Abstract
The nuclear enzyme poly(ADP-ribose) polymerase (PARP)-1 has an important role in regulating cell death and cellular responses to DNA repair. Pharmacological inhibitors of PARP have entered clinical testing as cytoprotective agents in cardiovascular diseases and as adjunct antitumor therapeutics. Initially, it was assumed that the regulation of PARP occurs primarily at the level of DNA breakage: recognition of DNA breaks was considered to be the primary regulator (activator) or the catalytic activity of PARP. Recent studies have provided evidence that PARP-1 activity can also be modulated by several endogenous factors, including various kinases, purines and caffeine metabolites. There is a gender difference in the contribution of PARP-1 to stroke and inflammatory responses, which is due, at least in part, to endogenous estrogen levels. Several tetracycline antibiotics are also potent PARP-1 inhibitors. In this article, we present an overview of novel PARP-1 modulators.
Introduction
Poly(ADP-ribose) polymerase (PARP)-1 is the best-studied isoform of a family of multifunctional nuclear enzymes. Activated PARP uses NAD+ and transfers ADP-ribose units to nuclear target proteins. Poly(ADP-ribosyl)ation is involved in the regulation of DNA repair, gene transcription, cell-cycle progression, cell death, chromatin function and genomic stability [1,2].
Pharmacological inhibitors of PARP have entered clinical testing as cytoprotective agents in cardiovascular diseases, and are being considered as both monotherapy and in combination with chemotherapy for cancer treatment [2]. The cytoprotective effects of PARP inhibitors comprise inhibition of the pathological overactivation of the enzyme and prevention of cell necrosis by overuse of cellular NAD+ pools and promotion of cellular energetic failure [1,2]. The use of PARP inhibitors as anticancer agents exploits the fact that the repair of certain types of cellular damage related to antitumor agents relies almost exclusively on the functional integrity of PARP [3]. Another distinct mode of action of PARP inhibitors relates to the downregulation of various pro-inflammatory signal transduction pathways [1,2] and suppression of chemokine, cytokine, adhesion molecule and free-radical-generating enzyme expression at the transcriptional level.
Initially, it was assumed that PARP (a constitutive enzyme) is regulated primarily by its ability to recognize broken DNA strands via its zinc fingers [1]. Endogenous oxidants and free radicals (e.g. peroxynitrite) were later added to the list of ‘classical’ triggers of DNA single-strand breakage (e.g. ionizing radiation and genotoxic compounds) [4]. Conditions that can produce reactive radicals and oxidants within the cell, including hypoxia–reoxygenation [5], elevated extracellular glucose concentration [6–9], Ca2+ [10] and angiotensin II [11,12], have been identified as endogenous activators of PARP. Allosteric regulation of auto(poly-ADP-ribosyl)ation by Mg2+, Ca2+, polyamines, ATP and the histones H1 and H3 has also been demonstrated [13]. Furthermore, the degree to which PARP is activated by DNA damage can be regulated by other factors. For example, PARP-1 phosphorylation by the mitogen-activated protein kinase (MAPK) extracellular-signal-regulated kinase (ERK)2 seems to be necessary for maximal PAPR-1 activation [14]. In cell cultures, ERK2 inhibition by either pharmacological agents or small interfering RNA downregulation attenuates PARP-1 activation after DNA damage [14]. This regulatory effect on PARP-1 might be a mechanism by which inhibitors of the ERK2 signaling cascade reduce cell death rates following ischemia–reperfusion [15]. Given the central role of PARP-1 in both cell death and inflammation, these results indicate that the effects of ERK2 on PARP-1 activity could be a general mechanism through which ERK2 influences cell survival. Similarly, calmodulin-dependent protein kinase (CaMK)IIδ might also activate PARP-1 by phosphorylation [16], and PARP can modulate AKT kinase (protein kinase B) [17] and JNK [18] kinase activities. Importantly, recent studies have identified the kinesin superfamily protein (KIF)4 [19], and sirtuin or silent mating-type information regulation 2 homolog (Sirt1) [20] as endogenous inhibitors of PARP-1.
Most of the these observations can be placed in the context of pathophysiological alterations: PARP and signal transduction pathways in the context of inflammation, PARP and re-oxygenation in the context of stroke and heart attacks, PARP and elevated glucose levels in the context of diabetic complications, and PARP and angiotensin II in the context of hypertension and cardiac hypertrophy. Until recently, however, essentially no information was available about the physiological regulation of PARP, for which we now provide an overview.
