Abstract
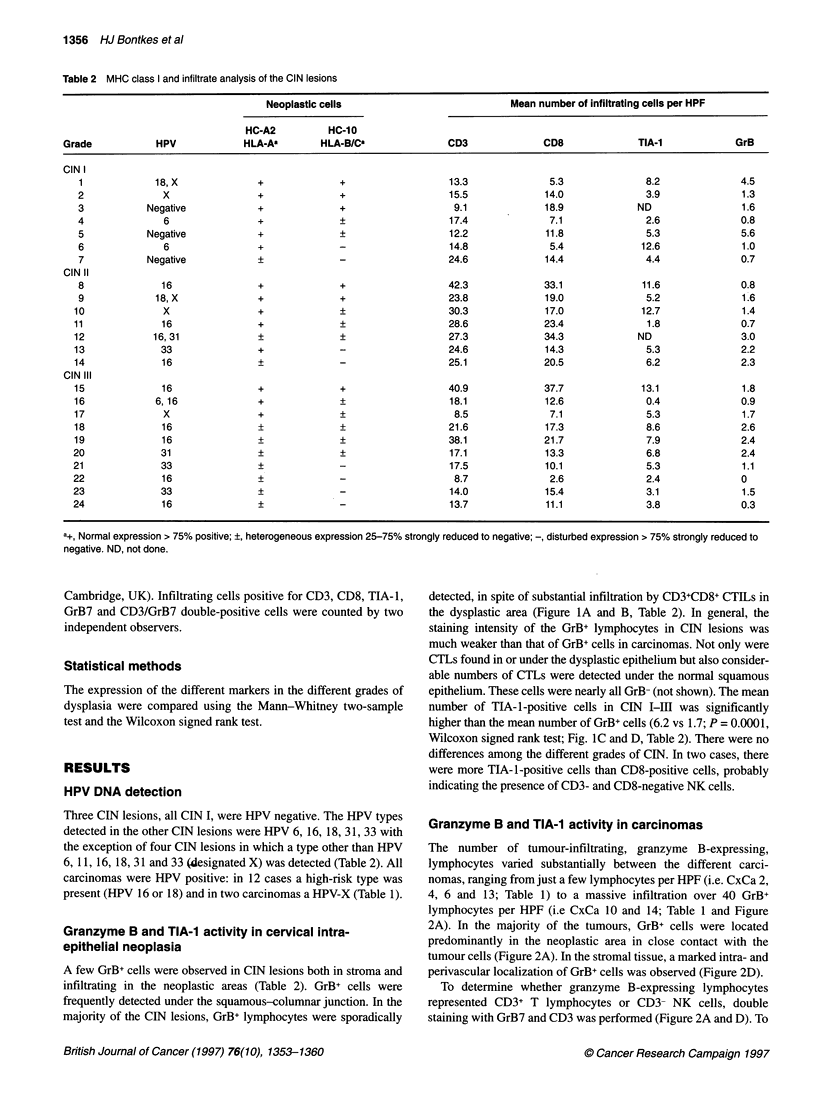
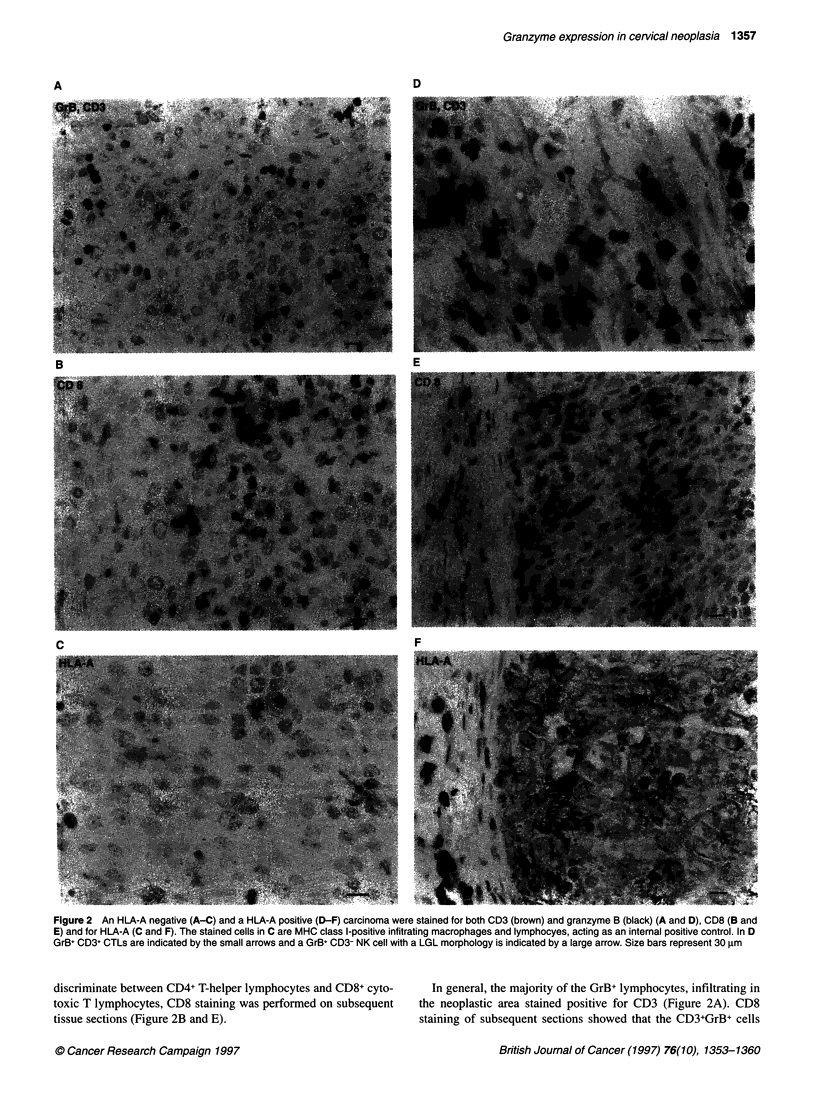
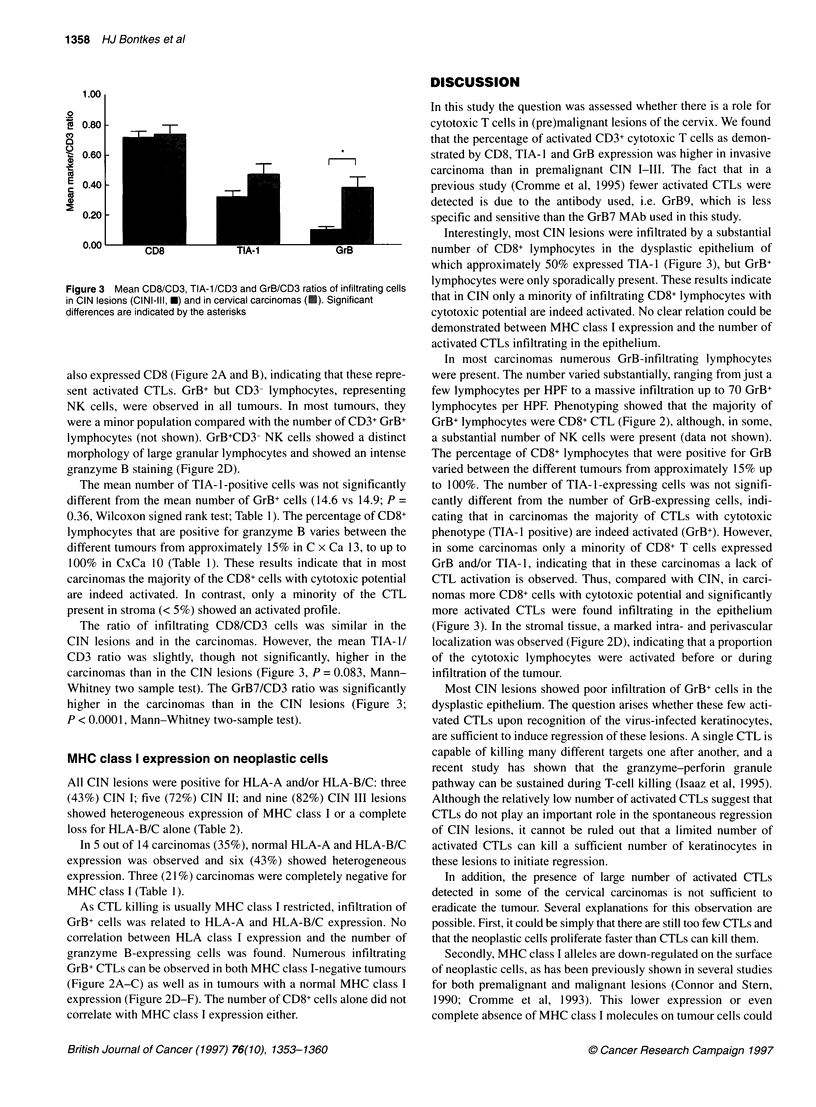
Cervical carcinomas are closely associated with high-risk human papillomavirus (HPV) types and are preceded by cervical intraepithelial neoplasia (CIN). Most CIN lesions regress spontaneously and will not evolve to invasive carcinoma. The cellular immune system mediated by cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells are thought to play an important role in the ultimate decline of CIN lesions. Although TIA-1 is constitutively expressed in the majority of circulating T cells and defines a subpopulation of CD8+ T cells with cytotoxic potential, granzyme B is only expressed in CTLs upon activation. In the present study we have evaluated the expression of these proteins by lymphocytes present in 24 randomly chosen CIN lesions with increasing degree of atypia and in 14 cervical squamous cell carcinomas. As major histocompatibility complex (MHC) class I expression is frequently down-regulated in HPV-induced lesions, thus possibly frustrating tumour cell recognition by infiltrating CTLs, these lesions were also analysed for MHC class I expression. The results indicated that in most CIN lesions only a minority of CTLs are activated, whereas in some carcinomas a massive infiltration of activated, i.e. granzyme B-positive, CTLs were observed. The percentage of activated CTLs was not related to expression of MHC class I on neoplastic cells. These results suggest that in some carcinomas proper activation of CTLs occurs but that most likely local factors or immunoselection of resistant neoplastic cells inhibit a proper response of CTLs to these neoplastic cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. TIA-1: structural and functional studies on a new class of cytolytic effector molecule. Curr Top Microbiol Immunol. 1995;198:131–143. doi: 10.1007/978-3-642-79414-8_8. [DOI] [PubMed] [Google Scholar]
- Coleman N., Birley H. D., Renton A. M., Hanna N. F., Ryait B. K., Byrne M., Taylor-Robinson D., Stanley M. A. Immunological events in regressing genital warts. Am J Clin Pathol. 1994 Dec;102(6):768–774. doi: 10.1093/ajcp/102.6.768. [DOI] [PubMed] [Google Scholar]
- Connor M. E., Stern P. L. Loss of MHC class-I expression in cervical carcinomas. Int J Cancer. 1990 Dec 15;46(6):1029–1034. doi: 10.1002/ijc.2910460614. [DOI] [PubMed] [Google Scholar]
