Abstract
Cannabinoids and their synthetic and endogenous analogs affect a broad range of physiological functions, including cardiovascular variables, the most important component of their effect being profound hypotension. The mechanisms of the cardiovascular effects of cannabinoids in vivo are complex and may involve modulation of autonomic outflow in both the central and peripheral nervous systems as well as direct effects on the myocardium and vasculature. Although several lines of evidence indicate that the cardiovascular depressive effects of cannabinoids are mediated by peripherally localized CB1 receptors, recent studies provide strong support for the existence of as-yet-undefined endothelial and cardiac receptor(s) that mediate certain endocannabinoid-induced cardiovascular effects. The endogenous cannabinoid system has been recently implicated in the mechanism of hypotension associated with hemorrhagic, endotoxic, and cardiogenic shock, and advanced liver cirrhosis. Furthermore, cannabinoids have been considered as novel antihypertensive agents. A protective role of endocannabinoids in myocardial ischemia has also been documented. In this chapter, we summarize current information on the cardiovascular effects of cannabinoids and highlight the importance of these effects in a variety of pathophysiological conditions.
Keywords: Cannabinoid, Anandamide, CB1 receptor, Blood pressure, Cardiac function, Vascular, Ischemia
1 Introduction
The biological effects of marijuana and its main psychoactive ingredient, Δ9-tetrahydrocannabinol (THC), are mediated by specific, G protein-coupled receptors (GPCRs) (Howlett et al. 1990). To date, two cannabinoid (CB) receptors have been identified by molecular cloning: the CB1 receptor, which is by far the most abundant of all neurotransmitter receptors in the brain (Matsuda et al. 1990), but is also present in various peripheral tissues including the heart and vasculature (Gebremedhin et al. 1999; Liu et al. 2000; Bonz et al. 2003), and the CB2 receptor, expressed primarily by immune (Munro et al. 1993) and hematopoietic cells (Valk and Delwel 1998). The natural ligands of these receptors are endogenous, lipid-like substances called endocannabinoids, which include arachidonoyl ethanolamide or anandamide and 2-arachidonoylglycerol (2-AG), as the two most widely studied members of this group (reviewed by Mechoulam et al. 1998).
Cannabinoids and their synthetic and endogenous analogs are best known for their prominent psychoactive properties, but their cardiovascular effects were also recognized as early as the 1960s. The most important component of these effects is a profound decrease in arterial blood pressure, cardiac contractility, and heart rate (Lake et al. 1997a,b; Hillard 2000; Kunos et al. 2000, 2002; Randall et al. 2002; Ralevic et al. 2002; Hiley and Ford 2004). Although several lines of evidence indicate that the cardiovascular depressive effects of cannabinoids are mediated by peripherally localized CB1 receptors, cannabinoids can also elicit vascular and cardiac effects, which are independent of CB1 and CB2 receptors, as discussed in detail later in this chapter.
Recent findings implicate the endogenous cannabinoid system in the pathomechanism of hypotension associated with various forms of shock, including hemorrhagic (Wagner et al. 1997), endotoxic (Varga et al. 1998; Wang et al. 2001; Liu et al. 2003; Bátkai et al. 2004a), and cardiogenic shock (Wagner et al. 2001a, 2003), as well as the hypotension associated with advanced liver cirrhosis (Bátkai et al. 2001; Ros et al. 2002). Furthermore, the possible use of cannabinoids as novel antihypertensive agents has been entertained (Birmingham 1973; Archer 1974; Varma et al. 1975; Crawford and Merritt 1979; Zaugg and Kynel 1983; Lake et al. 1997b; Bátkai et al. 2003 and 2004; Li et al. 2003). In addition, a protective role of endocannabinoids has been described in myocardial ischemia (reviewed in Hiley and Ford 2003, 2004).
The goal of this chapter is to summarize the cardiovascular effects of cannabinoids and to highlight the unique therapeutic potential of the pharmacological manipulation of the endocannabinergic system in a variety of pathological conditions.
