Abstract
Dysregulation of nitric oxide (NO) and increased oxidative and nitrosative stress are implicated in the pathogenesis of heart failure. Peroxynitrite is a reactive oxidant that is produced from the reaction of nitric oxide with superoxide anion and impairs cardiovascular function through multiple mechanisms, including activation of matrix metalloproteinases (MMPs) and nuclear enzyme poly(ADP-ribose) polymerase (PARP). Recent studies suggest that the neutralization of peroxynitrite or pharmacological inhibition of MMPs and PARP are promising new approaches in the experimental therapy of various forms of myocardial injury. In this article, the role of nitrosative stress and downstream mechanisms, including activation of MMPs and PARP, in various forms of heart failure are discussed and novel emerging therapeutic strategies offered by neutralization of peroxynitrite and inhibition of MMPs and PARP in these pathophysiological conditions are reviewed.
Role of increased oxidative stress in heart failure
Acute heart failure and chronic heart failure (CHF) are major causes of hospitalization, morbidity and mortality worldwide. Several different mechanisms can lead to cardiac pump failure (Figure 1). These mechanisms result in a mismatch between the load applied to the heart and the energy needed for contraction, which leads to reduced contractile efficiency (Figure 1).
Figure 1.
Progression of heart failure and the role of oxidative and nitrosative stress. The mechanisms that lead to heart failure are of multiple origins and include acute and chronic ischemic heart disease, cardiomyopathies, myocarditis and pressure overload. These diseases result in a mismatch between the load applied to the heart and the energy needed for contraction, leading to mechanoenergic uncoupling. Following initial insult, secondary mediators such as angiotensin II (Ang II), noradrenaline (NA), endothelin (ET) and pro-inflammatory cytokines [e.g. tumor necrosis factor α (TNF-α) and interleukin 6 (IL-6)], in concert with oxidative and nitrosative stress, act directly on the myocardium or indirectly via changes in hemodynamic loading conditions to cause endothelial and myocardial dysfunction, cardiac and vascular remodeling with hypertrophy, fibrosis, cardiac dilation and myocardial necrosis, leading eventually to heart failure. The adverse remodeling and increased peripheral resistance further aggravate heart failure. Abbreviations: MMP, matrix metalloproteinase; PARP, poly(ADP-ribose) polymerase.
The pathomechanism of heart failure is complex and involves the activation of numerous secondary pathways (e.g. pathways involving neurohormones, cytokines, oxidative stress and nitrosative stress), which leads to: (i) abnormalities in various signaling processes, cardiac receptors and Ca2+ homeostasis; (ii) contractile protein desensitization; and (iii) endothelial dysfunction. The resulting structural alterations can involve cardiac and vascular remodeling with hypertrophy, fibrosis, cardiac dilation and myocardial necrosis. The adverse remodeling and increased peripheral resistance further aggravate heart failure (Figure 1).
Experimental and clinical studies demonstrate increased production of reactive oxygen species (ROS) in the pathogenesis of acute heart failure and CHF. Plasma malondialdehyde-like activity (MDA), a marker of lipid peroxidation, is increased in patients with ischemic and non-ischemic dilated cardiomyopathy, and has an inverse relationship with ejection fraction (an index of cardiac function) and exercise capacity. The pericardial concentration of 8-iso-prostaglandin F2 (a marker for ROS production) in these patients also correlates closely with the degree of contractile dysfunction (reviewed in [1]).
The generation of ROS in the myocardium is triggered by ischemia and reperfusion and by exposure of the heart to inflammatory cytokines [e.g. tumor necrosis factor α (TNF-α) and interleukin 6 (IL-6)]. Impaired antioxidant defense mechanisms (superoxide dismutase, catalase and glutathione peroxidase) or reduced concentrations of endogenous antioxidants (vitamin E, ascorbic acid and glutathione) can also increase ROS levels within the myocardium. Sources of ROS in failing myocardium include, among many others, xanthine and NAD(P)H oxidoreductases, the mitochondrial electron transport chain and activated neutrophils [1–4].
Role of increased nitrosative stress and dysregulation of nitric oxide synthase in heart failure
Cardiomyocytes, endocardial endothelium, coronary endothelium and cardiac nerves are all sources of nitric oxide (NO) produced by Ca2+-dependent NO synthase (NOS) enzymes. NO serves several important physiological roles in the regulation of cardiac function, including coronary vasodilatation, inhibition of platelet and neutrophil adhesion and activation, modulation of cardiac contractile function, and inhibition of cardiac oxygen consumption (reviewed in [4–6]).
NO is necessary for normal cardiac physiology and has a protective role in the ischemic heart. This protective role is mediated by several mechanisms, including the stimulation of soluble guanylyl cyclase, which leads to a decrease of the concentration of intracellular Ca2+, and the termination of chain-propagating lipid radical reactions caused by oxidative stress [7]. However, reactive nitrogen species can also exert cytotoxic effects. Many of the toxic actions of NO are not due directly to NO itself but are mediated by the highly reactive oxidant peroxynitrite, which is produced following interaction of NO with superoxide anions (reviewed in [8,9]) (Table 1).
Table 1.
Cytotoxic processes initiated by peroxynitrite in vitro
| Action | Mechanism(s) |
|---|---|
| Lipid peroxidation | Peroxidation |
| Inhibition and depletion of antioxidant enzymes | Oxidation and nitration of glutathione and cysteine (required by antioxidant enzymes) |
| Activation of matrix metalloproteinases (MMPs) | S-Glutathiolation of pro-matrix metalloproteinases |
| Disturbances of signal transduction pathways | Oxidation and nitration of various proteins |
| Mitochondrial dysfunction | Inhibition of, for example, cytochromes and NADH-CoQ1 reductase (complex I) |
| Inhibition of cytosolic enzymes and membrane pumps | Oxidation and nitration of various proteins |
| Ca2+ dysregulation | Inhibition of ion pumps (e.g. Ca2+ pumps, Ca2+-activated K+ channels and Na+–K+-ATPase, leading to dysfunctional cell energetics |
| Contractile protein dysfunction | Oxidation and nitration of contractile proteins |
| Upregulation of adhesion receptors | Activation of NF-κB |
| DNA injury and PARP activation | Oxidation and nitration of various proteins |
| Inhibition of NAD-dependent enzymes | NAD oxidation, and NAD depletion via PARP |
| Apoptosis | Mitochondrial injury, DNA injury, caspase activation, signal transduction disturbances and Ca2+ dysregulation |
| Cell necrosis | Mitochondrial injury, energetic collapse, oxidation, nitration, antioxidant depletion and Ca2+ dysregulation |
NO is overproduced in the failing myocardium as a result of the increased expression and activity of the inducible isoform of NOS (iNOS) [9,10]. There is a correlation between myocardium-specific, chronic over-expression of iNOS and peroxynitrite generation and cardiac enlargement, conduction defects, sudden cardiac death and, less commonly, heart failure (HF) in mice [11]. Myocardial iNOS is induced in rats that have volume-overload HF, and increased iNOS activity in these rats leads to: (i) a loss of myocardial contractility; and (ii) β-adrenoceptor hyporesponsiveness [12]. NO derived from neuronal NOS is also present in the failing human heart [13]. It is likely that it is not the net amount of NO produced per se that determines cardiac cytotoxicity, but instead the type of reactive nitrogen species present (e.g. the toxic oxidant peroxynitrite produced following the interaction of NO and superoxide) [8,9,14]. The generation of peroxynitrite has been demonstrated in various forms of acute heart failure and CHF in both animals and humans (Table 2) [11,15–30].
