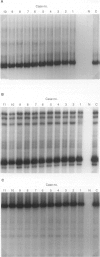
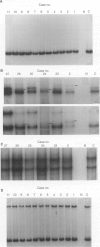
Abstract
Pituitary tumours develop at a high frequency in p27-knockout mice and retinoblastoma gene-knockout mice, which suggests that cell cycle regulatory genes, such as cyclin-dependent kinase inhibitor genes, are involved in the tumorigenesis of pituitary adenoma. Analysis of p21 and p27 gene abnormalities in human pituitary adenoma was performed in 28 pituitary adenomas by polymerase chain reaction-single-strand conformational polymorphism. No point mutations were detected in these genes. As no abnormalities of the p21 and p27genes were observed, and if these genes are indeed inactivated, it is likely to be via transcriptional or translational defects.
Full text
PDF

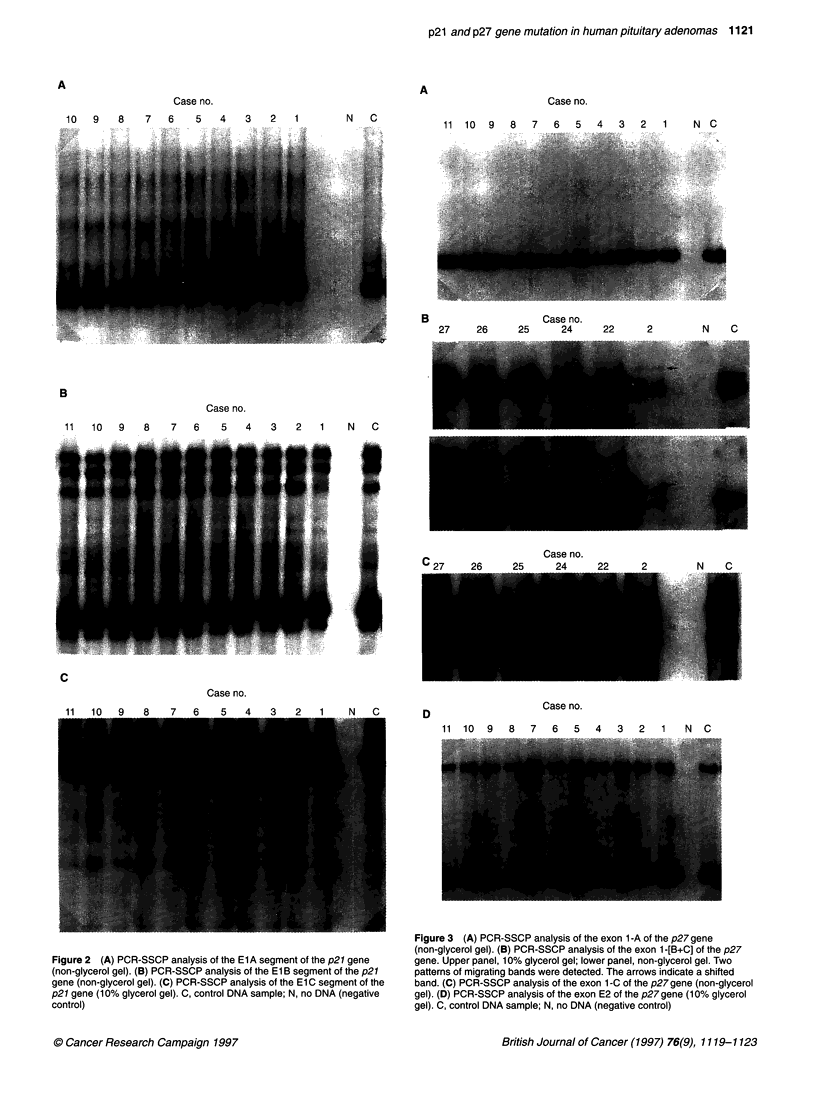


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bullrich F., MacLachlan T. K., Sang N., Druck T., Veronese M. L., Allen S. L., Chiorazzi N., Koff A., Heubner K., Croce C. M. Chromosomal mapping of members of the cdc2 family of protein kinases, cdk3, cdk6, PISSLRE, and PITALRE, and a cdk inhibitor, p27Kip1, to regions involved in human cancer. Cancer Res. 1995 Mar 15;55(6):1199–1205. [PubMed] [Google Scholar]
- Cryns V. L., Alexander J. M., Klibanski A., Arnold A. The retinoblastoma gene in human pituitary tumors. J Clin Endocrinol Metab. 1993 Sep;77(3):644–646. doi: 10.1210/jcem.77.3.7690360. [DOI] [PubMed] [Google Scholar]
- Deng C., Zhang P., Harper J. W., Elledge S. J., Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995 Aug 25;82(4):675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- Fero M. L., Rivkin M., Tasch M., Porter P., Carow C. E., Firpo E., Polyak K., Tsai L. H., Broudy V., Perlmutter R. M. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27(Kip1)-deficient mice. Cell. 1996 May 31;85(5):733–744. doi: 10.1016/s0092-8674(00)81239-8. [DOI] [PubMed] [Google Scholar]
- Hengst L., Reed S. I. Translational control of p27Kip1 accumulation during the cell cycle. Science. 1996 Mar 29;271(5257):1861–1864. doi: 10.1126/science.271.5257.1861. [DOI] [PubMed] [Google Scholar]
- Hu N., Gutsmann A., Herbert D. C., Bradley A., Lee W. H., Lee E. Y. Heterozygous Rb-1 delta 20/+mice are predisposed to tumors of the pituitary gland with a nearly complete penetrance. Oncogene. 1994 Apr;9(4):1021–1027. [PubMed] [Google Scholar]
- Ikeda H., Suzuki J., Sasano N., Niizuma H. The development and morphogenesis of the human pituitary gland. Anat Embryol (Berl) 1988;178(4):327–336. doi: 10.1007/BF00698663. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Yoshimoto T. Developmental changes in proliferative activity of cells of the murine Rathke's pouch. Cell Tissue Res. 1991 Jan;263(1):41–47. doi: 10.1007/BF00318398. [DOI] [PubMed] [Google Scholar]
- Ikeda Hidetoshi, Beauchamp Roberta L., Yoshimoto Takashi, Yandell David W. Detection of Heterozygous Mutation in the Retinoblastoma Gene in a Human Pituitary Adenoma Using PCR-SSCP Analysis and Direct Sequencing. Endocr Pathol. 1995 Autumn;6(3):189–196. doi: 10.1007/BF02739882. [DOI] [PubMed] [Google Scholar]
- Jacks T., Fazeli A., Schmitt E. M., Bronson R. T., Goodell M. A., Weinberg R. A. Effects of an Rb mutation in the mouse. Nature. 1992 Sep 24;359(6393):295–300. doi: 10.1038/359295a0. [DOI] [PubMed] [Google Scholar]