2 Cardiovascular Effects of Cannabinoids In Vivo
The in vivo cardiovascular effects of cannabinoids are complex and may involve modulation of the autonomic outflow in both the central and peripheral nervous systems as well as direct effects on the myocardium and vasculature. However, their peripheral actions appear to play the dominant role, at least upon systemic administration at the doses used by most investigators. Moreover, the effects of endocannabinoids are complicated by their rapid metabolism, which may liberate other vasoactive substances and their precursors (reviewed in Mechoulam et al. 1998; Kunos et al. 2002; Randall et al. 2002; Ralevic et al. 2002).
In humans, the acute effect of smoking cannabis usually manifests as an increase in heart rate with no significant change in blood pressure (Kanakis et al. 1976). However, chronic use of cannabis in man, as well as both acute and prolonged administration of THC to experimental animals, elicit a long-lasting decrease in blood pressure and heart rate (Rosenkratz 1974; Benowitz and Jones 1975). Because of the well-known effects of cannabinoids on central nervous system function, early studies of their cardiovascular actions concentrated on the ability of these compounds to inhibit sympathetic tone as the underlying mechanism. Indeed, cross-perfusion experiments in dogs have provided some evidence for a centrally mediated sympatho-inhibitory effect of THC, although additional peripheral sites of action could not be ruled out (Vollmer et al. 1974). Already at this early stage, the potential use of these compounds as antihypertensive agents was considered (Archer 1974), in the hope that their neurobehavioral and cardiovascular effects would turn out to be separable. That this may be possible to achieve was first suggested by a 1977 publication of the biological effects of abnormal cannabidiol, a synthetic analog of the neurobehaviorally inactive, plant-derived cannabinoid, cannabidiol (Adams et al. 1977). However, more than two decades have elapsed before this promising observation was followed up and extended (see below).
2.1 Role of CB1 Receptors in the Cardiovascular Effects of Cannabinoids
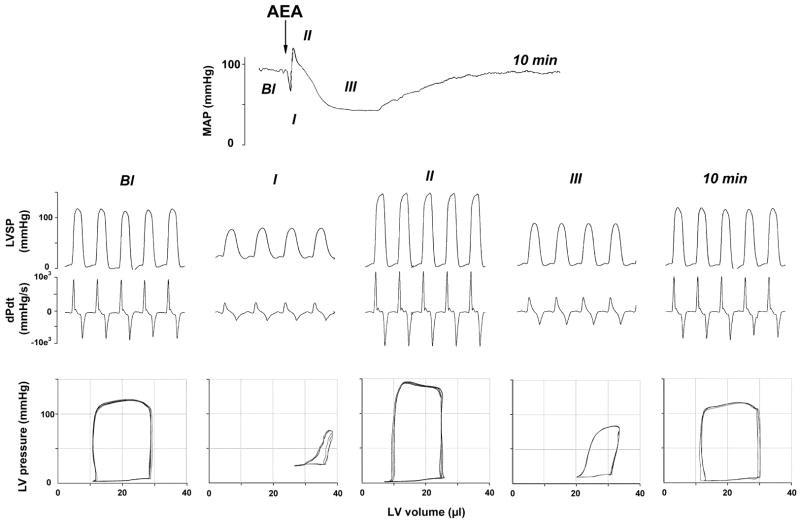
The discovery of anandamide, the first endocannabinoid (Devane et al. 1992), has raised the obvious question whether it possesses cardiovascular activity similar to THC. Upon its intravenous bolus injection into anesthetized rats and mice, anandamide was found to elicit a triphasic blood pressure response and bradycardia (Varga et al. 1995; Pacher et al. 2004; Bátkai et al. 2004b; see Fig. 1) similar to that reported earlier for THC (Siqueira et al. 1979). The first phase of the response consists of a precipitous drop in heart rate and blood pressure that lasts for a few seconds only. These effects are vagally mediated, as they are absent in animals after bilateral transection of the cervical vagus nerve, or after pretreatment with methylatropine (Varga et al. 1995). This vagal component is followed by a brief pressor response, which persists in the presence of α-adrenergic blockade and also in rats in which sympathetic tone is abolished by pithing, and is thus not sympathetically mediated (Varga et al. 1995). This pressor component is also unaffected by CB1 receptor antagonists and it persists in CB1 knockout mice (Járai et al. 1999; Pacher et al. 2004), indicating the lack of involvement of CB1 receptors. Recent observations using the radiolabeled microsphere technique in rats suggest that this pressor component may be due to vasoconstriction in certain vascular beds, such as the spleen (Wagner et al. 2001b). The third, and most prominent, phase in the effect of anandamide is hypotension associated with moderate bradycardia that last about 2–10 min. Interestingly, this third phase is absent in conscious normotensive rats (Stein et al. 1996; Lake et al. 1997a), but is present and more prolonged in conscious, spontaneously hypertensive rats (Lake et al. 1997b; Bátkai et al. 2004b). Since sympathetic tone is known to be low in conscious, undisturbed normotensive rats (Carruba et al. 1987), these observations appear to be compatible with a sympatho-inhibitory mechanism underlying anandamide-induced hypotension and bradycardia, as further discussed below. The finding that R-methanandamide, a metabolically stable analog of anandamide (Abadji et al. 1994), causes similar but more prolonged hypotension and bradycardia (Kunos et al. 2000) eliminates the possibility that the hypotensive and bradycardic effects of anandamide are mediated indirectly by a metabolite.
