Abstract
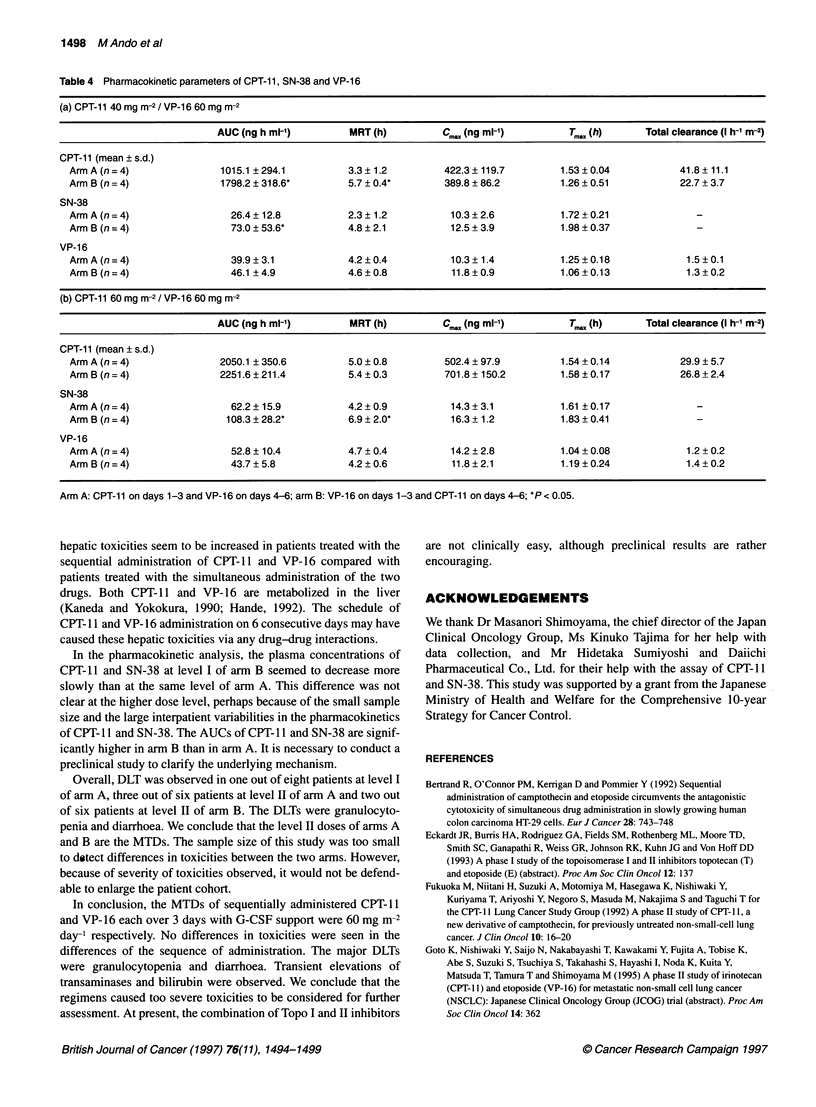
We conducted a phase I study of irinotecan (CPT-11) and etoposide (VP-16) given sequentially to untreated patients with metastatic non-small-cell lung cancer. Arm A: CPT-11 was given over 90 min on days 1-3 and VP-16 was given over 60 min on days 4-6. Arm B: VP-16 was given on days 1-3 and CPT-11 on days 4-6. G-CSF was given to all patients daily on days 7-17. Twenty-seven patients were entered randomly at the two arms. The major dose-limiting toxicities in arms A and B were granulocytopenia and diarrhoea. Transient elevations of transaminases and bilirubin were observed in both arms. The degree of the toxicities did not differ between the two arms. The maximum tolerated doses (MTDs) were 60 mg m-2 CPT-11 and 60 mg m-2 VP-16 in both arms. Of the 13 patients who received more than two cycles, two out of five achieved partial response (PR) at the first level of arm A and one out of four achieved PR at the second level of arm B. We conclude that these schedules of sequential CPT-11 and VP-16 administration were inappropriate because of severe toxicities.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertrand R., O'Connor P. M., Kerrigan D., Pommier Y. Sequential administration of camptothecin and etoposide circumvents the antagonistic cytotoxicity of simultaneous drug administration in slowly growing human colon carcinoma HT-29 cells. Eur J Cancer. 1992;28A(4-5):743–748. doi: 10.1016/0959-8049(92)90107-d. [DOI] [PubMed] [Google Scholar]
- Fukuoka M., Niitani H., Suzuki A., Motomiya M., Hasegawa K., Nishiwaki Y., Kuriyama T., Ariyoshi Y., Negoro S., Masuda N. A phase II study of CPT-11, a new derivative of camptothecin, for previously untreated non-small-cell lung cancer. J Clin Oncol. 1992 Jan;10(1):16–20. doi: 10.1200/JCO.1992.10.1.16. [DOI] [PubMed] [Google Scholar]
- Kaneda N., Yokokura T. Nonlinear pharmacokinetics of CPT-11 in rats. Cancer Res. 1990 Mar 15;50(6):1721–1725. [PubMed] [Google Scholar]
- Kano Y., Suzuki K., Akutsu M., Suda K., Inoue Y., Yoshida M., Sakamoto S., Miura Y. Effects of CPT-11 in combination with other anti-cancer agents in culture. Int J Cancer. 1992 Feb 20;50(4):604–610. doi: 10.1002/ijc.2910500420. [DOI] [PubMed] [Google Scholar]
