Abstract
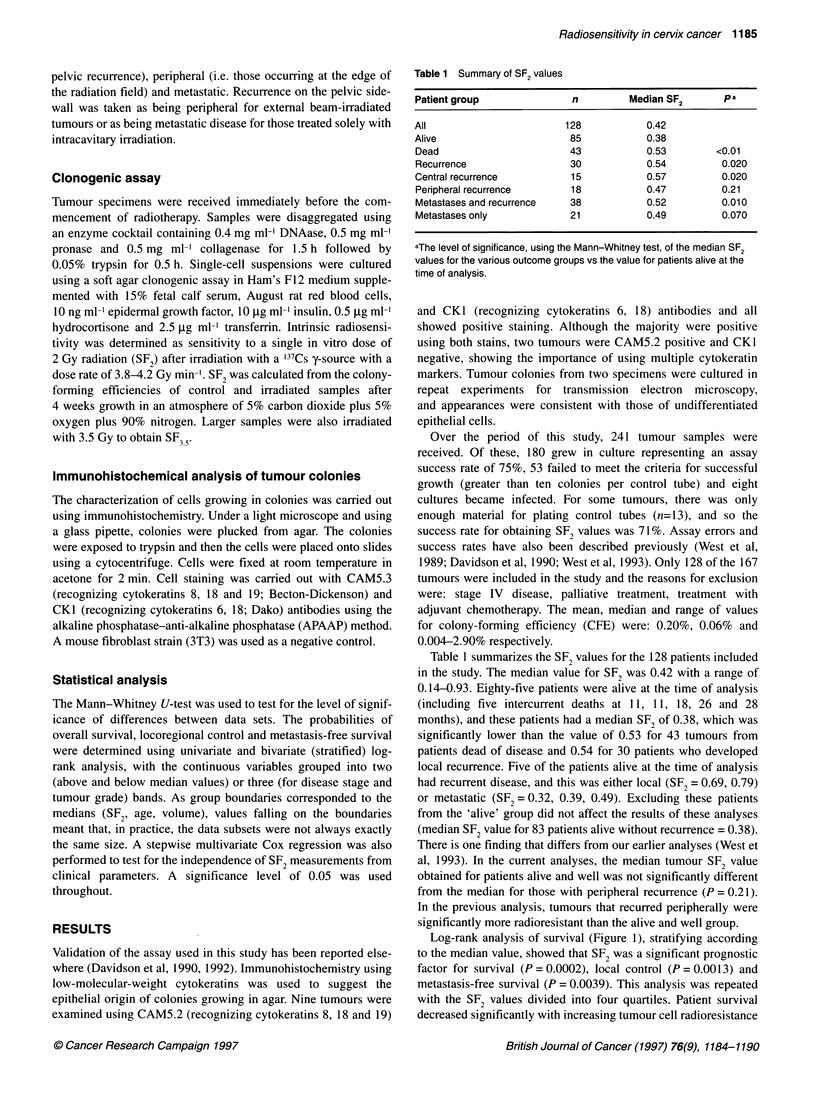
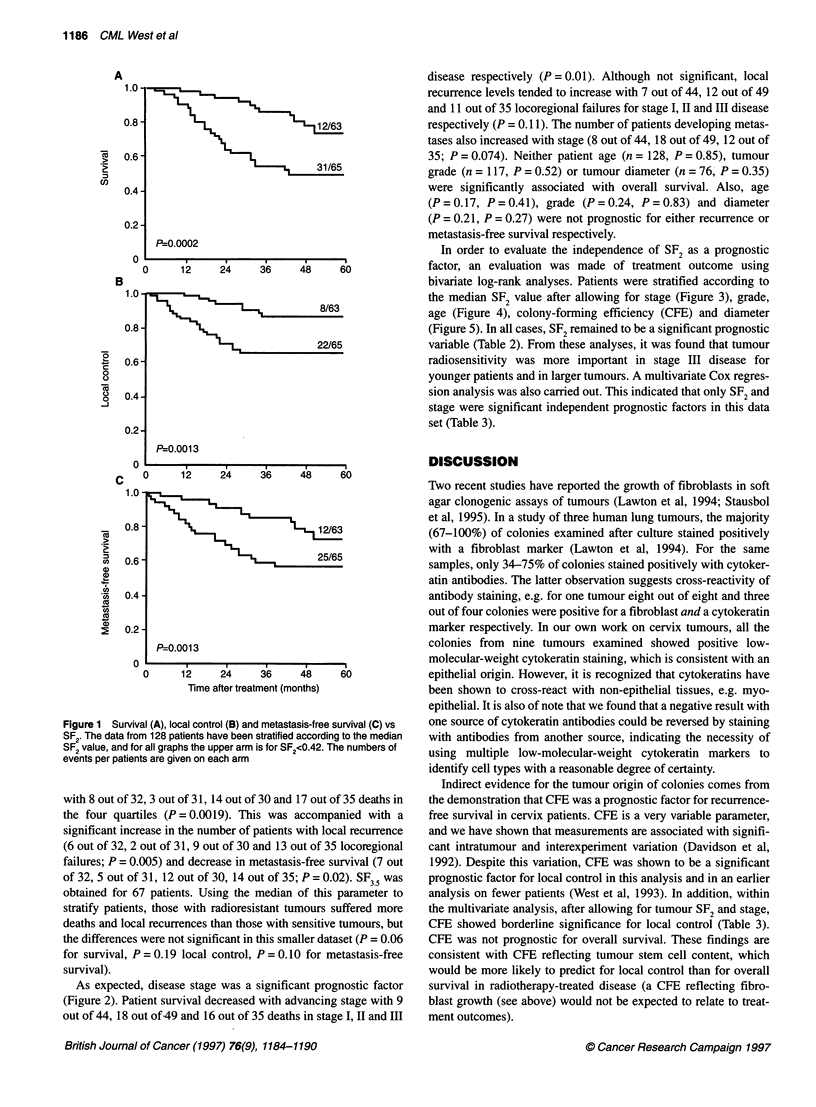
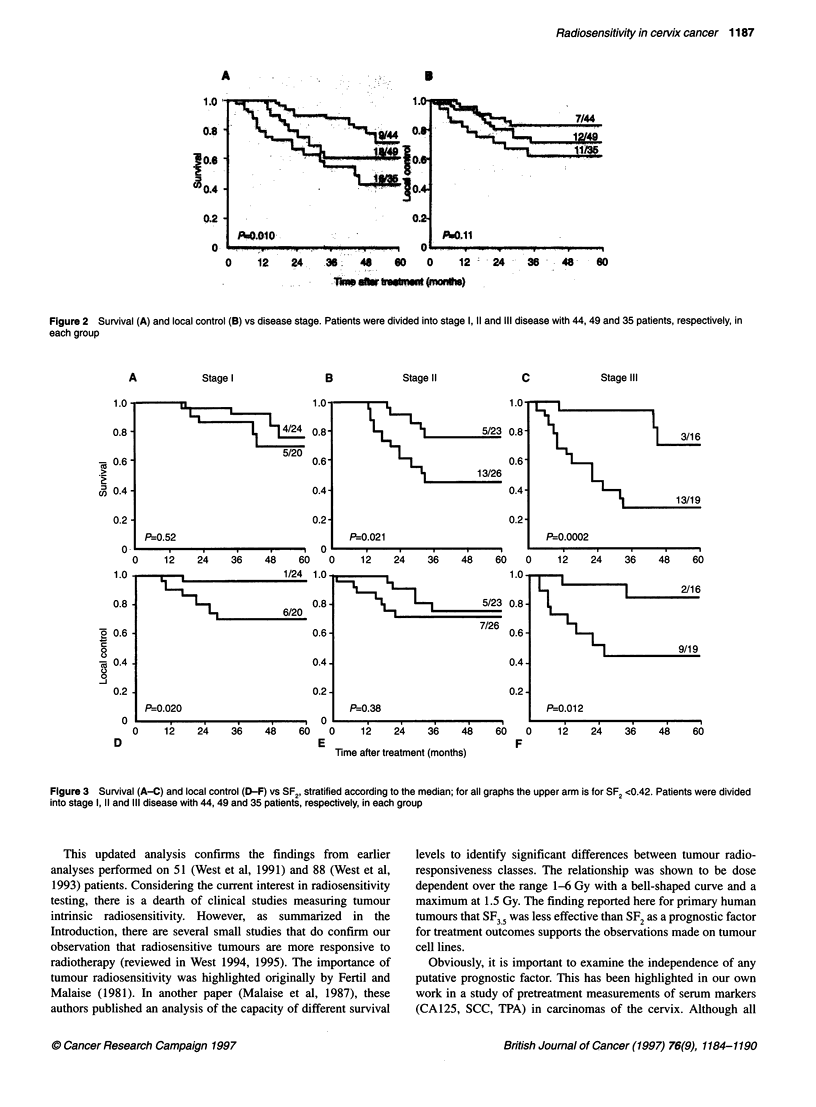
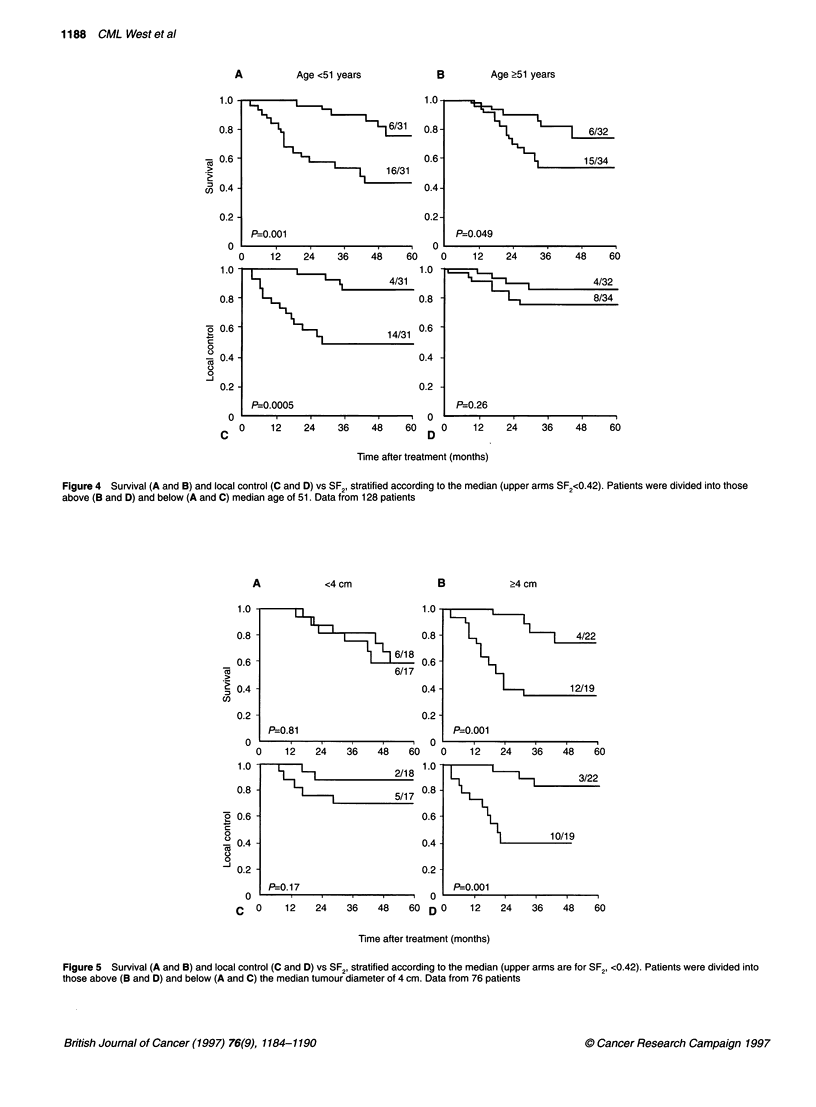
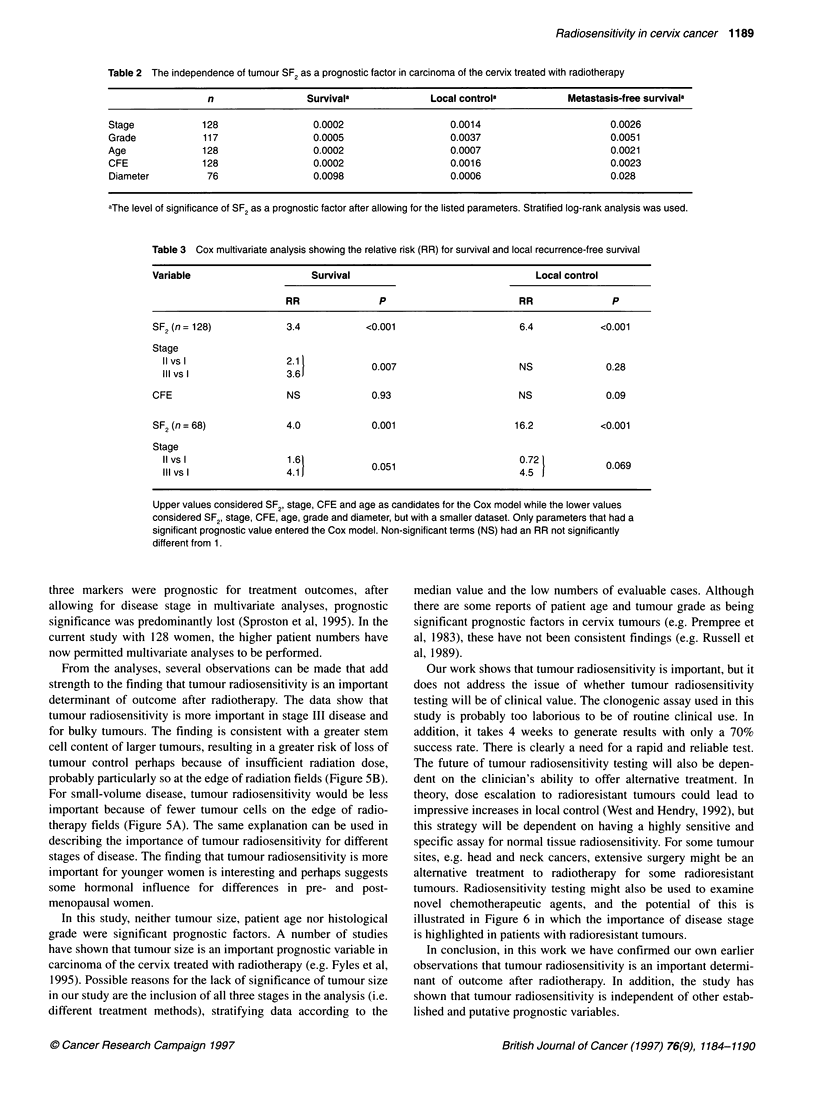
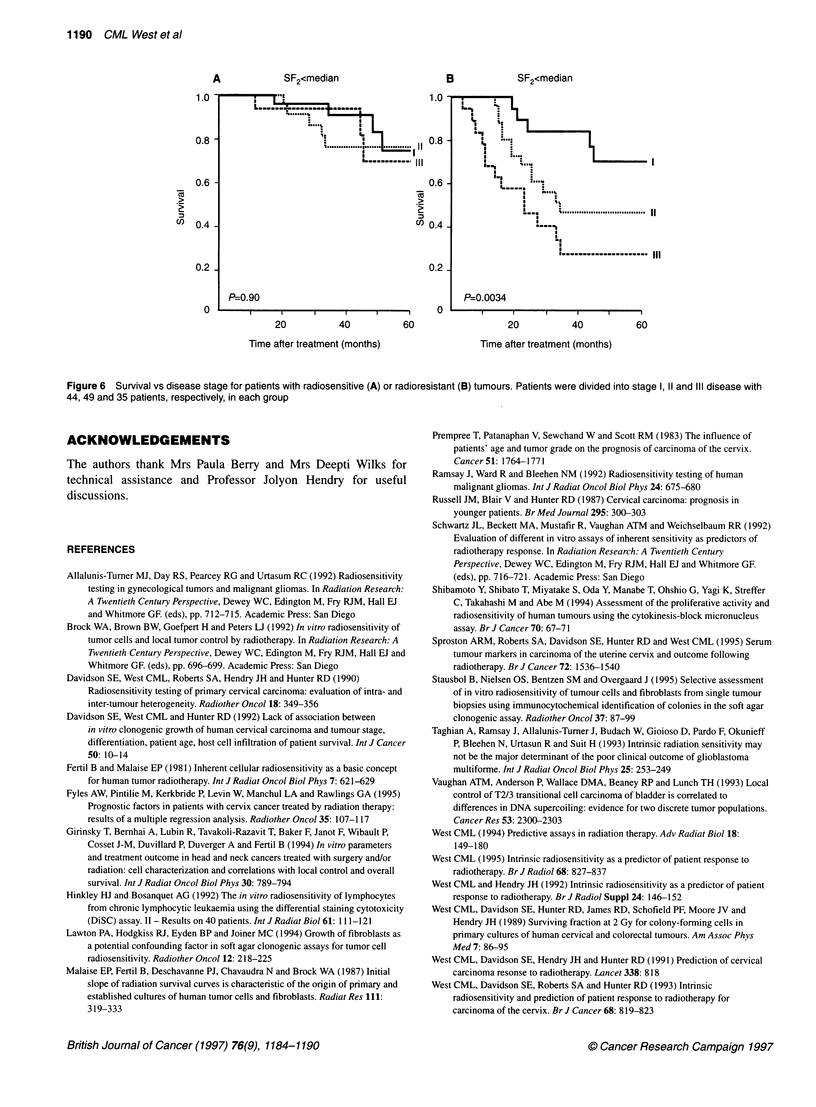
A study was made of the prognostic value of pretreatment measurements of tumour radiosensitivity (surviving fraction at 2 Gy, SF2) in 128 patients with stage I-III carcinomas of the uterine cervix undergoing radiotherapy. The median follow-up time was 47 months. In a univariate analysis stratifying patients according to the median value, radiosensitivity was a significant prognostic factor for overall survival, local control and metastasis-free survival. The 5-year survival rate for tumours with SF2 values below the median was 81% and was significantly greater than the rate of 51% for those with SF2 values above the median. In bivariate analyses, SF2 was shown to be independent of disease stage, tumour grade, patient age, colony-forming efficiency and tumour diameter. In a multivariate analysis, radiosensitivity was the most important variable and, after allowing for this, only stage was a significant independent predictor of treatment