Abstract
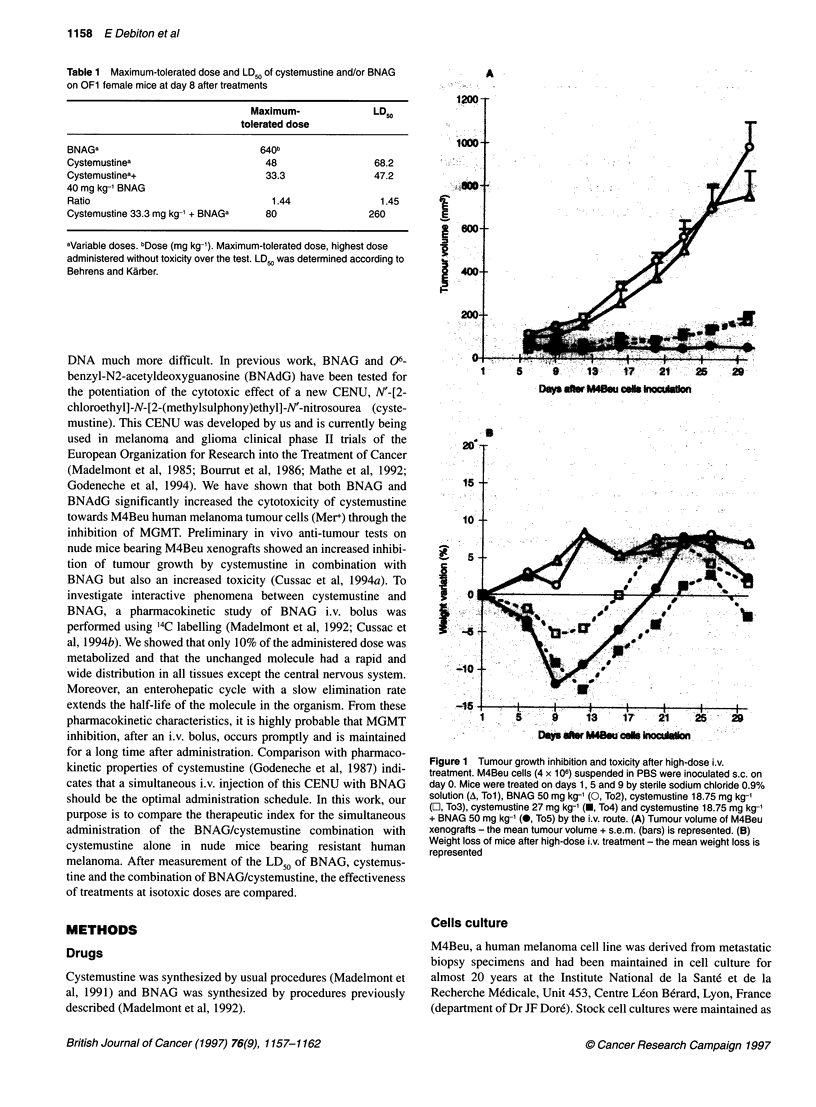
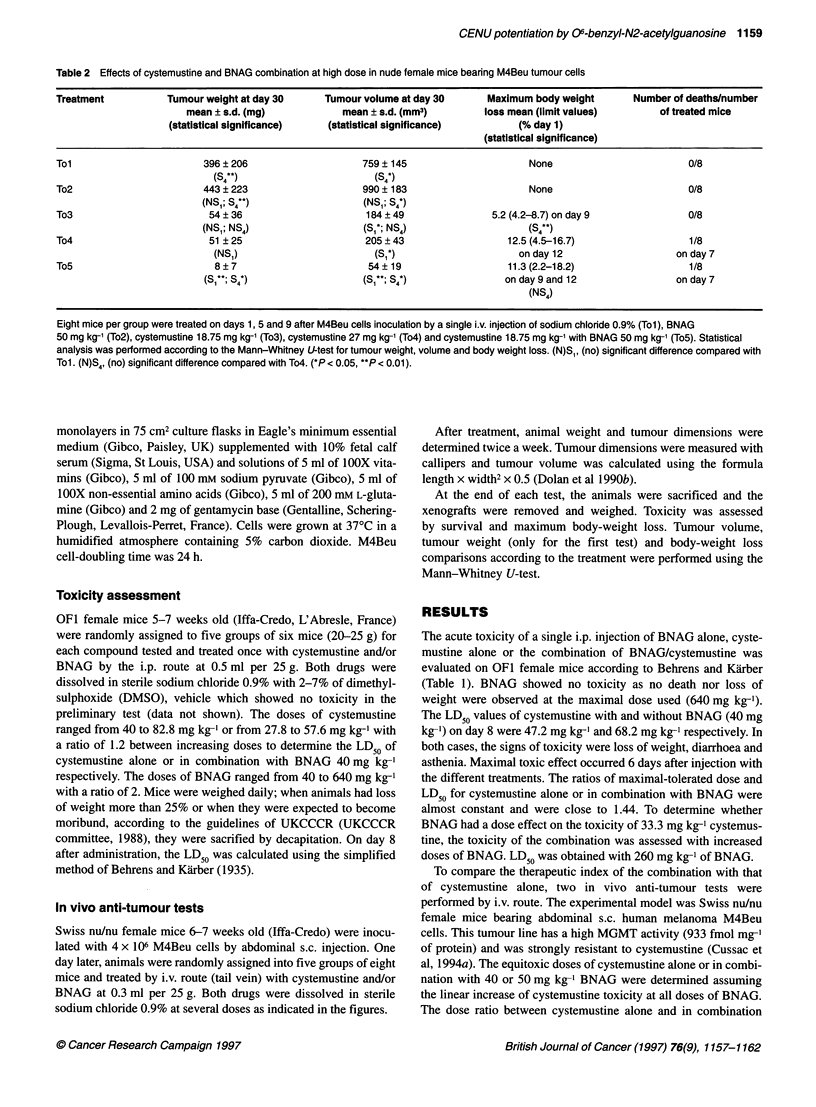
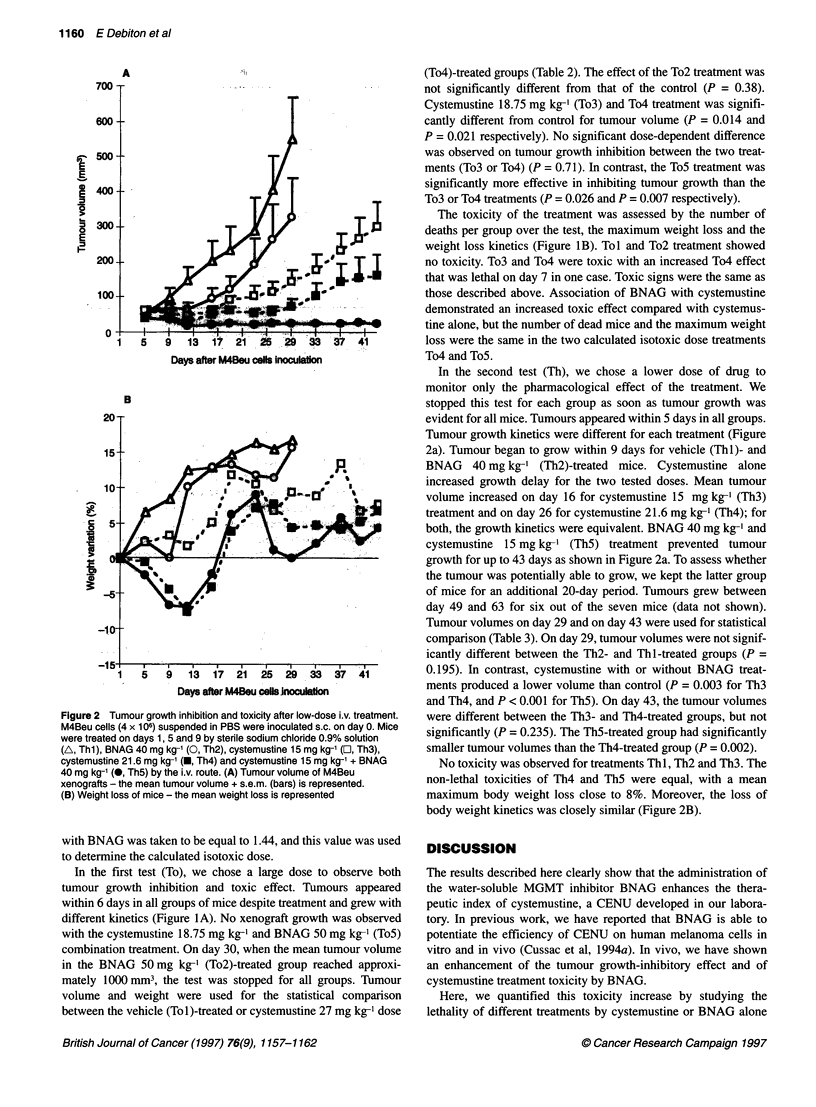
The exposure of cells to O6-benzyl-N2-acetylguanosine (BNAG) and several guanine derivatives is known to reduce the activity of O6-alkylguanine-DNA alkyltransferase (MGMT) and to enhance the sensitivity of Mer+ (methyl enzyme repair positive) tumour cells to chloroethylnitrosoureas (CENUs) in vitro and in vivo. High water solubility and the pharmacokinetic properties of BNAG make it a candidate for simultaneous administration with CENUs by the i.v. route in human clinical use. In vivo we have shown previously that BNAG significantly increases the efficiency of N'-[2-chloroethyl]-N-[2-(methylsulphonyl)ethyl]-N'-nitrosourea (cystemustine) against M4Beu melanoma cells (Mer+) through its cytostatic activity by the i.p. route, but also increases its toxicity. To investigate the toxicity of BNAG and cystemustine when administered simultaneously in mice, we compared the maximum tolerated dose and LD50 doses of cystemustine alone or in combination with 40 mg kg(-1) BNAG by the i.p. route. The toxicity of cystemustine was enhanced by a factor of almost 1.44 when combined with BNAG. To compare the therapeutic index of cystemustine alone and the cystemustine/BNAG combination, pharmacological tests were carried out in nude mice bearing Mer+ M4Beu human melanoma cells. Isotoxic doses were calculated using the 1.44 ratio. The treatments were administered three times by the i.v. route on days 1, 5 and 9 after s.c. inoculation of tumour cells. Although the toxicities of the treatments were equal, BNAG strongly enhanced tumour growth inhibition. These results demonstrate the increase of the therapeutic index of cystemustine by BNAG and justify the use of BNAG to enhance nitrosourea efficiency in vivo by i.v. co-injection.