Abstract
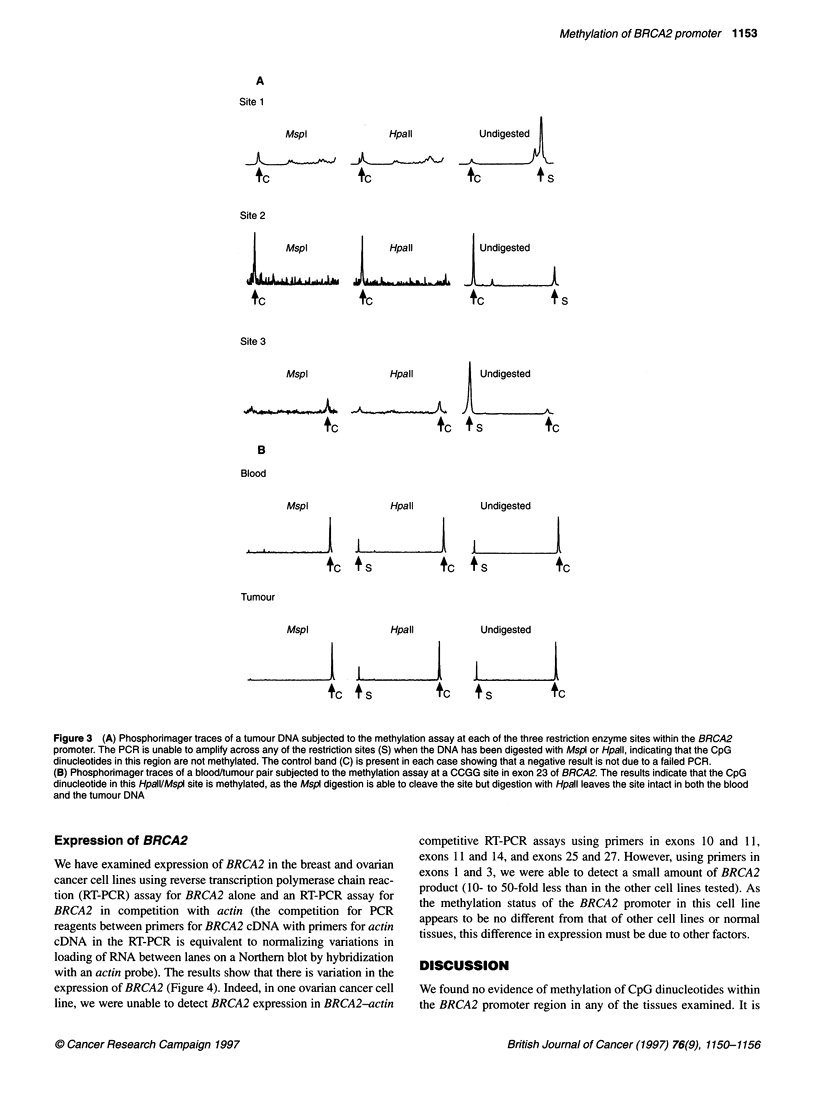
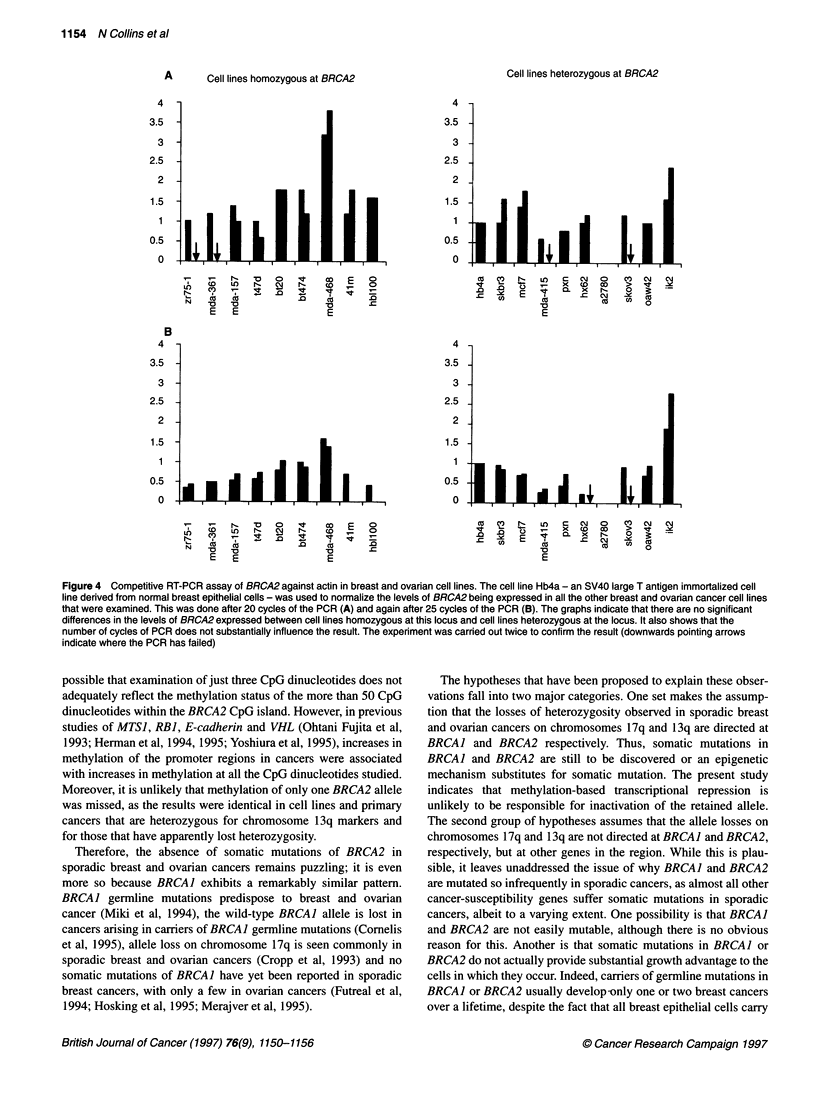
Germline mutations of the BRCA2 gene on chromosome 13q12-q13 predispose to the development of early-onset breast cancer and ovarian cancer. Loss of heterozygosity detected using chromosome 13q markers in the vicinity of BRCA2 is observed in most cancers arising in carriers of germline BRCA2 mutations and also in 30-50% of sporadic breast and ovarian cancers. However, somatic mutations of BRCA2 are extremely rare in sporadic cancers. We have examined the hypothesis that expression of the BRCA2 gene may be suppressed in sporadic breast cancers by a mechanism that is associated with increased methylation of cytosine residues in the promoter region. Using a HpaII/MspI digestion-polymerase chain reaction based assay, the presence of 5-methylcytosine in three CpG dinucleotides within the BRCA2 promoter was assessed in 18 breast or ovarian cancer cell lines, in an SV40 large T antigen immortalized cell line derived from normal breast epithelial cells, in 64 primary sporadic breast cancers and peripheral blood leucocytes from these cases and in a number of other normal human tissues. Methylation was not detected in any of the tissues examined, suggesting that this mechanism of transcriptional repression is unlikely to explain the absence of somatic mutations in sporadic cancers.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bird A. P. CpG-rich islands and the function of DNA methylation. Nature. 1986 May 15;321(6067):209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- Caldas C., Hahn S. A., da Costa L. T., Redston M. S., Schutte M., Seymour A. B., Weinstein C. L., Hruban R. H., Yeo C. J., Kern S. E. Frequent somatic mutations and homozygous deletions of the p16 (MTS1) gene in pancreatic adenocarcinoma. Nat Genet. 1994 Sep;8(1):27–32. doi: 10.1038/ng0994-27. [DOI] [PubMed] [Google Scholar]
- Cleton-Jansen A. M., Collins N., Lakhani S. R., Weissenbach J., Devilee P., Cornelisse C. J., Stratton M. R. Loss of heterozygosity in sporadic breast tumours at the BRCA2 locus on chromosome 13q12-q13. Br J Cancer. 1995 Nov;72(5):1241–1244. doi: 10.1038/bjc.1995.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins N., McManus R., Wooster R., Mangion J., Seal S., Lakhani S. R., Ormiston W., Daly P. A., Ford D., Easton D. F. Consistent loss of the wild type allele in breast cancers from a family linked to the BRCA2 gene on chromosome 13q12-13. Oncogene. 1995 Apr 20;10(8):1673–1675. [PubMed] [Google Scholar]
