Abstract
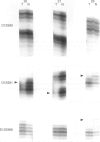
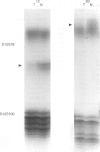
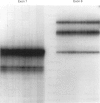
Identification of the key genetic alterations leading to ovarian cancer is in its infancy. Polymerase chain reaction (PCR)-based analysis of loss of heterozygosity (LOH) is a powerful method for detecting regions of altered tumour-suppressor genes. Focusing on chromosome 12, we examined 23 ovarian cancer samples for LOH using 31 highly polymorphic microsatellite markers and found the chromosomal localization of two putative tumour-suppressor genes. Two commonly deleted regions were 12p12.3-13.1 in 6/23 (26%) and 12q23-ter in 7/23 (30%) samples. LOH on chromosome 12 was more common in late-stage ovarian carcinomas. The region of LOH at 12p12.3-13.1 includes the genes that code for the ETS-family transcriptional factor, known as TEL, and the cyclin-dependent kinase inhibitor, known as p27Kip1. Mutational analysis of both TEL and p27Kip1 using single-strand conformation polymorphism (SSCP) showed no abnormalities, suggesting that the altered gene in this region is neither of these genes. Taken together, our data suggest that new tumour-suppressor genes in the region of chromosomes 12p12.3-13.1 and 12q23-ter may be involved in the development of ovarian cancer.
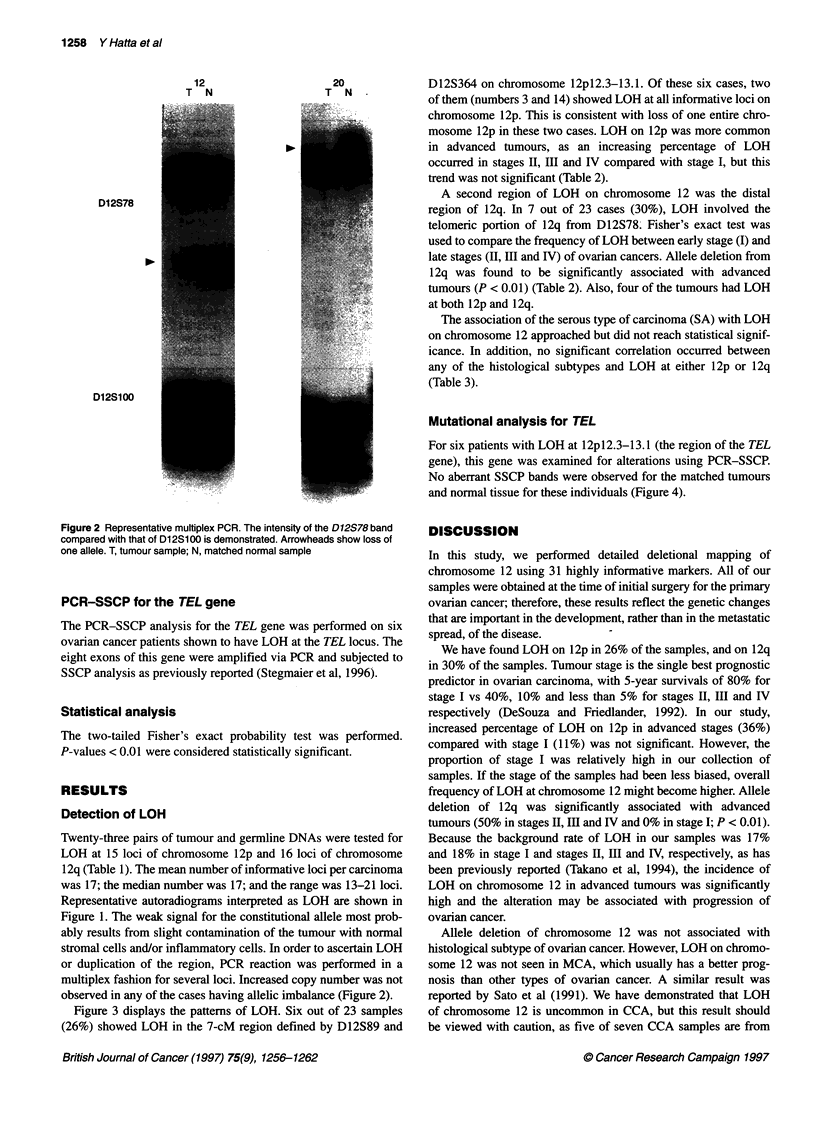
Full text
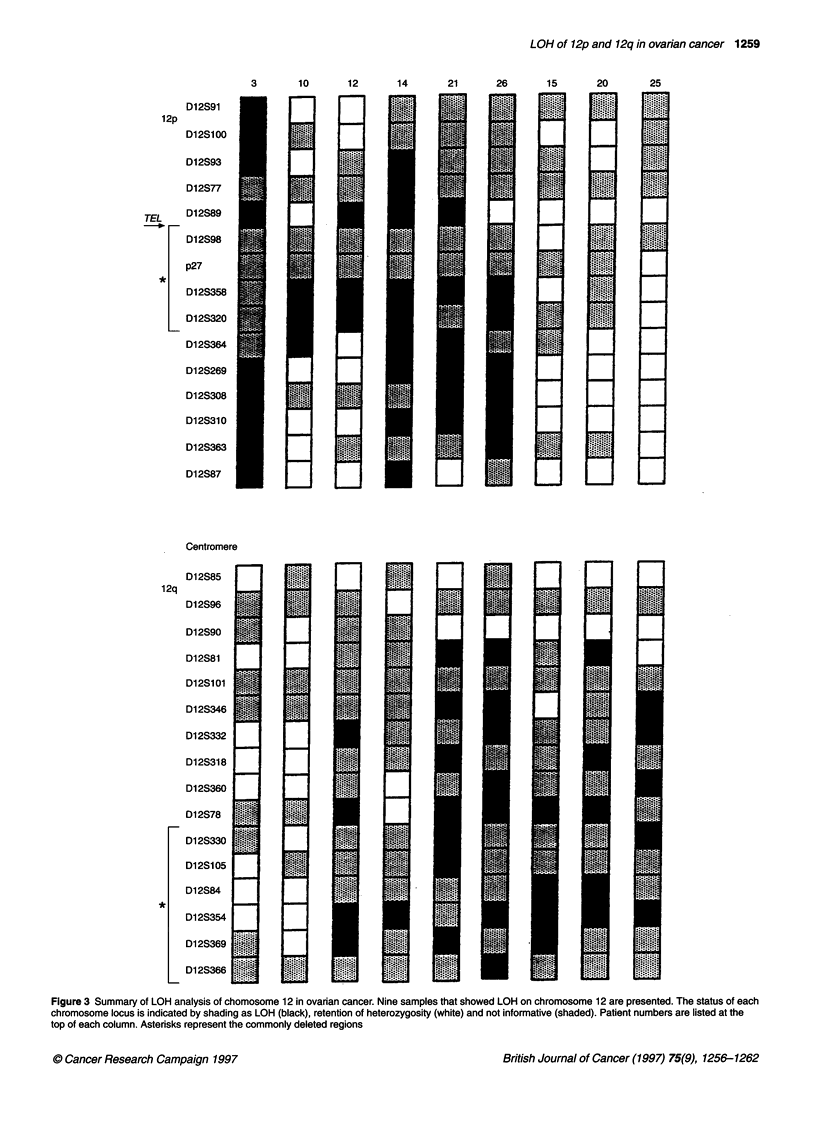
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berchuck A., Kamel A., Whitaker R., Kerns B., Olt G., Kinney R., Soper J. T., Dodge R., Clarke-Pearson D. L., Marks P. Overexpression of HER-2/neu is associated with poor survival in advanced epithelial ovarian cancer. Cancer Res. 1990 Jul 1;50(13):4087–4091. [PubMed] [Google Scholar]
- Buijs A., Sherr S., van Baal S., van Bezouw S., van der Plas D., Geurts van Kessel A., Riegman P., Lekanne Deprez R., Zwarthoff E., Hagemeijer A. Translocation (12;22) (p13;q11) in myeloproliferative disorders results in fusion of the ETS-like TEL gene on 12p13 to the MN1 gene on 22q11. Oncogene. 1995 Apr 20;10(8):1511–1519. [PubMed] [Google Scholar]
- Bullrich F., MacLachlan T. K., Sang N., Druck T., Veronese M. L., Allen S. L., Chiorazzi N., Koff A., Heubner K., Croce C. M. Chromosomal mapping of members of the cdc2 family of protein kinases, cdk3, cdk6, PISSLRE, and PITALRE, and a cdk inhibitor, p27Kip1, to regions involved in human cancer. Cancer Res. 1995 Mar 15;55(6):1199–1205. [PubMed] [Google Scholar]
- Børresen A. L. Oncogenesis in ovarian cancer. Acta Obstet Gynecol Scand Suppl. 1992;155:25–30. doi: 10.1111/j.1600-0412.1992.tb00004.x. [DOI] [PubMed] [Google Scholar]
