Abstract
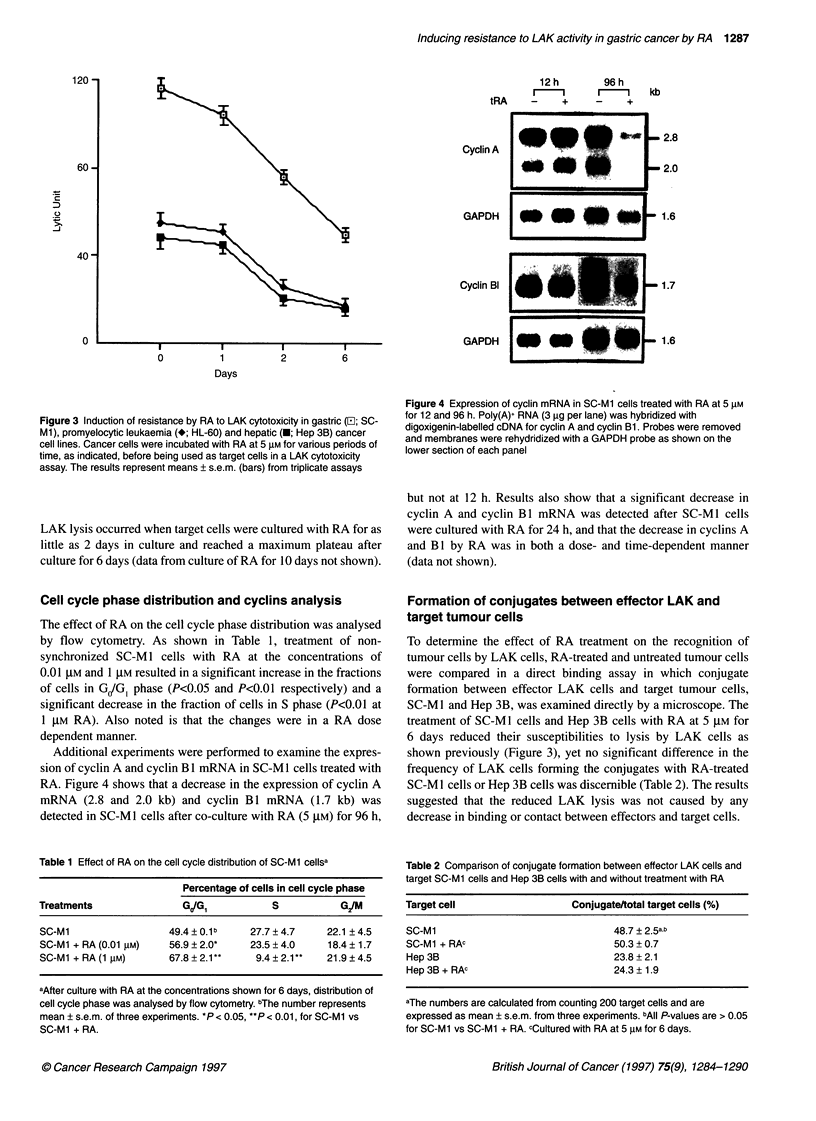
All-trans retinoic acid (RA) was previously shown to regulate the growth of gastric cancer cells derived from the cell line SC-M1. This study was designed to investigate the effect of RA on the sensitivity of SC-M1 cells to lymphokine-activated killer (LAK) activity. RA at the concentration range of 0.001-10 microM was shown to induce SC-M1 cells to exhibit resistance to LAK activity in a dose-dependent manner. A kinetics study indicated that a significantly increased resistance was detected after 2 days of co-culturing SC-M1 cells with RA and reached a maximum after 6 days of culture. Similar results were obtained from two other cancer cell lines: promyelocytic leukaemia HL-60 and hepatic cancer Hep 3B. A binding assay demonstrated that the binding efficacy between target SC-M1 cells and effector LAK cells was not altered by RA. Flow cytometric analyses revealed that RA exhibited no effect on the expression of cell surface molecules, including HLA class I and class II antigens, intercellular adhesion molecule-1 and -2, and lymphocyte function antigen-3. Cell cycle analysis revealed that culture of SC-M1 cells with RA resulted in an increase in G0/G1 phase and a decrease in S phase, accompanied by a decrease in cyclin A and cyclin B1 mRNA as determined by Northern blot analysis. Additionally, RA was shown to enhance the expression of retinoic acid receptor alpha (RAR alpha) in SC-M1 cells, and to have no effect on the expression of RARbeta or RARgamma. Taken together, these results indicate that RA can significantly increase gastric cancer cells SC-M1 to resist LAK cytotoxicity by means of a cytostatic effect through a mechanism relating to cell cycle regulation. The prevailing ideas, such as a decrease in effector to target cell binding, a reduced MHC class I antigen expression or an altered RARbeta expression, are not involved.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertini M. R., Gibson D. F., Robinson S. P., Howard S. P., Tans K. J., Lindstrom M. J., Robinson R. R., Tormey D. C., Jordan V. C., Sondel P. M. Influence of estradiol and tamoxifen on susceptibility of human breast cancer cell lines to lysis by lymphokine-activated killer cells. J Immunother (1991) 1992 Jan;11(1):30–39. doi: 10.1097/00002371-199201000-00004. [DOI] [PubMed] [Google Scholar]
- Apasov S., Redegeld F., Sitkovsky M. Cell-mediated cytotoxicity: contact and secreted factors. Curr Opin Immunol. 1993 Jun;5(3):404–410. doi: 10.1016/0952-7915(93)90060-6. [DOI] [PubMed] [Google Scholar]
- Athanassiades T. J. Adjuvant effect of vitamin A palmitate and analogs on cell-mediated immunity. J Natl Cancer Inst. 1981 Nov;67(5):1153–1156. [PubMed] [Google Scholar]
- Bouillon M., Audette M. Retinoic acid-stimulated intercellular adhesion molecule-1 expression on SK-N-SH cells: calcium/calmodulin-dependent pathway. Cancer Res. 1994 Aug 1;54(15):4144–4149. [PubMed] [Google Scholar]
