Abstract
Adenosine is an endogenous purine nucleoside that, following its release into the extracellular space, binds to specific adenosine receptors expressed on the cell surface. Adenosine appears in the extracellular space under metabolically stressful conditions, which are associated with ischemia, inflammation, and cell damage. There are 4 types of adenosine receptors (A1, A2A, A2B and A3) and all adenosine receptors are members of the G protein-coupled family of receptors. Adenosine receptors are expressed on monocytes and macrophages and through these receptors adenosine modulates monocyte and macrophage function. Since monocytes and macrophages are activated by the same danger signals that cause accumulation of extracellular adenosine, adenosine receptors expressed on macrophages represent a sensor system that provide monocytes and macrophages with information about the stressful environment. Adenosine receptors, thus, allow monocytes and macrophages to fine-tune their responses to stressful stimuli. Here, we review the consequences of adenosine receptor activation on monocyte/macrophage function. We will detail the effect of stimulating the various adenosine receptor subtypes on macrophage differentiation/proliferation, phagocytosis, and tissue factor (TF) expression. We will also summarize our knowledge of how adenosine impacts the production of extracellular mediators secreted by monocytes and macrophages in response to toll-like receptor (TLR) ligands and other inflammatory stimuli. Specifically, we will delineate how adenosine affects the production of superoxide, nitric oxide (NO), tumor necrosis factor-α, interleukin (IL)-12, IL-10, and vascular endothelial growth factor (VEGF). A deeper insight into the regulation of monocyte and macrophage function by adenosine receptors should assist in developing new therapies for inflammatory diseases.
Keywords: Infection, Autoimmunity, Asthma, Sepsis, Arthritis, Colitis
1. Introduction
Macrophages are a heterogeneous population of mononuclear phagocytes found ubiquitously in the body. These cells have a role in orchestrating and executing most homeostatic, immunological, and inflammatory processes (Mosser, 2003; Stout & Suttles, 2004). Owing to their ubiquitous tissue distribution these cells are ideally suited to mount an immediate attack against foreign elements prior to the migration of polymorphonuclear neutrophils and, thus, macrophages are major factors in the body’s first line of immune defense. Newly formed macrophages that are called monocytes are produced from bone marrow progenitor cells (Naito et al., 1996; Valledor et al., 1998). After differentiating as a result of exposure to local cytokines such as granulocyte-macrophage colony-stimulating factor, interleukin (IL)-3, and macrophage colony-stimulating factor, they leave the bone marrow and enter the blood. In the blood they come in contact with a plethora of regulatory molecules (including cytokines, chemokines, hormones, fatty acids, immunoglobulins), which impact their functional and phenotypic characteristics. Circulating monocytes represent a mobile source of functionally competent immune cell that upon appropriate stimuli can invade the tissue. This homing process takes place in response to chemotactic cytokines and other tissue-specific homing factors (Muller & Randolph, 1999). After their infiltration into tissues, monocytes go through further differentiation to become macrophages. It is becoming widely accepted that the distinct phenotypes of macrophages in different tissues reflect an effect of the actual tissue environment, rather than originating from a lineage specificity (Muller & Randolph, 1999).
The biological functions of activated monocytes/macrophages are numerous and well investigated (Gordon, 2003; Mosser, 2003; Stout & Suttles, 2004). As crucial elements of innate immune responses, monocytes/macrophages capture, process, and nonspecifically kill antigens, and then activate specific lymphocyte effector mechanisms. Lymphocyte activation crucially depends on the expression of macrophage cell surface molecules such as major histocompatibility complex and costimulatory molecules, as well as production of a wide range of mediators, which include cytokines and free radicals (Unanue, 1984). These activated lymphocytes representing the specific arm of the immune response in turn cooperate with macrophages to further destroy pathogens, as well as virus-infected cells, apoptotic host cells, and tumor cells.
Once the inflammatory/immune response has eliminated an injurious agent, the process of inflammatory resolution ensues, which is orchestrated by endogenous ‘pro-resolving’ mediators in a highly coordinated way (Duffield, 2003; Gilroy et al., 2004; Wells et al., 2005). These factors turn off leukocyte movement to the inflamed site, decrease vasodilatation and vascular permeability, and cause the safe removal of inflammatory neutrophils, exudate and fibrin, thereby restoring the integrity of the inflamed tissue to its prior physiological function. Macrophages have central roles in dictating inflammatory resolution and there is ample evidence that adenosine can interfere with features of the inflammatory resolution. Successful resolution will restrict excessive tissue injury and thwart the development of chronic, immune-mediated inflammation (Gilroy et al., 2004).
The aim of this Review article is to highlight the studies that have uncovered how adenosine interferes with the various facets of macrophage activation thereby modifying inflammatory/immune processes.
2. Adenosine receptors
Adenosine was first recognized as a physiologic regulator of coronary vascular tone by Drury and Szent-Gyorgyi (1929), however it was not until 1970 that Sattin and Rall showed that adenosine regulates cell function via occupancy of specific receptors on the cell surface (Sattin & Rall, 1970). It is now clear that there are at least 4 different subtypes of adenosine receptor, any one or combination of which may be expressed on the cell surface (Ralevic & Burnstock, 1998; Fredholm et al., 2001; Linden, 2001; Hasko & Cronstein, 2004; Fredholm et al., 2005). Four adenosine receptors have been cloned and the deduced sequence reveals that all 4 are members of the large family of 7-transmembrane spanning G protein coupled receptors. Three of the adenosine receptor subtypes, A1, A2A and A2B, are highly conserved throughout evolution (80–95% sequence homology) whereas A3 receptors vary significantly among species. In general, A1 and A3 receptors are coupled to pertussis toxin-inhibited Gi coupled signal transduction proteins or directly to ion channels whereas A2 receptors (A2A and A2B) are GαS-linked receptors and stimulate adenylyl cyclase and camp accumulation. Adenosine receptors or receptor-mediated effects have been demonstrated in virtually every tissue or organ examined (Hasko & Szabo, 1998; Ralevic & Burnstock, 1998; Fozard & Hannon, 1999; Fredholm et al., 2001; Linden, 2001; Fozard & McCarthy, 2002; Hasko et al., 2002; Hasko & Cronstein, 2004; Fredholm et al., 2005).
3. Adenosine metabolism
Most physiological effects of adenosine arise from its stimulation of cell surface adenosine receptors and the activation of downstream signaling pathways. Adenosine concentrations at its receptors are determined by a variety of processes, which include extracellular and intracellular adenosine generation, adenosine release from cells, cellular reuptake and metabolism. These processes are closely intertwined and strictly regulated. For, example, under hypoxic conditions, the increased intracellular dephosphorylation of adenosine 5′-triphosphate (ATP) to adenosine by the metabolic enzyme 5′-nucleotidase is accompanied by a suppression of the activity of the salvage enzyme adenosine kinase, which prevents the rephosphorylation of adenosine (Deussen, 2000). These processes lead to adenosine reaching high concentrations inside the cell and the release of adenosine into the extracellular space through nucleoside transporters (Hyde et al., 2001; Pastor-Anglada et al., 2001). The other major pathway that contributes to high extracellular adenosine concentrations during metabolic stress is release of precursor adenine nucleotides (ATP, ADP and AMP) from the cell. This is followed by extracellular degradation to adenosine by a cascade of ectonucleotidases, which include CD39 (nucleoside triphosphate diphosphohydrolase [NTPDase]) and CD73 (5′-ectonucleotidase) (Kaczmarek et al., 1996; Resta et al., 1998; Zimmermann, 1999; Eltzschig et al., 2004; Thompson et al., 2004; Sperlagh et al., 2006). Adenosine accumulation is limited by its catabolism to inosine by adenosine deaminase. Inosine is finally degraded to the stable end product uric acid (Jennings & Steenbergen, 1985; Hasko, Kuhel, Nemeth et al., 2000; Cristalli et al., 2001; Hasko et al., 2004).
There are several important producer cell types of extracellular adenosine. Neutrophils and endothelial cells release large amounts of adenosine at sites of metabolic distress, inflammation and infection (Cronstein et al., 1983; Gunther & Herring, 1991; Madara et al., 1993; Rounds et al., 1994). We recently documented that nerve terminals are a major contributor to extracellular adenosine accumulation in the ischemic spleen (Sperlagh et al., 2000) and ADP released by platelets can be a significant source of adenosine after dephosphorylation (Marcus et al., 1995). Activated macrophages can also serve as a major source of extracellular adenosine via ATP production. We recently reported that bacterial lipopolysaccharide (LPS) augmented the release of ATP from macrophages (Sperlagh et al., 1998). Because the inhibitory effects of exogenously added ATP on macrophage TNF-α production can be abrogated by administering exogenous adenosine deaminase (Hasko, Kuhel, Salzman et al., 2000), it can be proposed that endogenously released ATP is also degraded to adenosine by macrophages.
Lower concentrations of adenosine activate the high affinity A1, A2A, and A3 receptors, and high adenosine concentrations stimulate the low affinity A2B receptors. Thus, since the degree of metabolic distress can determine the concentration of extracellular adenosine, the more pronounced the metabolic distress is, the more likely it is that A2B receptors are activated. Further factors that determine the net effect of adenosine on macrophage function are adenosine receptor expression and coupling efficacy to intracellular signaling pathways, factors that are all very dynamically regulated.
4. Effect of adenosine on monocyte/macrophage maturation and proliferation
The differentiation, maturation, and proliferation of macrophages are tightly regulated processes that are important in determining the nature and degree of macrophage responsiveness to activating agents and stimuli. Evidence indicates that adenosine can alter the course of macrophage proliferation and differentiation. The first evidence supporting the concept that endogenous adenosine is capable of preventing human monocyte maturation was provided by demonstrating that adenosine deaminase activity is increased during early monocyte differentiation and that adenosine deaminase inhibition during this period delayed the maturation process (Fischer et al., 1976). High concentrations of exogenous adenosine seem to prevent monocyte development into macrophages and arrest monocyte development at a stage with high accessory function, a phenotype that is similar to dendritic cells (Najar et al., 1990; Fig. 1). Adenosine influences monocyte maturation also by promoting the formation of multinucleated giant cells via A1 receptor stimulation, whereas A2 receptor activation prevents the generation of giant cells (Merrill et al., 1997; Fig. 1). The specific intracellular mechanisms through which adenosine receptor signaling influences the maturation of monocytes are unclear and remain an important area for future study.