Gender-specific regulation of PARP-1
Early pioneering work by Jackowski and Kun in the early 1980s highlighted the role of thyroid hormones as regulators of PARP activity [21]. Administration of the thyroid hormone L-triiodothyronine in concentrations that induce cardiac ventricular enlargement was shown to inhibit the PARP-1 activity of cardiomyocyte nuclei, with a simultaneous augmentation of RNA synthesis [21]. Consequent studies have also demonstrated that hepatic PARP-1 activity in rats is controlled by thyroid hormones [22,23]. The concentrations of the thyroid hormones used were, in most cases, higher than the physiologically relevant concentrations. Nevertheless, the regulation of PARP-1 by these hormones merits renewed interest using the experimental tools that are now available.
Recent studies have expanded on the concept that endogenous physiological factors, conditions and mediators can regulate PARP-1. Several groups [24,25] have confirmed earlier observations [26] that PARP inhibition or PARP-1 deficiency is protective in stroke. Surprisingly, however, PARP inhibitors confer their protection in male mice only. By contrast, the absence of functional PARP-1 or pharmacological PARP inhibition is not beneficial to the outcome of ischemic stroke in female mice [24,25]. Similar observations have been noted in rodent models of shock or inflammation [27]. The endotoxin-induced inflammatory and vascular responses are cooperatively regulated by gender and PARP. Production of the inflammatory mediator tumor necrosis factor (TNF)-α, endotoxin-induced mortality and the development of endotoxin-induced endothelial dysfunction are markedly attenuated in female mice (compared with male mice), and they are also reduced by PARP inhibitors in male mice. However, pharmacological inhibition of PARP fails to provide further protection in female animals. In fact, PARP inhibition in male animals, and female gender provided comparable protection against several inflammatory and cardiovascular parameters that were investigated, although no combination effects of the two protective factors were noted [27]. Consistent with these findings, in circulating leukocytes, a potent PARP inhibitor regulated lipopolysaccharide (LPS)-induced PARP activation only in male animals [27]. In a subsequent series of investigations conducted in porcine models of thoracoabdominal aortic ischemia–reperfusion injury, the inhibition of cardiovascular collapse by PARP inhibitors was observed only in male animals [28].
What, then, is responsible for this marked gender difference? Although the issue requires further investigation, several studies indicate the possible importance of female sex hormones – at least in shock- or inflammation-related studies [27]. Several findings highlight the potential involvement of the main female sex hormone, 17-β-estradiol, for the observed effects: (i) the gender difference regarding LPS-induced TNF-α production is partially diminished in ovarectomized animals; (ii) poly(ADP-ribosyl)ation is attenuated by estrogen in male animals challenged with endotoxin in vivo; and (iii) there is a difference in the degree of PARP activation between cells incubated in male versus female rat serum.
However, estrogen does not seem to inhibit PARP activation directly. When recombinant PARP and estrogen are mixed, the catalytic activity of PARP does not seem to be affected. However, an interesting in vitro interaction has been reported among PARP, the estrogen receptor and DNA; this interaction is further reinforced by the presence of estrogen [27]. A model of interaction has been proposed between PARP-1 and the estrogen receptor α in which a stable complex might sequester PARP-1 to specific regions on DNA, making it difficult for the zinc fingers of the enzyme to access and recognize DNA breakpoints (without which its activation would be inhibited). It has been hypothesized that this action contributes to the observed effects of estrogen in vivo [27], although a direct link remains to be demonstrated. An additional mode of action might be the antioxidant property of the female sex hormones, which can exert cytoprotective effects, sometimes in surprisingly low (1–10 nmol/l) concentrations [29].
Pharmacological importance of gender-specific PARP-1 regulation
What is the applicability of this gender difference in PARP-dependent responses to physiological gender differences, to other animal models of disease and, ultimately, to humans? Clearly, PARP inhibitors are not always ineffective in female animals. Female nonobese diabetic (NOD) mice undergo autoimmune β-cell loss and a disease that resembles type 1 diabetes. In these mice, PARP inhibition is of major therapeutic benefit [7,30]. Also, PARP inhibitors are protective in female sheep subjected to shock, or burn and smoke inhalation damage [31]. Thus, the limit of the applicability of these gender-specific findings must be established.