- Kato J. Y., Matsuoka M., Polyak K., Massagué J., Sherr C. J. Cyclic AMP-induced G1 phase arrest mediated by an inhibitor (p27Kip1) of cyclin-dependent kinase 4 activation. Cell. 1994 Nov 4;79(3):487–496. doi: 10.1016/0092-8674(94)90257-7. [DOI] [PubMed] [Google Scholar]
- Kiyokawa H., Kineman R. D., Manova-Todorova K. O., Soares V. C., Hoffman E. S., Ono M., Khanam D., Hayday A. C., Frohman L. A., Koff A. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27(Kip1). Cell. 1996 May 31;85(5):721–732. doi: 10.1016/s0092-8674(00)81238-6. [DOI] [PubMed] [Google Scholar]
- Koopmann J., Maintz D., Schild S., Schramm J., Louis D. N., Wiestler O. D., von Deimling A. Multiple polymorphisms, but no mutations, in the WAF1/CIP1 gene in human brain tumours. Br J Cancer. 1995 Nov;72(5):1230–1233. doi: 10.1038/bjc.1995.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLachlan T. K., Sang N., Giordano A. Cyclins, cyclin-dependent kinases and cdk inhibitors: implications in cell cycle control and cancer. Crit Rev Eukaryot Gene Expr. 1995;5(2):127–156. doi: 10.1615/critreveukargeneexpr.v5.i2.20. [DOI] [PubMed] [Google Scholar]
- Nakayama K., Ishida N., Shirane M., Inomata A., Inoue T., Shishido N., Horii I., Loh D. Y., Nakayama K. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996 May 31;85(5):707–720. doi: 10.1016/s0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- Pietenpol J. A., Bohlander S. K., Sato Y., Papadopoulos N., Liu B., Friedman C., Trask B. J., Roberts J. M., Kinzler K. W., Rowley J. D. Assignment of the human p27Kip1 gene to 12p13 and its analysis in leukemias. Cancer Res. 1995 Mar 15;55(6):1206–1210. [PubMed] [Google Scholar]
- Pines J. Cyclins, CDKs and cancer. Semin Cancer Biol. 1995 Apr;6(2):63–72. doi: 10.1006/scbi.1995.0009. [DOI] [PubMed] [Google Scholar]
- Polyak K., Lee M. H., Erdjument-Bromage H., Koff A., Roberts J. M., Tempst P., Massagué J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994 Jul 15;78(1):59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- Ponce-Castañeda M. V., Lee M. H., Latres E., Polyak K., Lacombe L., Montgomery K., Mathew S., Krauter K., Sheinfeld J., Massague J. p27Kip1: chromosomal mapping to 12p12-12p13.1 and absence of mutations in human tumors. Cancer Res. 1995 Mar 15;55(6):1211–1214. [PubMed] [Google Scholar]
- Shiohara M., el-Deiry W. S., Wada M., Nakamaki T., Takeuchi S., Yang R., Chen D. L., Vogelstein B., Koeffler H. P. Absence of WAF1 mutations in a variety of human malignancies. Blood. 1994 Dec 1;84(11):3781–3784. [PubMed] [Google Scholar]
- Toyoshima H., Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell. 1994 Jul 15;78(1):67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- Ueki K., Ono Y., Henson J. W., Efird J. T., von Deimling A., Louis D. N. CDKN2/p16 or RB alterations occur in the majority of glioblastomas and are inversely correlated. Cancer Res. 1996 Jan 1;56(1):150–153. [PubMed] [Google Scholar]
- Woloschak M., Yu A., Xiao J., Post K. D. Frequent loss of the P16INK4a gene product in human pituitary tumors. Cancer Res. 1996 Jun 1;56(11):2493–2496. [PubMed] [Google Scholar]