- Butler W. B., Fontana J. A. Responses to retinoic acid of tamoxifen-sensitive and -resistant sublines of human breast cancer cell line MCF-7. Cancer Res. 1992 Nov 15;52(22):6164–6167. [PubMed] [Google Scholar]
- Chao T. Y., Hwang W. S., Yeh M. Y. Generation of lymphokine-activated killer (LAK) cell activity from malignant peritoneal effusions. Proc Natl Sci Counc Repub China B. 1995 Apr;19(2):92–98. [PubMed] [Google Scholar]
- Chao T. Y., Ohnishi H., Chu T. M. Indirect inhibition of generation of murine lymphokine-activated killer cell activity in splenocyte cultures by interferon-gamma. Immunology. 1990 May;70(1):116–120. [PMC free article] [PubMed] [Google Scholar]
- Cohen P. S., Letterio J. J., Gaetano C., Chan J., Matsumoto K., Sporn M. B., Thiele C. J. Induction of transforming growth factor beta 1 and its receptors during all-trans-retinoic acid (RA) treatment of RA-responsive human neuroblastoma cell lines. Cancer Res. 1995 Jun 1;55(11):2380–2386. [PubMed] [Google Scholar]
- Cordon-Cardo C. Mutations of cell cycle regulators. Biological and clinical implications for human neoplasia. Am J Pathol. 1995 Sep;147(3):545–560. [PMC free article] [PubMed] [Google Scholar]
- Correale P., Procopio A., Celio L., Caraglia M., Genua G., Coppola V., Pepe S., Normanno N., Vecchio I., Palmieri G. Phorbol 12-myristate 13-acetate induces resistance of human melanoma cells to natural-killer- and lymphokine-activated-killer-mediated cytotoxicity. Cancer Immunol Immunother. 1992;34(4):272–278. doi: 10.1007/BF01741796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degos L. Retinoic acid in acute promyelocytic leukemia: a model for differentiation therapy. Curr Opin Oncol. 1992 Feb;4(1):45–52. doi: 10.1097/00001622-199202000-00007. [DOI] [PubMed] [Google Scholar]
- Dillehay D. L., Walia A. S., Lamon E. W. Effects of retinoids on macrophage function and IL-1 activity. J Leukoc Biol. 1988 Nov;44(5):353–360. doi: 10.1002/jlb.44.5.353. [DOI] [PubMed] [Google Scholar]
- Dmitrovsky E., Markman M., Marks P. A. Clinical use of differentiating agents in cancer therapy. Cancer Chemother Biol Response Modif. 1990;11:303–320. [PubMed] [Google Scholar]
- Fady C., Gardner A., Gera J. F., Lichtenstein A. Interferon-gamma-induced increased sensitivity of HER2/neu-overexpressing tumor cells to lymphokine-activated killer cell lysis: importance of ICAM-1 in binding and post-binding events. Cancer Immunol Immunother. 1993 Oct;37(5):329–336. doi: 10.1007/BF01518456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fegan C., Bailey-Wood R., Coleman S., Phillips S. A., Neale L., Hoy T., Whittaker J. A. All trans retinoic acid enhances human LAK activity. Eur J Haematol. 1995 Feb;54(2):95–100. doi: 10.1111/j.1600-0609.1995.tb01774.x. [DOI] [PubMed] [Google Scholar]