Table 2.
Evidence for nitrosative stress and peroxynitrite in various forms of cardiac injurya
| Experimental model | Disease or trigger | Main findings | Refs |
|---|---|---|---|
| Mouse | Chronic myocardial ischemia | Increased myocardial iNOS expression, plasma nitrate and nitrite concentrations, and myocardial and plasma nitrotyrosine levels in wild-type compared with iNOS−/− mice; improved left ventricular function in iNOS−/− mice compared with wild-type mice | b |
| Mouse | Cardiac-specific iNOS overexpression | Increased peroxynitrite production, inflammation, cardiac fibrosis, hypertrophy, dilatation and sudden cardiac death compared with wild-type mice | [11] |
| Mouse | Doxorubicin | Increased myocardial iNOS expression, nitrotyrosine formation and matrix metalloproteinase activation, which correlates with cardiac dysfunction | [15,16]b |
| Mouse | LPBM5 retrovirus (murine AIDS model) | Progressive cardiac dysfunction correlated with increased myocardial inflammation and protein nitration | [17] |
| Mouse, rat heart | High glucose, STZ-induced diabetes | Increased levels of apoptosis, hydrogen peroxide, superoxide, angiotensin II, NO and nitrotyrosine formation and increased iNOS expression in myocytes | [18]b |
| Rat cardiac myofibrils, trabeculae | Peroxynitrite | Concentration-dependent nitration of myofibrillar creatine kinase; decreased activity of creatine kinase and impaired contractile force generation | [19,20]b |
| Rat | Chronic myocardial ischemia | Increased myocardial nitrotyrosine formation and decreased myofibrillar creatine kinase activity | [21]b |
| Rat heart | Global ischemia– reperfusion | Cardiac dysfunction; decreased efficiency of oxygen utilization and ATP and membrane-bound creatine kinase activity; increased iNOS expression; increased formation of superoxide and myocardial peroxynitrite; NOS inhibition, superoxide dismutase and urate (a peroxynitrite scavenger) greatly enhanced the recovery of contractile function in post-ischemic hearts | [22]b |
| Rat heart | Endotoxin | Enhanced generation of NO, superoxide and peroxynitrite in dysfunctional hearts from endotoxemic rats | [23,24] |
| Rat, mouse | Porcine cardiac myosin | Autoimmune myocarditis characterized by myocardial destruction, inflammation and increased iNOS expression and nitrotyrosine formation, which is decreased by the iNOS inhibitor aminoguanidine | b |
| Rat heart, rat cardiac myocytes | Mixture of pro- inflammatory cytokines | Increased production of superoxide and NO, increased peroxynitrite formation and increased activation of matrix metalloproteinase 2 (MMP-2); contractile dysfunction, which is prevented by superoxide and peroxynitrite scavengers, an MMP-2 antibody or MMP inhibitors | [25,26]b |
| Dog | Pacing, intracoronary inflammatory cytokine | Cardiac dysfunction, nitrotyrosine formation and cell death in myocytes, which is prevented by the iNOS inhibitor aminoguanidine or a superoxide scavenger | b |
| Dog, pig | Regional ischemia– reperfusion | Increased nitrotyrosine formation and cardiac dysfunction | [27]b |
| Human | Coronary artery disease, atrial fibrillation, cardiopulmonary bypass surgery | Increased NOS activity and nitrotyrosine formation in myocytes of patients | [28–30]b |
| Human | Type 2 diabetes mellitus, hypertension | Increased apoptosis, necrosis and formation of angiotensin II and nitrotyrosine in myocytes | b |
| Human | HIV | Increased myocardial nitrotyrosine formation in patients with HIV-induced dilated cardiomyopathy | [17] |
CHF is also characterized by impaired endothelium-dependent vasodilatation, which might contribute to increased peripheral vascular resistance. The pathogenesis of endothelial dysfunction associated with CHF involves enhanced oxidative stress from various local sources. A potential additional source of ROS is iNOS, which, paradoxically, can produce superoxide instead of NO if L-arginine (from which NO is normally produced) is depleted or there is a lack of the important cofactor tetrahydrobiopterin (BH4). The importance of this mechanism has been demonstrated in cultured cells, and in vascular tissues from CHF animals or humans [31,32], but has not yet been studied in hearts from CHF animals or patients.
Mechanisms of peroxynitrite-induced cardiac and vascular dysfunction
There are multiple possible downstream targets of peroxynitrite (Table 1, Figure 2) (reviewed in [8,9,14]). For example, peroxynitrite-induced tyrosine nitration can lead to dysfunctional nitrated proteins whereas peroxynitrite-induced oxidation of crucial sulfydryl groups can inhibit important enzymes in the mitochondrial respiratory chain. Indeed, peroxynitrite-mediated nitration of myofibrillar creatine kinase impairs myocardial contractility [10]. Furthermore, peroxynitrite-modified cellular proteins are subject to accelerated degradation via the proteasome and peroxynitrite inhibits a variety of ion pumps, including Ca2+ pumps, Ca2+-activated K+ channels and Na+–K+-ATPase [8,9].
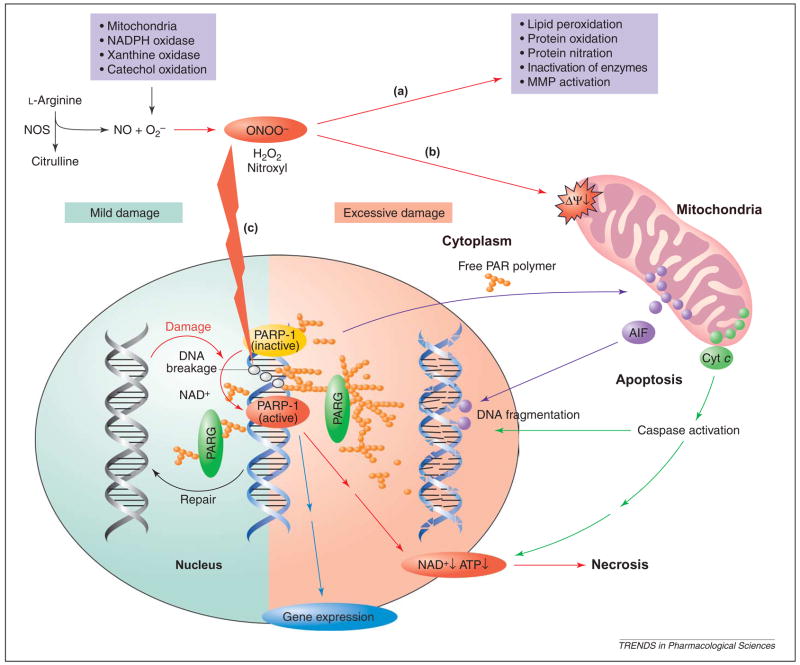
Figure 2.
