Abstract
Epidemiological studies demonstrated that even in the absence of other risk factors (e.g. diabetes, hypertension, hyperhomocysteinemia, hypercholesterolemia), advanced age itself significantly increases cardiovascular morbidity by enhancing vascular oxidative stress and inflammation. Because the population in the Western world is rapidly aging, there is a substantial need for pharmacological interventions that delay the functional decline of the cardiovascular system. Resveratrol is an atoxic phytoestrogen found in more than 70 plants including grapevine and berries. Recent data suggest that nutritional intake of resveratrol and other polyphenol compounds may contribute to the “French paradox”, the unexpectedly low cardiovascular morbidity in the Mediterranean population. There is increasing evidence that resveratrol exerts multifaceted anti-oxidant and/or anti-inflammatory effects in various disease models. Importantly, resveratrol was reported to slow aging and increase lifespan in simple organisms and has been suggested as a potential calorie restriction mimetic. Resveratrol has also been reported to activate NAD-dependent histone deacetylases (sirtuins), which may contribute to its anti-aging effects. This review focuses on the role of oxidative stress and inflammation in cardiovascular dysfunction in aging, and on emerging anti-aging therapeutic strategies offered by resveratrol and other polyphenol compounds.
Keywords: polyphenol, endothelium, heart, coronary circulation, senescence, inflammation, gene expression, NF-κB
INTRODUCTION
The population in the Western world is aging. By the end of this decade the number of senior citizens (65 years old or older) will reach 40 million people in the United States. Epidemiological studies suggest that even in the absence of other risk factors (e.g. diabetes, hypertension, hyperhomocysteinemia, hypercholesterolemia), advanced age itself significantly increases cardiovascular morbidity. Indeed, in 75–84 year old individuals, as compared to 35–44 year old people, there is a ~60 fold and ~80 fold increase in death rates for heart disease and cerebrovascular disease, respectively. In persons older than 85 these mortality rates show a ~200 to ~270 fold increase, respectively (according to the U.S. Department of Health and Human Services/Centers for Disease Control and National Center for Health Statistics). Better understanding of the molecular mechanisms underlying the aging process can, in the not-so-distant future, lead to pharmacological interventions that can significantly delay age-related decay of cardiovascular function.
Cardiovascular aging is characterized by a gradual deterioration of endothelial function and myocardial performance both in experimental animals and in humans [1–6], which begins to accelerate after mid-life. There is increasing evidence that oxidative/nitrosative stress (an increased production of O2·, H2O2 and ONOO−) and the consequent activation of numerous downstream effector pathways coupled with an interrelated chronic inflammation eventually lead to age-related cardiovascular dysfunction [2–4, 6–8].
Resveratrol is an atoxic phytoestrogen, which holds great promise as an anti-oxidant, anti-inflammatory and/or antitumor agent for various therapeutical indications [9–17]. The present review focuses on some of the mechanisms by which resveratrol may reverse or delay the symptoms of cardiovascular aging.
1. Resveratrol: Synthesis, Sources
Resveratrol is one of a group of compounds (called phytoalexins) that are produced in plants during times of environmental stress such as microbial (fungal) infection or UV irradiation [18]. Although the substrates (4-coumaroyl-CoA, malonyl-CoA) are present in all plants, most plants do not contain the enzyme (resveratrol synthase) necessary for the biosynthesis of the polyphenolic stilbene structure of resveratrol [19]. First isolated from the roots of the oriental medicinal plant Polygonum capsidatum, resveratrol has been identified in more than 70 species of plants, including mulberries, eucalyptus and peanuts. Grapevines (Vitis vinifera) are particularly good sources of resveratrol. Fresh grape skin contains about 50 to 100 μg of resveratrol per gram, while red wine concentrations range approximately from 0.1 to 14 mg/L with the highest concentrations reported in wines prepared from Pinot noir grapes [20, 21]. In addition to resveratrol, trans-piceid and delta-viniferin also constitute a significant proportion of stilbenes in wine dietary intake (Fig. 1). Other polyphenols that may exert biological effects similar to resveratrol include various anthocyanins and flavonols (e.g. quercetin, myricetin and kaempferol) [22]. The relatively high concentrations of resveratrol (and likely other polyphenols) in red wine is thought to be the explanation for the so-called “French-paradox” [23, 24].
Fig. 1.
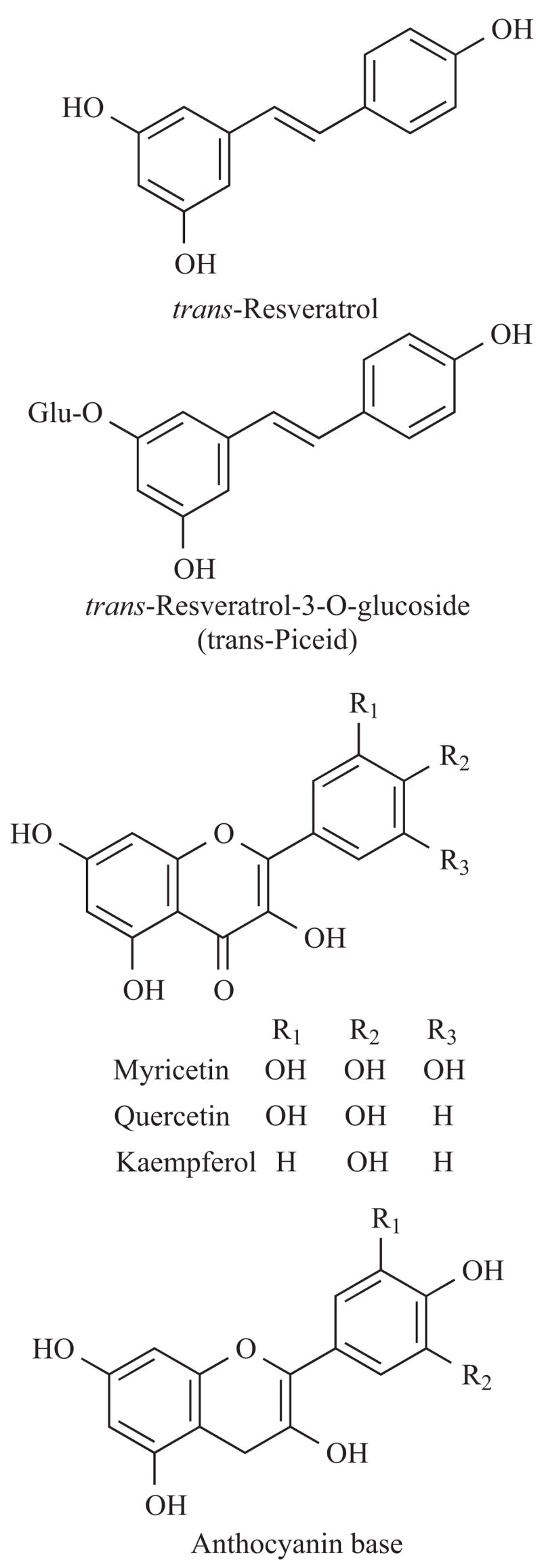
Chemical structure of resveratrol (trans–3, 4′, 5–trihydroxystilbene) and other dietary polyphenols.
