Abstract
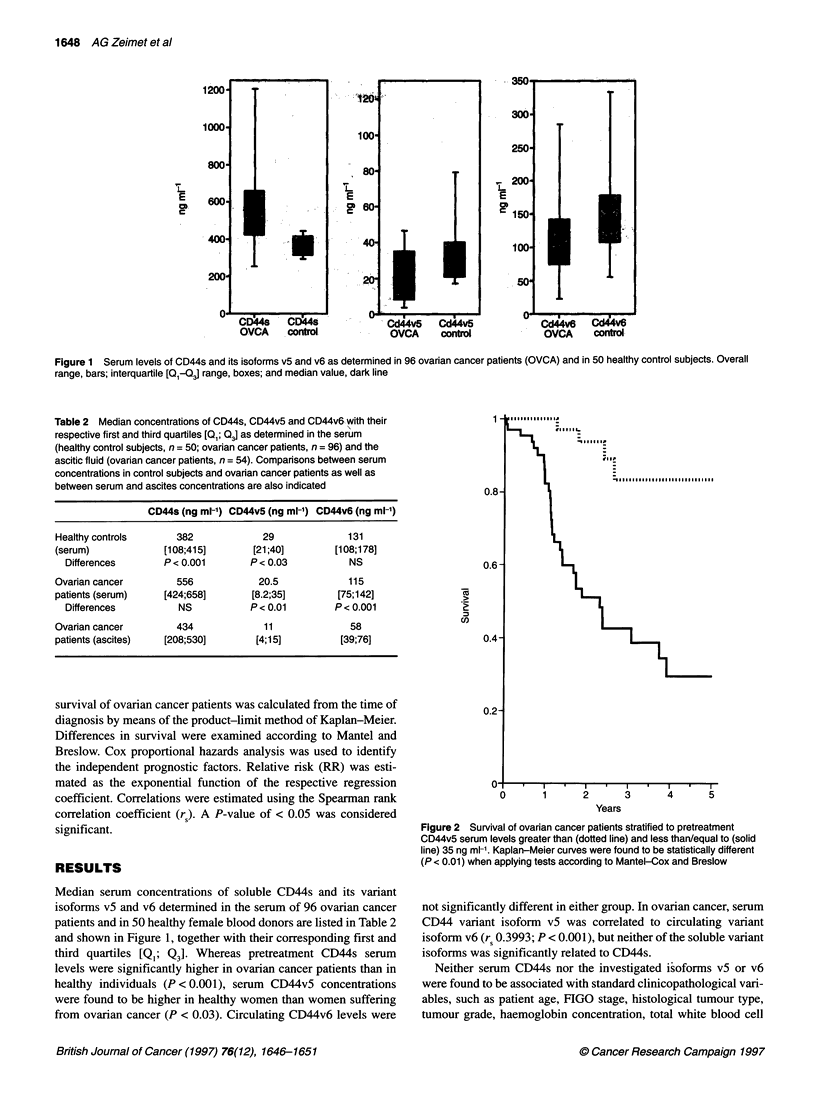
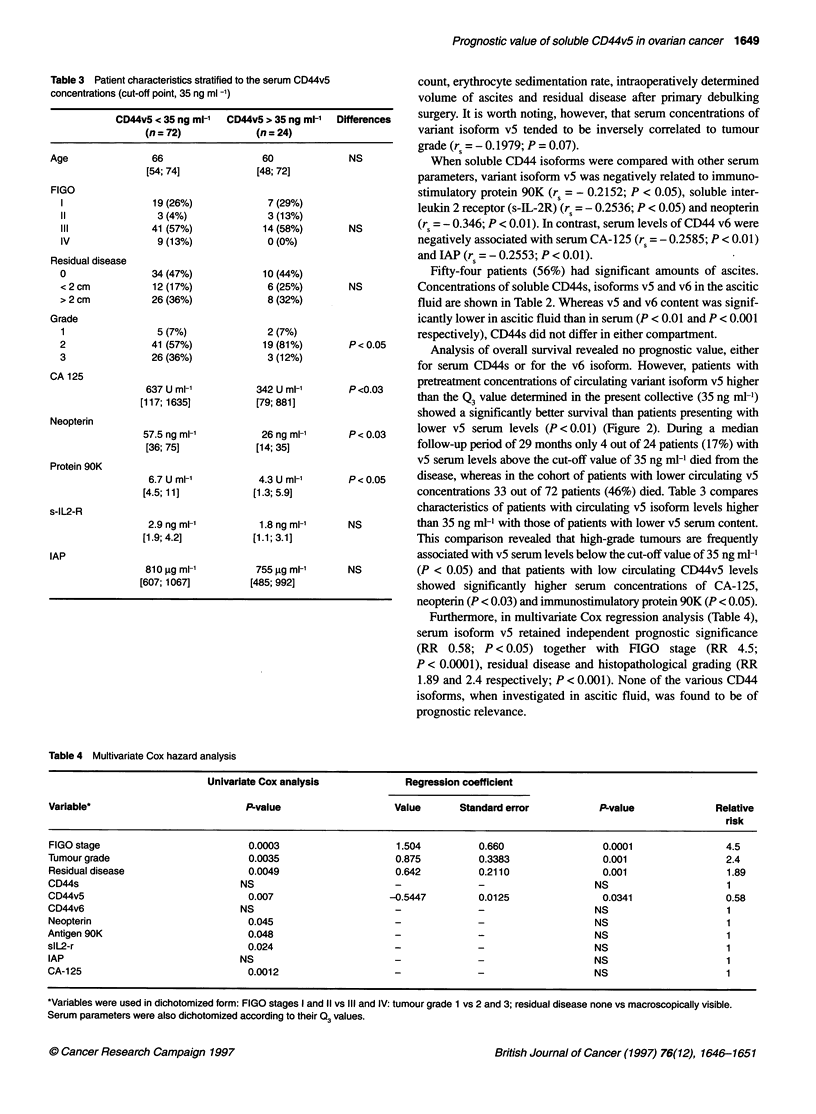
In 96 ovarian cancer patients, the present study investigates the clinical significance of pretreatment concentrations of soluble CD44 standard (CD44s) and its isoforms v5 and v6 determined in the serum and the ascitic fluid by means of recently developed enzyme-linked immunosorbent assays (ELISAs). Furthermore, CD44 serum concentrations in the ovarian cancer patients were compared with circulating CD44 levels in 50 healthy age-matched female blood donors. Whereas CD44s was found to be higher and CD44v5 to be lower in ovarian cancer patients than healthy control subjects, no statistical difference between the two cohorts was revealed for CD44 isoform v6. In the ascitic fluid samples, variant isoform v5 and v6 were demonstrated at lower concentrations than serum. Multivariate analysis of overall survival demonstrated that a high pretreatment serum level of soluble CD44 isoform v5 is independently associated with favourable clinical outcome in ovarian cancer. When circulating CD44 isoforms were compared with a panel of serum parameters known to be involved in the immunological network, an inverse correlation between serum CD44v5 levels and indicators of cellular immune system activation, such as soluble interleukin 2 receptor, immunostimulatory protein 90K and neopterin, became apparent.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arch R., Wirth K., Hofmann M., Ponta H., Matzku S., Herrlich P., Zöller M. Participation in normal immune responses of a metastasis-inducing splice variant of CD44. Science. 1992 Jul 31;257(5070):682–685. doi: 10.1126/science.1496383. [DOI] [PubMed] [Google Scholar]
- Cannistra S. A., Abu-Jawdeh G., Niloff J., Strobel T., Swanson L., Andersen J., Ottensmeier C. CD44 variant expression is a common feature of epithelial ovarian cancer: lack of association with standard prognostic factors. J Clin Oncol. 1995 Aug;13(8):1912–1921. doi: 10.1200/JCO.1995.13.8.1912. [DOI] [PubMed] [Google Scholar]