Proposed role of the oxidative and nitrosative stress–poly(ADP-ribose) polymerase 1 (PARP-1) pathway in oxidant-induced cellular dysfunction and necrosis. Nitric oxide (NO) and superoxide ( ) (derived from NADPH oxidase, mitochondria, xanthine oxidase and catechol oxidation) react to form peroxynitrite (ONOO−), which induces cell damage via lipid peroxidation, inactivation of enzymes and other proteins by oxidation and nitration, and activation of matrix metalloproteinases (MMPs) among others (a) (Table 1). Peroxynitrite also acts on mitochondria [decreasing the membrane potential (Ψ)], triggering the release of pro-apoptotic factors such as cytochrome c (Cyt c) and apoptosis-inducing factor (AIF) (b). These factors mediate caspase-dependent and caspase-independent apoptotic death pathways, respectively. Moreover, peroxynitrite, in concert with other oxidants [e.g. hydrogen peroxide (H2O2)], causes strand breaks in DNA, activating PARP-1 (c). Mild damage to DNA activates the DNA repair machinery. By contrast, once excessive oxidative and nitrosative stress-induced DNA damage occurs, as in various forms of myocardial reperfusion injury and heart failure, overactivated PARP-1 initiates an energy-consuming cycle by transferring ADP-ribose units (small orange spheres) from NAD+ to nuclear proteins, resulting in rapid depletion of the intracellular NAD+ and ATP pools, slowing the rate of glycolysis and mitochondrial respiration, and eventually leading to cellular dysfunction and death. Poly (ADP-ribose) glycohydrolase (PARG) degrades poly(ADP-ribose) (PAR) polymers, generating free PAR polymer and ADP-ribose. PARP-1 also regulates the expression of a variety of inflammatory mediators, which might facilitate the progression of heart failure.
Peroxynitrite also potently oxidizes various biomolecules including BH4, a crucial cofactor of NOS, to quinonoid 5,6-dihydrobiopterin. A large proportion of the quinonoid isomer readily loses its side-chain to form 7,8-dihydropterin, which does not serve as a cofactor for NOS. Low cellular levels of BH4 can promote a cycle of its own destruction mediated by NOS-dependent formation of peroxynitrite. This mechanism might contribute to vascular endothelial dysfunction. The generation of peroxynitrite also decreases the availability of NO for G-protein stimulation and vasodilatation, thus further contributing to endothelial dysfunction. In addition, peroxynitrite can inhibit superoxide dismutase, glutaredoxin and other antioxidant molecules and systems, which leads to positive feedback cycles of intracellular oxidant generation and oxidative injury [8,9]. As overviewed in subsequent sections, peroxynitrite can also activate matrix metalloproteinases (MMPs) [33,34] and the nuclear enzyme poly(ADP-ribose) polymerase (PARP) [8,35] in HF. Peroxynitrite also triggers the expression of P-selectin, intercellular adhesion molecule 1 (ICAM-1) and Mac-1 (CD11b/CD18), and mediates the cytokine-induced expression of IL-8 in human leukocytes via enhancement of nuclear factor κB (NF-κB) activation [8], thereby promoting pro-inflammatory responses.
Role of peroxynitrite-induced activation of matrix metalloproteinases
MMPs are a family of proteolytic enzymes that are best known for their ability to degrade and remodel the extracellular matrix. MMPs are now also recognized to have a variety of novel actions as proteases on substrates other than extracellular matrix proteins. They have important roles both under physiological (e.g. angiogenesis and remodeling of the endometrium) and pathophysiological (e.g. neutrophil infiltration, cancer cell invasion and connective tissue remodeling) conditions. In the heart, collagens are the major extracellular matrix proteins that surround and interconnect myocytes and muscular fibers to provide mechanical support. Loss of collagen leads to myocyte slippage, ventricular dilation, and progressive contractile dysfunction. Importantly, modulation of the balance between the synthesis and degradation of matrix proteins is implicated in the process of ventricular remodeling during CHF (reviewed in [34,36]). The type of MMP involved is important. For example, changes in gelatinases (MMP-2 and MMP-9) are not directly involved in fibrillar collagen breakdown or the cardiac remodeling produced by catabolism of type I and III collagens, whereas MMP-1 (interstitial collagenase), MMP-8 (neutrophilcollagenase),MMP-13(collagenasetype3)and MMP-14 [MT1 (membrane type 1)-MMP] are the only MMPs that cleave triple helical collagen [37].
MMPs are thought to be secreted in an inactive form from cells and activated at the cell membrane via a proteolytic mechanism. However, this paradigm of only extracellular activation and action of MMPs, particularly that of MMP-2, has been challenged by recent evidence.
Increased oxidative and nitrosative stress have been proposed to have an important role in the activation of MMPs both in vitro and in vivo (reviewed in [34,36,37]). Oxidative stress causes tissue injury through activation of pro-MMPs, which is triggered by the interaction of peroxynitrite with cytosolic glutathione and subsequent S-glutathiolation of a crucial cysteine residue in the autoinhibitory domain of the pro-MMP, resulting in a catalytically active pro-MMP [33]. MMP-2 is activated and released from the reperfused heart following ischemia, and inhibition of MMP-2 activity functionally protects the heart from ischemia–reperfusion injury [38]. Infusion of peroxynitrite into isolated rat hearts results in MMP-2 activation, which precedes the onset of mechanical dysfunction, an effect that is abolished by MMP inhibitors [39]. Upon ischemia–reperfusion injury MMP-2 was found to be localized to the sarcomeric thin myofilaments and was responsible for the proteolytic cleavage of troponin I [40], which contributes to the early reduction of myocardial contractility in acute myocardial reperfusion injury.
MMP-2 activity is increased in the myocardium of spontaneously hypertensive rats with CHF and correlates with ventricular dilation and age-dependent dysfunction. Inhibition of MMP-2 activity ameliorates these changes [41]. Targeted deletion of MMP-9 also attenuates left ventricular remodeling after experimental myocardial infarction in mice [42]. Additional evidence for the importance of MMP-2 and -9 in the heart is provided by the demonstration that, in a porcine model, MMP inhibitors ameliorate ventricular dysfunction induced by rapid ventricular pacing [43]. Peroxynitrite-dependent early activation of MMP-2 also occurs in rat hearts exposed to pro-inflammatory cytokines [26] and in a doxorubicin-induced murine heart failure model [15,16]. Contractile failure of the heart was reduced either by MMPs inhibitors [26] or by a peroxynitrite decomposition catalyst [15,16].
MMP-2 and MMP-9 have pivotal roles in a variety of human cardiovascular disorders. The activities of both these MMPs are increased in the ventricles of patients suffering from dilated cardiomyopathy [44] and in atrial biopsies of patients undergoing cardiopulmonary bypass [45]. The concentration of plasma MMP-9 might predict adverse cardiovascular events [46]. Furthermore, a particular polymorphism of MMP-9 (R279Q) is highly associated with the occurrence of stable angina in humans [46].