- Chien C. H., Chang K. T., Chow S. N. Amplification and expression of c-Ki-ras oncogene in human ovarian cancer. Proc Natl Sci Counc Repub China B. 1990 Jan;14(1):27–32. [PubMed] [Google Scholar]
- Cliby W., Ritland S., Hartmann L., Dodson M., Halling K. C., Keeney G., Podratz K. C., Jenkins R. B. Human epithelial ovarian cancer allelotype. Cancer Res. 1993 May 15;53(10 Suppl):2393–2398. [PubMed] [Google Scholar]
- Eccles D. M., Cranston G., Steel C. M., Nakamura Y., Leonard R. C. Allele losses on chromosome 17 in human epithelial ovarian carcinoma. Oncogene. 1990 Oct;5(10):1599–1601. [PubMed] [Google Scholar]
- Foulkes W. D., Campbell I. G., Stamp G. W., Trowsdale J. Loss of heterozygosity and amplification on chromosome 11q in human ovarian cancer. Br J Cancer. 1993 Feb;67(2):268–273. doi: 10.1038/bjc.1993.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulkes W. D., Ragoussis J., Stamp G. W., Allan G. J., Trowsdale J. Frequent loss of heterozygosity on chromosome 6 in human ovarian carcinoma. Br J Cancer. 1993 Mar;67(3):551–559. doi: 10.1038/bjc.1993.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulkes W., Black D., Solomon E., Trowsdale J. Allele loss on chromosome 17q in sporadic ovarian cancer. Lancet. 1991 Aug 17;338(8764):444–445. doi: 10.1016/0140-6736(91)91065-3. [DOI] [PubMed] [Google Scholar]
- Golub T. R., Barker G. F., Bohlander S. K., Hiebert S. W., Ward D. C., Bray-Ward P., Morgan E., Raimondi S. C., Rowley J. D., Gilliland D. G. Fusion of the TEL gene on 12p13 to the AML1 gene on 21q22 in acute lymphoblastic leukemia. Proc Natl Acad Sci U S A. 1995 May 23;92(11):4917–4921. doi: 10.1073/pnas.92.11.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub T. R., Barker G. F., Lovett M., Gilliland D. G. Fusion of PDGF receptor beta to a novel ets-like gene, tel, in chronic myelomonocytic leukemia with t(5;12) chromosomal translocation. Cell. 1994 Apr 22;77(2):307–316. doi: 10.1016/0092-8674(94)90322-0. [DOI] [PubMed] [Google Scholar]
- Hall A. V., Antoniou H., Wang Y., Cheung A. H., Arbus A. M., Olson S. L., Lu W. C., Kau C. L., Marsden P. A. Structural organization of the human neuronal nitric oxide synthase gene (NOS1). J Biol Chem. 1994 Dec 30;269(52):33082–33090. [PubMed] [Google Scholar]
- Hatta Y., Hirama T., Miller C. W., Yamada Y., Tomonaga M., Koeffler H. P. Homozygous deletions of the p15 (MTS2) and p16 (CDKN2/MTS1) genes in adult T-cell leukemia. Blood. 1995 May 15;85(10):2699–2704. [PubMed] [Google Scholar]
- Hatta Y., Hirama T., Takeuchi S., Lee E., Pham E., Miller C. W., Strohmeyer T., Wilczynski S. P., Melmed S., Koeffler H. P. Alterations of the p16 (MTS1) gene in testicular, ovarian, and endometrial malignancies. J Urol. 1995 Nov;154(5):1954–1957. [PubMed] [Google Scholar]