- Cromme F. V., Meijer C. J., Snijders P. J., Uyterlinde A., Kenemans P., Helmerhorst T., Stern P. L., van den Brule A. J., Walboomers J. M. Analysis of MHC class I and II expression in relation to presence of HPV genotypes in premalignant and malignant cervical lesions. Br J Cancer. 1993 Jun;67(6):1372–1380. doi: 10.1038/bjc.1993.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromme F.V., Walboomers J.M.M., Van Oostveen J.W., Stukart M.J., De Gruijl T.D., Kummer J.A., Leonhart A.M., Helmerhorst T.J.M., Meijer C.J.L.M. Lack of granzyme expression in T lymphocytes indicates poor cytotoxic T lymphocyte activation in human papillomavirus-associated cervical carcinomas. Int J Gynecol Cancer. 1995 Sep;5(5):366–373. doi: 10.1046/j.1525-1438.1995.05050366.x. [DOI] [PubMed] [Google Scholar]
- Frisan T., Sjöberg J., Dolcetti R., Boiocchi M., De Re V., Carbone A., Brautbar C., Battat S., Biberfeld P., Eckman M. Local suppression of Epstein-Barr virus (EBV)-specific cytotoxicity in biopsies of EBV-positive Hodgkin's disease. Blood. 1995 Aug 15;86(4):1493–1501. [PubMed] [Google Scholar]
- Giacomini P., Aguzzi A., Pestka S., Fisher P. B., Ferrone S. Modulation by recombinant DNA leukocyte (alpha) and fibroblast (beta) interferons of the expression and shedding of HLA- and tumor-associated antigens by human melanoma cells. J Immunol. 1984 Sep;133(3):1649–1655. [PubMed] [Google Scholar]
- Halpert R., Fruchter R. G., Sedlis A., Butt K., Boyce J. G., Sillman F. H. Human papillomavirus and lower genital neoplasia in renal transplant patients. Obstet Gynecol. 1986 Aug;68(2):251–258. [PubMed] [Google Scholar]
- Heusel J. W., Wesselschmidt R. L., Shresta S., Russell J. H., Ley T. J. Cytotoxic lymphocytes require granzyme B for the rapid induction of DNA fragmentation and apoptosis in allogeneic target cells. Cell. 1994 Mar 25;76(6):977–987. doi: 10.1016/0092-8674(94)90376-x. [DOI] [PubMed] [Google Scholar]
- Ho G. Y., Burk R. D., Klein S., Kadish A. S., Chang C. J., Palan P., Basu J., Tachezy R., Lewis R., Romney S. Persistent genital human papillomavirus infection as a risk factor for persistent cervical dysplasia. J Natl Cancer Inst. 1995 Sep 20;87(18):1365–1371. doi: 10.1093/jnci/87.18.1365. [DOI] [PubMed] [Google Scholar]
- Honma S., Tsukada S., Honda S., Nakamura M., Takakuwa K., Maruhashi T., Kodama S., Kanazawa K., Takahashi T., Tanaka K. Biological-clinical significance of selective loss of HLA-class-I allelic product expression in squamous-cell carcinoma of the uterine cervix. Int J Cancer. 1994 Jun 1;57(5):650–655. doi: 10.1002/ijc.2910570507. [DOI] [PubMed] [Google Scholar]