Fig. 1.
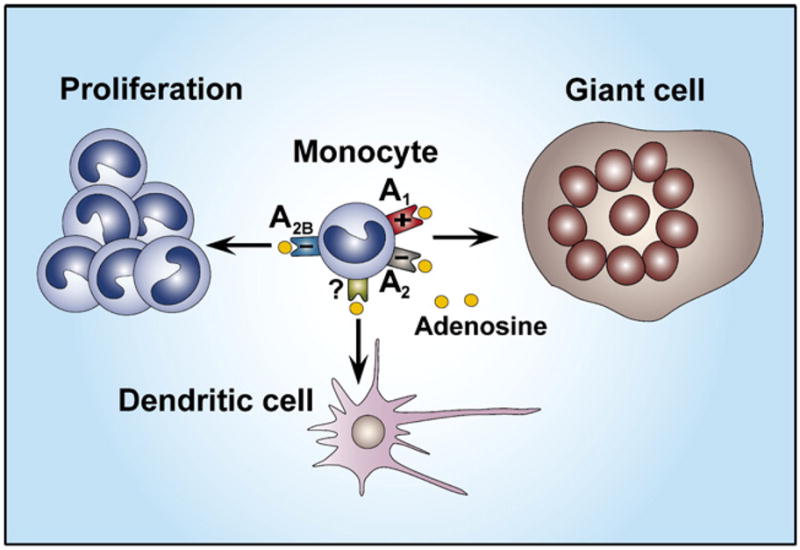
Adenosine suppresses macrophage proliferation through A2B receptors. Adenosine facilitates the formation of multinucleated giant cells via the activation of A1 receptors and inhibits it via A2 (A2A or A2B) receptors. Adenosine promotes monocyte differentiation toward a phenotype resembling dendritic cells.
We know little about how adenosine receptor occupancy impacts macrophage proliferation. Macrophage colony-stimulating factor-induced proliferation of mouse bone marrow macrophages is suppressed by adenosine, which is mediated through A2B receptors (Xaus, Valledor et al.,1999; Fig. 1). The mechanism of action of adenosine involves induction, in a PKA-dependent manner, of the expression of p27kip-1, a cyclin-dependent kinase inhibitor that leads to growth arrest at the G1 phase of the cell cycle.
In human monocytes, the non-selective adenosine receptor agonist 5′-N-ethylcarboxamidoadenosine (NECA) but not 2-p-(2-carboxyethyl)phenethylamino-5′-N-ethyl-carboxamidoadenosine (CGS 21680), a selective agonist of A2A receptors, down-regulated the proliferation of human peripheral blood mononuclear cells obtained from healthy subjects. On the other hand, the selective A1 receptor agonist N6-cyclopentyladenosine (CPA), but not other agonists, inhibited the proliferation of mononuclear cells in asthmatic patients (Landells et al., 2000). Because high, non-selective drug concentrations (10 μM) were used, the authors argued that their results did not support a major role for adenosine receptors in the regulation of mononuclear cell proliferation. Thus, further studies to examine the effect of extracellular adenosine on macrophage proliferation are warranted.
5. A1 and A2 receptors differentially modulate Fcγ receptor-mediated phagocytosis
Phagocytosis is the process by which macrophages ingest, degrade and eventually present peptides derived from particulate antigens. When a small particle is coated (opsonized) with IgG, the Fc regions of the IgG antibody molecules bind to Fc receptors that are expressed on the macrophage plasma membrane and trigger a phagocytic response (Swanson & Hoppe, 2004). The regulation of Fcγ receptor-mediated phagocytosis by adenosine was one of the first effects described for this nucleoside in modulating the activity of monocytes/macrophages (Pike et al., 1978). Adenosine, in the presence, but not in the absence, of the adenosine deaminase inhibitor erytho-9-(2-hydroxy-3-nonyl) adenine (EHNA) enhanced, in a dose-dependent manner, Fcγ-mediated phagocytosis by undifferentiated monocytes of IgG-coated sheep erythrocytes. The fact that the presence of EHNA was necessary for adenosine to affect this monocyte function indicates that monocytes contain high concentrations of adenosine deaminase, which metabolize adenosine leading to a loss of its activity. Contrary to the stimulatory effect on phagocytosis in human monocytes, adenosine in the presence of EHNA diminished Fcγ-mediated phagocytosis of erythrocytes by mouse peritoneal macrophages (Leonard et al., 1978; Sung & Silverstein, 1985) indicating that adenosine can have differential effects on phagocytosis depending on the cellular source. Later studies have helped to explain the opposing effects of adenosine on phagocytosis by undifferentiated monocytes and peritoneal macrophages which are a differentiated macrophage population. Eppell et al. (1989) demonstrated that adenosine in the absence of EHNA is unable to alter phagocytosis by undifferentiated monocytes; however, monocytes that were in culture for 2 days or more responded to adenosine by a diminished phagocytic response. The kinetics of this change was tightly associated with specific adenosine binding to the cells, suggesting that certain adenosine receptor(s) emerge on monocytes during in vitro differentiation into macrophages. The suppressive effect of adenosine on phagocytosis was due to A2 (A2A or A2B) receptor occupancy, because the order of potency of agonists was NECA>adenosine>N6-R-phenylisopropyladenosine (R-PIA, an A1 receptor agonist) when measured following 2 days of culture. Furthermore, the suppressive effect of adenosine on phagocytosis was prevented using a selective protein kinase A (PKA) inhibitor, further implicating A2 receptors, because A2 but not other adenosine receptors are positively coupled to the cAMP-PKA second messenger system. Although these results helped to elucidate why adenosine inhibits phagocytosis in macrophages (Leonard et al., 1978; Sung & Silverstein, 1985), they did not explain why adenosine in the presence of EHNA augments phagocytosis by fresh, undifferentiated monocytes (Pike et al., 1978). A subsequent study employing selective, stable adenosine receptor analogs in freshly isolated monocytes appeared to resolve this issue. Salmon et al. (1993) showed that in undifferentiated monocytes, the A1 receptor agonist CPA was capable of enhancing phagocytosis, indicating a role for A1 receptors in promoting Fcγ-receptor-mediated phagocytosis. These pharmacological results were confirmed by flow cytometry, because the A1 receptor was expressed on the surface of immature monocytes. Thus, when the degradation of adenosine is inhibited either by EHNA or A1 receptors are stimulated by a stable ligand, the stimulatory effect of A1 receptor activation on monocyte phagocytosis is unveiled. Concurrently, NECA moderated phagocytosis even in these fresh monocytes, which observation seems to challenge the earlier conclusion of Eppell et al. (1989) that A2 adenosine receptors are not present on these immature cells. It is possible that by using the stable adenosine receptor agonist NECA, the inhibitory effect of A2 receptors could be unmasked even in these immature monocytes. A2 receptor expression increases with time, because the degree of suppression of phagocytosis in fresh monocytes by NECA is less than that seen in more mature cells. Taken together, monocyte/macrophage phagocytosis is regulated in a contrasting and temporal manner by A1 and A2 receptors, where in fresh monocytes the stimulatory effect of A1 receptors is overcome by an A2 receptor-mediated suppression of phagocytosis in mature macrophages. Because the selectivity of the agents used is these studies that were carried out over 10 years ago is questionable, it is clear that additional studies on the involvement of the various adenosine receptor subtypes in regulating phagocytosis are warranted.
6. Adenosine inhibits monocyte/macrophage oxidative burst
Monocytes and macrophages secrete reactive oxygen species (mostly superoxide and H2O2) during phagocytosis or stimulation with a wide variety of agents, a process, which is crucial for the bactericidal activation of macrophages (Forman & Torres, 2002). Recent evidence documents that adenosine and adenosine analogs are potent inhibitors of the respiratory burst of monocyte/macrophages. Leonard and coworkers (1987) were the first to demonstrate that adenosine-treated human monocytes displayed diminished oxidative burst following stimulation with f-Met-Leu-Phe (fMLP) but not phorbol myristate acetate (PMA). Since adenosine did not decrease the PMA-induced respiratory burst, it was proposed that the mode of action of adenosine was not related to a direct effect on protein kinase C (PKC). Two later studies, one using fMLP (Broussas et al., 1999) and the other LPS (Thiele et al., 2004) to stimulate oxidative burst, confirmed that the effect of adenosine could be attributed primarily to A3 receptor stimulation. This idea was supported by the observations that the A3 receptor agonist N6-(3-iodobenzyl)-adenosine-5′-N-methyluronamide (IB-MECA) was more potent than the A2A agonist CGS 21680 or A1 receptor agonist R-PIA. mRNA for all 4 adenosine receptor subtypes was expressed in freshly isolated monocytes, and while the expression of A1, A2B, and A3 receptors did not vary during a 7-day cultivation period, the mRNA for A2A receptors was up-regulated after day 1 and disappeared by day 7 of culture (Thiele et al., 2004). In agreement with the pharmacological data that IB-MECA was more potent than CGS 21680, an agonist which elicits its cellular effects mainly by activating the cAMP-PKA pathway (Lupica et al., 1990), the cAMP-dependent protein kinase inhibitor KT5720 failed to reverse the inhibitory effect of adenosine on fMLP-induced monocyte respiratory burst (Broussas et al., 1999). The inhibitory effect of adenosine on human monocyte/macrophage respiratory burst was recapitulated using both mouse (Si et al., 1997) and rat (Edwards et al., 1994) peritoneal macrophages; however, there was no detailed analysis of the adenosine receptors involved in either study.