Fig. 1.
Hemodynamic effects of anandamide in anesthetized mice. Representative recordings of the effects of anandamide [20 mg/kg i.v., N-arachidonoyl-ethanolamine (AEA)] on mean arterial pressure (MAP, top panel), cardiac contractility (left ventricular systolic pressure (LVSP) and dP/dt (dPdt); middle panel) and pressure-volume relations (bottom panel) in a pentobarbital-anesthetized C57BL6 mouse. The five parts of the middle and bottom panels represent baseline conditions (Bl), phase I (I), phase II (II), and phase III (III) of the anandamide response, and recovery 10 min following the injection. The arrow indicates the injection of the drug. The decrease of the amplitude of PV loops and shift to the right indicate decrease of cardiac contractile performance
Several lines of evidence indicate that cannabinoid-induced hypotension is mediated by CB1 receptors. First, the hypotension is effectively antagonized by the CB1-selective antagonist SR141716 (Varga et al. 1995, 1996; Calignano et al. 1997). SR141716 can block hypotension induced by plant-derived and synthetic cannabinoids as well as anandamide (Lake et al. 1997a). However, when tested in anesthetized mice, the hypotensive effect of 2-AG was unexpectedly resistant to inhibition by SR141716, but could be antagonized by indomethacin, suggesting the involvement of a cyclo-oxygenase metabolite (Járai et al. 2000). Indeed, 2-AG was found to be rapidly (<30 s) degraded in mouse blood to generate arachidonic acid. Accordingly, when the metabolically stable analog 2-AG ether was tested, its hypotensive effect was antagonized by SR141716 and it was absent in CB1-deficient mice indicating that, similar to anandamide, it is an effective agonist of hypotensive CB1 receptors (Járai et al. 2000). 2-AG ether has been recently identified as an endogenous brain constituent (Hanus et al. 2001), thus it may also be involved in cardiovascular regulation.
The second line of evidence for CB1 receptor involvement is the strong, positive correlation between the concentrations of various cannabinoid agonists producing half-maximal hypotensive and bradycardic responses (EC50) and their affinity constants for binding to CB1 receptors in the brain (Lake et al. 1997a). The strongest evidence, however, is the total absence of cannabinoid-induced hypotension and bradycardia in mice lacking the CB1 receptor (Járai et al. 1999; Ledent et al. 1999). Interestingly, the isolated tachycardia in response to the acute intake of THC by human volunteers who are not chronic marijuana users is similarly inhibited by SR141716 (Huestis et al. 2001). In healthy young adults, the heart is under dominant vagal tone, and the tachycardic effect of THC is most likely due to inhibition of acetylcholine release from cardiac vagal efferents via presynaptic CB1 receptors (Szabo et al. 2001).