2. Vasculoprotection: Epidemiological Evidence
Epidemiological studies suggest that Mediterranean diets are associated with reduced risk of cardiovascular disease [25, 26]. It has been proposed that resveratrol is one of the most important dietary constituents involved in vasculoprotection. In line with this notion, epidemiological studies have linked moderate intake of resveratrol-containing red wine with a significant decrease in the risk of coronary artery disease. Resveratrol (and other natural plolyphenol compounds) are thought to have diverse antiatherogenic activities [27–31], such as the inhibition of LDL oxidation, platelet aggregation, regulation of vascular smooth muscle proliferation and modulation of NO production.
Because vascular aging is characterized by an increased ROS generation and pro-inflammatory phenotypic changes, the present review focuses primarily on the possible anti-platelet, anti-oxidant and anti-inflammatory mechanisms by which resveratrol may exert beneficial effects on the aged vasculature.
3. Anti-Platelet Action of Resveratrol
There is growing evidence that platelets play an important role in atherogenesis, plaque formation and plaque rupture. In clinical practice inhibition of platelet activity with aspirin significantly reduces the odds of serious atherothrombotic vascular events and death in high risk patients [32]. However, in many patients aspirin is not effective. Among the possible causes of aspirin treatment failures, aspirin resistance emerges as a major therapeutic challenge. The pathological mechanisms underlying aspirin resistance are not well understood and are likely multifaceted. One possible factor is age itself. Our own data shows that 26% of high risk cardiac patients under the age of 60 (5/19) were resistant to aspirin treatment. In contrast, among the elderly (>60 years old) aspirin resistance increased to affect 45% of patients (14/31; Stef and Veress, unpublished data, 2005).
There is increasing evidence that resveratrol suppresses platelet aggregation in humans (Fig. 2) and it has been suggested that this action may be responsible for its protection against coronary heart disease [33–37]. Resveratrol was shown to inhibit cyclooxygenase-1 [38], which likely contributes to its aspirin-like effects. Other potential mechanisms of the anti-platelet action of resveratrol involves inhibition of signal transduction pathways including MAP kinases [33] and polyphosphoinositide signaling [37]. Importantly, recent studies revealed that resveratrol effectively inhibits aggregation of platelets from patients with aspirin resistance (Stef and Veress, unpublished data, 2005). Structure-activity relationships on methoxy-resveratrol analogs showed that the m-hydroquinone moiety is essential for irreversible inactivation of cyclooxygenase-1 [38]. These findings encourage the search for structurally related compounds with better bioavailability for cardiovascular protection in elderly patients with aspirin resistance.
Fig. 2.
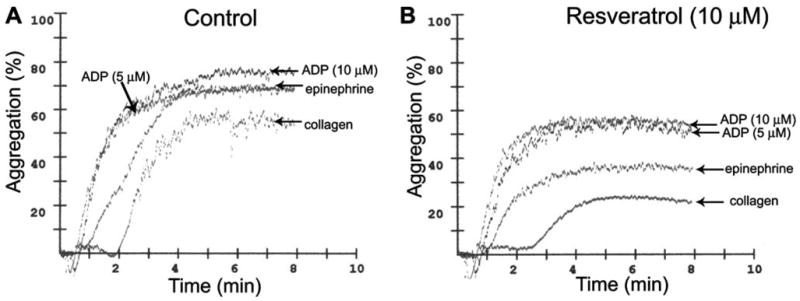
Original recordings showing ADP-, epinephrine- and collagen-induced aggregation in platelets of high risk human cardiac patients. B: Resveratrol significantly inhibits collagen- and epinephrine-induced aggregation, whereas it exerts a less pronounced effect on ADP-induced aggregation (optical aggregometry).
4. Anti-Oxidant Effects of Resveratrol
Since the publication of the free radical theory of aging [39] almost half a century ago by Harmon, substantial evidence has been published linking aging in various tissues with increased oxidative stress. These studies have demonstrated increased production of reactive oxygen species (ROS) in mesenteric [40] and small coronary arteries [7] of aged rats (reviewed in reference [6]). Additional studies have also described increased ROS production in the carotid arteries and aorta of aged rats [41, 42] and mice [43]. Aging-induced vascular oxidative stress is characterized by a down-regulation of antioxidants, such as ecSOD [40], an increased expression of iNOS [1, 43] and increased activity of NAD(P)H oxidases [7, 42, 44] and/or other oxidase mechanisms [45]. Increased oxidative stress in aging leads to functional inactivation of NO via increased O2·− levels yielding in an increase in formation of a reactive oxidant ONOO−, which may impair cardiovascular function through multiple mechanisms [7, 40, 43, 44, 46, 47]. Age-related decline in eNOS expression [7, 48–51] and/or a decreased intracellular L-arginine accessibility [52], further aggravate the already impaired NO bioavailability, thus leading to limited cardiac blood supply and altering myocardial O2 consumption and myocardial contractility [44]. Recent studies have also provided evidence that decreased endothelial NO production in aging enhances apoptosis of endothelial cells [51, 53].