- Cropp C. S., Champeme M. H., Lidereau R., Callahan R. Identification of three regions on chromosome 17q in primary human breast carcinomas which are frequently deleted. Cancer Res. 1993 Dec 1;53(23):5617–5619. [PubMed] [Google Scholar]
- Devilee P., van den Broek M., Kuipers-Dijkshoorn N., Kolluri R., Khan P. M., Pearson P. L., Cornelisse C. J. At least four different chromosomal regions are involved in loss of heterozygosity in human breast carcinoma. Genomics. 1989 Oct;5(3):554–560. doi: 10.1016/0888-7543(89)90023-2. [DOI] [PubMed] [Google Scholar]
- Foster K. A., Harrington P., Kerr J., Russell P., DiCioccio R. A., Scott I. V., Jacobs I., Chenevix-Trench G., Ponder B. A., Gayther S. A. Somatic and germline mutations of the BRCA2 gene in sporadic ovarian cancer. Cancer Res. 1996 Aug 15;56(16):3622–3625. [PubMed] [Google Scholar]
- Futreal P. A., Liu Q., Shattuck-Eidens D., Cochran C., Harshman K., Tavtigian S., Bennett L. M., Haugen-Strano A., Swensen J., Miki Y. BRCA1 mutations in primary breast and ovarian carcinomas. Science. 1994 Oct 7;266(5182):120–122. doi: 10.1126/science.7939630. [DOI] [PubMed] [Google Scholar]
- Gessler M., König A., Arden K., Grundy P., Orkin S., Sallan S., Peters C., Ruyle S., Mandell J., Li F. Infrequent mutation of the WT1 gene in 77 Wilms' Tumors. Hum Mutat. 1994;3(3):212–222. doi: 10.1002/humu.1380030307. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Zulueta M., Bender C. M., Yang A. S., Nguyen T., Beart R. W., Van Tornout J. M., Jones P. A. Methylation of the 5' CpG island of the p16/CDKN2 tumor suppressor gene in normal and transformed human tissues correlates with gene silencing. Cancer Res. 1995 Oct 15;55(20):4531–4535. [PubMed] [Google Scholar]
- Herman J. G., Latif F., Weng Y., Lerman M. I., Zbar B., Liu S., Samid D., Duan D. S., Gnarra J. R., Linehan W. M. Silencing of the VHL tumor-suppressor gene by DNA methylation in renal carcinoma. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):9700–9704. doi: 10.1073/pnas.91.21.9700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman J. G., Merlo A., Mao L., Lapidus R. G., Issa J. P., Davidson N. E., Sidransky D., Baylin S. B. Inactivation of the CDKN2/p16/MTS1 gene is frequently associated with aberrant DNA methylation in all common human cancers. Cancer Res. 1995 Oct 15;55(20):4525–4530. [PubMed] [Google Scholar]