Acute drug effects on blood pressure are the result of changes in peripheral vascular resistance, cardiac output, or both. Recent unpublished results in the authors’ laboratory, using the pressure-volume conductance system (Pacher et al. 2003 and 2004; Fig. 2) in pentobarbital-anesthetized mice in vivo, indicate that the hypotensive effect of (–)-11-OH-Δ9-THC dimethylheptyl (HU-210) results primarily from a decrease in cardiac contractility (Fig. 2). In contrast, anandamide reduces both cardiac contractility (Fig. 1) and total peripheral resistance (TPR) (Pacher et al. 2004; Bátkai et al. 2004b), which is in agreement with the cardiodepressant effects of HU-210 and anandamide observed in isolated Langendorff rat hearts and in isolated, electrically stimulated human atrial appendages in vitro (Bonz et al. 2003; Ford et al. 2002; Nahas and Trouve 1985; see below). These cardiodepressor effects may underlie the ability of anandamide and HU-210 to decrease cardiac output observed in studies using the radiolabeled microsphere technique (Wagner et al. 2001b). Wagner et al. also demonstrated that both HU-210 and anandamide produce major vasodilation in the coronary and cerebral circulation, which could be antagonized by SR141716 (Wagner et al. 2001b). Collectively, these findings suggest that cannabinoids cause cardiodepressor effects as well as coronary and cerebral vasodilation via SR141716-sensitive CB1 receptors.
Fig. 2.
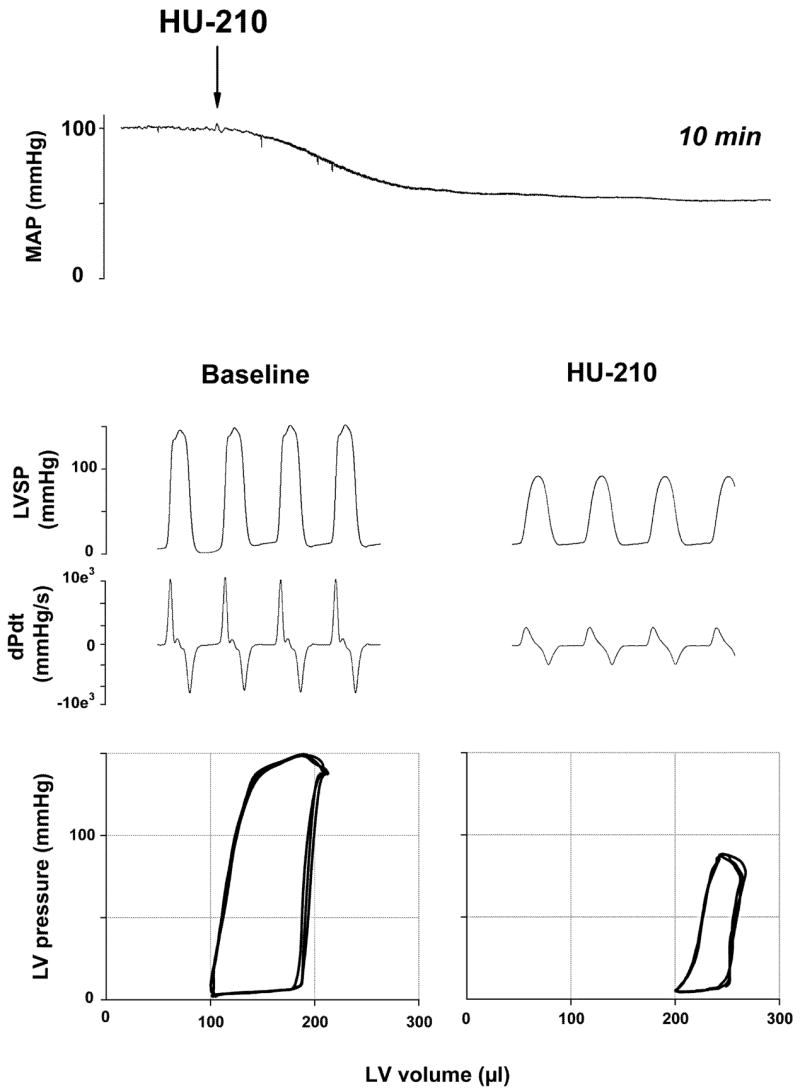
Hemodynamic effects of HU-210 in anesthetized rat. Representative recordings of the effects of HU-210 (30 μg/kg i.v.) on mean arterial pressure (MAP, top panel), cardiac contractility (LVSP and dP/dt; middle panel), and pressure-volume (PV) relations (bottom panel) in a pentobarbital-anesthetized rat. The arrow indicates the injection of the drug. The decrease of the amplitude of PV loops and their shift to the right are indicative of decreased cardiac contractile performance
Despite strong evidence for the exclusive role of CB1 receptors in the hypotensive effect of cannabinoids, there is growing evidence over the last few years that anandamide-induced vasodilation in the mesenteric, and possibly some other vascular beds, is independent of CB1 or CB2 receptors (see below, Sect. 3). This apparent paradox may be resolved by the observation that in anesthetized rats, abnormal cannabidiol (abn-cbd), a non-psychoactive cannabinoid with vasodilator activity (Adams et al. 1977; Járai et al. 1999), can elicit a significant increase in mesenteric blood flow in vivo as measured by Doppler sonography, without having a significant effect on blood pressure (S. Bátkai, P. Pacher, G. Kunos, unpublished observations). It is possible that the vasodilation elicited through local release of endocannabinoids in certain vascular beds is compensated by sympathetic vasoconstriction in others, resulting in no net effect on blood pressure.