- Foreman N. K., Rill D. R., Coustan-Smith E., Douglass E. C., Brenner M. K. Mechanisms of selective killing of neuroblastoma cells by natural killer cells and lymphokine activated killer cells. Potential for residual disease eradication. Br J Cancer. 1993 May;67(5):933–938. doi: 10.1038/bjc.1993.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galandrini R., Albi N., Zarcone D., Grossi C. E., Velardi A. Adhesion molecule-mediated signals regulate major histocompatibility complex-unrestricted and CD3/T cell receptor-triggered cytotoxicity. Eur J Immunol. 1992 Aug;22(8):2047–2053. doi: 10.1002/eji.1830220814. [DOI] [PubMed] [Google Scholar]
- Henkart P. A. Lymphocyte-mediated cytotoxicity: two pathways and multiple effector molecules. Immunity. 1994 Aug;1(5):343–346. doi: 10.1016/1074-7613(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Huang M. E., Ye Y. C., Chen S. R., Chai J. R., Lu J. X., Zhoa L., Gu L. J., Wang Z. Y. Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood. 1988 Aug;72(2):567–572. [PubMed] [Google Scholar]
- Katsanis E., Bausero M. A., Xu H., Orchard P. J., Xu Z., McIvor R. S., Brian A. A., Blazar B. R. Transfection of the mouse ICAM-1 gene into murine neuroblastoma enhances susceptibility to lysis, reduces in vivo tumorigenicity and decreases ICAM-2-dependent killing. Cancer Immunol Immunother. 1994 Feb;38(2):135–141. doi: 10.1007/BF01526209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T. H., Chu T. M. Enhancement of murine lymphokine-activated killer cell activity by retinoic acid. Cancer Res. 1990 May 15;50(10):3013–3018. [PubMed] [Google Scholar]
- Lotan R., Lotan D., Sacks P. G. Inhibition of tumor cell growth by retinoids. Methods Enzymol. 1990;190:100–110. doi: 10.1016/0076-6879(90)90014-r. [DOI] [PubMed] [Google Scholar]
- Love J. M., Gudas L. J. Vitamin A, differentiation and cancer. Curr Opin Cell Biol. 1994 Dec;6(6):825–831. doi: 10.1016/0955-0674(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Martin S. J., Bradley J. G., Cotter T. G. HL-60 cells induced to differentiate towards neutrophils subsequently die via apoptosis. Clin Exp Immunol. 1990 Mar;79(3):448–453. doi: 10.1111/j.1365-2249.1990.tb08110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nano R., Barni S., Capelli E., Prosperi E., Lavezzi L., Salvucci O. DNA-protein cell content of lymphokine activated killer (LAK) and target cells in coculture. Anticancer Res. 1995 May-Jun;15(3):751–754. [PubMed] [Google Scholar]
- Omenn G. S., Goodman G. E., Thornquist M. D., Balmes J., Cullen M. R., Glass A., Keogh J. P., Meyskens F. L., Valanis B., Williams J. H. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996 May 2;334(18):1150–1155. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- Palumbo A., Battaglio S., Napoli P., Bruno B., Omedè P., Boccadoro M., Pileri A. Retinoic acid inhibits the growth of human myeloma cells in vitro. Br J Haematol. 1995 Mar;89(3):555–560. doi: 10.1111/j.1365-2141.1995.tb08363.x. [DOI] [PubMed] [Google Scholar]