- Karato A., Sasaki Y., Shinkai T., Eguchi K., Tamura T., Ohe Y., Oshita F., Nishio M., Kunikane H., Arioka H. Phase I study of CPT-11 and etoposide in patients with refractory solid tumors. J Clin Oncol. 1993 Oct;11(10):2030–2035. doi: 10.1200/JCO.1993.11.10.2030. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H. Antagonism between camptothecin and topoisomerase II-directed chemotherapeutic agents in a human leukemia cell line. Cancer Res. 1991 Feb 15;51(4):1129–1136. [PubMed] [Google Scholar]
- Kim R., Hirabayashi N., Nishiyama M., Jinushi K., Toge T., Okada K. Experimental studies on biochemical modulation targeting topoisomerase I and II in human tumor xenografts in nude mice. Int J Cancer. 1992 Mar 12;50(5):760–766. doi: 10.1002/ijc.2910500516. [DOI] [PubMed] [Google Scholar]
- Masuda N., Fukuoka M., Kudoh S., Matsui K., Kusunoki Y., Takada M., Nakagawa K., Hirashima T., Tsukada H., Yana T. Phase I and pharmacologic study of irinotecan and etoposide with recombinant human granulocyte colony-stimulating factor support for advanced lung cancer. J Clin Oncol. 1994 Sep;12(9):1833–1841. doi: 10.1200/JCO.1994.12.9.1833. [DOI] [PubMed] [Google Scholar]
- Pommier Y. DNA topoisomerase I and II in cancer chemotherapy: update and perspectives. Cancer Chemother Pharmacol. 1993;32(2):103–108. doi: 10.1007/BF00685611. [DOI] [PubMed] [Google Scholar]
- Shimada Y., Yoshino M., Wakui A., Nakao I., Futatsuki K., Sakata Y., Kambe M., Taguchi T., Ogawa N. Phase II study of CPT-11, a new camptothecin derivative, in metastatic colorectal cancer. CPT-11 Gastrointestinal Cancer Study Group. J Clin Oncol. 1993 May;11(5):909–913. doi: 10.1200/JCO.1993.11.5.909. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi H., Fujiwara Y., Ohune T., Yamaoka N., Tamura K., Yamakido M. High-performance liquid chromatographic determination of irinotecan (CPT-11) and its active metabolite (SN-38) in human plasma. J Chromatogr B Biomed Appl. 1995 Aug 18;670(2):309–316. doi: 10.1016/0378-4347(95)00130-1. [DOI] [PubMed] [Google Scholar]
- Tobinai K., Kohno A., Shimada Y., Watanabe T., Tamura T., Takeyama K., Narabayashi M., Fukutomi T., Kondo H., Shimoyama M. Toxicity grading criteria of the Japan Clinical Oncology Group. The Clinical Trial Review Committee of the Japan Clinical Oncology Group. Jpn J Clin Oncol. 1993 Aug;23(4):250–257. [PubMed] [Google Scholar]
- Yamaoka K., Tanigawara Y., Nakagawa T., Uno T. A pharmacokinetic analysis program (multi) for microcomputer. J Pharmacobiodyn. 1981 Nov;4(11):879–885. doi: 10.1248/bpb1978.4.879. [DOI] [PubMed] [Google Scholar]