- Isaaz S., Baetz K., Olsen K., Podack E., Griffiths G. M. Serial killing by cytotoxic T lymphocytes: T cell receptor triggers degranulation, re-filling of the lytic granules and secretion of lytic proteins via a non-granule pathway. Eur J Immunol. 1995 Apr;25(4):1071–1079. doi: 10.1002/eji.1830250432. [DOI] [PubMed] [Google Scholar]
- Jenne D. E., Tschopp J. Granzymes, a family of serine proteases released from granules of cytolytic T lymphocytes upon T cell receptor stimulation. Immunol Rev. 1988 Mar;103:53–71. doi: 10.1111/j.1600-065x.1988.tb00749.x. [DOI] [PubMed] [Google Scholar]
- Keating P. J., Cromme F. V., Duggan-Keen M., Snijders P. J., Walboomers J. M., Hunter R. D., Dyer P. A., Stern P. L. Frequency of down-regulation of individual HLA-A and -B alleles in cervical carcinomas in relation to TAP-1 expression. Br J Cancer. 1995 Aug;72(2):405–411. doi: 10.1038/bjc.1995.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummer J. A., Kamp A. M., Tadema T. M., Vos W., Meijer C. J., Hack C. E. Localization and identification of granzymes A and B-expressing cells in normal human lymphoid tissue and peripheral blood. Clin Exp Immunol. 1995 Apr;100(1):164–172. doi: 10.1111/j.1365-2249.1995.tb03619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummer J. A., Kamp A. M., van Katwijk M., Brakenhoff J. P., Radosević K., van Leeuwen A. M., Borst J., Verweij C. L., Hack C. E. Production and characterization of monoclonal antibodies raised against recombinant human granzymes A and B and showing cross reactions with the natural proteins. J Immunol Methods. 1993 Jul 6;163(1):77–83. doi: 10.1016/0022-1759(93)90241-x. [DOI] [PubMed] [Google Scholar]
- Kägi D., Seiler P., Pavlovic J., Ledermann B., Bürki K., Zinkernagel R. M., Hengartner H. The roles of perforin- and Fas-dependent cytotoxicity in protection against cytopathic and noncytopathic viruses. Eur J Immunol. 1995 Dec;25(12):3256–3262. doi: 10.1002/eji.1830251209. [DOI] [PubMed] [Google Scholar]
- Lichtenheld M. G., Olsen K. J., Lu P., Lowrey D. M., Hameed A., Hengartner H., Podack E. R. Structure and function of human perforin. Nature. 1988 Sep 29;335(6189):448–451. doi: 10.1038/335448a0. [DOI] [PubMed] [Google Scholar]