There is accumulating evidence that resveratrol can exert anti-oxidant effects in biological systems via multiple direct and indirect mechanisms, including effects on ROS and NO production, lipid peroxidation, endogenous antioxidant systems, all of which may contribute to the cardiovascular benefits of the compound [12, 14, 15, 54–58]. Resveratrol, in a relatively high concentration [59, 60], was reported to elicit vasodilation both in large conduit arteries and in vessels of the microcirculation in animal models (Fig. 3A) [61–66]. A recent study demonstrated that resveratrol also evokes dilatation of the human vessels including the internal mammary artery [67]. Resveratrol-induced vasorelaxation seems to be preserved both in humans with established coronary heart disease [68] and in animal models of aging (Fig. 3A). There are also studies extant showing that resveratrol may improve vasodilation elicited by endothelium-dependent agents [67] and it was suggested that by restoring NO bioavailability it may exert beneficial effects in pathophysiological states associated with an increased oxidative stress [69]. Previous studies demonstrated that administration of antioxidants (such as superoxide dismutase and Tiron) can improve endothelium-dependent relaxations of aged arteries by restoring the bioavailability of NO [7, 70]. However, acute administration of resveratrol does not seem to affect endothelium-dependent dilations in aged vessels (Fig. 3B), likely because it is less effective in directly attenuating O2·− production in the endothelial and smooth muscle cells of aged arteries (Fig. 4A–B). It can be hypothesized that while exogenously administered resveratrol may scavenge extracellular ROS produced by cell membrane-associated oxidases (e.g. NAD(P)H oxidase) it is less effective against mitochondria-derived ROS in aging. Interestingly, resveratrol does not act primarily as an antioxidant in other biological systems either: pro-oxidant activity of resveratrol in vitro (increasing O2·− generation) has also been documented recently in human leukemia cells [21, 71]. Further studies are needed to elucidate whether chronic treatment with resveratrol effectively reduces ROS production in aged vessels (e.g. by up-regulating antioxidant enzymes) or if its vasculoprotective effects are mediated independent of its antioxidant properties.
Fig. 3.
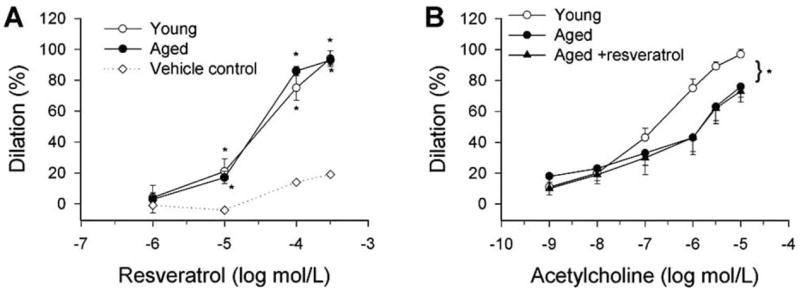
Resveratrol-induced dilations of isolated, perfused small mesenteric arteries (d: ~300 μm) of young (3 month old) and aged (29 month old) male F344 rats. B: Effect of resveratrol pretreatment (10−6 mol/L, for 30 min) on acetylcholine-induced dilations of young and aged arteries. The inner vascular diameter was measured by videomicroscopy as described [96–98]. The TXA2 mimetic U46619 (10−7 mol/L) was used for preconstriction. Data are mean±S.E.M. *p<0.05
Fig. 4.
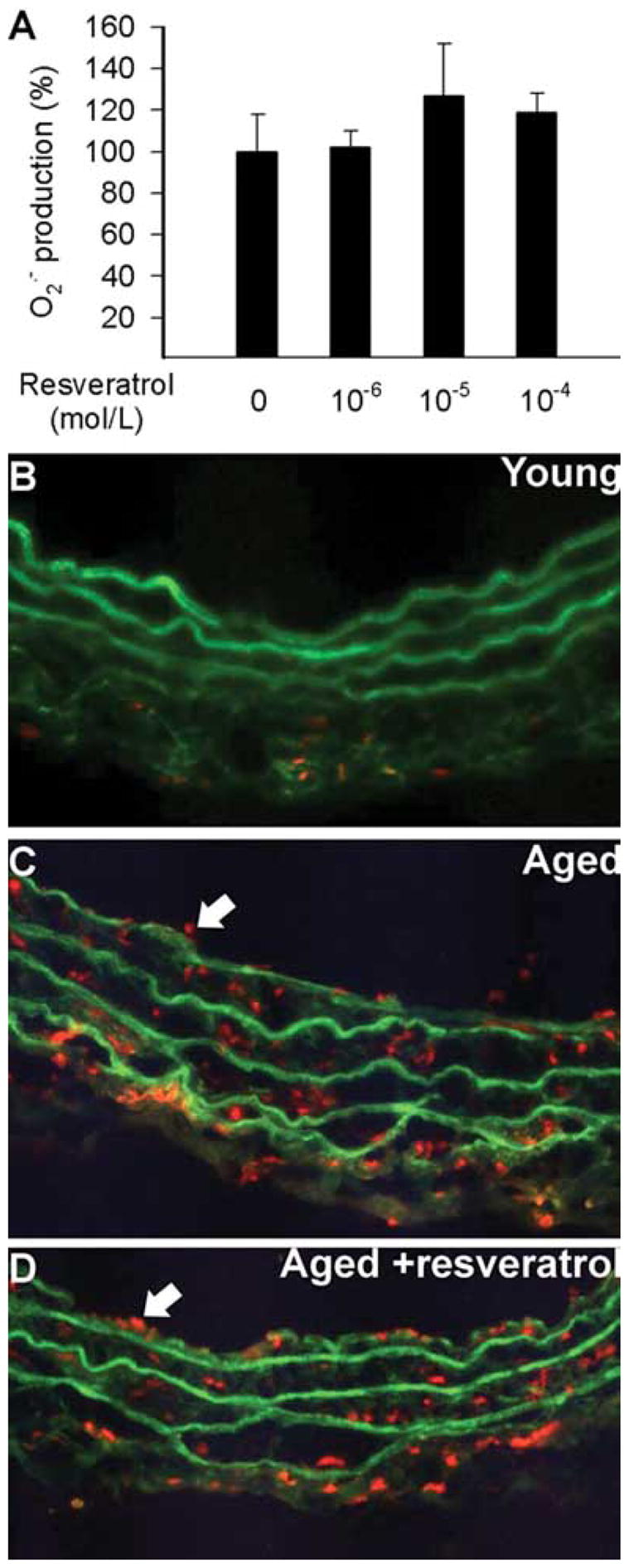
Superoxide production in cultured carotid arteries of 29 month old male F344 rats with or without resveratrol incubation (24 h, in sterile vessel culture; for a description of the technique see references [53, 87, 99, 100]). Data are mean ± S.E.M. (n=5–8 for each group). B–D: Fluorescent photomicrographs showing that compared to young vessels (B), there was a significantly increased O2·− production in the endothelial (arrows) and smooth muscle cells of aged arteries (C), as indicated by the intensive red fluorescent staining of the nuclei by ethidium bromide. The number and staining intensity of ethidium bromide-positive endothelial nuclei in aged vessels were not significantly decreased by short-term treatment with resveratrol (D). Green autofluorescence is shown for orientation purposes. Images are representative to 6 independent experiments.