7. Adenosine modulates nitric oxide production by monocyte/macrophages
Nitric oxide (NO) synthases (NOS) catalyze the oxidation of one of the guanidino nitrogens of L-arginine to the reactive nitrogen species NO (Southan & Szabo, 1996). Along with reactive oxygen species, NO is another important factor which contributes to the bactericidal activity of macrophages, especially in rodents. Of the several NOS isoforms that can catalyze NO synthesis, iNOS is the primary one that is responsible for antimicrobial activity by producing high levels of NO. Host expression of iNOS is first and foremost regulated at the transcriptional level and can be stimulated in response to microbial products or by cytokines such as IL-1, tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) (Nathan & Hibbs, 1991). We demonstrated that both the selective A1 receptor agonist 2-chloro-N6-cyclopentyladenosine (CCPA) and A2A agonist CGS 21680 suppressed NO production by LPS-stimulated RAW264.7 macrophages, both with low efficacy (IC50 > 100 μM) (Hasko et al., 1996). Subsequent studies determined that several adenosine receptor agonists (0–100 μM) increased NO production under very similar conditions (Hon et al., 1997; Min et al., 2000), which is difficult to reconcile with our results showing a lack of effect of adenosine receptor agonists at these concentrations. Conversely, treatment of IFN-γ-activated bone marrow macrophages with NECA decreased both iNOS induction and NO production in these cells (Xaus, Mirabet et al., 1999). Experiments employing radioligands and antibodies documented the presence of both A2B and A3 receptors on these bone marrow macrophages; however, a detailed pharmacological analysis was not carried out to clarify the receptor type responsible for the down-regulation of NO production. Although the receptor type that suppressed iNOS expression was not addressed directly in this study, the A2B receptor appears to be the primary receptor that influences the IFN-γ-induced activation of bone marrow-derived macrophages. This contention is based on the observation that NECA, but not selective A1, A2A, or A3 agonists attenuated the IFN-γ-induced up-regulation of major histocompatibility complex II expression on bone marrow-derived macrophages (Xaus, Mirabet et al., 1999). Taken together, further research will be required to examine the impact of adenosine on NO production by macrophages and to determine whether any modulatory effect results in altered bactericidal activity by these cells.
8. Adenosine receptor signaling regulates pattern recognition receptor-mediated cytokine production
Pattern recognition receptors (PRR) are a class of proteins, which are employed by the cells of the immune system to identify molecules common to microbial pathogens. They are key elements in innate immunity as well as influence the development of adaptive immunity. They include the toll-like receptors (TLR), members of the nucleotide-binding oligomerization domain (NOD; NOD1 and NOD2) proteins, scavenger receptors, and mannose-binding lectins. Among the molecules recognized by these PRR are LPS of Gram-negative bacteria, peptidoglycans and lipotechoic acids from Gram-positive bacteria, mannose residues, bacterial DNA, N-formylmethionine, viral double-stranded RNA and fungal glucans. The specific molecular sequences identified by a given PRR are its pathogen-associated molecular patterns (PAMP). Activation of PRR on monocytes and macrophages triggers a series of biological responses including cytokine secretion (Gordon, 2002; Janeway & Medzhitov, 2002).
There is a large body of evidence documenting that adenosine receptor stimulation can alter intracellular signaling pathways activated by PRR. The best-studied aspect of the interaction between the 2 pathways is the effect of adenosine receptor stimulation on cytokine production by monocytes/macrophages.
8.1. Adenosine suppresses TNF-α production by monocytes and macrophages
TNF-α is a well-known pro-inflammatory cytokine with a wide range of biological functions, which is secreted primarily by monocytes and macrophages (Hehlgans & Pfeffer, 2005). TNF-α plays an important role in the pathophysiology of chronic disease states such as rheumatoid arthritis (RA), Crohn’s disease, graft-versus-host disease (GVHD), and the cachexia accompanying cancer and acquired immunodeficiency syndrome (AIDS). Because TNF-α was one of the first cytokines to be discovered, there is a plethora of information available on the regulation of TNF-α production by adenosine receptors.
8.1.1. Human monocytes/macrophages
In initial studies (Le Vraux et al., 1993) adenosine receptor agonists were shown to suppress TNF-α production by LPS (TLR4 ligand)-challenged human monocytes with the following rank order of potency: NECA>R-PIA=CGS 21680>2-CADO (2-chloroadenosine)=CHA (N6-cyclohexyladenosine). Because NECA was the most potent agonist, a role for the A2B receptor can be considered, because if NECA is the most potent agonist in a system, it indicates a predominant role for A2B receptors (Feoktistov & Biaggioni, 1997). In another early study, NECA was more potent than CPA supporting the conclusion that A2 receptors were responsible for the suppressive effect of adenosine on TNF-α secretion (Bouma et al., 1994). However, selective A2A agonists were not tested in these studies, precluding a clear distinction between A2A and A2B receptors. In another study utilizing human monocytes, CGS 21680 was more potent than CPA and the selective A2 receptor antagonist 3,7-dimethyl-1-propargylxanthine (DMPX), but not A3 antagonist 1,3-dipropyl-8-phenylxanthine amine congener (XAC) reversed the effect of CGS 21680 (Prabhakar et al., 1995). These results might implicate A2A receptors, however, the role of A2B receptors can not be excluded because NECA and A2B antagonists were not tested. In a recent study utilizing the selective A2B receptor antagonist N-(4-cyano-phenyl)-2-[4-(2,6-dioxo-1,3-dipropyl-2,3,4,5,6,7-hexahydro-1H-purin-8-yl)-phenoxy]-acetamide (MRS 1754, 100 nM), Zhang et al. (2005) were unable to confirm the role of A2B receptors in moderating TNF-α production.
In subsequent studies following the discovery of A3 receptors, Sajjadi et al. (1996) employing PMA-differentiated U937 (human monocyte) cells found that the order of agonist potency in inhibiting LPS-induced TNF-α production was IB-MECA >2-CADO = I-ABA (N6-(4-amino-3-iodobenzyl) adenosine)> N6-benzyl NECA>NECA>CGS 21680>CHA, which is indicative of a principal role of A3 receptors. In addition, the selective A1/A3 receptor antagonist XAC, but not A1 antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX) or A2 antagonist DMPX prevented the suppressive effect of the selective A3 agonist I-ABA. Northern blot analysis revealed the presence of A3 receptors in these cells, although A1 and A2 receptors were also detected. The inhibitory effect of I-ABA on TNF-α secretion was correlated with a decrease in TNF-α mRNA accumulation, indicating a pre-translational effect. A comprehensive analysis of multiple intracellular signaling mechanisms found that the decrease in TNF-α production after I-ABA exposure was associated with changes in activation of the activator protein-1 (AP-1) transcription factor system, whereas the activity of I-ABA was independent of mitogen-activated protein (MAP) kinases and nuclear factor-κB (NF-κB), as well as PKA, PKC, and PLC. The inability of adenosine, at least at physiological concentrations, to attenuate LPS-induced NF-κB activation was confirmed by other studies (Majumdar & Aggarwal, 2003; Nemeth et al., 2003; Pinhal-Enfield et al., 2003).
Although, as illustrated in the aforementioned studies, A1 receptors do not have a role in diminishing TNF-α production in LPS-stimulated human monocytes, A1 receptors can decrease TNF-α release following different monocyte-activating stimuli. Upon stimulation with a combination of PMA and phytohemagglutinin (PHA), peripheral blood mononuclear cell TNF-α release was attenuated by A1 receptor activation (Mayne et al., 1999). Evidence to support this conclusion was based on the observation that the selective A1 receptor antagonist DMPX counteracted the inhibitory effect of the A1 agonist R-PIA on TNF-α secretion. Importantly, this response was confined to healthy patients but not individuals afflicted with multiple sclerosis, pointing to a possible role of dysregulated A1-receptor-mediated cytokine modulation as an etiological factor in multiple sclerosis. In addition to A1 receptors, A2A receptors might also decrease TNF-α release induced by PMA/PHA, because the inhibitory effect of CGS 21680 was abolished by a selective A2A antagonist in U937 cells (Fotheringham et al., 2004). The mechanism of action of CGS 21680 was post-transcriptional because it decreased the stability of TNF-α mRNA, an effect that was closely associated with a reduction in p38 MAPK activation, an important regulator of TNF-α mRNA stability (Kotlyarov et al., 1999).
8.1.2. Mouse monocytes/macrophages
Adenosine is a strong inhibitor of TNF-α production by monocytes and macrophages. Early studies with the mouse macrophage cell line RAW264.7 demonstrated that CCPA and CGS 21680 were equipotent in suppressing LPS-induced TNF-α secretion, leaving open the question of which adenosine receptors are dominant in mouse cells (Hasko et al., 1996). Recent studies using RAW264.7 cells showed that CGS 21680 was ineffective at reducing TNF-α production, but IB-MECA did suppress TNF-α levels (Martin et al., 2006), lending support to the notion that A3 receptors can regulate TNF-α production. Studies with the murine macrophage cell line J774.1 reinforced the idea that A3 receptors may be involved in the reduction of TNF-α production as the A3 receptor agonist N 6-(2-(4-aminophenyl)ethyl)-adenosine (APNEA) was more potent than various A1 and A2 agonists, and a selective A3 antagonist reversed the effect of APNEA (McWhinney et al., 1996). In addition, A3 receptor mRNAwas 10-fold more abundant than A1 or A2A receptor mRNA. The reason for the discrepancy between the findings of these studies regarding the role of A2A receptors is not clear but the differences may be a reflection of the different LPS concentrations and incubation times used.
Recent studies using a combination of KO and pharmacological approaches have helped to resolve these controversies. Our studies utilizing knockout mice for the A2A receptors showed that A2A receptors clearly contribute to the adenosine suppression of TNF-α production by LPS-stimulated murine macrophages (peritoneal), because the suppressive effect of adenosine on TNF-α production was impaired in macrophages isolated from A2A receptor KO animals when compared to WT controls (Hasko, Kuhel, Chen et al., 2000). In addition, CGS 21680 potently decreased TNF-α production by A2A WT macrophages but it failed to influence TNF-α release by KO cells (Hasko, Kuhel, Chen et al., 2000). On the other hand, A3 receptors are not involved in the suppressive effect of adenosine, because adenosine inhibited TNF-α production with the same potency by macrophages isolated from A3 receptor KO mice as by cells from WT mice (Kreckler et al., 2006). Moreover, the A2A receptor-independent portion of the adenosine effect that we observed (Hasko, Kuhel, Chen et al., 2000) appears to be secondary to A2B receptors, because MRS 1754 was able to completely antagonize this adenosine-induced A2A-independent effect (Kreckler et al., 2006).