Role of poly(ADP-ribose) polymerase (PARP) activation
Poly(ADP-ribose) polymerase 1 (PARP-1) is the major isoform of a family of nuclear enzymes with multiple regulatory functions. PARP cleaves NAD+to nicotinamide and ADP-ribose to form long branches of ADP-ribose polymers on nuclear target proteins. PARP activation exerts its pathophysiological effects via two principal mechanisms (reviewed in [35]). First, when PARP is activated by single-strand breaks in DNA, it catalyzes the cleavage of NAD+ into nicotinamide. As a result, in oxidatively or nitrosatively stressed cells NAD becomes depleted, impairing glycolysis, Krebs cycle and mitochondrial electron transport, eventually resulting in ATP depletion. Second, PARP-1 regulates the expression of various proteins, including iNOS, ICAM-1, and various cytokines and chemokines, at the transcriptional level. Pharmacological inhibition or genetic deletion of PARP attenuates the production of a variety of inflammatory mediators in vivo [35]. The relative contribution of the two mechanisms to acute heart failure and CHF has not yet been clarified completely. In acute hypoxic-reoxygenated hearts, there is a massive drop in NAD and ATP levels, compared with in control hearts, and PARP inhibitors accelerate the recovery of these energetic pools during reperfusion [47]. The effect of PARP inhibitors on myocardial energetics in CHF has not yet been studied. The triggers of PARP activation, in general, are DNA strand breakage and increased intracellular concentrations of Ca2+ [35]; both of these triggers have been demonstrated in acute heart failure and CHF [1,48,49].
Pharmacological inhibition or genetic deletion of PARP markedly improves the outcome of myocardial ischemia–reperfusion injury [35,48] and various forms of cardiomyopathy and CHF (Table 3) [15,21,50–68]. In diabetic cardiomyopathy models the depression of myocardial contractile function was associated with a significant increase in poly(ADP-ribosyl)ation in cardiac myocytes [54]. In rat models of CHF induced by chronic coronary artery ligation and advanced aging, increased nitrosative stress and poly(ADP-ribosyl)ation were found in both myocytes and endothelial cells. Pharmacological inhibition of PARP attenuated the myocardial hypertrophy and improved endothelial function in these rat models [21,62]. Using a dual approach of PARP-1 suppression by genetic deletion or pharmacological inhibition, the role of oxidative and nitrosative stress-induced PARP activation in the development of cardiac dysfunction induced by doxorubicin was also demonstrated [15,52,69]. Most recently, two studies demonstrated increased poly(ADP-ribosyl)ation and overexpression of PARP-1 in the myocardium of mice with aortic banding-induced CHF [49,53]. In these studies inhibition or genetic inactivation of PARP protected against a hypertrophy response and heart failure induced by aortic banding. There is also evidence for overexpression of PARP-1 in biopsies from human subjects with CHF [49]. Pharmacological inhibition of PARP or genetic PARP-1 deficiency also prevents the mitochondrial-to-nuclear translocation of the cell death factor apoptosis-inducing factor (AIF) in a banding-induced murine heart failure model [53].
Table 3.
Protection against cardiac dysfunction and injury by scavenging of peroxynitrite or by inhibition of PARPa
| Experimental model | Trigger of injury | Mode of peroxynitrite neutralization or PARP inhibitionb | Effects of peroxynitrite neutralization or PARP inhibition | Refs |
|---|---|---|---|---|
| Mouse, mouse heart | Global or regional ischemia–reperfusion | PARP−/− phenotype | Improved left ventricular function; reduced myocardial damage and apoptosis; reduced neutrophil infiltration; reduced circulating TNF-α, IL-10, nitrate, ICAM-1 and P-selectin expression, NAD+ consumption, NF-κB activation and phosphorylative activity of JNK and AP-1 activation; increased HSF-1 and HSP70 activation | [50,51]c |
| Mouse | Doxorubicin | PARP−/− phenotype, PJ34, FP15 | Improved contractile function; reduced myocyte death; decreased mortality | [15,52] |
| Mouse | Aortic banding | PARP−/− phenotype, INO1001 | Decreased hypertrophy and improved contractile function; improved survival | [49,53] |
| Mouse, rat | NOD diabetes, STZ diabetes, regional ischemia–reperfusion | PJ34, FP15, INO1001 | Improved cardiac and vascular function; reduced infarct size and mitochondrial-to-nuclear translocation of the cell death effector apoptosis- inducing factor (AIF) in myocardial infarction | [54–56] |
| Mouse, rat | Endotoxin | PARP−/− phenotype, PJ34 | Improved contractile function and decreased mortality | [57] |
| Mouse, rat | Angiotensin II infusion | PJ34, INO1001 | Improved endothelial function | [58] |
| Rat cardiomyoblasts, rat and human cardiomyocytes | Hydrogen peroxide, peroxynitrite, hypoxia reoxygenation | 3-AB, nicotinamide, 5-AIQ | Reduced cell death; improved mitochondrial respiration and cellular ATP levels | c |
| Rat heart | Mixture of pro- inflammatory cytokines | Tiron, FeTPPS | Improved cardiac function in cytokine-induced myocardial contractile failure | c |
| Rat, rat and rabbit heart | Regional and global ischemia–reperfusion | FP15, 3-AB, nicotinamide, 5-AIQ, BGP15, GPI6150 | Reduction of infarct size and left ventricular dysfunction; preservation of myocardial ATP stores | [15,22]c |
| Rat | Heart transplantation | 3-AB, PJ34, 5-AIQ | Decreased myocardial damage; reduced oxidative stress; improved cellular NAD+ and ATP levels; improved contractile and endothelial function; reduced ICAM-1 and P-selectin expression | [59–61] |
| Rat | Chronic myocardial ischemia and advanced aging | PJ34, INO1001 | Improved left ventricular and vascular function; reduced hypertrophy | [21,62] |
| Rat | Endotoxin | iNOS inhibitor mercaptoethylguanidine and peroxynitrite decomposition catalyst FeTPPS | Improved contractile function and decreased myocardial peroxynitrite accumulation; decreased degradation of the NF-κB inhibitory protein IκB; decreased levels of plasma TNF-α; decreased vascular endothelial cell–leukocyte activation | [63] |
| Rat heart | High glucose | M40403 | Decreased QT interval prolongation; decreased coronary perfusion pressure and lipid peroxidation; decreased nitrotyrosine formation and PARP activation | [64] |
| Dog | Cardiopulmonary bypass; crystalloid cardioplegia and extra-corporal circulation | PJ34, INO1001 | Better recovery of left and right ventricular systolic function; increased coronary blood flow; improved vascular and pulmonary function | [65–67] |
| Pig | Regional ischemia– reperfusion | FP15, 3-AB, PJ34 | Reduced infarct size; improved left ventricular function | [68]c |
Abbreviations: AP-1, activator protein 1; HSF-1, heat shock transcription factor 1; HSP70, heat shock protein 70; ICAM-1, intercellular adhesion molecule 1; IL-10, interleukin 10; iNOS, inducible nitric oxide synthase; JNK, Jun N-terminal kinase; NF-κB, nuclear factor κB; NOD, non-obese diabetic; PARP, poly(ADP-ribose) polymerase; STZ, streptozotocin; TNF-α, tumor necrosis factor α.