5. Anti-Inflammatory Effects of Resveratrol
There is increasing evidence that resveratrol exerts multifaceted anti-inflammatory effects in various disease models (for a review see references [12, 21]). Ischemia followed by reperfusion has been shown to markedly increase leukocyte adherence and vascular transmigration in the mesenteric microcirculation [72]. A recent study has demonstrated that intravenous administration of resveratrol attenuates these deleterious effects of ischemia/reperfusion [72]. Resveratrol was also shown to attenuate the proinflammatory effects invoked by PAF [72]. Importantly, resveratrol may also confer vasculoprotection by regulating the expression of pro-inflammatory and pro-atherogenic genes in endothelial cells.
Local leukocyte recruitment into the vessel wall is an early step in atherogenesis and is controlled by the expression of cell adhesion molecules. It is significant that resveratrol was shown in vitro to decrease endothelial VCAM and ICAM-1 expression and attenuate monocyte adhesiveness to the endothelium [73–76]. Because of the potent anti-inflammatory action of resveratrol, there is a lot of interest in investigating the effect of resveratrol and its derivatives on transcription factors that regulate the expression of inflammatory mediators, such as adhesion molecules, cytokines (e.g. TNFα, IL-1β, IL-6), and iNOS. These transcriptional mechanisms include C/EBP, fos/jun, AP-1, and NF-κB. Several lines of evidence suggest that inhibition of NF-κB by resveratrol underlies many of the anti-inflammatory effects of resveratrol [73, 77]. NF-κB is a redox-sensitive transcription factor that is expressed by both endothelial and smooth muscle cells. Activation of NF-κB is thought to induce the transcription of a large range of genes implicated in inflammation, including cytokines (e.g. TNFα, IL-6 and IL-1β), chemokines and adhesion molecules [78–80]. It is also generally believed that chronic activation of NF-κB predispose arteries to atherosclerosis [81]. There is evidence for a pro-inflammatory shift in vascular [7, 8, 53] and cardiac [82] gene expression profiles (including an up-regulation of TNFα, IL-6 and iNOS) with aging. It has been proposed that high levels of inflammatory cytokines (in particular TNFα) play a role in the development of cardiac and vascular dysfunction. Numerous studies demonstrated that increased levels of ROS may activate NF-κB in endothelial, smooth muscle cells and other cell types, leading to the up-regulation of adhesion molecules, iNOS, TNFα and other cytokines. Of note, there are studies suggesting that NF-κB binding increases in aging [83], which is likely responsible for the increased expression of iNOS found in aged coronary vessels [7], carotid arteries and aortas [84]. Importantly, resveratrol treatment significantly decreases iNOS expression in aged vessels (Fig. 5) and aged hearts (Ungvari, unpublished observation 2005). Resveratrol was also shown to down-regulate iNOS in other cell types [85, 86]. Up-regulation of iNOS is thought to play a central role in vascular oxidative stress, endothelial dysfunction and pro-atherogenic processes [87]. Thus, it is logical to hypothesize that in the aged circulation, inhibition of NF-κB activation and iNOS expression by resveratrol exerts vasculoprotective effects. A second pro-inflammatory transcription factor, AP-1 (activator protein 1) may also be inhibited by resveratrol [77]. AP-1 similarly to NF-κB, is important in the regulation of many inflammatory genes that are induced by oxidative stress and its inhibition may contribute to the anti-inflammatory properties of resveratrol. Thus, further studies are definitely needed to establish a role for resveratrol as treatment to inhibit NF-κB and AP-1 activation and cardiovascular inflammation in aging.
Fig. 5.
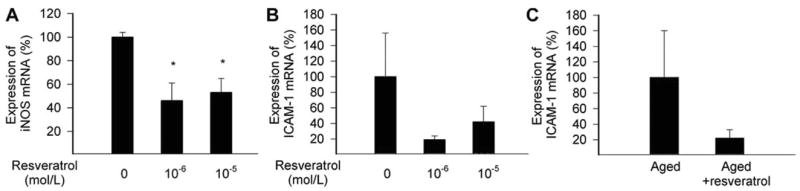
Expression of iNOS (A) and ICAM-1 (B) mRNA in cultured carotid arteries of 29 month old male F344 rats with or without resveratrol incubation (24 h, in sterile vessel culture; for a description of the technique see references [53, 87, 99, 100]). Quantification of mRNA expression was performed by real-time PCR, as described [53, 87, 99, 100]. C: Expression of ICAM-1 mRNA in carotid arteries of 29 month old male F344 rats with or without resveratrol treatment (p.o. 3 mg/kg/day, for 1 week). Data are mean ± S.E.M. (n=5 for each group).
6. Anti-Aging Effects of Resveratrol
Resveratrol was reported to slow aging and increase lifespan in simple eukaryotes (S. cerevisiae, C. elegans, D. melanogaster) and has been suggested as a potential calorie restriction mimetic [88, 89]. In a series of landmark studies Dr. David Sinclair’s Laboratory has shown that resveratrol is a sirtuin activator, and this property has been proposed to account for its anti-aging effects [88, 89]. Sirtuins are NAD+-dependent histone deacetylases (named after the silent information regulator 2 [Sir2], which acts to extend lifespan in yeast and C. elegans; it is of note that recent data raised the possibility that resveratrol is a substrate-specific activator of sirtuins [90]). Despite its absolute requirement for NAD+, the regulation of sirtuins by NAD+ biosynthesis pathways is poorly understood in mammals. It is thought that intracellular events that affect NAD+ levels or NAD+/NADH ratios, such as energy production through respiration, may affect physiological and pathological processes and lifespan through sirtuin-dependent pathways. Interestingly, there is data that resveratrol can inhibit the mitochondrial respiratory chain [56], which may affect NAD+/NADH ratios. Despite the obvious importance of sirtuins in the regulation of lifespan in lower organisms, there is a paucity of data regarding the role of sirtuins and the effects of resveratrol in mammalian aging. Studies are currently underway in Dr. Sinclair’s Laboratory to characterize the effect of resveratrol on murine lifespan. Future studies should also investigate the effect of chronic resveratrol treatment on age-related decline in cardiovascular function.