outcome. These data indicate that, in carcinoma of the cervix treated with radiotherapy, pretreatment tumour intrinsic radiosensitivity is an important prognostic parameter and contributes to prognosis independently of other established and putative parameters.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davidson S. E., West C. M., Hunter R. D. Lack of association between in vitro clonogenic growth of human cervical carcinoma and tumour stage, differentiation, patient age, host cell infiltration or patient survival. Int J Cancer. 1992 Jan 2;50(1):10–14. doi: 10.1002/ijc.2910500104. [DOI] [PubMed] [Google Scholar]
- Davidson S. E., West C. M., Roberts S. A., Hendry J. H., Hunter R. D. Radiosensitivity testing of primary cervical carcinoma: evaluation of intra- and inter-tumour heterogeneity. Radiother Oncol. 1990 Aug;18(4):349–356. doi: 10.1016/0167-8140(90)90115-d. [DOI] [PubMed] [Google Scholar]
- Fertil B., Malaise E. P. Inherent cellular radiosensitivity as a basic concept for human tumor radiotherapy. Int J Radiat Oncol Biol Phys. 1981 May;7(5):621–629. doi: 10.1016/0360-3016(81)90377-1. [DOI] [PubMed] [Google Scholar]
- Fyles A. W., Pintilie M., Kirkbride P., Levin W., Manchul L. A., Rawlings G. A. Prognostic factors in patients with cervix cancer treated by radiation therapy: results of a multiple regression analysis. Radiother Oncol. 1995 May;35(2):107–117. doi: 10.1016/0167-8140(95)01535-o. [DOI] [PubMed] [Google Scholar]
- Girinsky T., Bernheim A., Lubin R., Tavakoli-Razavi T., Baker F., Janot F., Wibault P., Cosset J. M., Duvillard P., Duverger A. In vitro parameters and treatment outcome in head and neck cancers treated with surgery and/or radiation: cell characterization and correlations with local control and overall survival. Int J Radiat Oncol Biol Phys. 1994 Nov 15;30(4):789–794. doi: 10.1016/0360-3016(94)90350-6. [DOI] [PubMed] [Google Scholar]
- Hinkley H. J., Bosanquet A. G. The in vitro radiosensitivity of lymphocytes from chronic lymphocytic leukaemia using the differential staining cytotoxicity (DiSC) assay. II--Results on 40 patients. Int J Radiat Biol. 1992 Jan;61(1):111–121. doi: 10.1080/09553009214550681. [DOI] [PubMed] [Google Scholar]
- Lawton P. A., Hodgkiss R. J., Eyden B. P., Joiner M. C. Growth of fibroblasts as a potential confounding factor in soft agar clonogenic assays for tumour cell radiosensitivity. Radiother Oncol. 