- Iwabuchi H., Sakamoto M., Sakunaga H., Ma Y. Y., Carcangiu M. L., Pinkel D., Yang-Feng T. L., Gray J. W. Genetic analysis of benign, low-grade, and high-grade ovarian tumors. Cancer Res. 1995 Dec 15;55(24):6172–6180. [PubMed] [Google Scholar]
- Jaakkola S., Salmikangas P., Nylund S., Partanen J., Armstrong E., Pyrhönen S., Lehtovirta P., Nevanlinna H. Amplification of fgfr4 gene in human breast and gynecological cancers. Int J Cancer. 1993 May 28;54(3):378–382. doi: 10.1002/ijc.2910540305. [DOI] [PubMed] [Google Scholar]
- Jacobs I. J., Smith S. A., Wiseman R. W., Futreal P. A., Harrington T., Osborne R. J., Leech V., Molyneux A., Berchuck A., Ponder B. A. A deletion unit on chromosome 17q in epithelial ovarian tumors distal to the familial breast/ovarian cancer locus. Cancer Res. 1993 Mar 15;53(6):1218–1221. [PubMed] [Google Scholar]
- Jones M. H., Nakamura Y. Deletion mapping of chromosome 3p in female genital tract malignancies using microsatellite polymorphisms. Oncogene. 1992 Aug;7(8):1631–1634. [PubMed] [Google Scholar]
- Kamb A., Gruis N. A., Weaver-Feldhaus J., Liu Q., Harshman K., Tavtigian S. V., Stockert E., Day R. S., 3rd, Johnson B. E., Skolnick M. H. A cell cycle regulator potentially involved in genesis of many tumor types. Science. 1994 Apr 15;264(5157):436–440. doi: 10.1126/science.8153634. [DOI] [PubMed] [Google Scholar]
- Kawamata N., Morosetti R., Miller C. W., Park D., Spirin K. S., Nakamaki T., Takeuchi S., Hatta Y., Simpson J., Wilcyznski S. Molecular analysis of the cyclin-dependent kinase inhibitor gene p27/Kip1 in human malignancies. Cancer Res. 1995 Jun 1;55(11):2266–2269. [PubMed] [Google Scholar]
- Knudson A. G., Jr Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971 Apr;68(4):820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. Y., Mattei M. G., Zaleska-Rutczynska Z., Hooft van Huijsduijnen R., Figueroa F., Nadeau J., Benoist C., Mathis D. One subunit of the transcription factor NF-Y maps close to the major histocompatibility complex in murine and human chromosomes. Genomics. 1991 Nov;11(3):630–634. doi: 10.1016/0888-7543(91)90070-u. [DOI] [PubMed] [Google Scholar]
- Lloberas J., Maki R. A., Celada A. Repression of major histocompatibility complex I-A beta gene expression by dbpA and dbpB (mYB-1) proteins. Mol Cell Biol. 1995 Sep;15(9):5092–5099. doi: 10.1128/mcb.15.9.5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani R., Li X. Y., Pessara U., Hooft van Huisjduijnen R., Benoist C., Mathis D. Dominant negative analogs of NF-YA. J Biol Chem. 1994 Aug 12;269(32):20340–20346. [PubMed] [Google Scholar]