The following drugs were used in these studies: (i) early PARP inhibitors of low potency [3-aminobenzamide (3-AB) and nicotinamide]; (ii) a potent new generation of selective PARP inhibitors {e.g. GPI6150 (1,11b-dihydro-[2H]benzopyrano[4,3,2-de]isoquinolin-3-one), BGP15 (O-(3-piperidino-2-hydroxy-1-propyl)nicotinic amidoxime), 5-AIQ (5-aminoisoquinolinone), PJ34 (N-(6-oxo-5,6-dihydro-phenanthridin-2-yl)-N,N-dimethylacetamide HCl) and INO1001 (isoindolinone-based PARP inhibitor)}; (iii) antioxidants (tiron); (iv) peroxynitrite decomposition catalysts {FeTPPS [5,10,15,20-tetrakis(4-sulfonatophenyl)porphyrinato iron (III)], FP15 [FeCl tetrakis-2-(triethylene glycol monomethyl ether) pyridyl porphyrin]}; and (v) the superoxide dismutase (SOD) mimetic M40403 [a manganese (II) complex with a bis(cyclohexylpyridine-substituted) macrocyclic ligand].
Angiotensin II might also be important in PARP activation and the pathogenesis of heart failure. Angiotensin II is a known factor in the pathogenesis of CHF, and can induce endothelial peroxynitrite formation [70] and PARP activation [58]. Angiotensin II triggers the activation of PARP in cultured endothelial cells in vitro and chronic infusion of sub-pressor doses of angiotensin II triggers endothelial dysfunction in vivo, which can be prevented or reversed by PARP inhibition [58]. A recent study also implicates PARP in regulating the expression of endothelin receptors [71], and the dysregulation of endothelin signaling is known to be important in CHF.
Although the exact molecular triggers of PARP activation in CHF are unknown, we propose that following initial damage (e.g. myocardial infarction) the compensatory increased sympathetic activity, activated renin–angiotensin system and increased workload of the non-infarcted regions of the myocardium leads to myocardial hypertrophy, myocardial ischemia or functional hypoxia, increased oxidative and nitrosative stress and PARP activation, which in turn results in alterations in cellular energetics and the activation of pro-inflammatory pathways (Figures 1 and 2).
Possibilities for future pharmacological interventions for the experimental therapy of heart failure
Although there is strong experimental evidence for the role of ROS and reactive nitrogen species in the development of the structural and functional changes of the failing myocardium, the results of clinical trials with antioxidants such as vitamin C, vitamin E and coenzyme Q10 are equivocal [1]. The reason for these findings might be related to the type of antioxidant used (relatively low reaction rate with the reactive species or non-catalytic antioxidant) and the dose of the antioxidant used (less than the doses used in preclinical animal studies).
Approaches that use more-effective antioxidants (catalytic antioxidants with high reaction rates) or that specifically target myocardial oxidant-producing enzymes or specific downstream targets of oxidative or nitrosative stress might be more fruitful approaches in the future. For example, xanthine oxidase, which is an important source of superoxide in the failing heart, can be inhibited by pharmacological agents (e.g. allopurinol and oxypurinol). Both preclinical and clinical studies demonstrate that this approach improves cardiac efficiency and might become a future therapeutic modality. Furthermore, the data obtained so far in short-term clinical studies with allopurinol in CHF patients are encouraging (reviewed in [3]). In theory, mitochondrial uncoupling proteins might reduce superoxide formation in mitochondria in the heart [72], but such an approach might induce several unwanted effects. The pathogenetic role of NADPH oxidase in CHF might be exploited with apocynin (a weak nonselective NADPH oxidase inhibitor) [73] or with yet to be identified more-potent or more-selective NADPH oxidase inhibitors.
Peroxynitrite decomposition catalysts appear to improve cardiac function and overall outcome in animal models of CHF. For example, the metalloporphyrin peroxynitrite decomposition catalyst FP15 reduced myocardial necrosis in rat [15] and pig [68] models of acute myocardial infarction, and improved mechanical dysfunction in rat hearts exposed to pro-inflammatory cytokines [74]. Furthermore, catalytic decomposition of peroxynitrite significantly improved cardiac function in models of diabetic and doxorubicin-induced cardiomyopathy [15,55,75].
Both ROS and reactive nitrogen species trigger downstream cellular suicide pathways. As discussed earlier, MMP activation and PARP activation might be two examples of such pathways, and in both cases there are significant preclinical data demonstrating their efficacy in HF models (see earlier and Table 3). With respect to MMP inhibitors, there are several compounds (including the tetracycline-class antibiotics, which possess the ability to inhibit MMP activity independently of their antibacterial action [38]) that might be used clinically and might be suitable for controlled clinical investigations. In fact, recent preclinical studies demonstrate the cardioprotective effects of doxycycline in myocardial infarction [76,77], and in a small clinical trial a sub-antimicrobial dose of doxycycline (the most potent tetracycline inhibitor of MMP) attenuated the levels of several inflammatory markers in post-myocardial infarction patients [78]. In addition, potent pharmacological PARP inhibitors have recently undergone clinical investigations for cardioprotective indications [79].
It must be kept in mind that, at present, CHF patients receive a variety of therapeutic agents, some of which have known antioxidant effects (e.g. the β-adrenoceptor antagonist carvedilol). In the case of diabetic heart failure, it must also be kept in mind that acute (in addition to chronic) hyperglycemia are strong triggers of ROS and reactive nitrogen species formation in the cardiovascular system [75,80]. Therefore, tight control of hyperglycemia in diabetics might be one of the best ways to prevent the production of reactive species and the initiation of downstream pathways of myocardial injury. Whether conventional interventions (e.g. antioxidant therapies, statins, β-adrenoceptor antagonists, angiotensin-converting enzyme inhibitors, insulin or oral anti-diabetic agents) are able to suppress the activation of PARP or MMPs during CHF remains to be investigated. Overall, we expect that recent advances in our understanding of the role of reactive species in the pathogenesis of heart failure will, in the not-so-distant future, yield novel therapeutic approaches (monotherapy or combination approaches) that will be exploited for the benefit of patients with heart failure.