1994 Sep;32(3):218–225. doi: 10.1016/0167-8140(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Malaise E. P., Fertil B., Deschavanne P. J., Chavaudra N., Brock W. A. Initial slope of radiation survival curves is characteristic of the origin of primary and established cultures of human tumor cells and fibroblasts. Radiat Res. 1987 Aug;111(2):319–333. [PubMed] [Google Scholar]
- Prempree T., Patanaphan V., Sewchand W., Scott R. M. The influence of patients' age and tumor grade on the prognosis of carcinoma of the cervix. Cancer. 1983 May 1;51(9):1764–1771. doi: 10.1002/1097-0142(19830501)51:9<1764::aid-cncr2820510934>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Ramsay J., Ward R., Bleehen N. M. Radiosensitivity testing of human malignant gliomas. Int J Radiat Oncol Biol Phys. 1992;24(4):675–680. doi: 10.1016/0360-3016(92)90713-r. [DOI] [PubMed] [Google Scholar]
- Russell J. M., Blair V., Hunter R. D. Cervical carcinoma: prognosis in younger patients. Br Med J (Clin Res Ed) 1987 Aug 1;295(6593):300–303. doi: 10.1136/bmj.295.6593.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibamoto Y., Shibata T., Miyatake S., Oda Y., Manabe T., Ohshio G., Yagi K., Streffer C., Takahashi M., Abe M. Assessment of the proliferative activity and radiosensitivity of human tumours using the cytokinesis-block micronucleus assay. Br J Cancer. 