- Friedrichs K., Franke F., Lisboa B. W., Kügler G., Gille I., Terpe H. J., Hölzel F., Maass H., Günthert U. CD44 isoforms correlate with cellular differentiation but not with prognosis in human breast cancer. Cancer Res. 1995 Nov 15;55(22):5424–5433. [PubMed] [Google Scholar]
- Guo Y. J., Liu G., Wang X., Jin D., Wu M., Ma J., Sy M. S. Potential use of soluble CD44 in serum as indicator of tumor burden and metastasis in patients with gastric or colon cancer. Cancer Res. 1994 Jan 15;54(2):422–426. [PubMed] [Google Scholar]
- Günthert U., Hofmann M., Rudy W., Reber S., Zöller M., Haussmann I., Matzku S., Wenzel A., Ponta H., Herrlich P. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell. 1991 Apr 5;65(1):13–24. doi: 10.1016/0092-8674(91)90403-l. [DOI] [PubMed] [Google Scholar]
- Günthert U., Stauder R., Mayer B., Terpe H. J., Finke L., Friedrichs K. Are CD44 variant isoforms involved in human tumour progression? Cancer Surv. 1995;24:19–42. [PubMed] [Google Scholar]
- Heider K. H., Dämmrich J., Skroch-Angel P., Müller-Hermelink H. K., Vollmers H. P., Herrlich P., Ponta H. Differential expression of CD44 splice variants in intestinal- and diffuse-type human gastric carcinomas and normal gastric mucosa. Cancer Res. 1993 Sep 15;53(18):4197–4203. [PubMed] [Google Scholar]
- Jalkanen S., Bargatze R. F., de los Toyos J., Butcher E. C. Lymphocyte recognition of high endothelium: antibodies to distinct epitopes of an 85-95-kD glycoprotein antigen differentially inhibit lymphocyte binding to lymph node, mucosal, or synovial endothelial cells. J Cell Biol. 1987 Aug;105(2):983–990. doi: 10.1083/jcb.105.2.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann M., Heider K. H., Sinn H. P., von Minckwitz G., Ponta H., Herrlich P. CD44 variant exon epitopes in primary breast cancer and length of survival. Lancet. 1995 Mar 11;345(8950):615–619. doi: 10.1016/s0140-6736(95)90521-9. [DOI] [PubMed] [Google Scholar]
- Khaldoyanidi S., Achtnich M., Hehlmann R., Zöller M. Expression of CD44 variant isoforms in peripheral blood leukocytes in malignant lymphoma and leukemia: inverse correlation between expression and tumor progression. Leuk Res. 1996 Oct;20(10):839–851. doi: 10.1016/s0145-2126(96)00048-3. [DOI] [PubMed] [Google Scholar]