An area that requires more-extensive investigation is myocardial infarction and gender. It is known from human epidemiological studies that females are protected – to a significant degree because of estrogen – against cardiovascular disease, and this protection disappears after menopause [32]. It remains to be tested whether this protection is related to a ‘baseline’ PARP inhibitory effect of female sex hormones.
New modulators of PARP-1 activity
Vitamin D might be an additional endogenous regulator of PARP [33]. Using purified PARP enzyme, the active form of vitamin D3, 1,25-dihydroxyvitamin D3, was identified as an inhibitor of PARP activity with an IC50 of 0.2 μM and 3 μM in cell-free and cell-based PARP assays, respectively [33]. However, vitamin D3 itself had no effect on PARP activity at more than 100 times this concentration in the cell-free assay. Vitamin D3 has well-documented anti-inflammatory effects in various experimental models of disease (for review, see Refs [34,35]). However, the extent to which this action influences the wide-ranging effects of vitamin D3 is yet to be determined.
ATP [13,36] and certain purines, including hypoxanthine and inosine, are endogenous inhibitors of PARP [37]. However, it is unclear whether the concentration range of these substances is within the physiological or pathophysiological range. Recent studies demonstrate that many other xanthine derivatives (metabolites of caffeine, including 1-methylxanthine and 1,7-dimethylxantine) have considerable PARP inhibitory activity and are more potent than hypoxanthine as endogenous inhibitors of this enzyme (caffeine itself has only modest inhibitory effects) [38]. Likewise, theophylline (which is present in tea and is used to treat lung pathologies because of its bronchodilator and antioxidant–anti-inflammatory properties) exerts PARP inhibitory effects in human pulmonary epithelial cells [39]. The plasma levels of these compounds [40] are in a range that is consistent with the PARP inhibitory property of the compounds, such that drinking coffee and tea might affect cellular PARP activity in vivo.
Another line of investigation demonstrates that some common antibiotics of tetracycline class, including doxycycline and minocycline, are relatively potent inhibitors of PARP, with inhibition occurring in the high nanomolar range. Thus, these compounds are less potent than are the latest generation of PARP inhibitors but they are comparable in potency to earlier compounds of the isoquinolinone and phenanthridinone classes [41]. The inhibition by tetracycline antibiotics – which is similar to that caused by most of the ‘professional’ PARP inhibitor compounds – is competitive and occurs by preventing the PARP substrate NAD from binding to the active center of the enzyme [41]. Minocycline has recently received attention as a possible therapy for neurodegenerative disease and it has been reported to exert cytoprotective and anti-inflammatory effects in vitro [42,43]. Minocycline can reduce rates of neuronal death after excitotoxicity and ionizing radiation in culture [44,45] and in animal models of stroke [45–48], Parkinson’s disease [49,50], Huntington’s disease [51] and amyotrophic lateral sclerosis [52]. The neuroprotective effects of minocycline have been attributed to both reduced inflammation and a direct effect on neuronal survival [44,45,48,52,53]. All of these actions are consistent with its function as a PARP inhibitor.
The identification of doxycycline as a PARP inhibitor [41] represents the third proposed therapeutic mode of action for this compound – the first being the original antibiotic effect and the second being the inhibitory effect on matrix metalloproteinases (MMPs) [54]. Doxycycline is of major benefit in multiple cardiovascular diseases, including myocardial ischemia–reperfusion and heart failure [55,56]; either its MMP inhibitory effect or its PARP inhibitory effect (or a combination of the two, or another unidentified action) could be its mode of action.
Concluding remarks
Some implications of the findings discussed relate to the current clinical trials of PARP inhibitors. If some of the endogenous compounds mentioned are inhibitors of the enzyme, plasma levels of these molecules might influence physiological or pathophysiological parameters and, ultimately, the outcome of the trials. For instance, possible gender differences in cancer trials with PARP inhibitors might require investigation (this issue is less important in cardiovascular trials with PARP inhibitors because most women develop stroke or heart attack only after menopause, when the regulation of PARP activity by estrogen is expected to be diminished). In many clinical trials, coffee drinking is discouraged. The observation that caffeine metabolites can suppress PARP activity could necessitate the exclusion of coffee from clinical trials involving PARP inhibitors.
The safety and risks associated with chronic or repeated administration of PARP inhibitors are currently under debate because of the role of PARP in the maintenance of genomic integrity [2,57]. The recent studies demonstrating that PARP activity is dynamically regulated by a multitude of factors and compounds (some of which –e.g. coffee and tetracycline antibiotics – are considered to be safe and well tolerated) favor a more permissive view of the chronic use of PARP inhibitors, perhaps at doses and concentrations at which inhibition is only partial.