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baer J. C., Freeman A. A., Newlands E. S., Watson A. J., Rafferty J. A., Margison G. P. Depletion of O6-alkylguanine-DNA alkyltransferase correlates with potentiation of temozolomide and CCNU toxicity in human tumour cells. Br J Cancer. 1993 Jun;67(6):1299–1302. doi: 10.1038/bjc.1993.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourut C., Chenu E., Godenèche D., Madelmont J. C., Maral R., Mathé G., Meyniel G. Cytostatic action of two nitrosoureas derived from cysteamine. Br J Pharmacol. 1986 Nov;89(3):539–546. doi: 10.1111/j.1476-5381.1986.tb11154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cussac C., Mounetou E., Rapp M., Madelmont J. C., Maurizis J. C., Labarre P., Chollet P., Chabard J. L., Godeneche D., Baudry J. P. Disposition and metabolism of O6-alkylguanine-DNA alkyltransferase inhibitor in nude mice bearing human melanoma. Drug Metab Dispos. 1994 Jul-Aug;22(4):637–642. [PubMed] [Google Scholar]
- Cussac C., Rapp M., Mounetou E., Madelmont J. C., Maurizis J. C., Godeneche D., Dupuy J. M., Sauzieres J., Baudry J. P., Veyre A. Enhancement by O6-benzyl-N-acetylguanosine derivatives of chloroethylnitrosourea antitumor action in chloroethylnitrosourea-resistant human malignant melanocytes. J Pharmacol Exp Ther. 1994 Dec;271(3):1353–1358. [PubMed] [Google Scholar]
- D'Incalci M., Citti L., Taverna P., Catapano C. V. Importance of the DNA repair enzyme O6-alkyl guanine alkyltransferase (AT) in cancer chemotherapy. Cancer Treat Rev. 1988 Dec;15(4):279–292. doi: 10.1016/0305-7372(88)90026-6. [DOI] [PubMed] [Google Scholar]
- Dolan M. E., Mitchell R. B., Mummert C., Moschel R. C., Pegg A. E. Effect of O6-benzylguanine analogues on sensitivity of human tumor cells to the cytotoxic effects of alkylating agents. Cancer Res. 1991 Jul 1;51(13):3367–3372. [PubMed] [Google Scholar]
