72 +/- 0.14 g; P<0.005 from control) over a 24 h period. This weight loss was attenuated in mice pretreated with the monoclonal antibody (0.06 +/- 0.26 g over 24 h) and occurred without a reduction in food and water intake. There was no change in body water composition, and the major contribution to the decrease in body weight was a decrease in the non-fat carcass dry weight (mainly lean body mass). The plasma levels of glucose and most amino acids were also significantly depressed. The decrease in lean body mass was accounted for by an increase (by 50%) in protein degradation and a decrease (by 50%) in protein synthesis in gastrocnemius muscle. Protein degradation was significantly decreased and protein synthesis increased to control values in mice pretreated with the monoclonal antibody. Protein degradation initiated in vitro with the proteolysis-inducing factor was abolished in mice pretreated with eicosapentaenoic acid (EPA), which had been shown to prevent muscle wastage in mice bearing the MAC16 tumour. Protein degradation was associated with a significant elevation of prostaglandin E2 production by isolated soleus muscle, which was inhibited by both the monoclonal antibody and EPA. These results suggest that this material may be the humoral factor mediating changes in skeletal muscle protein homeostasis during the process of cancer cachexia in animals bearing the MAC16 tumour, and could potentially be involved in other cases of cachexia.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck S. A., Smith K. L., Tisdale M. J. Anticachectic and antitumor effect of eicosapentaenoic acid and its effect on protein turnover. Cancer Res. 1991 Nov 15;51(22):6089–6093. [PubMed] [Google Scholar]
- Beck S. A., Tisdale M. J. Nitrogen excretion in cancer cachexia and its modification by a high fat diet in mice. Cancer Res. 1989 Jul 15;49(14):3800–3804. [PubMed] [Google Scholar]
- Beck S. A., Tisdale M. J. Production of lipolytic and proteolytic factors by a murine tumor-producing cachexia in the host. Cancer Res. 1987 Nov 15;47(22):5919–5923. [PubMed] [Google Scholar]
- Brennan M. F. Uncomplicated starvation versus cancer cachexia. Cancer Res. 1977 Jul;37(7 Pt 2):2359–2364. [PubMed] [Google Scholar]
- Clark C. M., Goodlad G. A. Depletion of proteins of phasic and tonic muscles in tumour-bearing rats. Eur J Cancer. 1971 Feb;7(1):3–9. doi: 10.1016/0014-2964(71)90088-0. [DOI] [PubMed] [Google Scholar]
- Clarke E. F., Lewis A. M., Waterhouse C. Peripheral amino acid levels in patients with cancer. Cancer. 1978 Dec;42(6):2909–2913. doi: 10.1002/1097-0142(197812)42:6<2909::aid-cncr2820420654>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Cohn S. H., Gartenhaus W., Sawitsky A., Rai K., Zanzi I., Vaswani A., Ellis K. J., Yasumura S., Cortes E., Vartsky D. Compartmental body composition of cancer patients by measurement of total body nitrogen, potassium, and water. Metabolism. 1981 Mar;30(3):222–229. doi: 10.1016/0026-0495(81)90145-1. [DOI] [PubMed] [Google Scholar]
- Dewys W. D., Begg C., Lavin P. T., Band P. R., Bennett J. M., Bertino J. R., Cohen M. H., Douglass H. O., Jr, Engstrom P. F., Ezdinli E. Z. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med. 1980 Oct;69(4):491–497. doi: 10.1016/s0149-2918(05)80001-3. [DOI] [PubMed] [Google Scholar]
- Flores E. A., Bistrian B. R., Pomposelli J. J., Dinarello C. A., Blackburn G. L., Istfan N. W. Infusion of tumor necrosis factor/cachectin promotes muscle catabolism in the rat. A synergistic effect with interleukin 1. J Clin Invest. 1989 May;83(5):1614–1622. doi: 10.1172/JCI114059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Martínez C., López-Soriano F. J., Argilés J. M. Interleukin-6 does not activate protein breakdown in rat skeletal muscle. Cancer Lett. 1994 Jan 15;76(1):1–4. doi: 10.1016/0304-3835(94)90126-0. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., Kettelhut I. C., Furuno K., Fagan J. M., Baracos V. Activation of protein breakdown and prostaglandin E2 production in rat skeletal muscle in fever is signaled by a macrophage product distinct from interleukin 1 or other known monokines. J Clin Invest. 1988 May;81(5):1378–1383. doi: 10.1172/JCI113466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselgren P. O., Zamir O., James J. H., Fischer J. E. Prostaglandin E2 does not regulate total or myofibrillar protein breakdown in incubated skeletal muscle from normal or septic rats. Biochem J. 1990 Aug 15;270(1):45–50. doi: 10.1042/bj2700045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellerstein M. K., Meydani S. N., Meydani M., Wu K., Dinarello C. A. Interleukin-1-induced anorexia in the rat. Influence of prostaglandins. J Clin Invest. 