Like the sirtuins, the nuclear enzyme poly(ADP-ribose) polymerase (PARP) also utilizes NAD+ in a stochiometric manner for its multiple regulatory functions [91]. When DNA damage occurs, PARP-1 (the most abundant and most studied isoform of PARP) cleaves nicotinamide adenine dinucleotide (NAD+) to nicotinamide and ADP-ribose to form long branches of ADP-ribose polymers on the glutamic acid residues of a number of target proteins including histones. Poly(ADP-ribosyl)ation is involved in the regulation of many cellular processes such as DNA repair, gene transcription, cell cycle progression, cell death, chromatin function, and genomic stability (reviewed in reference [92]). Moderate PARP-1 activation facilitates the efficient repair of DNA damage resulting from reactive oxygen and nitrogen species such as H2O2 and ONOO−. In contrast, when excessive and sustained activation of PARP-1 occurs, such as under conditions of tissue ischemia and/or reperfusion, substantial depletion of intracellular NAD+ results. As NADH functions as an electron carrier in the mitochondrial respiratory chain, NAD+ depletion rapidly leads to falling intracellular ATP levels, eventually leading to cellular dysfunction and death.
During the last decade a growing number of experimental studies have demonstrated beneficial effects of both PARP inhibitors and the genetic deletion of the PARP-1 enzyme in various animal models of increased cardiovascular oxidative stress and inflammation [92]. The proven cardioprotective effects of PARP inhibitors in diabetes [93] are particularly of interest, because diabetes is known to be associated with accelerated vascular aging. There are also reports that chronic administration of PARP inhibitors improved cardiac and vascular dysfunction in aged rats (reviewed in references [6, 94, 95]). It is thought that inhibition of PARP decreases NAD+ consumption and raises nuclear NAD+ levels, which in turn is likely to activate the sirtuins. Additionally, the mechanism behind the protective effects of PARP-1 inhibitors may involve prevention of the upregulation of various proinflammatory pathways (cytokines, adhesion receptors, mononuclear cell infiltration [6, 47]). Because of the aforementioned considerations, we propose that resveratrol and PARP-1 inhibitors are likely to have additive cardio- and/or vasculoprotective effects in aging (and in disease states associated with accelerated vascular aging, such as diabetes [93]), which should be unveiled in upcoming studies.
CONCLUSION
In conclusion, aging is associated with oxidative/nitrosative stress and inflammatory changes in the phenotype of blood vessels and the heart. Whether conventional treatments (e.g. statins) with antioxidant and/or anti-inflammatory properties are able to reverse or delay the considerable aging-induced functional decline of the cardiovascular system remains a subject of current debate. Because resveratrol (and likely other polyphenol compounds) exert significant anti-inflammatory effects and extends lifespan in experimental aging models we can expect that resveratrol research will yield novel therapeutic approaches that will be exploited for the benefit of elderly patients (Fig. 6).
Fig. 6.
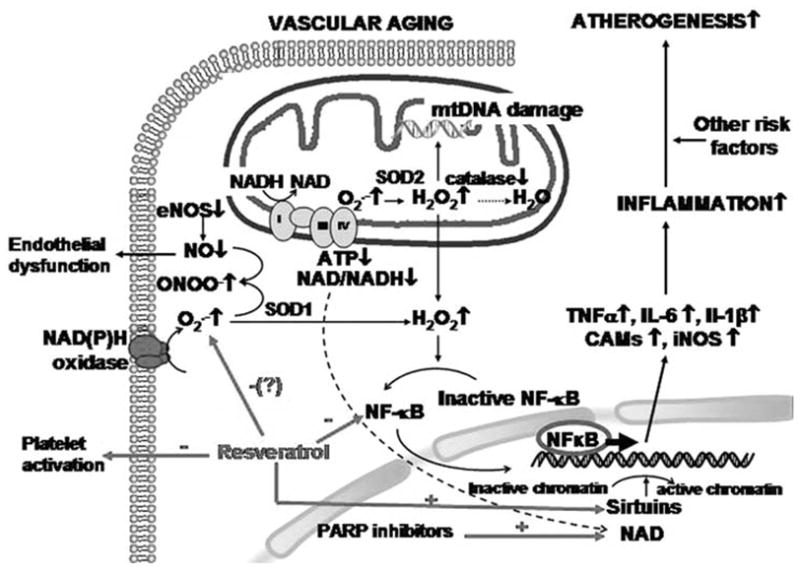
Proposed scheme for the mechanisms by which aging promotes oxidative stress, endothelial dysfunction and pro-inflammatory phenotypic alterations in blood vessels. The model predicts that aging is associated with an increased ROS generation by NAD(P)H oxidase and/or mitochondrial sources, which activates redox-sensitive transcription factors (NF-κB) upregulating inflammatory gene expression. The resulting pro-inflammatory phenotype of arteries will promote atherogenesis, especially if other risk factors (e.g. hypertension, hyperhomocysteinemia, hypercholesterolemia) are also present. We propose that resveratrol inhibits the ROS - NF-κB axis, inhibits platelet activation, increases NO bioavailability and/or activates sirtuins thereby helps to maintain a youthful vascular phenotype. It is likely that inhibition of PARP-1 will increase NAD+ levels, which serves as a co-factor for sirtuin activation. Thus, it can be predicted that resveratrol and PARP inhibitors exert additive anti-aging actions.
Acknowledgments
This work was supported by grants from the American Heart Association 0430108N and 0435140N and American Federation for Aging Research (to AC and ZU), Phillip Morris USA Inc and Phillip Morris International and (to ZU and JW), the California Grape Commission (to JW) and by the Intramural Research Program of the NIH, NIAAA (to PP).