In light of the data showing the PARP inhibitory effect of tetracyclines (coupled with the established safety profile of doxycycline and minocycline), the possible therapeutic benefit of the combination of doxycycline or minocycline with anticancer chemotherapeutics could be explored, as with the recent attempts to investigate the combination therapy of novel ultrapotent PARP inhibitors with chemotherapeutic agents such as temozolomide.
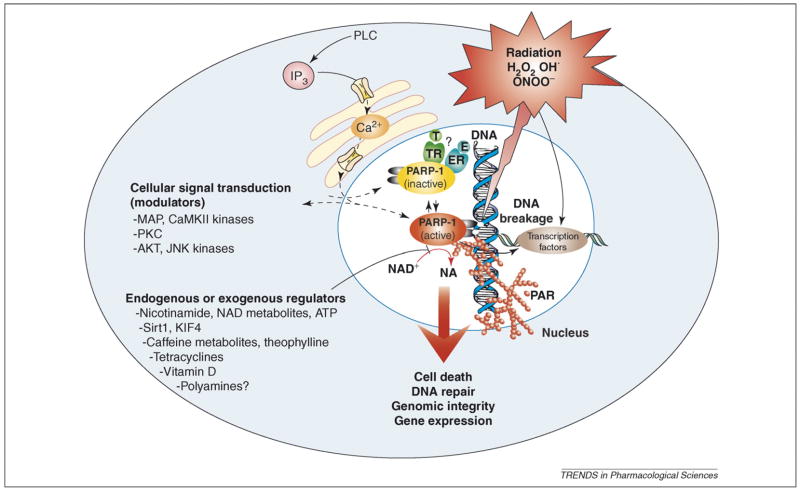
In conclusion, a growing body of data demonstrates that PARP activity is dynamically regulated by many activators and inhibitors (Figure 1). These observations necessitate the revision of some of the views of PARP as a regulator of health and disease, and highlight new points regarding the design and interpretation of clinical trials involving PARP inhibitors.
Figure 1.
Endogenous and exogenous regulators and modulators of PARP-1 activity. Endogenous factors can affect PARP-1 activity either by forming a complex with PARP-1 or by inhibiting the binding of NAD+ to the active site of the enzyme. The former might include estrogen (E) and thyroid hormones (T), and the latter might include nicotinamide (NA), NAD+ metabolites, caffeine metabolites and vitamin D. PARP-1 activity can also be modulated through phosphorylation by kinases (e.g. MAPK and CaMKIIδ, and PKC), by Sirt1 and through binding to KIF4. PARP can also modulate kinase (e.g. AKT and JNK) activity. Exogenous factors such as caffeine and its endogenously formed metabolites, theophylline and tetracycline antibiotics might also modulate PARP activity. Overall, PARP seems to be subject to multiple lines of endogenous regulators, and it is conceivable that the processes regulated by PARP (e.g. DNA repair and cellular NAD homeostasis) are under similarly dynamic control by a multitude of factors and influences. Abbreviations: ER, estrogen receptor; TR, thyroid hormone receptor.
Acknowledgments
This work was supported in part by the Intramural Research Program of the National Institutes of Health and National Institute on Alcohol Abuse and Alcoholism (to P.P.) and by grant R01 from the National Institutes of Health (to C.S. and R.S.).