- Koretz K., Möller P., Lehnert T., Hinz U., Otto H. F., Herfarth C. Effect of CD44v6 on survival in colorectal carcinoma. Lancet. 1995 Feb 4;345(8945):327–328. doi: 10.1016/s0140-6736(95)90320-8. [DOI] [PubMed] [Google Scholar]
- Lein M., Weiss S., Jung K., Schnorr D., Loening S. Soluble CD44 variants in serum of patients with prostate cancer and other urological malignancies. Prostate. 1996 Nov;29(5):334–335. doi: 10.1002/(SICI)1097-0045(199611)29:5<334::AID-PROS9>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Marth C., Dreps A., Natoli C., Zeimet A. G., Lang T., Widschwendter M., Daxenbichler G., Ullrich A., Iacobelli S. Effects of type-I and -II interferons on 90K antigen expression in ovarian carcinoma cells. Int J Cancer. 1994 Dec 15;59(6):808–813. doi: 10.1002/ijc.2910590617. [DOI] [PubMed] [Google Scholar]
- Marth C., Weger A., Müller-Holzner E., Zeimet A. G., Moncayo-Naveda H., Daxenbichler G., Reibnegger G., Fuchs D., Wachter H., Dapunt O. The prognostic value of nuclear roundness and neopterin in ovarian cancer. Eur J Cancer. 1993;29A(13):1863–1868. doi: 10.1016/0959-8049(93)90539-r. [DOI] [PubMed] [Google Scholar]
- Mayer B., Jauch K. W., Günthert U., Figdor C. G., Schildberg F. W., Funke I., Johnson J. P. De-novo expression of CD44 and survival in gastric cancer. Lancet. 1993 Oct 23;342(8878):1019–1022. doi: 10.1016/0140-6736(93)92879-x. [DOI] [PubMed] [Google Scholar]
- Miyake K., Underhill C. B., Lesley J., Kincade P. W. Hyaluronate can function as a cell adhesion molecule and CD44 participates in hyaluronate recognition. J Exp Med. 1990 Jul 1;172(1):69–75. doi: 10.1084/jem.172.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder J. W., Kruyt P. M., Sewnath M., Oosting J., Seldenrijk C. A., Weidema W. F., Offerhaus G. J., Pals S. T. Colorectal cancer prognosis and expression of exon-v6-containing CD44 proteins. Lancet. 1994 Nov 26;344(8935):1470–1472. doi: 10.1016/s0140-6736(94)90290-9. [DOI] [PubMed] [Google Scholar]
- Ristamäki R., Joensuu H., Salmi M., Jalkanen S. Serum CD44 in malignant lymphoma: an association with treatment response. Blood. 1994 Jul 1;84(1):238–243. [PubMed] [Google Scholar]