References
- 1.Ferrari R, et al. Oxidative stress during myocardial ischaemia and heart failure. Curr Pharm Des. 2004;10:1699–1711. doi: 10.2174/1381612043384718. [DOI] [PubMed] [Google Scholar]
- 2.Heymes C, et al. Increased myocardial NADPH oxidase activity in human heart failure. J Am Coll Cardiol. 2003;41:2164–2171. doi: 10.1016/s0735-1097(03)00471-6. [DOI] [PubMed] [Google Scholar]
- 3.Berry CE, Hare JM. Xanthine oxidoreductase and cardiovascular disease: molecular mechanisms and pathophysiological implications. J Physiol. 2004;555:589–606. doi: 10.1113/jphysiol.2003.055913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hare JM, Stamler JS. NO/redox disequilibrium in the failing heart and cardiovascular system. J Clin Invest. 2005;115:509–517. doi: 10.1172/JCI200524459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Massion PB, et al. Nitric oxide and cardiac function: ten years after, and continuing. Circ Res. 2003;93:388–398. doi: 10.1161/01.RES.0000088351.58510.21. [DOI] [PubMed] [Google Scholar]
- 6.Paulus WJ, Bronzwaer JG. Nitric oxide’s role in the heart: control of beating or breathing? Am J Physiol Heart Circ Physiol. 2004;287:H8–H13. doi: 10.1152/ajpheart.01147.2003. [DOI] [PubMed] [Google Scholar]
- 7.Rubbo H, et al. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. J Biol Chem. 1994;269:26066–26075. [PubMed] [Google Scholar]
- 8.Szabo C. Multiple pathways of peroxynitrite cytotoxicity. Toxicol Lett. 2003:140–141. 105–112. doi: 10.1016/s0378-4274(02)00507-6. [DOI] [PubMed] [Google Scholar]
- 9.Turko IV, Murad F. Protein nitration in cardiovascular diseases. Pharmacol Rev. 2002;54:619–634. doi: 10.1124/pr.54.4.619. [DOI] [PubMed] [Google Scholar]
- 10.Mihm MJ, et al. Peroxynitrite induced nitration and inactivation of myofibrillar creatine kinase in experimental heart failure. Cardiovasc Res. 2001;49:798–807. doi: 10.1016/s0008-6363(00)00307-2. [DOI] [PubMed] [Google Scholar]
- 11.Mungrue IN, et al. Cardiomyocyte overexpression of iNOS in mice results in peroxynitrite generation, heart block, and sudden death. J Clin Invest. 2002;109:735–743. doi: 10.1172/JCI13265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gealekman O, et al. Role of myocardial inducible nitric oxide synthase in contractile dysfunction and beta-adrenergic hyporesponsiveness in rats with experimental volume-overload heart failure. Circulation. 2002;105:236–243. doi: 10.1161/hc0202.102015. [DOI] [PubMed] [Google Scholar]
- 13.Damy T, et al. Increased neuronal nitric oxide synthase-derived NO production in the failing human heart. Lancet. 2004;363:1365–1367. doi: 10.1016/S0140-6736(04)16048-0. [DOI] [PubMed] [Google Scholar]
- 14.Ferdinandy P, Schulz R. Nitric oxide, superoxide, and peroxynitrite in myocardial ischaemia-reperfusion injury and preconditioning. Br J Pharmacol. 2003;138:532–543. doi: 10.1038/sj.bjp.0705080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pacher P, et al. Potent metalloporphyrin peroxynitrite decomposition catalyst protects against the development of doxorubicin-induced cardiac dysfunction. Circulation. 2003;107:896–904. doi: 10.1161/01.cir.0000048192.52098.dd. [DOI] [PubMed] [Google Scholar]
- 16.Bai P, et al. Matrix metalloproteinase activation is an early event in doxorubicin-induced cardiotoxicity. Oncol Rep. 2004;11:505–509. [PubMed] [Google Scholar]
- 17.Chaves AA, et al. Cardiomyopathy in a murine model of AIDS: evidence of reactive nitrogen species and corroboration in human HIV/AIDS cardiac tissues. Cardiovasc Res. 2003;60:108–118. doi: 10.1016/s0008-6363(03)00431-0. [DOI] [PubMed] [Google Scholar]
- 18.Ceriello A, et al. Acute hyperglycemia induces nitrotyrosine formation and apoptosis in perfused heart from rat. Diabetes. 2002;51:1076–1082. doi: 10.2337/diabetes.51.4.1076. [DOI] [PubMed] [Google Scholar]
- 19.Mihm MJ, Bauer JA. Peroxynitrite-induced inhibition and nitration of cardiac myofibrillar creatine kinase. Biochimie. 2002;84:1013–1019. doi: 10.1016/s0300-9084(02)00005-6. [DOI] [PubMed] [Google Scholar]
- 20.Mihm MJ, et al. Effects of peroxynitrite on isolated cardiac trabeculae: selective impact on myofibrillar energetic controllers. Biochimie. 2003;85:587–596. doi: 10.1016/s0300-9084(03)00090-7. [DOI] [PubMed] [Google Scholar]
- 21.Pacher P, et al. Pharmacologic inhibition of poly(adenosine diphosphate-ribose) polymerase may represent a novel therapeutic approach in chronic heart failure. J Am Coll Cardiol. 2002;40:1006–1016. doi: 10.1016/s0735-1097(02)02062-4. [DOI] [PubMed] [Google Scholar]
- 22.Lee WH, et al. Influence of peroxynitrite on energy metabolism and cardiac function in a rat ischemia-reperfusion model. Am J Physiol Heart Circ Physiol. 2003;285:H1385–H1395. doi: 10.1152/ajpheart.00808.2002. [DOI] [PubMed] [Google Scholar]
- 23.Khadour FH, et al. Enhanced NO and superoxide generation in dysfunctional hearts from endotoxemic rats. Am J Physiol Heart Circ Physiol. 2002;283:H1108–H1115. doi: 10.1152/ajpheart.00549.2001. [DOI] [PubMed] [Google Scholar]
- 24.Iqbal M, et al. Time course of nitric oxide, peroxynitrite and antioxidants in the endotoxemic heart. Crit Care Med. 2002;30:1291–1296. doi: 10.1097/00003246-200206000-00021. [DOI] [PubMed] [Google Scholar]
- 25.Keira N, et al. Lethal effect of cytokine-induced nitric oxide and peroxynitrite on cultured rat cardiac myocytes. J Mol Cell Cardiol. 2002;34:583–596. doi: 10.1006/jmcc.2002.1539. [DOI] [PubMed] [Google Scholar]
- 26.Gao CQ, et al. Matrix metalloproteinase-2 mediates cytokine-induced myocardial contractile dysfunction. Cardiovasc Res. 2003;57:426–433. doi: 10.1016/s0008-6363(02)00719-8. [DOI] [PubMed] [Google Scholar]
- 27.Baker CSR, et al. Repetitive myocardial stunning in pigs is associated with an increased formation of reactive nitrogen species. Heart. 2002;87:77–78. doi: 10.1136/heart.87.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayashi Y, et al. Leukocyte-depleted terminal blood cardioplegia provides superior myocardial protective effects in association with myocardium-derived nitric oxide and peroxynitrite production for patients undergoing prolonged aortic crossclamping for more than 120 minutes. J Thorac Cardiovasc Surg. 2003;126:1813–1821. doi: 10.1016/s0022-5223(03)01282-0. [DOI] [PubMed] [Google Scholar]