- Dolan M. E., Moschel R. C., Pegg A. E. Depletion of mammalian O6-alkylguanine-DNA alkyltransferase activity by O6-benzylguanine provides a means to evaluate the role of this protein in protection against carcinogenic and therapeutic alkylating agents. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5368–5372. doi: 10.1073/pnas.87.14.5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan M. E., Stine L., Mitchell R. B., Moschel R. C., Pegg A. E. Modulation of mammalian O6-alkylguanine-DNA alkyltransferase in vivo by O6-benzylguanine and its effect on the sensitivity of a human glioma tumor to 1-(2-chloroethyl)-3-(4-methylcyclohexyl)-1-nitrosourea. Cancer Commun. 1990;2(11):371–377. doi: 10.3727/095535490820873985. [DOI] [PubMed] [Google Scholar]
- Friedman H. S., Dolan M. E., Moschel R. C., Pegg A. E., Felker G. M., Rich J., Bigner D. D., Schold S. C., Jr Enhancement of nitrosourea activity in medulloblastoma and glioblastoma multiforme. J Natl Cancer Inst. 1992 Dec 16;84(24):1926–1931. doi: 10.1093/jnci/84.24.1926. [DOI] [PubMed] [Google Scholar]
- Gerson S. L., Willson J. K. O6-alkylguanine-DNA alkyltransferase. A target for the modulation of drug resistance. Hematol Oncol Clin North Am. 1995 Apr;9(2):431–450. [PubMed] [Google Scholar]
- Gerson S. L., Zborowska E., Norton K., Gordon N. H., Willson J. K. Synergistic efficacy of O6-benzylguanine and 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) in a human colon cancer xenograft completely resistant to BCNU alone. Biochem Pharmacol. 1993 Jan 26;45(2):483–491. doi: 10.1016/0006-2952(93)90086-c. [DOI] [PubMed] [Google Scholar]
- Godeneche D., Rapp M., Thierry A., Laval F., Madelmont J. C., Chollet P., Veyre A. DNA damage induced by a new 2-chloroethyl nitrosourea on malignant melanoma cells. Cancer Res. 1990 Sep 15;50(18):5898–5903. [PubMed] [Google Scholar]