1989 Jul;84(1):228–235. doi: 10.1172/JCI114145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes M. N., Black P., Ashford A. J., Pain V. M. Protein metabolism in the tumour-bearing mouse. Rates of protein synthesis in host tissues and in an Ehrlich ascites tumour at different stages in tumour growth. Biochem J. 1989 Dec 15;264(3):713–719. doi: 10.1042/bj2640713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundholm K., Bylund A. C., Holm J., Scherstén T. Skeletal muscle metabolism in patients with malignant tumor. Eur J Cancer. 1976 Jun;12(6):465–473. doi: 10.1016/0014-2964(76)90036-0. [DOI] [PubMed] [Google Scholar]
- Lundholm K., Edström S., Karlberg I., Ekman L., Scherstén T. Relationship of food intake, body composition, and tumor growth to host metabolism in nongrowing mice with sarcoma. Cancer Res. 1980 Jul;40(7):2516–2522. [PubMed] [Google Scholar]
- McDevitt T. M., Tisdale M. J. Tumour-associated hypoglycaemia in a murine cachexia model. Br J Cancer. 1992 Nov;66(5):815–820. doi: 10.1038/bjc.1992.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moley J. F., Aamodt R., Rumble W., Kaye W., Norton J. A. Body cell mass in cancer-bearing and anorexic patients. JPEN J Parenter Enteral Nutr. 1987 May-Jun;11(3):219–222. doi: 10.1177/0148607187011003219. [DOI] [PubMed] [Google Scholar]
- Norton J. A., Gorschboth C. M., Wesley R. A., Burt M. E., Brennan M. F. Fasting plasma amino acid levels in cancer patients. Cancer. 1985 Sep 1;56(5):1181–1186. doi: 10.1002/1097-0142(19850901)56:5<1181::aid-cncr2820560535>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- O'Keefe S. J., Ogden J., Ramjee G., Rund J. Contribution of elevated protein turnover and anorexia to cachexia in patients with hepatocellular carcinoma. Cancer Res. 1990 Feb 15;50(4):1226–1230. [PubMed] [Google Scholar]
- Palmer R. M. Prostaglandins and the control of muscle protein synthesis and degradation. Prostaglandins Leukot Essent Fatty Acids. 1990 Feb;39(2):95–104. doi: 10.1016/0952-3278(90)90017-f. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Wahle K. W. Protein synthesis and degradation in isolated muscle. Effect of omega 3 and omega 6 fatty acids. Biochem J. 1987 Mar 1;242(2):615–618. doi: 10.1042/bj2420615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeds P. J., Hay S. M., Glennie R. T., Mackie W. S., Garlick P. J. The effect of indomethacin on the stimulation of protein synthesis by insulin in young post-absorptive rats. Biochem J. 1985 Apr 1;227(1):255–261. doi: 10.1042/bj2270255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodemann H. P., Goldberg A. L. Arachidonic acid, prostaglandin E2 and F2 alpha influence rates of protein turnover in skeletal and cardiac muscle. J Biol Chem. 1982 Feb 25;257(4):1632–1638. [PubMed] [Google Scholar]
- Rotman E. I., Brostrom M. A., Brostrom C. O. Inhibition of protein synthesis in intact mammalian cells by arachidonic acid. Biochem J. 1992 Mar 1;282(Pt 2):487–494. doi: 10.1042/bj2820487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. L., Tisdale M. J. Increased protein degradation and decreased protein synthesis in skeletal muscle during cancer cachexia. Br J Cancer. 1993 Apr;67(4):680–685. doi: 10.1038/bjc.1993.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. L., Tisdale M. J. Mechanism of muscle protein degradation in cancer cachexia. Br J Cancer. 1993 Aug;68(2):314–318. doi: 10.1038/bjc.1993.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strelkov A. B., Fields A. L., Baracos V. E. Effects of systemic inhibition of prostaglandin production on protein metabolism in tumor-bearing rats. Am J Physiol. 1989 Aug;257(2 Pt 1):C261–C269. doi: 10.1152/ajpcell.1989.257.2.C261. [DOI] [PubMed] [Google Scholar]
- Tessitore L., Costelli P., Baccino F. M. Pharmacological interference with tissue hypercatabolism in tumour-bearing rats. Biochem J. 1994 Apr 1;299(Pt 1):71–78. doi: 10.1042/bj2990071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov P., Cariuk P., McDevitt T., Coles B., Fearon K., Tisdale M. Characterization of a cancer cachectic factor. Nature. 1996 Feb 22;379(6567):739–742. doi: 10.1038/379739a0. [DOI] [PubMed] [Google Scholar]
- WAALKES T. P., UDENFRIEND S. A fluorometric method for the estimation of tyrosine in plasma and tissues. J Lab Clin Med. 1957 Nov;50(5):733–736. [PubMed] [Google Scholar]