In summary, TNF-α production by monocytes/macrophages can be subject to inhibition by A1, A2A, and A3 receptors and the receptor subtype involved depends on many factors, which include the source of cell, the species, and the inflammatory stimulus used. Remarkably, the inhibitory effect of adenosine on TNF-α production by macrophages is not limited to TLR4-mediated (LPS) induction of this cytokine because adenosine down-regulates TNF-α production when induced by agonists of TLR2, TLR3, TLR4, TLR7 and TLR9 (Pinhal-Enfield et al., 2003). Although it is probable that adenosine receptor occupancy inhibits a common major intracellular pathway that leads to TNF-α production in response to TLR activation, the nature of this intracellular target is unclear at this point. Nevertheless, the fact that the various adenosine receptors all inhibit TNF-α production suggests that endogenous adenosine may represent a crucial negative feedback signal on inflammatory processes.
8.2. Adenosine inhibits IL-12 production by monocytes and macrophages
The IL-12 family of heterodimeric cytokines includes IL-12, and the recently discovered IL-23, and IL-27. IL-12 itself was identified in 1989, as a soluble factor that could stimulate natural killer (NK) cells to produce IFN-γ (Kobayashi et al., 1989). This discovery paved the way for studies in which it was determined that macrophages and dendritic cells produced this cytokine in response to certain bacterial and parasitic infections and that this, in turn, led to the polarization of naive T cells to produce a T helper 1-cell response (Hunter, 2005). IL-12 is a covalently linked heterodimer composed of a light chain (IL-12p35) and a heavy chain (IL-12p40). The IL-12p40 component of IL-12 can also dimerize with IL-23p19 to form IL-23. The last member of this family of cytokines is IL-27, which is composed of Epstein-Barr-virus-induced molecule 3 (EBI3) and IL-27p28. IL-12 and IL-23 are important factors that promote cell-mediated immunity and inflammation, whereas IL-27 appears to have a role in limiting the intensity and duration of adaptive immune responses.
Studies so far have assessed the effect of adenosine receptor ligation on IL-12 and IL-12p40 production. Using A2A receptor knockout mice, our group (Hasko, Kuhel, Chen et al., 2000) documented that adenosine down-regulates IL-12 p40 production by LPS-stimulated mouse peritoneal macrophages and that this effect is dependent, in part, on A2A receptors. Further evidence implicating A2A receptors came from studies with human monocytes, in which CGS 21680 potently blunted LPS-induced IL-12 (both IL-12p40 and IL-12) production, which effect was reversed by A2A antagonists. The effect of A2A stimulation is suppressing IL-12 production was linked to increased intracellular cAMP concentrations (Link et al., 2000; Khoa et al., 2001). A2A receptor stimulation lead to decreased levels of IL-12 p40 mRNA, implying a pre-translational mechanism of action (Link et al., 2000). Khoa et al. (2001) reported recently that the degree to which IL-12 production is down-regulated following A2A receptor stimulation is strongly influenced by the inflammatory environment that the cells encounter. The pro-inflammatory cytokines TNF-α and IL-1 augmented responsiveness to A2A receptor stimulation resulting in a more impaired IL-12 production after administering CGS 21680. On the other hand, the presence of IFN-γ attenuated the inhibitory effect of A2A receptor stimulation on IL-12 secretion. Corresponding with these observed functional changes in response to treatment with pro-inflammatory cytokines or IFN-γ was the expression of A2A receptor mRNA and protein, which was found to be increased or decreased, respectively.
A3 receptor stimulation can also negatively regulate IL-12 production, because the selective A3 receptor agonist IB-MECA moderates IL-12 production both in LPS-treated mice (Hasko et al., 1998) and by human monocytes (la Sala et al., 2005). IB-MECA activated the phosophatidyl inositol-3-kinase (PI3K) pathway, and the activity of both PI3K and Akt was required for its suppressive effect (la Sala et al., 2005).
Taken together, since IL-12 has an eminent role in mediating a strong T helper 1-type inflammatory response, the suppression of IL-12 production by adenosine is probably one of the pivotal mechanisms by which adenosine receptor activation exerts its strong anti-inflammatory effects that are observed in animal models of autoimmunity (Hasko & Cronstein, 2004; Sitkovsky et al., 2004). Further studies are warranted to uncover how adenosine regulates the production of IL-23 and IL-27.
8.3. Adenosine facilitates IL-10 release by monocytes and macrophages
IL-10 is the founding member of a growing family of structurally related cytokines, which includes IL-19, IL-20, IL-22, IL-24, and IL-26 (Kotenko, 2002). IL-10 is a relatively unique cytokine in that it has been documented to have potent immune inhibitory activity. IL-10 was initially described as a T helper 2 product that reduced the production of cytokines by T helper 1 T cell clones (Mosmann et al., 1990). Subsequently, it has become clear that IL-10 is also produced by cells of the monocyte/macrophage lineage (de Waal Malefyt et al., 1991; Barsig et al., 1995; Moore et al., 2001). IL-10 was also described as cytokine synthesis inhibitory factor, because IL-10 can inhibit the secretion of a variety of proinflammatory cytokines, including TNF-α and IL-12. IL-10 synthesis comes after the induction of proinflammatory cytokines allowing termination of proinflammatory responses and the resolution of inflammation (Moore et al., 2001).
One of the most consistent actions of adenosine across various experimental systems is its ability to increase IL-10 production by monocytes/macrophages (Hasko et al., 1996; Le Moine et al., 1996; Hasko, Kuhel, Chen et al., 2000; Link et al., 2000; Khoa et al., 2001; Nemeth et al., 2005) (Fig. 2). In human monocytes activated with TNF-α, H2O2, or LPS, adenosine up-regulated IL-10 production, an effect that was not mimicked by administering NECA, 2-CADO, or R-PIA (Le Moine et al., 1996). In addition, the non-selective adenosine receptor antagonist theophylline was unable to reverse the stimulatory effect of adenosine, which led the authors to conclude that the stimulatory effect of adenosine on IL-10 secretion was not receptor-mediated. In spite of these observations the involvement of cell surface adenosine receptors mediating the stimulatory effect of adenosine on IL-10 production can not be precluded for several reasons. Firstly, selective A2A or A3 agonists and selective adenosine receptor antagonists were not examined in this study. Secondly, theophylline, used at high concentration (100 μM), is also a potent inhibitor of phosphodiesterases (Barry, 1988), and phosphodiesterase inhibition has been shown to increase IL-10 production (Mascali et al., 1996), thereby potentially masking its adenosine receptor antagonistic property. Thirdly, the adenosine uptake blocker dipyridamole was also incapable of preventing the stimulatory effect of adenosine on IL-10 production, secluding an intracellular, receptor-independent effect of adenosine. A subsequent study using selective adenosine receptor agonists documented that adenosine receptor agonists potentiated IL-10 production by LPS-activated monocytes (Link et al., 2000). In addition, adenosine receptor agonists markedly up-regulated LPS-induced IL-10 production in human whole blood, and the order of potency of agonists indicated a predominant role of A2A receptors (Link et al., 2000). The A2A antagonist (8-(3-chlorostyryl)caffeine) CSC, but not antagonists of the other adenosine receptors blocked the stimulatory effect of the agonist CGS 21680. Adenosine receptor activation up-regulated also Staphylococcus aureus-induced IL-10 production. A2A receptor stimulation enhanced IL-10 production also in THP-1 monocytes (Khoa et al., 2001). IL-1 and TNF-α pretreatment potentiated the stimulatory effect of adenosine and CGS 21680 on LPS-induced IL-10 production, whereas IFN-γ treatment almost completely abolished this effect.
Fig. 2.
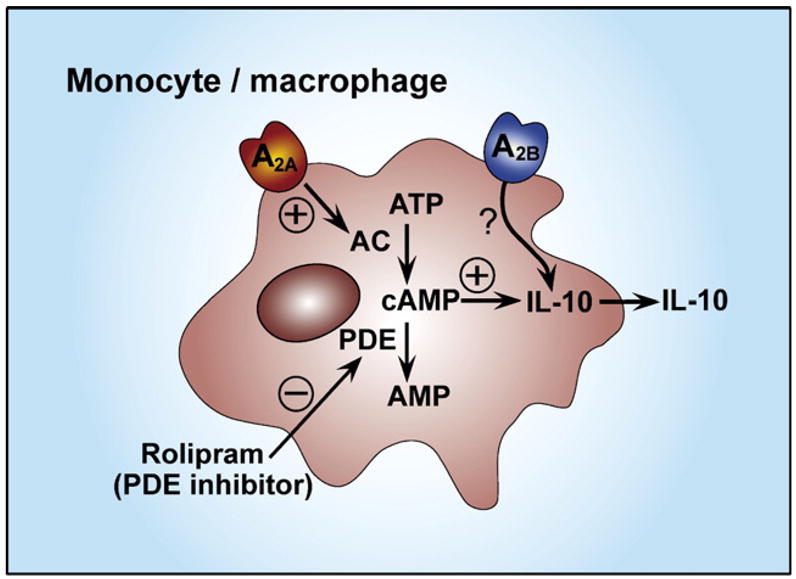
A2A receptor activation augments IL-10 production by monocytes and macrophages through a cyclic adenosine monophosphate (cAMP)-mediated pathway. A2A receptor activation stimulates adenylate cyclase (AC) leading to elevated intracellular cAMP levels. Phosphodiesterase (PDE) inhibition with rolipram can mimic the effect of A2A receptor stimulation in augmenting IL-10 production. A2B receptor activation also facilitates IL-10 production.
In a study employing murine peritoneal macrophages, we found that adenosine augmented IL-10 production (Hasko, Kuhel, Chen et al., 2000) to a similar extent (approximately by 100%) as in human monocytes. The receptor subtype(s) responsible for this stimulatory effect of adenosine on IL-10 production by peritoneal macrophages have not been defined. On the other hand, we recently found that the stimulatory effect of adenosine on IL-10 production in RAW264.7 mouse macrophages was mediated through the A2B receptor, because the order of potency of selective agonists was NECA>IB-MECA>CCPA=CGS 21680 (Nemeth et al., 2005). Also, the selective A2B antagonist, alloxazine, prevented the effect of adenosine. The possible role of A2B receptor was further highlighted by the fact that RAW264.7 cells expressed the A2B receptor, which was further increased following LPS treatment.