- Robertson M. J., Caligiuri M. A., Manley T. J., Levine H., Ritz J. Human natural killer cell adhesion molecules. Differential expression after activation and participation in cytolysis. J Immunol. 1990 Nov 15;145(10):3194–3201. [PubMed] [Google Scholar]
- Shyu R. Y., Jiang S. Y., Huang S. L., Chang T. C., Wu K. L., Roffler S. R., Yeh M. Y. Growth regulation by all-trans-retinoic acid and retinoic acid receptor messenger ribonucleic acids expression in gastric cancer cells. Eur J Cancer. 1995;31A(2):237–243. doi: 10.1016/0959-8049(94)00481-j. [DOI] [PubMed] [Google Scholar]
- Smith M. A., Parkinson D. R., Cheson B. D., Friedman M. A. Retinoids in cancer therapy. J Clin Oncol. 1992 May;10(5):839–864. doi: 10.1200/JCO.1992.10.5.839. [DOI] [PubMed] [Google Scholar]
- Strickland S., Sawey M. J. Studies on the effect of retinoids on the differentiation of teratocarcinoma stem cells in vitro and in vivo. Dev Biol. 1980 Jul;78(1):76–85. doi: 10.1016/0012-1606(80)90319-x. [DOI] [PubMed] [Google Scholar]
- Teichmann J. V., Ludwig W. D., Thiel E. GM-CSF-mediated proliferation induction improves the susceptibility of leukemia cells to lymphokine-activated killer cells. Int J Hematol. 1992 Jun;55(3):255–264. [PubMed] [Google Scholar]
- Triozzi P. L., Eicher D. M., Smoot J., Rinehart J. J. Modulation of leukemic cell sensitivity to lymphokine-activated killer cytolysis: role of intercellular adhesion molecule-1. Exp Hematol. 1992 Oct;20(9):1072–1076. [PubMed] [Google Scholar]
- Villa M. L., Ferrario E., Trabattoni D., Formelli F., De Palo G., Magni A., Veronesi U., Clerici E. Retinoids, breast cancer and NK cells. Br J Cancer. 1993 Nov;68(5):845–850. doi: 10.1038/bjc.1993.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrell R. P., Jr, Frankel S. R., Miller W. H., Jr, Scheinberg D. A., Itri L. M., Hittelman W. N., Vyas R., Andreeff M., Tafuri A., Jakubowski A. Differentiation therapy of acute promyelocytic leukemia with tretinoin (all-trans-retinoic acid). N Engl J Med. 1991 May 16;324(20):1385–1393. doi: 10.1056/NEJM199105163242002. [DOI] [PubMed] [Google Scholar]
- Warrell R. P., Jr, de Thé H., Wang Z. Y., Degos L. Acute promyelocytic leukemia. N Engl J Med. 1993 Jul 15;329(3):177–189. doi: 10.1056/NEJM199307153290307. [DOI] [PubMed] [Google Scholar]
- Zou C. P., Clifford J. L., Xu X. C., Sacks P. G., Chambon P., Hong W. K., Lotan R. Modulation by retinoic acid (RA) of squamous cell differentiation, cellular RA-binding proteins, and nuclear RA receptors in human head and neck squamous cell carcinoma cell lines. Cancer Res. 1994 Oct 15;54(20):5479–5487. [PubMed] [Google Scholar]
- Zychlinsky A., Zheng L. M., Liu C. C., Young J. D. Cytolytic lymphocytes induce both apoptosis and necrosis in target cells. J Immunol. 1991 Jan 1;146(1):393–400. [PubMed] [Google Scholar]
- de Fries R. U., Golub S. H. Characteristics and mechanism of IFN-gamma-induced protection of human tumor cells from lysis by lymphokine-activated killer cells. J Immunol. 1988 May 15;140(10):3686–3693. [PubMed] [Google Scholar]