- 29.Mehlhorn U, et al. Nitrotyrosine and 8-isoprostane formation indicate free radical-mediated injury in hearts of patients subjected to cardioplegia. J Thorac Cardiovasc Surg. 2003;125:178–183. doi: 10.1067/mtc.2003.97. [DOI] [PubMed] [Google Scholar]
- 30.Baker CS, et al. Immunocytochemical evidence for inducible nitric oxide synthase and cyclooxygenase-2 expression with nitrotyrosine formation in human hibernating myocardium. Basic Res Cardiol. 2002;97:409–415. doi: 10.1007/s003950200050. [DOI] [PubMed] [Google Scholar]
- 31.Miller AA, et al. Inducible nitric oxide synthase-derived superoxide contributes to hypereactivity in small mesenteric arteries from a rat model of chronic heart failure. Br J Pharmacol. 2000;131:29–36. doi: 10.1038/sj.bjp.0703528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colombo PC, et al. Endothelial cell activation in patients with decompensated heart failure. Circulation. 2005;111:58–62. doi: 10.1161/01.CIR.0000151611.89232.3B. [DOI] [PubMed] [Google Scholar]
- 33.Okamoto T, et al. Activation of matrix metalloproteinases by peroxynitrite-induced protein S-glutathiolation via disulfide S-oxide formation. J Biol Chem. 2001;276:29596–29602. doi: 10.1074/jbc.M102417200. [DOI] [PubMed] [Google Scholar]
- 34.Lalu MM, et al. Peroxynitrite in myocardial ischemia-reperfusion injury. Heart Fail Rev. 2002;7:359–369. doi: 10.1023/a:1020766502316. [DOI] [PubMed] [Google Scholar]
- 35.Virag L, Szabo C. The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol Rev. 2002;54:375–429. doi: 10.1124/pr.54.3.375. [DOI] [PubMed] [Google Scholar]
- 36.Spinale FG. Matrix metalloproteinases: regulation and dysregulation in the failing heart. Circ Res. 2002;90:520–530. doi: 10.1161/01.res.0000013290.12884.a3. [DOI] [PubMed] [Google Scholar]
- 37.Siwik DA, Colucci WS. Regulation of matrix metalloproteinases by cytokines and reactive oxygen/nitrogen species in the myocardium. Heart Fail Rev. 2004;9:43–51. doi: 10.1023/B:HREV.0000011393.40674.13. [DOI] [PubMed] [Google Scholar]
- 38.Cheung PY, et al. Matrix etalloproteinase-2 contributes to ischemia–reperfusion injury in the heart. Circulation. 2000;101:1833–1839. doi: 10.1161/01.cir.101.15.1833. [DOI] [PubMed] [Google Scholar]
- 39.Wang W, et al. Peroxynitrite-induced myocardial injury is mediated through matrix metalloproteinase-2. Cardiovasc Res. 2002;53:165–174. doi: 10.1016/s0008-6363(01)00445-x. [DOI] [PubMed] [Google Scholar]
- 40.Wang W, et al. Intracellular action of matrix metalloproteinase-2 accounts for acute myocardial ischemia and reperfusion injury. Circulation. 2002;106:1543–1549. doi: 10.1161/01.cir.0000028818.33488.7b. [DOI] [PubMed] [Google Scholar]
- 41.Peterson JT, et al. Matrix metalloproteinase inhibition attenuates left ventricular remodeling and dysfunction in a rat model of progressive heart failure. Circulation. 2001;103:2303–2309. doi: 10.1161/01.cir.103.18.2303. [DOI] [PubMed] [Google Scholar]
- 42.Ducharme A, et al. Targeted deletion of matrix metallo-proteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J Clin Invest. 2000;106:55–62. doi: 10.1172/JCI8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.King MK, et al. Selective matrix metalloproteinase inhibition with developing heart failure: effects on left ventricular function and structure. Circ Res. 2003;92:177–185. doi: 10.1161/01.res.0000052312.41419.55. [DOI] [PubMed] [Google Scholar]
- 44.Spinale FG, et al. A matrix metalloproteinase induction/activation system exists in the human left ventricular myocardium and is upregulated in heart failure. Circulation. 2000;102:1944–1949. doi: 10.1161/01.cir.102.16.1944. [DOI] [PubMed] [Google Scholar]
- 45.Lalu MM, et al. Ischemia-reperfusion injury activates matrix metalloproteinases in the human heart. Eur Heart J. 2005;26:27–35. doi: 10.1093/eurheartj/ehi007. [DOI] [PubMed] [Google Scholar]
- 46.Blankenberg S, et al. Plasma concentrations and genetic variation of matrix metalloproteinase 9 and prognosis of patients with cardiovascular disease. Circulation. 2003;107:1579–1585. doi: 10.1161/01.CIR.0000058700.41738.12. [DOI] [PubMed] [Google Scholar]
- 47.Docherty JC, et al. An inhibitor of poly (ADP-ribose) synthetase activity reduces contractile dysfunction and preserves high energy phosphate levels during reperfusion of the ischaemic rat heart. Br J Pharmacol. 1999;127:1518–1524. doi: 10.1038/sj.bjp.0702705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Szabo G, et al. Poly(ADP-ribose) polymerase activation in the reperfused myocardium. Cardiovasc Res. 2004;61:471–480. doi: 10.1016/j.cardiores.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 49.Pillai JB, et al. increased expression of poly (ADP) ribose polymerase-1 contributes to caspase-independent myocyte cell death during heart failure. Am J Physiol Heart Circ Physiol. 2005;288:H486–H496. doi: 10.1152/ajpheart.00437.2004. [DOI] [PubMed] [Google Scholar]
- 50.Zingarelli B, et al. Absence of poly(ADP-ribose)polymerase-1 alters nuclear factor-kappa B activation and gene expression of apoptosis regulators after reperfusion injury. Mol Med. 2003;9:143–153. doi: 10.2119/2003-00011.zingarelli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zingarelli B, et al. Differential regulation of activator protein-1 and heat shock factor-1 in myocardial ischemia and reperfusion injury: role of poly(ADP-ribose) polymerase-1. Am J Physiol Heart Circ Physiol. 2004;286:H1408–H1415. doi: 10.1152/ajpheart.00953.2003. [DOI] [PubMed] [Google Scholar]
- 52.Pacher P, et al. Activation of poly(ADP-ribose) polymerase contributes to development of doxorubicin-induced heart failure. J Pharmacol Exp Ther. 2002;300:862–867. doi: 10.1124/jpet.300.3.862. [DOI] [PubMed] [Google Scholar]
- 53.Xiao CY, et al. Poly(ADP-ribose) polymerase promotes cardiac remodeling, contractile failure and translocation of apoptosis-inducing factor in a murine experimental model of aortic banding and heart failure. J Pharmacol Exp Ther. 2005;312:891–898. doi: 10.1124/jpet.104.077164. [DOI] [PubMed] [Google Scholar]
- 54.Pacher P, et al. The role of poly(ADP-ribose) polymerase activation in the development of myocardial and endothelial dysfunction in diabetes. Diabetes. 2002;51:514–521. doi: 10.2337/diabetes.51.2.514. [DOI] [PubMed] [Google Scholar]