In conclusion, the facilitation of IL-10 production may contribute to the anti-inflammatory and immunosuppressive effects of A2A and A2B receptor stimulation.
9. Adenosine decreases tissue factor expression
Tissue factor (TF) is a transmembrane glycoprotein that initiates the coagulation cascade and may also participate in angiogenesis; its regulation is responsive to inflammation and mechanical forces. Baseline (resting) monocyte TF activity is extremely low, but a several-fold increase occurs upon incubation for some hours (4–6 h) with several stimulators, such as LPS or certain pro-inflammatory cytokines (Osterud, 1998; Morrissey, 2004).
The platelet inhibitory drug dilazep exerts its anti-thrombotic effects by augmenting the adenosine level in the extracellular fluid through inhibition of adenosine transporters (Parks et al., 1985). In addition to its direct effects on platelets that are mediated via binding to A2A receptors (Ledent et al., 1997), dilazep was reported to also block TF expression on monocytes and thereby inhibit blood coagulation (Deguchi et al., 1997; Zhou et al., 2004) implicating adenosine as a modulator of TF expression. Adenosine inhibits TF expression on LPS-stimulated human monocytes through the activation of A3 receptors, because IB-MECA is the most potent agonist in reducing TF expression and the mixed A1/A3 antagonist XAC, but not selective A1, A2A, or A2B antagonists prevented the effect of adenosine (Broussas et al., 2002).
10. Adenosine regulates vascular endothelial growth factor production
Vascular endothelial growth factor (VEGF), also known as vascular permeability factor, is a potent inducer of angiogenesis and vascular permeability (Semenza, 2003). VEGF is required for endothelial cell differentiation during vasculogenesis, and for the sprouting of new capillaries from pre-existing vessels. Among all the pro-angiogenic factors, VEGF is considered the most essential for the differentiation of the vascular system. VEGF is thus an important mediator of both inflammation and repair, and is critical for the resolution of injury through the process of wound healing. Macrophages are primary producers of VEGF in wound healing, as well as in chronic inflammation (Semenza, 2003). Because VEGF is a crucial factor for tumor angiogenesis and tumor growth, it is an important target for clinical therapy in cancer (Lewis et al., 1995).
There is accumulating evidence that adenosine can facilitate angiogenesis, in part, by promoting VEGF production (Adair, 2005). Stimulation of A2 receptors in U-937 human macrophages elicits VEGF mRNA accumulation (Hashimoto et al., 1994). Moreover, hypoxia, which is a major trigger of VEGF expression in most cell types (Semenza, 2003), augmented VEGF mRNA expression in U-937 cells, a process that could be prevented by adenosine deaminase and DMPX, indicating that hypoxia triggers the release of adenosine and the adenosine released so increases VEGF mRNA abundance. By contrast, although both hypoxia and A2A stimulation acted as potent inducers of VEGF production by murine peritoneal macrophages, the effect of hypoxia in inducing VEGF secretion did not require adenosine and adenosine receptors (Leibovich et al., 2002). A2A receptor stimulation and LPS through TLR4 activation synergistically facilitated the production of VEGF (Leibovich et al., 2002). In a recent study our group documented that this synergistic interaction in inducing VEGF production was not confined to TLR4, and we observed a similar synergistic interaction between A2A receptors and TLR2, TLR7, and TLR9 (Pinhal-Enfield et al., 2003). The mechanistic basis for this synergistic activity between A2A receptors and TLRs remains to be explored.
11. Therapeutic implications
Macrophages/monocytes have emerged as a cell type that has a key role in shaping inflammation, immunity, wound healing, and cancer. Adenosine receptor activation can influence macrophage function depending on the receptor subtypes expressed and the environment in which macrophages are exposed to extracellular adenosine. The fact that the distinct adenosine receptors can selectively regulate discrete macrophage functions makes adenosine receptors a promising target for pharmacological interventions in a wide range of disease states that involve macrophage activation. For example, the therapy of autoimmune processes or ischemia–reperfusion injury, diseases that are associated with exuberant inflammation may capitalize on the use of adenosine receptor ligands that can suppress the pro-inflammatory activity of macrophages. Protective effects of adenosine receptor stimulation (mainly A2A and A3) have been observed in models of ischemia–reperfusion (Day et al., 2003, 2005; Yang et al., 2005), as well as autoimmune diseases, such as RA (Szabo et al., 1998), multiple sclerosis (Tsutsui et al., 2004), colitis (Odashima et al., 2005), and hepatitis (Ohta & Sitkovsky, 2001). A further possibility for the therapeutic use of the adenosine receptor system is to exploit the ability of A2A receptor agonists to suppress inflammation and promote wound healing in disease processes such as diabetic ulcers (Montesinos et al., 1997). On the other hand, adenosine receptor ligands that have the ability to facilitate the inflammatory/immune responses of macrophages represent potential therapies for infectious diseases. A good example for this notion is our recent observation that A2A receptor blockade can promote antibacterial immunity and bacterial clearance in septic animals (Nemeth et al., 2006). Finally, there is convincing evidence that selective adenosine receptor antagonists (A2B and A3) are potential therapeutics for asthma and chronic lung disease (Fozard & Hannon, 1999; Fozard & McCarthy, 2002; Blackburn, 2003; Fozard, 2003; Mohsenin & Blackburn, 2006; Spicuzza et al., 2006). Hopefully, the success of adenosinergic ligands as therapeutic interventions in experimental disease models will be replicated in human ailments in the not so distant future.
Acknowledgments
This work was supported by the National Institutes of Health Grant R01 GM66189 (G. Hasko), National Research and Development Program, Hungary (1A-036-2004, G. Hasko) and in part by the Intramural Research Program of NIH-NIAAA (P. Pacher).
Abbreviations
- 2Cl-IB-MECA
2-cloro-N6-(3-iodobenzyl)-adenosine-5′-N-methyluronamide
- ATP
adenosine 5′-triphosphate
- CCPA
2-chloro-N6-cyclopentyladenosine
- CGS 21680
2-p-(2-carboxyethyl)phenethylamino-5′-N-ethyl-carboxamidoadenosine
- CPA
N6-cyclopentyladenosine
- CSC
8-(3-chlorostyryl)caffeine
- DPCPX
8-cyclopentyl-1,3-dipropylxanthine
- IB-MECA
N6-(3-iodobenzyl)-adenosine-5′-N-methyluronamide
- IFN
interferon
- IL
interleukin
- LPS
bacterial lipopolysaccharide
- MRS 1220
(9-chloro-2-(2-furanyl)-5-[(phenylacetyl)amino][1,2,4]-triazolo[1,5-c]quinazoline
- NO
nitric oxide
- PACPX
1,3-dipropyl-8-(2-amino-4-chlorophenyl)-xanthine
- TF
tissue factor
- VEGF
vascular endothelial growth factor
- ZM241385
4-(2-[7-amino-2-(2-furyl)-{1,2,4}-triazolo{2,3-a}{1,3,5} triazin-5-ylamino]ethyl)phenol
References
- Adair TH. Growth regulation of the vascular system: an emerging role for adenosine. Am J Physiol Regul Integr Comp Physiol. 2005;289:R283–R296. doi: 10.1152/ajpregu.00840.2004. [DOI] [PubMed] [Google Scholar]
- Barry SR. Dual effects of theophylline on spontaneous transmitter release from frog motor nerve terminals. J Neurosci. 1988;8:4427–4433. doi: 10.1523/JNEUROSCI.08-12-04427.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsig J, Kusters S, Vogt K, Volk HD, Tiegs G, Wendel A. Lipopolysaccharide-induced interleukin-10 in mice: role of endogenous tumor necrosis factor-alpha. Eur J Immunol. 1995;25:2888–2893. doi: 10.1002/eji.1830251027. [DOI] [PubMed] [Google Scholar]
- Blackburn MR. Too much of a good thing: adenosine overload in adenosine-deaminase-deficient mice. Trends Pharmacol Sci. 2003;24:66–70. doi: 10.1016/S0165-6147(02)00045-7. [DOI] [PubMed] [Google Scholar]
- Bouma MG, Stad RK, van den Wildenberg FA, Buurman WA. Differential regulatory effects of adenosine on cytokine release by activated human monocytes. J Immunol. 1994;153:4159–4168. [PubMed] [Google Scholar]
- Broussas M, Cornillet-Lefebvre P, Potron G, Nguyen P. Inhibition of fMLP-triggered respiratory burst of human monocytes by adenosine: involvement of A3 adenosine receptor. J Leukoc Biol. 1999;66:495–501. [PubMed] [Google Scholar]
- Broussas M, Cornillet-Lefebvre P, Potron G, Nguyen P. Adenosine inhibits tissue factor expression by LPS-stimulated human monocytes: involvement of the A3 adenosine receptor. Thromb Haemost. 2002;88:123–130. [PubMed] [Google Scholar]