- Liu C. C., Rafii S., Granelli-Piperno A., Trapani J. A., Young J. D. Perforin and serine esterase gene expression in stimulated human T cells. Kinetics, mitogen requirements, and effects of cyclosporin A. J Exp Med. 1989 Dec 1;170(6):2105–2118. doi: 10.1084/jem.170.6.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long E. O., Colonna M., Lanier L. L. Inhibitory MHC class I receptors on NK and T cells: a standard nomenclature. Immunol Today. 1996 Feb;17(2):100–100. doi: 10.1016/0167-5699(96)80590-1. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I., Stankovics J., Gallyas F. A highly sensitive one-step method for silver intensification of the nickel-diaminobenzidine endproduct of peroxidase reaction. J Histochem Cytochem. 1989 Oct;37(10):1563–1565. doi: 10.1177/37.10.2674275. [DOI] [PubMed] [Google Scholar]
- Müller C., Tschopp J. Resistance of CTL to perforin-mediated lysis. Evidence for a lymphocyte membrane protein interacting with perforin. J Immunol. 1994 Sep 15;153(6):2470–2478. [PubMed] [Google Scholar]
- Nishioka W. K., Welsh R. M. Susceptibility to cytotoxic T lymphocyte-induced apoptosis is a function of the proliferative status of the target. J Exp Med. 1994 Feb 1;179(2):769–774. doi: 10.1084/jem.179.2.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudejans J. J., Jiwa N. M., Kummer J. A., Horstman A., Vos W., Baak J. P., Kluin P. M., van der Valk P., Walboomers J. M., Meijer C. J. Analysis of major histocompatibility complex class I expression on Reed-Sternberg cells in relation to the cytotoxic T-cell response in Epstein-Barr virus-positive and -negative Hodgkin's disease. Blood. 1996 May 1;87(9):3844–3851. [PubMed] [Google Scholar]
- Poppema S., Visser L. Absence of HLA class I expression by Reed-Sternberg cells. Am J Pathol. 1994 Jul;145(1):37–41. [PMC free article] [PubMed] [Google Scholar]
- Remmink A. J., Walboomers J. M., Helmerhorst T. J., Voorhorst F. J., Rozendaal L., Risse E. K., Meijer C. J., Kenemans P. The presence of persistent high-risk HPV genotypes in dysplastic cervical lesions is associated with progressive disease: natural history up to 36 months. Int J Cancer. 1995 May 4;61(3):306–311. doi: 10.1002/ijc.2910610305. [DOI] [PubMed] [Google Scholar]
- Rivoltini L., Barracchini K. C., Viggiano V., Kawakami Y., Smith A., Mixon A., Restifo N. P., Topalian S. L., Simonis T. B., Rosenberg S. A. Quantitative correlation between HLA class I allele expression and recognition of melanoma cells by antigen-specific cytotoxic T lymphocytes. Cancer Res. 1995 Jul 15;55(14):3149–3157. [PMC free article] [PubMed] [Google Scholar]