- 55.Szabo C, et al. Pathogenetic role of peroxynitrite in the development of diabetes and diabetic vascular complications: studies with FP15, a novel potent peroxynitrite decomposition catalyst. Mol Med. 2002;8:571–580. [PMC free article] [PubMed] [Google Scholar]
- 56.Xiao CY, et al. Poly(ADP-ribose) polymerase contributes to the development of myocardial infarction in diabetic rats and regulates the nuclear translocation of apoptosis-inducing factor. J Pharmacol Exp Ther. 2004;310:498–504. doi: 10.1124/jpet.104.066803. [DOI] [PubMed] [Google Scholar]
- 57.Pacher P, et al. Role of poly(ADP-ribose) polymerase activation in endotoxin-induced cardiac collapse in rodents. Biochem Pharmacol. 2002;64:1785–1791. doi: 10.1016/s0006-2952(02)01421-1. [DOI] [PubMed] [Google Scholar]
- 58.Szabo C, et al. Angiotensin II-mediated endothelial dysfunction: role of poly(ADP-ribose) polymerase activation. Mol Med. 2004;10:28–35. doi: 10.2119/2004-00001.szabo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fiorillo C, et al. Poly(ADP-ribose) polymerase activation and cell injury in the course of rat heart heterotopic transplantation. Free Radic Res. 2002;36:79–87. doi: 10.1080/10715760210168. [DOI] [PubMed] [Google Scholar]
- 60.Fiorillo C, et al. Beneficial effects of poly (ADP-ribose) polymerase inhibition against the reperfusion injury in heart transplantation. Free Radic Res. 2003;37:331–339. doi: 10.1080/1071576021000055262. [DOI] [PubMed] [Google Scholar]
- 61.Szabo G, et al. Poly(ADP-ribose) polymerase inhibition reduces reperfusion injury after heart transplantation. Circ Res. 2002;90:100–106. doi: 10.1161/hh0102.102657. [DOI] [PubMed] [Google Scholar]
- 62.Pacher P, et al. A new, potent poly(ADP-ribose) polymerase inhibitor improves cardiac and vascular dysfunction associated with advanced aging. J Pharmacol Exp Ther. 2004;311:485–491. doi: 10.1124/jpet.104.069658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lancel S, et al. Peroxynitrite decomposition catalysts prevent myocardial dysfunction and inflammation in endotoxemic rats. J Am Coll Cardiol. 2004;43:2348–2358. doi: 10.1016/j.jacc.2004.01.047. [DOI] [PubMed] [Google Scholar]
- 64.Di Filippo C, et al. M40403 prevents myocardial injury induced by acute hyperglycaemia in perfused rat heart. Eur J Pharmacol. 2004;497:65–74. doi: 10.1016/j.ejphar.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 65.Szabo G, et al. Poly-ADP-ribose polymerase inhibition protects against myocardial and endothelial reperfusion injury after hypothermic cardiac arrest. J Thorac Cardiovasc Surg. 2003;126:651–658. doi: 10.1016/s0022-5223(02)73235-2. [DOI] [PubMed] [Google Scholar]
- 66.Szabo G, et al. Role of poly(ADP-ribose) polymerase activation in the pathogenesis of cardiopulmonary dysfunction in a canine model of cardiopulmonary bypass. Eur J Cardiothorac Surg. 2004;25:825–832. doi: 10.1016/j.ejcts.2004.01.031. [DOI] [PubMed] [Google Scholar]
- 67.Szabo G, et al. INO-1001 a novel poly(ADP-ribose) polymerase (PARP) inhibitor improves cardiac and pulmonary function after crystalloid cardioplegia and extracorporal circulation. Shock. 2004;21:426–432. doi: 10.1097/00024382-200405000-00005. [DOI] [PubMed] [Google Scholar]
- 68.Bianchi C, et al. A novel peroxynitrite decomposer catalyst (FP-15) reduces myocardial infarct size in an in vivo peroxynitrite decomposer and acute ischemia-reperfusion in pigs. Ann Thorac Surg. 2002;74:1201–1207. doi: 10.1016/s0003-4975(02)03953-x. [DOI] [PubMed] [Google Scholar]
- 69.Szenczi O, et al. Poly(ADP-ribose) polymerase regulates myocardial calcium handling in doxorubicin-induced heart failure. Biochem Pharmacol. 2005;69:725–732. doi: 10.1016/j.bcp.2004.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wattanapitayakul SK, et al. Endothelial dysfunction and peroxynitrite formation are early events in angiotensin-induced cardiovascular disorders. FASEB J. 2000;14:271–278. doi: 10.1096/fasebj.14.2.271. [DOI] [PubMed] [Google Scholar]
- 71.Minchenko AG, et al. Diabetes-induced overexpression of endothelin-1 and endothelin receptors in the rat renal cortex is mediated via poly(ADP-ribose) polymerase activation. FASEB J. 2003;17:1514–1516. doi: 10.1096/fj.03-0013fje. [DOI] [PubMed] [Google Scholar]
- 72.Murakami K, et al. Perindopril effect on uncoupling protein and energy metabolism in failing rat hearts. Hypertension. 2002;40:251–255. doi: 10.1161/01.hyp.0000029094.85023.01. [DOI] [PubMed] [Google Scholar]
- 73.Takayama T, et al. Contribution of vascular NAD(P)H oxidase to endothelial dysfunction in heart failure and the therapeutic effects of HMG-CoA reductase inhibitor. Circ J. 2004;68:1067–1075. doi: 10.1253/circj.68.1067. [DOI] [PubMed] [Google Scholar]
- 74.Ferdinandy P, et al. Peroxynitrite is a major contributor to cytokine-induced contractile failure. Circ Res. 2000;87:241–247. doi: 10.1161/01.res.87.3.241. [DOI] [PubMed] [Google Scholar]
- 75.Pacher P, et al. Role of nitrosative stress and peroxynitrite in the pathogenesis of diabetic complications. Emerging new therapeutical strategies. Curr Med Chem. 2005;12:267–275. doi: 10.2174/0929867053363207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Villarreal FJ, et al. Early short-term treatment with doxycycline modulates postinfarction left ventricular remodeling. Circulation. 2003;108:1487–1492. doi: 10.1161/01.CIR.0000089090.05757.34. [DOI] [PubMed] [Google Scholar]
- 77.Camp TM, et al. Doxycycline ameliorates ischemic and border-zone remodeling and endothelial dysfunction after myocardial infarction in rats. J Heart Lung Transplant. 2004;23:729–736. doi: 10.1016/j.healun.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 78.Brown DL, et al. Clinical and biochemical results of the metalloproteinase inhibition with subantimicrobial doses of doxycycline to prevent acute coronary syndromes (MIDAS) pilot trial. Arterioscler Thromb Vasc Biol. 2004;24:733–738. doi: 10.1161/01.ATV.0000121571.78696.dc. [DOI] [PubMed] [Google Scholar]
- 79.Southan GJ, Szabo C. Poly(ADP-ribose) polymerase inhibitors. Curr Med Chem. 2003;10:321–340. doi: 10.2174/0929867033368376. [DOI] [PubMed] [Google Scholar]
- 80.Ceriello A. New insights on oxidative stress and diabetic complications may lead to a “causal” antioxidant therapy. Diabetes Care. 2003;26:1589–1596. doi: 10.2337/diacare.26.5.1589. [DOI] [PubMed] [Google Scholar]