References
- 1.Yang B, Larson DF, Watson RR. Life Sci. 2004;75:655–67. doi: 10.1016/j.lfs.2003.09.076. [DOI] [PubMed] [Google Scholar]
- 2.Lakatta EG. Circulation. 2003;107:490–7. doi: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- 3.Lakatta EG, Levy D. Circulation. 2003;107:346–54. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- 4.Lakatta EG, Levy D. Circulation. 2003;107:139–46. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 5.Sussman MA, Anversa P. Annu Rev Physiol. 2004;66:29–48. doi: 10.1146/annurev.physiol.66.032102.140723. [DOI] [PubMed] [Google Scholar]
- 6.Csiszar A, Pacher P, Kaley G, Ungvari Z. Curr Vasc Pharmacol. 2005;3:285–91. doi: 10.2174/1570161054368616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Csiszar A, Ungvari Z, Edwards JG, Kaminski PM, Wolin MS, Koller A, Kaley G. Circ Res. 2002;90:1159–66. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- 8.Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. FASEB J. 2003;17:1183–5. doi: 10.1096/fj.02-1049fje. [DOI] [PubMed] [Google Scholar]
- 9.Aziz MH, Reagan-Shaw S, Wu J, Longley BJ, Ahmad N. FASEB J. 2005;19:1193–5. doi: 10.1096/fj.04-3582fje. [DOI] [PubMed] [Google Scholar]
- 10.Hsieh TC, Wang Z, Hamby CV, Wu JM. Biochem Biophys Res Commun. 2005;334:223–30. doi: 10.1016/j.bbrc.2005.06.073. [DOI] [PubMed] [Google Scholar]
- 11.Hsieh TC, Wu JM. Anticancer Res. 2000;20:225–8. [PubMed] [Google Scholar]
- 12.de la Lastra CA, Villegas I. Mol Nutr Food Res. 2005;49:405–30. doi: 10.1002/mnfr.200500022. [DOI] [PubMed] [Google Scholar]
- 13.Martin AR, Villegas I, La Casa C, de la Lastra CA. Biochem Pharmacol. 2004;67:1399–410. doi: 10.1016/j.bcp.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 14.Bradamante S, Barenghi L, Villa A. Cardiovasc Drug Rev. 2004;22:169–88. doi: 10.1111/j.1527-3466.2004.tb00139.x. [DOI] [PubMed] [Google Scholar]
- 15.Bradamante S, Barenghi L, Piccinini F, Bertelli AA, De Jonge R, Beemster P, De Jong JW. Eur J Pharmacol. 2003;465:115–23. doi: 10.1016/s0014-2999(03)01441-9. [DOI] [PubMed] [Google Scholar]
- 16.Olas B, Nowak P, Kolodziejczyk J, Ponczek M, Wachowicz B. J Nutr Biochem. 2006;17(2):96–102. doi: 10.1016/j.jnutbio.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 17.Signorelli P, Ghidoni R. J Nutr Biochem. 2005;16:449–66. doi: 10.1016/j.jnutbio.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 18.Versari A, Parpinello GP, Tornielli GB, Ferrarini R, Giulivo C. J Agric Food Chem. 2001;49:5531–6. doi: 10.1021/jf010672o. [DOI] [PubMed] [Google Scholar]
- 19.Austin MB, Bowman ME, Ferrer JL, Schroder J, Noel JP. Chem Biol. 2004;11:1179–94. doi: 10.1016/j.chembiol.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 20.Mark L, Nikfardjam MS, Avar P, Ohmacht R. J Chromatogr Sci. 2005;43:445–9. doi: 10.1093/chromsci/43.9.445. [DOI] [PubMed] [Google Scholar]
- 21.Pervaiz S. FASEB J. 2003;17:1975–85. doi: 10.1096/fj.03-0168rev. [DOI] [PubMed] [Google Scholar]
- 22.Padilla E, Ruiz E, Redondo S, Gordillo-Moscoso A, Slowing K, Tejerina T. Eur J Pharmacol. 2005;517:84–91. doi: 10.1016/j.ejphar.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 23.Kopp P. Eur J Endocrinol. 1998;138:619–20. doi: 10.1530/eje.0.1380619. [DOI] [PubMed] [Google Scholar]
- 24.Zern TL, Fernandez ML. J Nutr. 2005;135:2291–4. doi: 10.1093/jn/135.10.2291. [DOI] [PubMed] [Google Scholar]
- 25.Keys A, Menotti A, Karvonen MJ, Aravanis C, Blackburn H, Buzina R, Djordjevic BS, Dontas AS, Fidanza F, Keys MH. Am J Epidemiol. 1986;124:903–15. doi: 10.1093/oxfordjournals.aje.a114480. [DOI] [PubMed] [Google Scholar]
- 26.de Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mamelle N. Circulation. 1999;99:779–85. doi: 10.1161/01.cir.99.6.779. [DOI] [PubMed] [Google Scholar]
- 27.Wang Z, Zou J, Cao K, Hsieh TC, Huang Y, Wu JM. Int J Mol Med. 2005;16:533–40. [PubMed] [Google Scholar]
- 28.Wang Z, Zou J, Huang Y, Cao K, Xu Y, Wu JM. Chin Med J (Engl) 2002;115:378–80. [PubMed] [Google Scholar]
- 29.Zou J, Huang Y, Chen Q, Wei E, Cao K, Wu JM. Chin Med J (Engl) 2000;113:99–102. [PubMed] [Google Scholar]
- 30.Wang Z, Huang Y, Zou J, Cao K, Xu Y, Wu JM. Int J Mol Med. 2002;9:77–9. [PubMed] [Google Scholar]
- 31.Zou J, Huang Y, Cao K, Yang G, Yin H, Len J, Hsieh TC, Wu JM. Life Sci. 2000;68:153–63. doi: 10.1016/s0024-3205(00)00925-5. [DOI] [PubMed] [Google Scholar]
- 32.Antithrombotic Trialists' Collabaoration. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirk RI, Deitch JA, Wu JM, Lerea KM. Blood Cells Mol Dis. 2000;26:144–50. doi: 10.1006/bcmd.2000.0289. [DOI] [PubMed] [Google Scholar]
- 34.Olas B, Wachowicz B. Thromb Res. 2002;106:143–8. doi: 10.1016/s0049-3848(02)00101-9. [DOI] [PubMed] [Google Scholar]
- 35.Olas B, Wachowicz B, Saluk-Juszczak J, Zielinski T. Thromb Res. 2002;107:141–5. doi: 10.1016/s0049-3848(02)00273-6. [DOI] [PubMed] [Google Scholar]
- 36.Olas B, Wachowicz B. Platelets. 2005;16:251–60. doi: 10.1080/09537100400020591. [DOI] [PubMed] [Google Scholar]
- 37.Olas B, Wachowicz B, Holmsen H, Fukami MH. Biochim Biophys Acta. 2005;1714:125–33. doi: 10.1016/j.bbamem.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 38.Szewczuk LM, Forti L, Stivala LA, Penning TM. J Biol Chem. 2004;279, 227:27–37. doi: 10.1074/jbc.M314302200. [DOI] [PubMed] [Google Scholar]