2.2 Role of Central Versus Peripheral Mechanisms in the Cardiovascular Effects of Cannabinoids
Early work with THC suggested that cannabinoids lower blood pressure through a centrally mediated sympatho-inhibitory mechanism (Vollmer et al. 1974). However, the hypotension elicited by anandamide in urethane-anesthetized rats is not associated with any change in the activity of sympathetic premotor neurons in the medullary vasomotor center or in the activity of sympathetic postganglionic nerves (Varga et al. 1996), which ruled out centrally mediated sympatho-inhibition or ganglionic blockade as possible underlying mechanisms, at least for anandamide. Intra-cerebroventricular administration in rabbits of the potent synthetic cannabinoid WIN55,212-2 was found to increase rather than decrease sympathetic tone, which also argues against a central mechanism for the hypotensive effect (Niederhoffer and Szabo 2000). Yet, the pressor response triggered by electrical stimulation of the vasomotor center was reversibly inhibited by anandamide, whereas the effect of exogenous phenylephrine was unaffected, suggesting a presynaptic-inhibitory effect of norepinephrine release from peripheral sympathetic nerve terminals (Varga et al. 1996). Indeed, stimulation of presynaptic CB1 receptors inhibits norepinephrine release both in vitro (Ishac et al. 1996; Deutsch et al. 1997; Schlicker et al. 1997; Christopoulos et al. 2001; Vizi et al. 2001) and in vivo (Malinowska et al. 1997; Niederhoffer and Szabo 2000). However, when sympathetic tone is eliminated by ganglionic blockade and vascular tone is restored by vasopressin infusion, the hypotensive response to the potent synthetic cannabinoid HU-210 remains unchanged, although its bradycardic effect is lost (Wagner et al. 2001b). This suggests that cannabinoid-induced bradycardia may be due to inhibition of sympathetic tone to the heart, but the hypotensive response is due to direct vasodilation, as also indicated by its presence in rats following chemical sympathetic denervation (Vidrio et al. 1996).
3 Cardiovascular Effects of Cannabinoids In Vitro
3.1 Direct Vasorelaxant Effects of Cannabinoids
An early report on the effect of anandamide on cerebral blood flow indicated that the observed vasodilator response could be inhibited by indomethacin (Ellis et al. 1995). The obvious implication of this finding was that anandamide causes vasorelaxation indirectly through the generation of arachidonic acid and its subsequent metabolism by cyclooxygenase. Although THC also produced an indomethacin-sensitive response, subsequent studies could not document an effect of cyclooxygenase inhibition on anandamide-induced vasorelaxation in other blood vessels, including the mesenteric and coronary vasculature (Randall et al. 1996; Randall and Kendall 1997; Plane et al. 1996; White and Hiley 1997), ruling out increased prostanoid formation as a major mechanism for the vasodilatory effect.