- Cristalli G, Costanzi S, Lambertucci C, Lupidi G, Vittori S, Volpini R, et al. Adenosine Deaminase: Functional Implications and Different Classes of Inhibitors. 2001 doi: 10.1002/1098-1128(200103)21:2<105::aid-med1002>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Cronstein BN, Kramer SB, Weissmann G, Hirschhorn R. Adenosine: a physiological modulator of superoxide anion generation by human neutrophils. J Exp Med. 1983;158:1160–1177. doi: 10.1084/jem.158.4.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day YJ, Huang L, McDuffie MJ, Rosin DL, Ye H, Chen JF, et al. Renal protection from ischemia mediated by A2A adenosine receptors on bone marrow-derived cells. J Clin Invest. 2003;112:883–891. doi: 10.1172/JCI15483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day YJ, Li Y, Rieger JM, Ramos SI, Okusa MD, Linden J. A2A adenosine receptors on bone marrow-derived cells protect liver from ischemia–reperfusion injury. J Immunol. 2005;174:5040–5046. doi: 10.4049/jimmunol.174.8.5040. [DOI] [PubMed] [Google Scholar]
- de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi H, Takeya H, Wada H, Gabazza EC, Hayashi N, Urano H, et al. Dilazep, an antiplatelet agent, inhibits tissue factor expression in endothelial cells and monocytes. Blood. 1997;90:2345–2356. [PubMed] [Google Scholar]
- Deussen A. Metabolic flux rates of adenosine in the heart. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:351–363. doi: 10.1007/s002100000318. [DOI] [PubMed] [Google Scholar]
- Drury AN, Szent-Gyorgyi A. The physiological activity of adenine compounds with special reference to their action upon the mammalian heart. J Physiol. 1929;68:213–237. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffield JS. The inflammatory macrophage: a story of Jekyll and Hyde. Clin Sci (Lond) 2003;104:27–38. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- Edwards CK, III, Watts LM, Parmely MJ, Linnik MD, Long RE, Borcherding DR. Effect of the carbocyclic nucleoside analogue MDL 201,112 on inhibition of interferon-gamma-induced priming of Lewis (LEW/N) rat macrophages for enhanced respiratory burst and MHC class II Ia+ antigen expression. J Leukoc Biol. 1994;56:133–144. doi: 10.1002/jlb.56.2.133. [DOI] [PubMed] [Google Scholar]
- Eltzschig HK, Thompson LF, Karhausen J, Cotta RJ, Ibla JC, Robson SC, et al. Endogenous adenosine produced during hypoxia attenuates neutrophil accumulation: coordination by extracellular nucleotide metabolism. Blood. 2004;104:3986–3992. doi: 10.1182/blood-2004-06-2066. [DOI] [PubMed] [Google Scholar]
- Eppell BA, Newell AM, Brown EJ. Adenosine receptors are expressed during differentiation of monocytes to macrophages in vitro. Implications for regulation of phagocytosis. J Immunol. 1989;143:4141–4145. [PubMed] [Google Scholar]
- Feoktistov I, Biaggioni I. Adenosine A2B receptors. Pharmacol Rev. 1976;49:381–402. [PubMed] [Google Scholar]
- Fischer D, Van der Weyden MB, Snyderman R, Kelley WN. A role for adenosine deaminase in human monocyte maturation. J Clin Invest. 1976;58:399–407. doi: 10.1172/JCI108484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman HJ, Torres M. Reactive oxygen species and cell signaling: respiratory burst in macrophage signaling. Am J Respir Crit Care Med. 2002;166:S4–S8. doi: 10.1164/rccm.2206007. [DOI] [PubMed] [Google Scholar]
- Fotheringham JA, Mayne MB, Grant JA, Geiger JD. Activation of adenosine receptors inhibits tumor necrosis factor-alpha release by decreasing TNF-alpha mRNA stability and p38 activity. Eur J Pharmacol. 2004;497:87–95. doi: 10.1016/j.ejphar.2004.06.029. [DOI] [PubMed] [Google Scholar]
- Fozard JR. The case for a role for adenosine in asthma: almost convincing? Curr Opin Pharmacol. 2003;3:264–269. doi: 10.1016/s1471-4892(03)00039-0. [DOI] [PubMed] [Google Scholar]
- Fozard JR, Hannon JP. Adenosine receptor ligands: potential as therapeutic agents in asthma and COPD. Pulm Pharmacol Ther. 1999;12:111–114. doi: 10.1006/pupt.1999.0191. [DOI] [PubMed] [Google Scholar]
- Fozard JR, McCarthy C. Adenosine receptor ligands as potential therapeutics in asthma. Curr Opin Investig Drugs. 2002;3:69–77. [PubMed] [Google Scholar]
- Fredholm BB, Ijzerman AP, Jacobson KA, Klotz KN, Linden J. International union of pharmacology: XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, Chen JF, Masino SA, Vaugeois JM. Actions of adenosine at its receptors in the CNS: insights from knockouts and drugs. Annu Rev Pharmacol Toxicol. 2005;45:385–412. doi: 10.1146/annurev.pharmtox.45.120403.095731. [DOI] [PubMed] [Google Scholar]
- Gilroy DW, Lawrence T, Perretti M, Rossi AG. Inflammatory resolution: new opportunities for drug discovery. Nat Rev Drug Discov. 2004;3:401–416. doi: 10.1038/nrd1383. [DOI] [PubMed] [Google Scholar]
- Gordon S. Pattern recognition receptors: doubling up for the innate immune response. Cell. 2002;111:927–930. doi: 10.1016/s0092-8674(02)01201-1. [DOI] [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Gunther GR, Herring MB. Inhibition of neutrophil superoxide production by adenosine released from vascular endothelial cells. Ann Vasc Surg. 1991;5:325–330. doi: 10.1007/BF02015292. [DOI] [PubMed] [Google Scholar]
- Hashimoto E, Kage K, Ogita T, Nakaoka T, Matsuoka R, Kira Y. Adenosine as an endogenous mediator of hypoxia for induction of vascular endothelial growth factor mRNA in U-937 cells. Biochem Biophys Res Commun. 1994;204:318–324. doi: 10.1006/bbrc.1994.2462. [DOI] [PubMed] [Google Scholar]
- Hasko G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends Immunol. 2004;25:33–39. doi: 10.1016/j.it.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Hasko G, Szabo C. Regulation of cytokine and chemokine production by transmitters and co-transmitters of the autonomic nervous system. Biochem Pharmacol. 1998;56:1079–1087. doi: 10.1016/s0006-2952(98)00153-1. [DOI] [PubMed] [Google Scholar]
- Hasko G, Szabo C, Nemeth ZH, Kvetan V, Pastores SM, Vizi ES. Adenosine receptor agonists differentially regulate IL-10, TNF-alpha, and nitric oxide production in RAW 264.7 macrophages and in endotoxemic mice. J Immunol. 1996;157:4634–4640. [PubMed] [Google Scholar]
- Hasko G, Nemeth ZH, Vizi ES, Salzman AL, Szabo C. An agonist of adenosine A3 receptors decreases interleukin-12 and interferon-gamma production and prevents lethality in endotoxemic mice. Eur J Pharmacol. 1998;358:261–268. doi: 10.1016/s0014-2999(98)00619-0. [DOI] [PubMed] [Google Scholar]
- Hasko G, Kuhel DG, Chen JF, Schwarzschild MA, Deitch EA, Mabley JG, et al. Adenosine inhibits IL-12 and TNF-α production via adenosine A2a receptor-dependent and independent mechanisms. FA-SEB J. 2000;14:2065–2074. doi: 10.1096/fj.99-0508com. [DOI] [PubMed] [Google Scholar]
- Hasko G, Kuhel DG, Nemeth ZH, Mabley JG, Stachlewitz RF, Virag L, et al. Inosine inhibits inflammatory cytokine production by a posttranscriptional mechanism and protects against endotoxin-induced shock. J Immunol. 2000;164:1013–1019. doi: 10.4049/jimmunol.164.2.1013. [DOI] [PubMed] [Google Scholar]
- Hasko G, Kuhel DG, Salzman AL, Szabo C. ATP suppression of interleukin-12 and tumour necrosis factor-alpha release from macrophages. Br J Pharmacol. 2000;129:909–914. doi: 10.1038/sj.bjp.0703134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasko G, Deitch EA, Szabo C, Nemeth ZH, Vizi ES. Adenosine: a potential mediator of immunosuppression in multiple organ failure. Curr Opin Pharmacol. 2002;2:440–444. doi: 10.1016/s1471-4892(02)00172-8. [DOI] [PubMed] [Google Scholar]
- Hasko G, Sitkovsky MV, Szabo C. Immunomodulatory and neuroprotective effects of inosine. Trends Pharmacol Sci. 2004;25:152–157. doi: 10.1016/j.tips.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Hehlgans T, Pfeffer K. The intriguing biology of the tumour necrosis factor/tumour necrosis factor receptor superfamily: players, rules and the games. Immunology. 2005;115:1–20. doi: 10.1111/j.1365-2567.2005.02143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hon WM, Moochhala S, Khoo HE. Adenosine and its receptor agonists potentiate nitric oxide synthase expression induced by lipopolysaccharide in RAW 264.7 murine macrophages. Life Sci. 1997;60:1327–1335. doi: 10.1016/s0024-3205(97)00078-7. [DOI] [PubMed] [Google Scholar]
- Hunter CA. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat Rev Immunol. 2005;5:521–531. doi: 10.1038/nri1648. [DOI] [PubMed] [Google Scholar]
- Hyde RJ, Cass CE, Young JD, Baldwin SA. The ENT family of eukaryote nucleoside and nucleobase transporters: recent advances in the investigation of structure/function relationships and the identification of novel isoforms. Mol Membr Biol. 2001;18:53–63. [PubMed] [Google Scholar]
- Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- Jennings RB, Steenbergen C., Jr Nucleotide metabolism and cellular damage in myocardial ischemia. Annu Rev Physiol. 1985;47:727–749. doi: 10.1146/annurev.ph.47.030185.003455. [DOI] [PubMed] [Google Scholar]
- Kaczmarek E, Koziak K, Sevigny J, Siegel JB, Anrather J, Beaudoin AR, et al. Identification and characterization of CD39/vascular ATP diphosphohydrolase. J Biol Chem. 1996;271:33116–33122. doi: 10.1074/jbc.271.51.33116. [DOI] [PubMed] [Google Scholar]