- McLean T. W., Ringold S., Neuberg D., Stegmaier K., Tantravahi R., Ritz J., Koeffler H. P., Takeuchi S., Janssen J. W., Seriu T. TEL/AML-1 dimerizes and is associated with a favorable outcome in childhood acute lymphoblastic leukemia. Blood. 1996 Dec 1;88(11):4252–4258. [PubMed] [Google Scholar]
- Morosétti R., Kawamata N., Gombart A. F., Miller C. W., Hatta Y., Hirama T., Said J. W., Tomonaga M., Koeffler H. P. Alterations of the p27KIP1 gene in non-Hodgkin's lymphomas and adult T-cell leukemia/lymphoma. Blood. 1995 Sep 1;86(5):1924–1930. [PubMed] [Google Scholar]
- Okamoto A., Demetrick D. J., Spillare E. A., Hagiwara K., Hussain S. P., Bennett W. P., Forrester K., Gerwin B., Serrano M., Beach D. H. Mutations and altered expression of p16INK4 in human cancer. Proc Natl Acad Sci U S A. 1994 Nov 8;91(23):11045–11049. doi: 10.1073/pnas.91.23.11045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos P., Ridge S. A., Boucher C. A., Stocking C., Wiedemann L. M. The novel activation of ABL by fusion to an ets-related gene, TEL. Cancer Res. 1995 Jan 1;55(1):34–38. [PubMed] [Google Scholar]
- Pejovic T., Heim S., Mandahl N., Baldetorp B., Elmfors B., Flodérus U. M., Furgyik S., Helm G., Himmelmann A., Willén H. Chromosome aberrations in 35 primary ovarian carcinomas. Genes Chromosomes Cancer. 1992 Jan;4(1):58–68. doi: 10.1002/gcc.2870040108. [DOI] [PubMed] [Google Scholar]
- Perez R. P., Godwin A. K., Hamilton T. C., Ozols R. F. Ovarian cancer biology. Semin Oncol. 1991 Jun;18(3):186–204. [PubMed] [Google Scholar]
- Pietenpol J. A., Bohlander S. K., Sato Y., Papadopoulos N., Liu B., Friedman C., Trask B. J., Roberts J. M., Kinzler K. W., Rowley J. D. Assignment of the human p27Kip1 gene to 12p13 and its analysis in leukemias. Cancer Res. 1995 Mar 15;55(6):1206–1210. [PubMed] [Google Scholar]
- Polyak K., Lee M. H., Erdjument-Bromage H., Koff A., Roberts J. M., Tempst P., Massagué J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994 Jul 15;78(1):59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- Ponce-Castañeda M. V., Lee M. H., Latres E., Polyak K., Lacombe L., Montgomery K., Mathew S., Krauter K., Sheinfeld J., Massague J. p27Kip1: chromosomal mapping to 12p12-12p13.1 and absence of mutations in human tumors. Cancer Res. 1995 Mar 15;55(6):1211–1214. [PubMed] [Google Scholar]
- Romana S. P., Mauchauffé M., Le Coniat M., Chumakov I., Le Paslier D., Berger R., Bernard O. A. The t(12;21) of acute lymphoblastic leukemia results in a tel-AML1 gene fusion. Blood. 1995 Jun 15;85(12):3662–3670. [PubMed] [Google Scholar]