1994 Jul;70(1):67–71. doi: 10.1038/bjc.1994.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sproston A. R., Roberts S. A., Davidson S. E., Hunter R. D., West C. M. Serum tumour markers in carcinoma of the uterine cervix and outcome following radiotherapy. Br J Cancer. 1995 Dec;72(6):1536–1540. doi: 10.1038/bjc.1995.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stausbøl-Grøn B., Nielsen O. S., Møller Bentzen S., Overgaard J. Selective assessment of in vitro radiosensitivity of tumour cells and fibroblasts from single tumour biopsies using immunocytochemical identification of colonies in the soft agar clonogenic assay. Radiother Oncol. 1995 Nov;37(2):87–99. doi: 10.1016/0167-8140(95)98589-d. [DOI] [PubMed] [Google Scholar]
- Taghian A., Ramsay J., Allalunis-Turner J., Budach W., Gioioso D., Pardo F., Okunieff P., Bleehen N., Urtasun R., Suit H. Intrinsic radiation sensitivity may not be the major determinant of the poor clinical outcome of glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 1993 Jan 15;25(2):243–249. doi: 10.1016/0360-3016(93)90345-v. [DOI] [PubMed] [Google Scholar]
- Vaughan A. T., Anderson P., Wallace D. M., Beaney R. P., Lynch T. H. Local control of T2/3 transitional cell carcinoma of bladder is correlated to differences in DNA supercoiling: evidence for two discrete tumor populations. Cancer Res. 1993 May 15;53(10 Suppl):2300–2303. [PubMed] [Google Scholar]
- West C. M., Davidson S. E., Hendry J. H., Hunter R. D. Prediction of cervical carcinoma response to radiotherapy. Lancet. 1991 Sep 28;338(8770):818–818. doi: 10.1016/0140-6736(91)90700-y. [DOI] [PubMed] [Google Scholar]
- West C. M., Davidson S. E., Roberts S. A., Hunter R. D. Intrinsic radiosensitivity and prediction of patient response to radiotherapy for carcinoma of the cervix. Br J Cancer. 1993 Oct;68(4):819–823. doi: 10.1038/bjc.1993.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West C. M., Hendry J. H. Intrinsic radiosensitivity as a predictor of patient response to radiotherapy. BJR Suppl. 1992;24:146–152. [PubMed] [Google Scholar]
- West C. M. Invited review: intrinsic radiosensitivity as a predictor of patient response to radiotherapy. Br J Radiol. 1995 Aug;68(812):827–837. doi: 10.1259/0007-1285-68-812-827. [DOI] [PubMed] [Google Scholar]