References
- 1.Virag L, Szabo C. The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol Rev. 2002;54:375–429. doi: 10.1124/pr.54.3.375. [DOI] [PubMed] [Google Scholar]
- 2.Jagtap P, Szabo C. Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat Rev Drug Discov. 2005;4:421–440. doi: 10.1038/nrd1718. [DOI] [PubMed] [Google Scholar]
- 3.Graziani G, et al. PARP-1 inhibition to treat cancer, ischemia, inflammation. Pharmacol Res. 2005;52:1–4. doi: 10.1016/j.phrs.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Szabo C, et al. DNA strand breakage, activation of poly(ADP-ribose) synthetase, and cellular energy depletion are involved in the cytotoxicity of macrophages and smooth muscle cells exposed to peroxynitrite. Proc Natl Acad Sci U S A. 1996;93:1753–1758. doi: 10.1073/pnas.93.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilad E, et al. Protection by inhibition of poly(ADP-ribose) synthetase against oxidant injury in cardiac myoblasts in vitro. J Mol Cell Cardiol. 1997;29:2585–2597. doi: 10.1006/jmcc.1997.0496. [DOI] [PubMed] [Google Scholar]
- 6.Garcia Soriano F, et al. Diabetic endothelial dysfunction: the role of poly(ADP-ribose) polymerase activation. Nat Med. 2001;7:108–113. doi: 10.1038/83241. [DOI] [PubMed] [Google Scholar]
- 7.Pacher P, et al. The role of poly(ADP-ribose) polymerase activation in the development of myocardial and endothelial dysfunction in diabetes. Diabetes. 2002;51:514–521. doi: 10.2337/diabetes.51.2.514. [DOI] [PubMed] [Google Scholar]
- 8.Du X, et al. Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J Clin Invest. 2003;112:1049–1057. doi: 10.1172/JCI18127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pacher P, Szabo C. Role of poly(ADP-ribose) polymerase-1 activation in the pathogenesis of diabetic complications: endothelial dysfunction, as a common underlying theme. Antioxid Redox Signal. 2005;7:1568–1580. doi: 10.1089/ars.2005.7.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yakovlev AG, et al. A role of the Ca2+/Mg2+-dependent endonuclease in apoptosis and its inhibition by poly(ADP-ribose) polymerase. J Biol Chem. 2000;275:21302–21308. doi: 10.1074/jbc.M001087200. [DOI] [PubMed] [Google Scholar]
- 11.Szabo C, et al. Angiotensin II-mediated endothelial dysfunction: role of poly(ADP-ribose) polymerase activation. Mol Med. 2004;10:28–35. doi: 10.2119/2004-00001.szabo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pillai JB, et al. Poly(ADP-ribose) polymerase-1-deficient mice are protected from angiotensin II-induced cardiac hypertrophy. Am J Physiol Heart Circ Physio. 2006;291:H1545–H1553. doi: 10.1152/ajpheart.01124.2005. [DOI] [PubMed] [Google Scholar]
- 13.Kun E, et al. Regulation of the enzymatic catalysis of poly(ADP-ribose) polymerase by dsDNA, polyamines, Mg2+, Ca2+, histones H1 and H3, and ATP. Biochemistry. 2004;43:210–216. doi: 10.1021/bi0301791. [DOI] [PubMed] [Google Scholar]
- 14.Kauppinen TM, et al. Direct phosphorylation and regulation of poly(ADP-ribose) polymerase-1 by extracellular signal-regulated kinases 1/2. Proc Natl Acad Sci U S A. 2006;103:7136–7141. doi: 10.1073/pnas.0508606103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alessandrini A, et al. MEK1 protein kinase inhibition protects against damage resulting from focal cerebral ischemia. Proc Natl Acad Sci U S A. 1999;96:12866–12869. doi: 10.1073/pnas.96.22.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim MY, et al. NAD+-dependent modulation of chromatin structure and transcription by nucleosome binding properties of PARP-1. Cell. 2004;119:803–814. doi: 10.1016/j.cell.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Veres B, et al. Decrease of the inflammatory response and induction of the Akt/protein kinase B pathway by poly-(ADP-ribose) polymerase 1 inhibitor in endotoxin-induced septic shock. Biochem Pharmacol. 2003;65:1373–1382. doi: 10.1016/s0006-2952(03)00077-7. [DOI] [PubMed] [Google Scholar]
- 18.Xu Y, et al. Poly(ADP-ribose) polymerase-1 signaling to mitochondria in necrotic cell death requires RIP1/TRAF2-mediated JNK1 activation. J Biol Chem. 2006;281:8788–8795. doi: 10.1074/jbc.M508135200. [DOI] [PubMed] [Google Scholar]