- Romana S. P., Poirel H., Leconiat M., Flexor M. A., Mauchauffé M., Jonveaux P., Macintyre E. A., Berger R., Bernard O. A. High frequency of t(12;21) in childhood B-lineage acute lymphoblastic leukemia. Blood. 1995 Dec 1;86(11):4263–4269. [PubMed] [Google Scholar]
- Russell S. E., Hickey G. I., Lowry W. S., White P., Atkinson R. J. Allele loss from chromosome 17 in ovarian cancer. Oncogene. 1990 Oct;5(10):1581–1583. [PubMed] [Google Scholar]
- Saito S., Saito H., Koi S., Sagae S., Kudo R., Saito J., Noda K., Nakamura Y. Fine-scale deletion mapping of the distal long arm of chromosome 6 in 70 human ovarian cancers. Cancer Res. 1992 Oct 15;52(20):5815–5817. [PubMed] [Google Scholar]
- Sato T., Saito H., Morita R., Koi S., Lee J. H., Nakamura Y. Allelotype of human ovarian cancer. Cancer Res. 1991 Oct 1;51(19):5118–5122. [PubMed] [Google Scholar]
- Shurtleff S. A., Buijs A., Behm F. G., Rubnitz J. E., Raimondi S. C., Hancock M. L., Chan G. C., Pui C. H., Grosveld G., Downing J. R. TEL/AML1 fusion resulting from a cryptic t(12;21) is the most common genetic lesion in pediatric ALL and defines a subgroup of patients with an excellent prognosis. Leukemia. 1995 Dec;9(12):1985–1989. [PubMed] [Google Scholar]
- Slamon D. J., Godolphin W., Jones L. A., Holt J. A., Wong S. G., Keith D. E., Levin W. J., Stuart S. G., Udove J., Ullrich A. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989 May 12;244(4905):707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- Stegmaier K., Pendse S., Barker G. F., Bray-Ward P., Ward D. C., Montgomery K. T., Krauter K. S., Reynolds C., Sklar J., Donnelly M. Frequent loss of heterozygosity at the TEL gene locus in acute lymphoblastic leukemia of childhood. Blood. 1995 Jul 1;86(1):38–44. [PubMed] [Google Scholar]
- Stegmaier K., Takeuchi S., Golub T. R., Bohlander S. K., Bartram C. R., Koeffler H. P. Mutational analysis of the candidate tumor suppressor genes TEL and KIP1 in childhood acute lymphoblastic leukemia. Cancer Res. 1996 Mar 15;56(6):1413–1417. [PubMed] [Google Scholar]
- Takeuchi S., Bartram C. R., Miller C. W., Reiter A., Seriu T., Zimmerann M., Schrappe M., Mori N., Slater J., Miyoshi I. Acute lymphoblastic leukemia of childhood: identification of two distinct regions of deletion on the short arm of chromosome 12 in the region of TEL and KIP1. Blood. 1996 Apr 15;87(8):3368–3374. [PubMed] [Google Scholar]
- Takeuchi S., Bartram C. R., Seriu T., Miller C. W., Tobler A., Janssen J. W., Reiter A., Ludwig W. D., Zimmermann M., Schwaller J. Analysis of a family of cyclin-dependent kinase inhibitors: p15/MTS2/INK4B, p16/MTS1/INK4A, and p18 genes in acute lymphoblastic leukemia of childhood. Blood. 1995 Jul 15;86(2):755–760. [PubMed] [Google Scholar]