- Khoa ND, Montesinos MC, Reiss AB, Delano D, Awadallah N, Cronstein BN. Inflammatory cytokines regulate function and expression of adenosine A(2A) receptors in human monocytic THP-1 cells. J Immunol. 2001;167:4026–4032. doi: 10.4049/jimmunol.167.7.4026. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Fitz L, Ryan M, Hewick RM, Clark SC, Chan S, et al. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989;170:827–845. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotenko SV. The family of IL-10-related cytokines and their receptors: related, but to what extent? Cytokine Growth Factor Rev. 2002;13:223–240. doi: 10.1016/s1359-6101(02)00012-6. [DOI] [PubMed] [Google Scholar]
- Kotlyarov A, Neininger A, Schubert C, Eckert R, Birchmeier C, Volk HD, et al. MAPKAP kinase 2 is essential for LPS-induced TNF-alpha biosynthesis. Nat Cell Biol. 1999;1:94–97. doi: 10.1038/10061. [DOI] [PubMed] [Google Scholar]
- Kreckler LM, Wan TC, Ge ZD, Auchampach JA. Adenosine inhibits tumor necrosis factor-α release from mouse peritoneal macrophages via A2A and A2B but not the A3 adenosine receptor. J Pharmacol Exp Ther. 2006;317:172–180. doi: 10.1124/jpet.105.096016. [DOI] [PubMed] [Google Scholar]
- la Sala A, Gadina M, Kelsall BL. Gi-protein-dependent inhibition of IL-12 production is mediated by activation of the phosphatidylinositol 3-kinase-protein 3 kinase b/akt pathway and JNK. J Immunol. 2005;175:2994–2999. doi: 10.4049/jimmunol.175.5.2994. [DOI] [PubMed] [Google Scholar]
- Landells LJ, Jensen MW, Orr LM, Spina D, O’Connor BJ, Page CP. The role of adenosine receptors in the action of theophylline on human peripheral blood mononuclear cells from healthy and asthmatic subjects. Br J Pharmacol. 2000;129:1140–1144. doi: 10.1038/sj.bjp.0703177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Moine O, Stordeur P, Schandene L, Marchant A, de Groote D, Goldman M, et al. Adenosine enhances IL-10 secretion by human monocytes. J Immunol. 1996;156:4408–4414. [PubMed] [Google Scholar]
- Le Vraux V, Chen YL, Masson I, De Sousa M, Giroud JP, Florentin I, et al. Inhibition of human monocyte TNF production by adenosine receptor agonists. Life Sci. 1993;52:1917–1924. doi: 10.1016/0024-3205(93)90632-d. [DOI] [PubMed] [Google Scholar]
- Ledent C, Vaugeois JM, Schiffmann SN, Pedrazzini T, El Yacoubi M, Vanderhaeghen JJ, et al. Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature. 1997;388:674–678. doi: 10.1038/41771. [DOI] [PubMed] [Google Scholar]
- Leibovich SJ, Chen JF, Pinhal-Enfield G, Belem PC, Elson G, Rosania A, et al. Synergistic up-regulation of vascular endothelial growth factor expression in murine macrophages by adenosine A(2A) receptor agonists and endotoxin. Am J Pathol. 2002;160:2231–2244. doi: 10.1016/S0002-9440(10)61170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard EJ, Skeel A, Chiang PK, Cantoni GL. The action of the adenosylhomocysteine hydrolase inhibitor, 3-deazaadenosine, on phagocytic function of mouse macrophages and human monocytes. Biochem Biophys Res Commun. 1978;84:102–109. doi: 10.1016/0006-291x(78)90269-3. [DOI] [PubMed] [Google Scholar]
- Leonard EJ, Shenai A, Skeel A. Dynamics of chemotactic peptide-induced superoxide generation by human monocytes. Inflammation. 1987;11:229–240. doi: 10.1007/BF00916023. [DOI] [PubMed] [Google Scholar]
- Lewis CE, Leek R, Harris A, McGee JO. Cytokine regulation of angiogenesis in breast cancer: the role of tumor-associated macrophages. J Leukoc Biol. 1995;57:747–751. doi: 10.1002/jlb.57.5.747. [DOI] [PubMed] [Google Scholar]
- Linden J. Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Annu Rev Pharmacol Toxicol. 2001;41:775–787. doi: 10.1146/annurev.pharmtox.41.1.775. [DOI] [PubMed] [Google Scholar]
- Link AA, Kino T, Worth JA, McGuire JL, Crane ML, Chrousos GP, et al. Ligand-activation of the adenosine A2a receptors inhibits IL-12 production by human monocytes. J Immunol. 2000;164:436–442. doi: 10.4049/jimmunol.164.1.436. [DOI] [PubMed] [Google Scholar]
- Lupica CR, Cass WA, Zahniser NR, Dunwiddie TV. Effects of the selective adenosine A2 receptor agonist CGS 21680 on in vitro electrophysiology, cAMP formation and dopamine release in rat hippocampus and striatum. J Pharmacol Exp Ther. 1990;252:1134–1141. [PubMed] [Google Scholar]
- Madara JL, Patapoff TW, Gillece-Castro B, Colgan SP, Parkos CA, Delp C, et al. 5′-Adenosine monophosphate is the neutrophil-derived paracrine factor that elicits chloride secretion from T84 intestinal epithelial cell monolayers. J Clin Invest. 1993;91:2320–2325. doi: 10.1172/JCI116462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar S, Aggarwal BB. Adenosine suppresses activation of nuclear factor-κB selectively induced by tumor necrosis factor in different cell types. Oncogene. 2003;22:1206–1218. doi: 10.1038/sj.onc.1206184. [DOI] [PubMed] [Google Scholar]
- Marcus AJ, Safier LB, Broekman MJ, Islam N, Fliessbach JH, Hajjar KA, et al. Thrombosis and inflammation as multicellular processes: significance of cell-cell interactions. Thromb Haemost. 1995;74:213–217. [PubMed] [Google Scholar]
- Martin L, Pingle SC, Hallam DM, Rybak LP, Ramkumar V. Activation of the adenosine A3 receptor in RAW 264.7 cells inhibits lipopolysaccharide-stimulated tumor necrosis factor-alpha release by reducing calcium-dependent activation of nuclear factor-κB and extracellular signal-regulated kinase 1/2. J Pharmacol Exp Ther. 2006;316:71–78. doi: 10.1124/jpet.105.091868. [DOI] [PubMed] [Google Scholar]
- Mascali JJ, Cvietusa P, Negri J, Borish L. Anti-inflammatory effects of theophylline: modulation of cytokine production. Ann Allergy Asthma Immun. 1996;77:34–38. doi: 10.1016/S1081-1206(10)63476-X. [DOI] [PubMed] [Google Scholar]
- Mayne M, Shepel PN, Jiang Y, Geiger JD, Power C. Dysregulation of adenosine A1 receptor-mediated cytokine expression in peripheral blood mononuclear cells from multiple sclerosis patients. Ann Neurol. 1999;45:633–639. doi: 10.1002/1531-8249(199905)45:5<633::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- McWhinney CD, Dudley MW, Bowlin TL, Peet NP, Schook L, Bradshaw M, et al. Activation of adenosine A3 receptors on macrophages inhibits tumor necrosis factor-alpha. Eur J Pharmacol. 1996;310:209–216. doi: 10.1016/0014-2999(96)00272-5. [DOI] [PubMed] [Google Scholar]
- Merrill JT, Shen C, Schreibman D, Coffey D, Zakharenko O, Fisher R, et al. Adenosine A1 receptor promotion of multinucleated giant cell formation by human monocytes: a mechanism for methotrexate-induced nodulosis in rheumatoid arthritis. Arthritis Rheum. 1997;40:1308–1315. doi: 10.1002/1529-0131(199707)40:7<1308::AID-ART16>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Min HW, Moochhala S, Eng KH. Adenosine and its receptor agonists regulate nitric oxide production and RAW 264.7 macrophages via both receptor binding and its downstream metabolites-inosine. Life Sci. 2000;66:1781–1793. doi: 10.1016/s0024-3205(00)00502-6. [DOI] [PubMed] [Google Scholar]
- Mohsenin A, Blackburn MR. Adenosine signaling in asthma and chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2006;12:54–59. doi: 10.1097/01.mcp.0000199002.46038.cb. [DOI] [PubMed] [Google Scholar]
- Montesinos MC, Gadangi P, Longaker M, Sung J, Levine J, Nilsen D, et al. Wound healing is accelerated by agonists of adenosine A2 (G alpha s-linked) receptors. J Exp Med. 1997;186:1615–1620. doi: 10.1084/jem.186.9.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Morrissey JH. Tissue factor: a key molecule in hemostatic and nonhemostatic systems. Int J Hematol. 2004;79:103–108. doi: 10.1532/ijh97.03167. [DOI] [PubMed] [Google Scholar]
- Mosmann TR, Schumacher JH, Fiorentino DF, Leverah J, Moore KW, Bond MW. Isolation of monoclonal antibodies specific for IL-4, IL-5, IL-6, and a new Th2-specific cytokine (IL-10), cytokine synthesis inhibitory factor, by using a solid phase radioimmunoadsorbent assay. J Immunol. 1990;145:2938–2945. [PubMed] [Google Scholar]
- Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73:209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- Muller WA, Randolph GJ. Migration of leukocytes across endothelium and beyond: molecules involved in the transmigration and fate of monocytes. J Leukoc Biol. 1999;66:698–704. doi: 10.1002/jlb.66.5.698. [DOI] [PubMed] [Google Scholar]
- Naito M, Umeda S, Yamamoto T, Moriyama H, Umezu H, Hasegawa G, et al. Development, differentiation, and phenotypic heterogeneity of murine tissue macrophages. J Leukoc Biol. 1996;59:133–138. doi: 10.1002/jlb.59.2.133. [DOI] [PubMed] [Google Scholar]
- Najar HM, Ruhl S, Bru-Capdeville AC, Peters JH. Adenosine and its derivatives control human monocyte differentiation into highly accessory cells versus macrophages. J Leukoc Biol. 1990;47:429–439. doi: 10.1002/jlb.47.5.429. [DOI] [PubMed] [Google Scholar]
- Nathan CF, Hibbs JB., Jr Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol. 1991;3:65–70. doi: 10.1016/0952-7915(91)90079-g. [DOI] [PubMed] [Google Scholar]
- Nemeth ZH, Leibovich SJ, Deitch EA, Vizi ES, Szabo C, Hasko G. cDNA microarray analysis reveals a nuclear factor-kappaB-independent regulation of macrophage function by adenosine. J Pharmacol Exp Ther. 2003;306:1042–1049. doi: 10.1124/jpet.103.052944. [DOI] [PubMed] [Google Scholar]