- 19.Midorikawa R, et al. KIF4 motor regulates activity-dependent neuronal survival by suppressing PARP-1 enzymatic activity. Cell. 2006;125:371–383. doi: 10.1016/j.cell.2006.02.039. [DOI] [PubMed] [Google Scholar]
- 20.Kolthur-Seetharam U, et al. Control of AIF-mediated cell death by the functional interplay of SIRT1 and PARP-1 in response to DNA damage. Cell Cycle. 2006;5:873–877. doi: 10.4161/cc.5.8.2690. [DOI] [PubMed] [Google Scholar]
- 21.Jackowski G, Kun E. The effect of in vivo treatment with triiodothyronine on the in vitro synthesis of protein–poly(ADP)-ribose adducts by isolated cardiocyte nuclei and the separation of poly(ADP)-ribosylated proteins by phenol extraction and electrophoresis. J Biol Chem. 1983;258:12587–12593. [PubMed] [Google Scholar]
- 22.Cesarone CF, et al. Hepatic poly(ADP-ribose) polymerase activity in rat is controlled by thyroid hormones. Biochem Biophys Res Commun. 1994;203:1548–1553. doi: 10.1006/bbrc.1994.2362. [DOI] [PubMed] [Google Scholar]
- 23.Giannoni P, et al. In vitro effect of 3,5,3′-triiodothyronine on poly(ADP-ribosyl)ation of DNA topoisomerase I. Ital J Biochem. 1995;44:129–136. [PubMed] [Google Scholar]
- 24.Hagberg H, et al. PARP-1 gene disruption in mice preferentially protects males from perinatal brain injury. J Neurochem. 2004;90:1068–1075. doi: 10.1111/j.1471-4159.2004.02547.x. [DOI] [PubMed] [Google Scholar]
- 25.McCullough LD, et al. Ischemic nitric oxide and poly(ADP-ribose) polymerase-1 in cerebral ischemia: male toxicity, female protection. J Cereb Blood Flow Metab. 2005;25:502–512. doi: 10.1038/sj.jcbfm.9600059. [DOI] [PubMed] [Google Scholar]
- 26.Eliasson MJ, et al. Poly(ADP-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia. Nat Med. 1997;3:1089–1095. doi: 10.1038/nm1097-1089. [DOI] [PubMed] [Google Scholar]
- 27.Mabley JG, et al. Gender differences in the endotoxin-induced inflammatory and vascular responses: potential role of poly(ADP-ribose) polymerase activation. J Pharmacol Exp Ther. 2005;315:812–820. doi: 10.1124/jpet.105.090480. [DOI] [PubMed] [Google Scholar]
- 28.Hauser B, et al. The PARP-1 inhibitor ino-1001 facilitates hemodynamic stabilization without affecting DNA repair in porcine thoracic aortic cross-clamping-induced ischemia/reperfusion. Shock. 2006;25:633–640. doi: 10.1097/01.shk.0000209561.61951.2e. [DOI] [PubMed] [Google Scholar]
- 29.Kuohung W, et al. Tamoxifen, esterified estradiol, and physiologic concentrations of estradiol inhibit oxidation of low-density lipoprotein by endothelial cells. Am J Obstet Gynecol. 2001;184:1060–1063. doi: 10.1067/mob.2001.115229. [DOI] [PubMed] [Google Scholar]
- 30.Suarez-Pinzon WL, et al. Poly(ADP-ribose) polymerase inhibition prevents spontaneous and recurrent autoimmune diabetes in NOD mice by inducing apoptosis of islet-infiltrating leukocytes. Diabetes. 2003;52:1683–1688. doi: 10.2337/diabetes.52.7.1683. [DOI] [PubMed] [Google Scholar]
- 31.Shimoda K, et al. Effect of poly(ADP-ribose) synthetase inhibition on burn and smoke inhalation injury in sheep. Am J Physiol Lung Cell Mol Physiol. 2003;285:L240–L249. doi: 10.1152/ajplung.00319.2002. [DOI] [PubMed] [Google Scholar]
- 32.Mehilli J, et al. Gender and myocardial salvage after reperfusion treatment in acute myocardial infarction. J Am Coll Cardiol. 2005;45:828–831. doi: 10.1016/j.jacc.2004.11.054. [DOI] [PubMed] [Google Scholar]
- 33.Mabley JG, et al. Vitamine D3 is an inhibitor of poly(ADP)-ribose synthase. FASEB J. 2003;17:1123–1124. [Google Scholar]
- 34.Yee YK, et al. Vitamin D receptor modulators for inflammation and cancer. Mini Rev Med Chem. 2005;5:761–778. doi: 10.2174/1389557054553785. [DOI] [PubMed] [Google Scholar]
- 35.Nagpal S, et al. Noncalcemic actions of vitamin D receptor ligands. Endocr Rev. 2005;26:662–687. doi: 10.1210/er.2004-0002. [DOI] [PubMed] [Google Scholar]
- 36.Bauer PI, et al. The influence of ATP on poly(ADP-ribose) metabolism. Int J Mol Med. 2005;16:321–324. [PubMed] [Google Scholar]