- Godenèche D., Madelmont J. C., Labarre P., Plagne R., Meyniel G. Disposition of new sulphur-containing 2-(chloroethyl)nitrosoureas in rats. Xenobiotica. 1987 Jan;17(1):59–70. doi: 10.3109/00498258709047175. [DOI] [PubMed] [Google Scholar]
- Lee S. M., Betticher D. C., Thatcher N. Melanoma: chemotherapy. Br Med Bull. 1995 Jul;51(3):609–630. doi: 10.1093/oxfordjournals.bmb.a072982. [DOI] [PubMed] [Google Scholar]
- Lemoine A., Lucas C., Ings R. M. Metabolism of the chloroethylnitrosoureas. Xenobiotica. 1991 Jun;21(6):775–791. doi: 10.3109/00498259109039517. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Sedgwick B., Sekiguchi M., Nakabeppu Y. Regulation and expression of the adaptive response to alkylating agents. Annu Rev Biochem. 1988;57:133–157. doi: 10.1146/annurev.bi.57.070188.001025. [DOI] [PubMed] [Google Scholar]
- Madelmont J. C., Godeneche D., Parry D., Duprat J., Chabard J. L., Plagne R., Mathe G., Meyniel G. New cysteamine (2-chloroethyl)nitrosoureas. Synthesis and preliminary antitumor results. J Med Chem. 1985 Sep;28(9):1346–1350. doi: 10.1021/jm00147a040. [DOI] [PubMed] [Google Scholar]
- Mathé G., Misset J. L., Triana B. K., Godenèche D., Madelmont J. C., Meyniel G. Phase I trial of cystemustine, a new cysteamine (2-chloroethyl) nitrosourea: an intrapatient escalation scheme. Drugs Exp Clin Res. 1992;18(4):155–158. [PubMed] [Google Scholar]
- Mineura K., Izumi I., Watanabe K., Kowada M. Influence of O6-methylguanine-DNA methyltransferase activity on chloroethylnitrosourea chemotherapy in brain tumors. Int J Cancer. 1993 Aug 19;55(1):76–81. doi: 10.1002/ijc.2910550115. [DOI] [PubMed] [Google Scholar]
- Mineura K., Izumi I., Watanabe K., Kowada M., Kohda K., Koyama K., Terashima I., Ikenaga M. Enhancing effect of O6-alkylguanine derivatives on chloroethylnitrosourea cytotoxicity toward tumor cells. Int J Cancer. 1994 Sep 1;58(5):706–712. doi: 10.1002/ijc.2910580515. [DOI] [PubMed] [Google Scholar]