- Nemeth ZH, Lutz CS, Csoka B, Deitch EA, Leibovich SJ, Gause WC, et al. Adenosine augments IL-10 production by macrophages through an A2B receptor-mediated posttranscriptional mechanism. J Immunol. 2005;175:8260–8270. doi: 10.4049/jimmunol.175.12.8260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth ZH, Csoka B, Wilmanski J, Xu D, Lu Q, Ledent C, et al. Adenosine A2A receptor inactivation increases survival in polymicrobial sepsis. J Immunol. 2006;176:5616–5626. doi: 10.4049/jimmunol.176.9.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odashima M, Bamias G, Rivera-Nieves J, Linden J, Nast CC, Moskaluk CA, et al. Activation of A2A adenosine receptor attenuates intestinal inflammation in animal models of inflammatory bowel disease. Gastroenterology. 2005;129:26–33. doi: 10.1053/j.gastro.2005.05.032. [DOI] [PubMed] [Google Scholar]
- Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- Osterud B. Tissue factor expression by monocytes: regulation and pathophysiological roles. Blood Coagul Fibrinolysis. 1998;9:S9–S14. [PubMed] [Google Scholar]
- Parks RE, Jr, Dawicki DD, Agarwal KC, Chen SF, Stoeckler JD. Role of nucleoside transport in drug action. The adenosine deaminase inhibitor, deoxycoformycin, and the antiplatelet drugs, dipyridamole and dilazep. Ann NY Acad Sci. 1985;451:188–203. doi: 10.1111/j.1749-6632.1985.tb27110.x. [DOI] [PubMed] [Google Scholar]
- Pastor-Anglada M, Casado FJ, Valdes R, Mata J, Garcia-Manteiga J, Molina M. Complex regulation of nucleoside transporter expression in epithelial and immune system cells. Mol Membr Biol. 2001;18:81–85. doi: 10.1080/096876800110033783. [DOI] [PubMed] [Google Scholar]
- Pike MC, Kredich NM, Snyderman R. Requirement of S-adenosyl-L-methionine-mediated methylation for human monocyte chemotaxis. Proc Natl Acad Sci U S A. 1978;75:3928–3932. doi: 10.1073/pnas.75.8.3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinhal-Enfield G, Ramanathan M, Hasko G, Vogel SN, Salzman AL, Boons GJ, et al. An angiogenic switch in macrophages involving synergy between Toll-like receptors 2, 4, 7, and 9 and adenosine A(2A) receptors. Am J Pathol. 2003;163:711–721. doi: 10.1016/S0002-9440(10)63698-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar U, Brooks DP, Lipshlitz D, Esser KM. Inhibition of LPS-induced TNF alpha production in human monocytes by adenosine (A2) receptor selective agonists. Int J Immunopharmacol. 1995;17:221–224. doi: 10.1016/0192-0561(94)00096-7. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Resta R, Yamashita Y, Thompson LF. Ecto-enzyme and signaling functions of lymphocyte CD73. Immunol Rev. 1998;161:95–109. doi: 10.1111/j.1600-065x.1998.tb01574.x. [DOI] [PubMed] [Google Scholar]
- Rounds S, Hsieh L, Agarwal KC. Effects of endotoxin injury on endothelial cell adenosine metabolism. J Lab Clin Med. 1994;123:309–317. [PubMed] [Google Scholar]
- Sajjadi FG, Takabayashi K, Foster AC, Domingo RC, Firestein GS. Inhibition of TNF-alpha expression by adenosine: role of A3 adenosine receptors. J Immunol. 1996;156:3435–3442. [PubMed] [Google Scholar]
- Salmon JE, Brogle N, Brownlie C, Edberg JC, Kimberly RP, Chen BX, et al. Human mononuclear phagocytes express adenosine A1 receptors. A novel mechanism for differential regulation of Fc gamma receptor function. J Immunol. 1993;151:2775–2785. [PubMed] [Google Scholar]
- Sattin A, Rall TW. The effect of adenosine and adenine nucleotides on the cyclic adenosine-3′,5′-phosphate content of guinea pig cerebral cortex slices. Mol Pharmacol. 1970;6:13–23. [PubMed] [Google Scholar]
- Semenza GL. Angiogenesis in ischemic and neoplastic disorders. Annu Rev Med. 2003;54:17–28. doi: 10.1146/annurev.med.54.101601.152418. [DOI] [PubMed] [Google Scholar]
- Si QS, Nakamura Y, Kataoka K. Adenosine inhibits superoxide production in rat peritoneal macrophages via elevation of cAMP level. Immunopharmacology. 1997;36:1–7. doi: 10.1016/s0162-3109(96)00158-0. [DOI] [PubMed] [Google Scholar]
- Sitkovsky MV, Lukashev D, Apasov S, Kojima H, Koshiba M, Caldwell C, et al. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu Rev Immunol. 2004;22:657–682. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- Southan GJ, Szabo C. Selective pharmacological inhibition of distinct nitric oxide synthase isoforms. Biochem Pharmacol. 1996;51:383–394. doi: 10.1016/0006-2952(95)02099-3. [DOI] [PubMed] [Google Scholar]
- Sperlagh B, Hasko G, Nemeth Z, Vizi ES. ATP released by LPS increases nitric oxide production in raw 264.7 macrophage cell line via P2Z/P2X7 receptors. Neurochem Int. 1998;33:209–215. doi: 10.1016/s0197-0186(98)00025-4. [DOI] [PubMed] [Google Scholar]
- Sperlagh B, Doda M, Baranyi M, Hasko G. Ischemic-like condition releases norepinephrine and purines from different sources in superfused rat spleen strips. J Neuroimmunol. 2000;111:45–54. doi: 10.1016/s0165-5728(00)00365-9. [DOI] [PubMed] [Google Scholar]
- Sperlagh B, Vizi ES, Wirkner K, Illes P. P2X7 receptors in the nervous system. Prog Neurobiol. 2006;78:327–346. doi: 10.1016/j.pneurobio.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Spicuzza L, Di Maria G, Polosa R. Adenosine in the airways: implications and applications. Eur J Pharmacol. 2006;533:77–88. doi: 10.1016/j.ejphar.2005.12.056. [DOI] [PubMed] [Google Scholar]
- Stout RD, Suttles J. Functional plasticity of macrophages: reversible adaptation to changing microenvironments. J Leukoc Biol. 2004;76:509–513. doi: 10.1189/jlb.0504272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung SJ, Silverstein SC. Inhibition of macrophage phagocytosis by methylation inhibitors. Lack of correlation of protein carboxymethylation and phospholipid methylation with phagocytosis. J Biol Chem. 1985;260:546–554. [PubMed] [Google Scholar]
- Swanson JA, Hoppe AD. The coordination of signaling during Fc receptor-mediated phagocytosis. J Leukoc Biol. 2004;76:1093–1103. doi: 10.1189/jlb.0804439. [DOI] [PubMed] [Google Scholar]
- Szabo C, Scott GS, Virag L, Egnaczyk G, Salzman AL, Shanley TP, et al. Suppression of macrophage inflammatory protein (MIP)- 1alpha production and collagen-induced arthritis by adenosine receptor agonists. Br J Pharmacol. 1998;125:379–387. doi: 10.1038/sj.bjp.0702040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele A, Kronstein R, Wetzel A, Gerth A, Nieber K, Hauschildt S. Regulation of adenosine receptor subtypes during cultivation of human monocytes: role of receptors in preventing lipopolysaccharide-triggered respiratory burst. Infect Immun. 2004;72:1349–1357. doi: 10.1128/IAI.72.3.1349-1357.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson LF, Eltzschig HK, Ibla JC, Van De Wiele CJ, Resta R, Morote-Garcia JC, et al. Crucial role for ecto-5′-nucleotidase (CD73) in vascular leakage during hypoxia. J Exp Med. 2004;200:1395–1405. doi: 10.1084/jem.20040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui S, Schnermann J, Noorbakhsh F, Henry S, Yong VW, Winston BW, et al. A1 adenosine receptor upregulation and activation attenuates neuroinflammation and demyelination in a model of multiple sclerosis. J Neurosci. 2004;24:1521–1529. doi: 10.1523/JNEUROSCI.4271-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue ER. Antigen-presenting function of the macrophage. Annu Rev Immunol. 1984;2:395–428. doi: 10.1146/annurev.iy.02.040184.002143. [DOI] [PubMed] [Google Scholar]
- Valledor AF, Borras FE, Cullell-Young M, Celada A. Transcription factors that regulate monocyte/macrophage differentiation. J Leukoc Biol. 1998;63:405–417. doi: 10.1002/jlb.63.4.405. [DOI] [PubMed] [Google Scholar]
- Wells CA, Ravasi T, Hume DA. Inflammation suppressor genes: please switch out all the lights. J Leukoc Biol. 2005;78:9–13. doi: 10.1189/jlb.1204710. [DOI] [PubMed] [Google Scholar]
- Xaus J, Mirabet M, Lloberas J, Soler C, Lluis C, Franco R, et al. IFN-gamma up-regulates the A2B adenosine receptor expression in macrophages: a mechanism of macrophage deactivation. J Immunol. 1999;162:3607–3614. [PubMed] [Google Scholar]
- Xaus J, Valledor AF, Cardo M, Marques L, Beleta J, Palacios JM, et al. Adenosine inhibits macrophage colony-stimulating factor-dependent proliferation of macrophages through the induction of p27kip-1 expression. J Immunol. 1999;163:4140–4149. [PubMed] [Google Scholar]
- Yang Z, Day YJ, Toufektsian MC, Ramos SI, Marshall M, Wang XQ, et al. Infarct-sparing effect of A2A-adenosine receptor activation is due primarily to its action on lymphocytes. Circulation. 2005;111:2190–2197. doi: 10.1161/01.CIR.0000163586.62253.A5. [DOI] [PubMed] [Google Scholar]
- Zhang JG, Hepburn L, Cruz G, Borman RA, Clark KL. The role of adenosine A2A and A2B receptors in the regulation of TNF-α production by human monocytes. Biochem Pharmacol. 2005;69:883–889. doi: 10.1016/j.bcp.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Zhou H, Wolberg AS, Roubey RA. Characterization of monocyte tissue factor activity induced by IgG antiphospholipid antibodies and inhibition by dilazep. Blood. 2004;104:2353–2358. doi: 10.1182/blood-2004-01-0145. [DOI] [PubMed] [Google Scholar]
- Zimmermann H. Nucleotides and cd39: principal modulatory players in hemostasis and thrombosis. Nat Med. 1999;5:987–988. doi: 10.1038/12419. [DOI] [PubMed] [Google Scholar]