- Salles G., Zain M., Jiang W. M., Boussiotis V. A., Shipp M. A. Alternatively spliced CD44 transcripts in diffuse large-cell lymphomas: characterization and comparison with normal activated B cells and epithelial malignancies. Blood. 1993 Dec 15;82(12):3539–3547. [PubMed] [Google Scholar]
- Seiter S., Arch R., Reber S., Komitowski D., Hofmann M., Ponta H., Herrlich P., Matzku S., Zöller M. Prevention of tumor metastasis formation by anti-variant CD44. J Exp Med. 1993 Feb 1;177(2):443–455. doi: 10.1084/jem.177.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliutz G., Tempfer C., Winkler S., Kohlberger P., Reinthaller A., Kainz C. Immunohistochemical and serological evaluation of CD44 splice variants in human ovarian cancer. Br J Cancer. 1995 Dec;72(6):1494–1497. doi: 10.1038/bjc.1995.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauder R., Eisterer W., Thaler J., Günthert U. CD44 variant isoforms in non-Hodgkin's lymphoma: a new independent prognostic factor. Blood. 1995 May 15;85(10):2885–2899. [PubMed] [Google Scholar]
- Terpe H. J., Stark H., Prehm P., Günthert U. CD44 variant isoforms are preferentially expressed in basal epithelial of non-malignant human fetal and adult tissues. Histochemistry. 1994 Feb;101(2):79–89. doi: 10.1007/BF00269353. [DOI] [PubMed] [Google Scholar]
- Uhl-Steidl M., Müller-Holzner E., Zeimet A. G., Adolf G. R., Daxenbichler G., Marth C., Dapunt O. Prognostic value of CD44 splice variant expression in ovarian cancer. Oncology. 1995 Sep-Oct;52(5):400–406. doi: 10.1159/000227497. [DOI] [PubMed] [Google Scholar]
- Yu Q., Toole B. P. A new alternatively spliced exon between v9 and v10 provides a molecular basis for synthesis of soluble CD44. J Biol Chem. 1996 Aug 23;271(34):20603–20607. doi: 10.1074/jbc.271.34.20603. [DOI] [PubMed] [Google Scholar]
- Zeimet A. G., Natoli C., Herold M., Fuchs D., Windbichler G., Daxenbichler G., Iacobelli S., Dapunt O., Marth C. Circulating immunostimulatory protein 90K and soluble interleukin-2-receptor in human ovarian cancer. Int J Cancer. 1996 Sep 27;68(1):34–38. doi: 10.1002/(SICI)1097-0215(19960927)68:1<34::AID-IJC7>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]