- Mitchell R. B., Moschel R. C., Dolan M. E. Effect of O6-benzylguanine on the sensitivity of human tumor xenografts to 1,3-bis(2-chloroethyl)-1-nitrosourea and on DNA interstrand cross-link formation. Cancer Res. 1992 Mar 1;52(5):1171–1175. [PubMed] [Google Scholar]
- Moschel R. C., McDougall M. G., Dolan M. E., Stine L., Pegg A. E. Structural features of substituted purine derivatives compatible with depletion of human O6-alkylguanine-DNA alkyltransferase. J Med Chem. 1992 Nov 13;35(23):4486–4491. doi: 10.1021/jm00101a028. [DOI] [PubMed] [Google Scholar]
- Nagane M., Asai A., Shibui S., Nomura K., Matsutani M., Kuchino Y. Expression of O6-methylguanine-DNA methyltransferase and chloroethylnitrosourea resistance of human brain tumors. Jpn J Clin Oncol. 1992 Jun;22(3):143–149. [PubMed] [Google Scholar]
- Pegg A. E., Byers T. L. Repair of DNA containing O6-alkylguanine. FASEB J. 1992 Mar;6(6):2302–2310. doi: 10.1096/fasebj.6.6.1544541. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Dolan M. E., Moschel R. C. Structure, function, and inhibition of O6-alkylguanine-DNA alkyltransferase. Prog Nucleic Acid Res Mol Biol. 1995;51:167–223. doi: 10.1016/s0079-6603(08)60879-x. [DOI] [PubMed] [Google Scholar]
- Schold S. C., Jr, Kokkinakis D. M., Rudy J. L., Moschel R. C., Pegg A. E. Treatment of human brain tumor xenografts with O6-benzyl-2'-deoxyguanosine and BCNU. Cancer Res. 1996 May 1;56(9):2076–2081. [PubMed] [Google Scholar]
- Tong W. P., Kirk M. C., Ludlum D. B. Mechanism of action of the nitrosoureas--V. Formation of O6-(2-fluoroethyl)guanine and its probable role in the crosslinking of deoxyribonucleic acid. Biochem Pharmacol. 1983 Jul 1;32(13):2011–2015. doi: 10.1016/0006-2952(83)90420-3. [DOI] [PubMed] [Google Scholar]