- Hosking L., Trowsdale J., Nicolai H., Solomon E., Foulkes W., Stamp G., Signer E., Jeffreys A. A somatic BRCA1 mutation in an ovarian tumour. Nat Genet. 1995 Apr;9(4):343–344. doi: 10.1038/ng0495-343. [DOI] [PubMed] [Google Scholar]
- Kerangueven F., Allione F., Noguchi T., Adélaïde J., Sobol H., Jacquemier J., Birnbaum D. Patterns of loss of heterozygosity at loci from chromosome arm 13q suggests a possible involvement of BRCA2 in sporadic breast tumors. Genes Chromosomes Cancer. 1995 Aug;13(4):291–294. doi: 10.1002/gcc.2870130410. [DOI] [PubMed] [Google Scholar]
- Lancaster J. M., Wooster R., Mangion J., Phelan C. M., Cochran C., Gumbs C., Seal S., Barfoot R., Collins N., Bignell G. BRCA2 mutations in primary breast and ovarian cancers. Nat Genet. 1996 Jun;13(2):238–240. doi: 10.1038/ng0696-238. [DOI] [PubMed] [Google Scholar]
- Lekanne Deprez R. H., Bianchi A. B., Groen N. A., Seizinger B. R., Hagemeijer A., van Drunen E., Bootsma D., Koper J. W., Avezaat C. J., Kley N. Frequent NF2 gene transcript mutations in sporadic meningiomas and vestibular schwannomas. Am J Hum Genet. 1994 Jun;54(6):1022–1029. [PMC free article] [PubMed] [Google Scholar]
- Li Y., Bollag G., Clark R., Stevens J., Conroy L., Fults D., Ward K., Friedman E., Samowitz W., Robertson M. Somatic mutations in the neurofibromatosis 1 gene in human tumors. Cell. 1992 Apr 17;69(2):275–281. doi: 10.1016/0092-8674(92)90408-5. [DOI] [PubMed] [Google Scholar]
- Merajver S. D., Pham T. M., Caduff R. F., Chen M., Poy E. L., Cooney K. A., Weber B. L., Collins F. S., Johnston C., Frank T. S. Somatic mutations in the BRCA1 gene in sporadic ovarian tumours. Nat Genet. 1995 Apr;9(4):439–443. doi: 10.1038/ng0495-439. [DOI] [PubMed] [Google Scholar]
- Miki Y., Katagiri T., Kasumi F., Yoshimoto T., Nakamura Y. Mutation analysis in the BRCA2 gene in primary breast cancers. Nat Genet. 1996 Jun;13(2):245–247. doi: 10.1038/ng0696-245. [DOI] [PubMed] [Google Scholar]
- Nigro J. M., Baker S. J., Preisinger A. C., Jessup J. M., Hostetter R., Cleary K., Bigner S. H., Davidson N., Baylin S., Devilee P. Mutations in the p53 gene occur in diverse human tumour types. Nature. 1989 Dec 7;342(6250):705–708. doi: 10.1038/342705a0. [DOI] [PubMed] [Google Scholar]
- Ohtani-Fujita N., Fujita T., Aoike A., Osifchin N. E., Robbins P. D., Sakai T. CpG methylation inactivates the promoter activity of the human retinoblastoma tumor-suppressor gene. Oncogene. 1993 Apr;8(4):1063–1067. [PubMed] [Google Scholar]