- 37.Virag L, Szabo C. Purines inhibit poly(ADP-ribose) polymerase activation and modulate oxidant-induced cell death. FASEB J. 2001;15:99–107. doi: 10.1096/fj.00-0299com. [DOI] [PubMed] [Google Scholar]
- 38.Geraets L, et al. Caffeine metabolites are inhibitors of the nuclear enzyme poly(ADP-ribose)polymerase-1 at physiological concentrations. Biochem Pharmacol. 2006;72:902–910. doi: 10.1016/j.bcp.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 39.Moonen HJ, et al. Theophylline prevents NAD+ depletion via PARP-1 inhibition in human pulmonary epithelial cells. Biochem Biophys Res Commun. 2005;338:1805–1810. doi: 10.1016/j.bbrc.2005.10.159. [DOI] [PubMed] [Google Scholar]
- 40.Tang-Liu DD, et al. Disposition of caffeine and its metabolites in man. J Pharmacol Exp Ther. 1983;224:180–185. [PubMed] [Google Scholar]
- 41.Alano CC, et al. Minocycline inhibits poly(ADP-ribose) polymerase-1 at nanomolar concentrations. Proc Natl Acad Sci U S A. 2006;103:9685–9690. doi: 10.1073/pnas.0600554103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Domercq M, Matute C. Neuroprotection by tetracyclines. Trends Pharmacol Sci. 2004;25:609–612. doi: 10.1016/j.tips.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 43.Elewa HF, et al. Minocycline for short-term neuroprotection. Pharmacotherapy. 2006;26:515–521. doi: 10.1592/phco.26.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tikka T, et al. Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J Neurosci. 2001;21:2580–2588. doi: 10.1523/JNEUROSCI.21-08-02580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morimoto N, et al. Minocycline inhibits oxidative stress and decreases in vitro and in vivo ischemic neuronal damage. Brain Res. 2005;1044:8–15. doi: 10.1016/j.brainres.2005.02.062. [DOI] [PubMed] [Google Scholar]
- 46.Yrjanheikki J, et al. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci U S A. 1998;95:15769–15774. doi: 10.1073/pnas.95.26.15769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yrjanheikki J, et al. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci U S A. 1999;96:13496–13500. doi: 10.1073/pnas.96.23.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fox C, et al. Minocycline confers early but transient protection in the immature brain following focal cerebral ischemia–reperfusion. J Cereb Blood Flow Metab. 2005;25:1138–1149. doi: 10.1038/sj.jcbfm.9600121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Du Y, et al. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson’s disease. Proc Natl Acad Sci U S A. 2001;98:14669–14674. doi: 10.1073/pnas.251341998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu DC, et al. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J Neurosci. 2002;22:1763–1771. doi: 10.1523/JNEUROSCI.22-05-01763.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen M, et al. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat Med. 2000;6:797–801. doi: 10.1038/77528. [DOI] [PubMed] [Google Scholar]
- 52.Zhu S, et al. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature. 2002;417:74–78. doi: 10.1038/417074a. [DOI] [PubMed] [Google Scholar]
- 53.Kraus RL, et al. Antioxidant properties of minocycline: neuroprotection in an oxidative stress assay and direct radical-scavenging activity. J Neurochem. 2005;94:819–827. doi: 10.1111/j.1471-4159.2005.03219.x. [DOI] [PubMed] [Google Scholar]
- 54.Peterson JT. Matrix metalloproteinase inhibitor development and the remodeling of drug discovery. Heart Fail Rev. 2004;9:63–79. doi: 10.1023/B:HREV.0000011395.11179.af. [DOI] [PubMed] [Google Scholar]
- 55.Wang W, et al. Peroxynitrite-induced myocardial injury is mediated through matrix metalloproteinase-2. Cardiovasc Res. 2002;53:165–174. doi: 10.1016/s0008-6363(01)00445-x. [DOI] [PubMed] [Google Scholar]
- 56.Villarreal FJ, et al. Early short-term treatment with doxycycline modulates postinfarction left ventricular remodeling. Circulation. 2003;108:1487–1492. doi: 10.1161/01.CIR.0000089090.05757.34. [DOI] [PubMed] [Google Scholar]
- 57.Graziani G, Szabo C. Clinical perspectives of PARP inhibitors. Pharmacol Res. 2005;52:109–118. doi: 10.1016/j.phrs.2005.02.013. [DOI] [PubMed] [Google Scholar]