- Takeuchi S., Bartram C. R., Wada M., Reiter A., Hatta Y., Seriu T., Lee E., Miller C. W., Miyoshi I., Koeffler H. P. Allelotype analysis of childhood acute lymphoblastic leukemia. Cancer Res. 1995 Nov 15;55(22):5377–5382. [PubMed] [Google Scholar]
- Takeuchi S., Mori N., Koike M., Slater J., Park S., Miller C. W., Miyoshi I., Koeffler H. P. Frequent loss of heterozygosity in region of the KIP1 locus in non-small cell lung cancer: evidence for a new tumor suppressor gene on the short arm of chromosome 12. Cancer Res. 1996 Feb 15;56(4):738–740. [PubMed] [Google Scholar]
- Toyoshima H., Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell. 1994 Jul 15;78(1):67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- Viel A., Giannini F., Tumiotto L., Sopracordevole F., Visentin M. C., Boiocchi M. Chromosomal localisation of two putative 11p oncosuppressor genes involved in human ovarian tumours. Br J Cancer. 1992 Dec;66(6):1030–1036. doi: 10.1038/bjc.1992.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodarska I., Mecucci C., Marynen P., Guo C., Franckx D., La Starza R., Aventin A., Bosly A., Martelli M. F., Cassiman J. J. TEL gene is involved in myelodysplastic syndromes with either the typical t(5;12)(q33;p13) translocation or its variant t(10;12)(q24;p13). Blood. 1995 May 15;85(10):2848–2852. [PubMed] [Google Scholar]
- Xu W., Gorman P., Sheer D., Bates G., Kishimoto J., Lizhi L., Emson P. Regional localization of the gene coding for human brain nitric oxide synthase (NOS1) to 12q24.2-->24.31 by fluorescent in situ hybridization. Cytogenet Cell Genet. 1993;64(1):62–63. doi: 10.1159/000133562. [DOI] [PubMed] [Google Scholar]
- Yang-Feng T. L., Han H., Chen K. C., Li S. B., Claus E. B., Carcangiu M. L., Chambers S. K., Chambers J. T., Schwartz P. E. Allelic loss in ovarian cancer. Int J Cancer. 1993 Jun 19;54(4):546–551. doi: 10.1002/ijc.2910540405. [DOI] [PubMed] [Google Scholar]
- Yang-Feng T. L., Li S., Han H., Schwartz P. E. Frequent loss of heterozygosity on chromosomes Xp and 13q in human ovarian cancer. Int J Cancer. 1992 Oct 21;52(4):575–580. doi: 10.1002/ijc.2910520414. [DOI] [PubMed] [Google Scholar]
- Zheng J. P., Robinson W. R., Ehlen T., Yu M. C., Dubeau L. Distinction of low grade from high grade human ovarian carcinomas on the basis of losses of heterozygosity on chromosomes 3, 6, and 11 and HER-2/neu gene amplification. Cancer Res. 1991 Aug 1;51(15):4045–4051. [PubMed] [Google Scholar]
- de Souza P. L., Friedlander M. L. Prognostic factors in ovarian cancer. Hematol Oncol Clin North Am. 1992 Aug;6(4):761–782. [PubMed] [Google Scholar]