- 39.Harman D. J Gerontol. 1956:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 40.Sun D, Huang A, Yan EH, Wu Z, Yan C, Kaminski PM, Oury TD, Wolin MS, Kaley G. Am J Physiol Heart Circ Physiol. 2004;286:H2249–56. doi: 10.1152/ajpheart.00854.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hamilton CA, Brosnan MJ, McIntyre M, Graham D, Dominiczak AF. Hypertension. 2001;37:529–34. doi: 10.1161/01.hyp.37.2.529. [DOI] [PubMed] [Google Scholar]
- 42.van der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, Palacios-Callender M, Erusalimsky JD, Quaschning T, Malinski T, Gygi D, Ullrich V, Lüscher TF. J Exp Med. 2000;192:1731–44. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Francia P, delli Gatti C, Bachschmid M, Martin-Padura I, Savoia C, Migliaccio E, Pelicci PG, Schiavoni M, Luscher TF, Volpe M, Cosentino F. Circulation. 2004;110:2889–95. doi: 10.1161/01.CIR.0000147731.24444.4D. [DOI] [PubMed] [Google Scholar]
- 44.Adler A, Messina E, Sherman B, Wang Z, Huang H, Linke A, Hintze TH. Am J Physiol Heart Circ Physiol. 2003;285:H1015–22. doi: 10.1152/ajpheart.01047.2002. [DOI] [PubMed] [Google Scholar]
- 45.Bachschmid M, van der Loo B, Schuler K, Labugger R, Thurau S, Eto M, Kilo J, Holz R, Luscher TF, Ullrich V. Arch Gerontol Geriatr. 2004;38:181–90. doi: 10.1016/j.archger.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 46.Ungvari Z, Gupte SA, Recchia FA, Batkai S, Pacher P. Curr Vasc Pharmacol. 2005;3:221–9. doi: 10.2174/1570161054368607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pacher P, Schulz R, Liaudet L, Szabo C. Trends Pharmacol Sci. 2005;26:302–10. doi: 10.1016/j.tips.2005.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tanabe T, Maeda S, Miyauchi T, Iemitsu M, Takanashi M, Irukayama-Tomobe Y, Yokota T, Ohmori H, Matsuda M. Acta Physiol Scand. 2003;178:3–10. doi: 10.1046/j.1365-201X.2003.01100.x. [DOI] [PubMed] [Google Scholar]
- 49.Woodman CR, Price EM, Laughlin MH. J Appl Physiol. 2002;93:1685–90. doi: 10.1152/japplphysiol.00461.2002. [DOI] [PubMed] [Google Scholar]
- 50.Matsushita H, Chang E, Glassford AJ, Cooke JP, Chiu CP, Tsao PS. Circ Res. 2001;89:793–8. doi: 10.1161/hh2101.098443. [DOI] [PubMed] [Google Scholar]
- 51.Hoffmann J, Haendeler J, Aicher A, Rossig L, Vasa M, Zeiher AM, Dimmeler S. Circ Res. 2001;89:709–15. doi: 10.1161/hh2001.097796. [DOI] [PubMed] [Google Scholar]
- 52.Berkowitz DE, White R, Li D, Minhas KM, Cernetich A, Kim S, Burke S, Shoukas AA, Nyhan D, Champion HC, Hare JM. Circulation. 2003;108:2000–6. doi: 10.1161/01.CIR.0000092948.04444.C7. [DOI] [PubMed] [Google Scholar]
- 53.Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Physiol Genomics. 2004;17:21–30. doi: 10.1152/physiolgenomics.00136.2003. [DOI] [PubMed] [Google Scholar]
- 54.Martinez J, Moreno JJ. Biochem Pharmacol. 2000;59:865–70. doi: 10.1016/s0006-2952(99)00380-9. [DOI] [PubMed] [Google Scholar]
- 55.Hattori R, Otani H, Maulik N, Das DK. Am J Physiol Heart Circ Physiol. 2002;282:H1988–95. doi: 10.1152/ajpheart.01012.2001. [DOI] [PubMed] [Google Scholar]
- 56.Zini R, Morin C, Bertelli A, Bertelli AA, Tillement JP. Drugs Exp Clin Res. 1999;25:87–97. [PubMed] [Google Scholar]
- 57.Zou JG, Wang ZR, Huang YZ, Cao KJ, Wu JM. Int J Mol Med. 2003;11:317–20. [PubMed] [Google Scholar]
- 58.Wu JM, Wang ZR, Hsieh TC, Bruder JL, Zou JG, Huang YZ. Int J Mol Med. 2001;8:3–17. doi: 10.3892/ijmm.8.1.3. [DOI] [PubMed] [Google Scholar]
- 59.Bertelli AA, Giovannini L, Stradi R, Urien S, Tillement JP, Bertelli A. Drugs Exp Clin Res. 1998;24:51–5. [PubMed] [Google Scholar]
- 60.Bertelli AA, Giovannini L, Stradi R, Bertelli A, Tillement JP. Int J Tissue React. 1996;18:67–71. [PubMed] [Google Scholar]
- 61.Li HF, Tian ZF, Qiu XQ, Wu JX, Zhang P, Jia ZJ. Physiol Res. 2006 doi: 10.33549/physiolres.930826. (in press) [DOI] [PubMed] [Google Scholar]
- 62.El-Mowafy AM. Biochem Biophys Res Commun. 2002;291:1218–24. doi: 10.1006/bbrc.2002.6598. [DOI] [PubMed] [Google Scholar]
- 63.Naderali EK, Smith SL, Doyle PJ, Williams G. Clin Sci (Lond) 2001;100:55–60. [PubMed] [Google Scholar]
- 64.Naderali EK, Doyle PJ, Williams G. Clin Sci (Lond) 2000;98:537–43. [PubMed] [Google Scholar]
- 65.Jager U, Nguyen-Duong H. Arzneimittelforschung. 1999;49:207–11. doi: 10.1055/s-0031-1300403. [DOI] [PubMed] [Google Scholar]
- 66.Chen CK, Pace-Asciak CR. Gen Pharmacol. 1996;27:363–6. doi: 10.1016/0306-3623(95)02001-2. [DOI] [PubMed] [Google Scholar]
- 67.Rakici O, Kiziltepe U, Coskun B, Aslamaci S, Akar F. Int J Cardiol. 2005;105:209–15. doi: 10.1016/j.ijcard.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 68.Cruz MN, Luksha L, Logman H, Poston L, Agewall S, Kublickiene K. Am J Physiol Heart Circ Physiol. 2005 doi: 10.1152/ajpheart.01065.2005. (in press) [DOI] [PubMed] [Google Scholar]