- Powell S. M., Zilz N., Beazer-Barclay Y., Bryan T. M., Hamilton S. R., Thibodeau S. N., Vogelstein B., Kinzler K. W. APC mutations occur early during colorectal tumorigenesis. Nature. 1992 Sep 17;359(6392):235–237. doi: 10.1038/359235a0. [DOI] [PubMed] [Google Scholar]
- Shuin T., Kondo K., Torigoe S., Kishida T., Kubota Y., Hosaka M., Nagashima Y., Kitamura H., Latif F., Zbar B. Frequent somatic mutations and loss of heterozygosity of the von Hippel-Lindau tumor suppressor gene in primary human renal cell carcinomas. Cancer Res. 1994 Jun 1;54(11):2852–2855. [PubMed] [Google Scholar]
- T'Ang A., Varley J. M., Chakraborty S., Murphree A. L., Fung Y. K. Structural rearrangement of the retinoblastoma gene in human breast carcinoma. Science. 1988 Oct 14;242(4876):263–266. doi: 10.1126/science.3175651. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Chiu H. C., Bandera C. A., Behbakht K., Liu P. C., Couch F. J., Weber B. L., LiVolsi V. A., Furusato M., Rebane B. A. Mutations of the BRCA2 gene in ovarian carcinomas. Cancer Res. 1996 Jun 15;56(12):2738–2741. [PubMed] [Google Scholar]
- Tavtigian S. V., Simard J., Rommens J., Couch F., Shattuck-Eidens D., Neuhausen S., Merajver S., Thorlacius S., Offit K., Stoppa-Lyonnet D. The complete BRCA2 gene and mutations in chromosome 13q-linked kindreds. Nat Genet. 1996 Mar;12(3):333–337. doi: 10.1038/ng0396-333. [DOI] [PubMed] [Google Scholar]
- Teng D. H., Bogden R., Mitchell J., Baumgard M., Bell R., Berry S., Davis T., Ha P. C., Kehrer R., Jammulapati S. Low incidence of BRCA2 mutations in breast carcinoma and other cancers. Nat Genet. 1996 Jun;13(2):241–244. doi: 10.1038/ng0696-241. [DOI] [PubMed] [Google Scholar]
- Wooster R., Bignell G., Lancaster J., Swift S., Seal S., Mangion J., Collins N., Gregory S., Gumbs C., Micklem G. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995 Dec 21;378(6559):789–792. doi: 10.1038/378789a0. [DOI] [PubMed] [Google Scholar]
- Yang-Feng T. L., Han H., Chen K. C., Li S. B., Claus E. B., Carcangiu M. L., Chambers S. K., Chambers J. T., Schwartz P. E. Allelic loss in ovarian cancer. Int J Cancer. 1993 Jun 19;54(4):546–551. doi: 10.1002/ijc.2910540405. [DOI] [PubMed] [Google Scholar]
- Yoshiura K., Kanai Y., Ochiai A., Shimoyama Y., Sugimura T., Hirohashi S. Silencing of the E-cadherin invasion-suppressor gene by CpG methylation in human carcinomas. Proc Natl Acad Sci U S A. 1995 Aug 1;92(16):7416–7419. doi: 10.1073/pnas.92.16.7416. [DOI] [PMC free article] [PubMed] [Google Scholar]