- Sale G. E., Beauchamp M., Myerson D. Immunohistologic staining of cytotoxic T and NK cells in formalin-fixed paraffin-embedded tissue using microwave TIA-1 antigen retrieval. Transplantation. 1994 Jan;57(2):287–289. [PubMed] [Google Scholar]
- Stam N. J., Vroom T. M., Peters P. J., Pastoors E. B., Ploegh H. L. HLA-A- and HLA-B-specific monoclonal antibodies reactive with free heavy chains in western blots, in formalin-fixed, paraffin-embedded tissue sections and in cryo-immuno-electron microscopy. Int Immunol. 1990;2(2):113–125. doi: 10.1093/intimm/2.2.113. [DOI] [PubMed] [Google Scholar]
- Ter Harmsel B., Smedts F., Kuijpers J., Jeunink M., Trimbos B., Ramaekers F. BCL-2 immunoreactivity increases with severity of CIN: a study of normal cervical epithelia, CIN, and cervical carcinoma. J Pathol. 1996 May;179(1):26–30. doi: 10.1002/(SICI)1096-9896(199605)179:1<26::AID-PATH516>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Tian Q., Streuli M., Saito H., Schlossman S. F., Anderson P. A polyadenylate binding protein localized to the granules of cytolytic lymphocytes induces DNA fragmentation in target cells. Cell. 1991 Nov 1;67(3):629–639. doi: 10.1016/0092-8674(91)90536-8. [DOI] [PubMed] [Google Scholar]
- Wang Y. C., Zhu L., McHugh R., Sell K. W., Selvaraj P. Expression of heat-stable antigen on tumor cells provides co-stimulation for tumor-specific T cell proliferation and cytotoxicity in mice. Eur J Immunol. 1995 May;25(5):1163–1167. doi: 10.1002/eji.1830250505. [DOI] [PubMed] [Google Scholar]
- Xie J., Gallagher G. The ability of transforming growth factor-beta 1 to preferentially inhibit the induction of cytotoxicity in human T cells is determined by the nature of the activating signals. Anticancer Res. 1994 Jul-Aug;14(4A):1595–1598. [PubMed] [Google Scholar]
- van den Brule A. J., Meijer C. J., Bakels V., Kenemans P., Walboomers J. M. Rapid detection of human papillomavirus in cervical scrapes by combined general primer-mediated and type-specific polymerase chain reaction. J Clin Microbiol. 1990 Dec;28(12):2739–2743. doi: 10.1128/jcm.28.12.2739-2743.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H. Molecular pathogenesis of cancer of the cervix and its causation by specific human papillomavirus types. Curr Top Microbiol Immunol. 1994;186:131–156. doi: 10.1007/978-3-642-78487-3_8. [DOI] [PubMed] [Google Scholar]