- 69.Miatello R, Vazquez M, Renna N, Cruzado M, Zumino AP, Risler N. Am J Hypertens. 2005;18:864–70. doi: 10.1016/j.amjhyper.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 70.Csiszar A, Pacher P, Kaley G, Ungvari Z. Curr Cardiovasc Pharmacol. 2005;3(3):285–91. doi: 10.2174/1570161054368616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ahmad KA, Clement MV, Pervaiz S. Ann N Y Acad Sci. 2003;1010:365–73. doi: 10.1196/annals.1299.067. [DOI] [PubMed] [Google Scholar]
- 72.Shigematsu S, Ishida S, Hara M, Takahashi N, Yoshimatsu H, Sakata T, Korthuis RJ. Free Radic Biol Med. 2003;34:810–7. doi: 10.1016/s0891-5849(02)01430-2. [DOI] [PubMed] [Google Scholar]
- 73.Carluccio MA, Siculella L, Ancora MA, Massaro M, Scoditti E, Storelli C, Visioli F, Distante A, De Caterina R. Arterioscler Thromb Vasc Biol. 2003;23:622–9. doi: 10.1161/01.ATV.0000062884.69432.A0. [DOI] [PubMed] [Google Scholar]
- 74.Pendurthi UR, Rao LV. Thromb Res. 2002;106:243–8. doi: 10.1016/s0049-3848(02)00141-x. [DOI] [PubMed] [Google Scholar]
- 75.Bertelli AA, Baccalini R, Battaglia E, Falchi M, Ferrero ME. Therapie. 2001;56:613–6. [PubMed] [Google Scholar]
- 76.Ferrero ME, Bertelli AE, Fulgenzi A, Pellegatta F, Corsi MM, Bonfrate M, Ferrara F, De Caterina R, Giovannini L, Bertelli A. Am J Clin Nutr. 1998;68:1208–14. doi: 10.1093/ajcn/68.6.1208. [DOI] [PubMed] [Google Scholar]
- 77.Manna SK, Mukhopadhyay A, Aggarwal BB. J Immunol. 2000;164:6509–19. doi: 10.4049/jimmunol.164.12.6509. [DOI] [PubMed] [Google Scholar]
- 78.Tedgui A, Mallat Z. Circ Res. 2001;88:877–87. doi: 10.1161/hh0901.090440. [DOI] [PubMed] [Google Scholar]
- 79.Libermann TA, Baltimore D. Mol Cell Biol. 1990;10:2327–34. doi: 10.1128/mcb.10.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang YH, Lin JX, Vilcek J. Mol Cell Biol. 1990;10:3818–23. doi: 10.1128/mcb.10.7.3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hajra L, Evans AI, Chen M, Hyduk SJ, Collins T, Cybulsky MI. Proc Natl Acad Sci USA. 2000;97:9052–7. doi: 10.1073/pnas.97.16.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee CK, Allison DB, Brand J, Weindruch R, Prolla TA. Proc Natl Acad Sci USA. 2002;99:14988–93. doi: 10.1073/pnas.232308999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Helenius M, Hanninen M, Lehtinen SK, Salminen A. J Mol Cell Cardiol. 1996;28:487–98. doi: 10.1006/jmcc.1996.0045. [DOI] [PubMed] [Google Scholar]
- 84.Cernadas MR, Sanchez de Miguel L, Garcia-Duran M, Gonzalez-Fernandez F, Millas I, Monton M, Rodrigo J, Rico L, Fernandez P, de Frutos T, Rodriguez-Feo JA, Guerra J, Caramelo C, Casado S, Lopez F. Circ Res. 1998;83:279–86. doi: 10.1161/01.res.83.3.279. [DOI] [PubMed] [Google Scholar]
- 85.Tsai SH, Lin-Shiau SY, Lin JK. Br J Pharmacol. 1999;126:673–80. doi: 10.1038/sj.bjp.0702357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Donnelly LE, Newton R, Kennedy GE, Fenwick PS, Leung RH, Ito K, Russell RE, Barnes PJ. Am J Physiol Lung Cell Mol Physiol. 2004;287:L774–83. doi: 10.1152/ajplung.00110.2004. [DOI] [PubMed] [Google Scholar]
- 87.Ungvari Z, Csiszar A, Edwards JG, Kaminski PM, Wolin MS, Kaley G, Koller A. Arterioscler Thromb Vasc Biol. 2003;23:418–24. doi: 10.1161/01.ATV.0000061735.85377.40. [DOI] [PubMed] [Google Scholar]
- 88.Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Nature. 2004;430:686–9. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 89.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Nature. 2003;425:191–6. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 90.Kaeberlein M, McDonagh T, Heltweg B, Hixon J, Westman EA, Caldwell SD, Napper A, Curtis R, DiStefano PS, Fields S, Bedalov A, Kennedy BK. J Biol Chem. 2005;280:17038–45. doi: 10.1074/jbc.M500655200. [DOI] [PubMed] [Google Scholar]
- 91.Burkle A, Beneke S, Muiras ML. Exp Gerontol. 2004;39:1599–1601. doi: 10.1016/j.exger.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 92.Southan GJ, Szabo C. Curr Med Chem. 2003;10:321–40. doi: 10.2174/0929867033368376. [DOI] [PubMed] [Google Scholar]
- 93.Pacher P, Obrosova IG, Mabley JG, Szabo C. Curr Med Chem. 2005;12:267–75. doi: 10.2174/0929867053363207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pacher P, Mabley JG, Soriano FG, Liaudet L, Komjati K, Szabo C. Br J Pharmacol. 2002;135:1347–50. doi: 10.1038/sj.bjp.0704627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pacher P, Vaslin A, Benko R, Mabley JG, Liaudet L, Hasko G, Marton A, Batkai S, Kollai M, Szabo C. J Pharmacol Exp Ther. 2004;311:485–91. doi: 10.1124/jpet.104.069658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ungvari Z, Csiszar A, Bagi Z, Koller A. Am J Pathol. 2002;161:145–53. doi: 10.1016/S0002-9440(10)64166-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ungvari Z, Koller A. Hypertension. 2000;36:856–861. doi: 10.1161/01.hyp.36.5.856. [DOI] [PubMed] [Google Scholar]
- 98.Ungvari Z, Csiszar A, Koller A. Am J Physiol Heart Circ Physiol. 2002;282:H1760–7. doi: 10.1152/ajpheart.00676.2001. [DOI] [PubMed] [Google Scholar]
- 99.Ungvari Z, Csiszar A, Kaminski PM, Wolin MS, Koller A. Am J Pathol. 2004;165:219–26. doi: 10.1016/S0002-9440(10)63290-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Csiszar A, Smith KE, Koller A, Kaley G, Edwards JG, Ungvari Z. Circulation. 2005;111:2364–72. doi: 10.1161/01.CIR.0000164201.40634.1D. [DOI] [PubMed] [Google Scholar]