The vasorelaxant effect of anandamide displays tissue and interspecies differences. Anandamide has been found to relax rat hepatic and guinea pig basilar arteries (Zygmunt et al. 1999), bovine coronary arteries (Pratt et al. 1998), but not rat carotid arteries (Holland et al. 1999) or the rat aorta (Darker et al. 1998). Anandamide has been reported to mediate vasodilation in kidney afferent arterioles through the endothelial release of nitric oxide (NO) (Deutsch et al. 1997). Anandamide was also found to release NO in a variety of human blood vessels as well as the right atrium (Bilfinger et al. 1998). In contrast, in other studies the anandamide-induced vasorelaxation was insensitive to inhibition of NO synthase (Randall et al. 1996; White and Hiley 1997; Járai et al. 1999). Both anandamide and the CB agonist HU-210 caused up-regulation of the expression and activity of the inducible NO synthase in human umbilical vein endothelial cells (HUVEC), which is unlikely to contribute to the acute vasodilatory effect, but may play an important role in terminating the action of endogenous anandamide by affecting its cellular uptake (Maccarrone et al. 2000).
The interest in the vasodilator action of endocannabinoids was further stimulated by a report in 1996 that the mesenteric vasodilation attributable to an endothelium-derived hyperpolarizing factor (EDHF) is sensitive to inhibition by SR141716 (Randall et al. 1996). The corollary of this finding was that EDHF might be an endocannabinoid released from the vascular endothelium and acting at SR141716-sensitive CB receptors on vascular smooth muscle cells, which it would hyperpolarize and relax (Randall et al. 1996). Interestingly, carbachol was found to induce 2-AG production by the rat aortic endothelium (Mechoulam et al. 1998b), which is compatible with 2-AG being an EDHF.
Inhibition of EDHF-induced vasorelaxation by SR141716 was confirmed in some (White and Hiley 1997) but not other (Chataigneau et al. 1998; Fulton and Quilley 1998; Niederhoffer and Szabo 1999a; Pratt et al. 1998) studies. A possible source of these discrepancies may be the different species and vascular preparations tested. Additionally, the finding that the vasodilator action of anandamide has both an endothelium-dependent and an endothelium-independent component, and only the former is sensitive to inhibition by SR141716 (Chaytor et al. 1999; Mukhopadhyay et al. 2002; Wagner et al. 1999), also argues against anandamide itself being EDHF, although it leaves open the possibility that anandamide or another endocannabinoid acting at an SR141716-sensitive receptor on vascular endothelial cells may release an EDHF (Járai et al. 1999). This latter possibility is compatible with findings that anandamide triggers calcium transients in cultured vascular endothelial cells (Fimiani et al. 1999; Mombouli et al. 1999), and that the anandamide-induced hyperpolarization of the rat hepatic artery is endothelium dependent (Zygmunt et al. 1997). Interestingly, the mesenteric vasodilation caused by the non-psychotropic cannabinoid abn-cbd is inhibited by the same combination of calcium-activated potassium channel toxins (apamin+charybdotoxin; Járai et al. 1999; Ho and Hiley, 2003) that were reported to inhibit EDHF-induced vasodilation (Randall and Kendall 1998), although no such inhibition was observed in rat hepatic arteries (Zygmunt et al. 1997), and in some other preparations the effect of anandamide was inhibited by iberiotoxin, which blocks a different (large conductance) calcium-activated potassium channel (Ishioka et al. 1999; Begg et al. 2001; White et al. 2001). The findings with abn-cbd would suggest that cannabinoids might release EDHF via activation of an endothelial site distinct from CB1 receptors (which recognizes abn-cbd, see below). Indeed, activation of bona fide CB1 receptors may have an opposite effect, i.e., inhibition of the release of EDHF, as indicated by the findings of Fleming et al. (1999) in porcine coronary and rabbit carotid and mesenteric arteries.
Another mechanism by which anandamide elicits SR141716-sensitive, endothelium-dependent vasodilation may be through an intracellular site of action at gap junctions. Evidence for this mechanism is the ability of various gap junction inhibitors as well as the anandamide transport inhibitor AM404 to antagonize anandamide-induced mesenteric vasodilation and the ability of SR141716 to inhibit dye transfer through gap junctions (Chaytor et al. 1999). Together, these findings form the basis of the hypothesis that anandamide induces vasodilation at an intracellular site in endothelial cells where it would facilitate the gap junctional transfer of an EDHF to vascular smooth muscle (Chaytor et al. 1999). However, in other studies the vasodilator effect of anandamide was inhibited by some but not other gap junction inhibitors, and the ones that were inhibitory (18α-glycyrrhetinic acid, ouabain) also blocked Na+, K+-ATPases at the concentrations used, suggesting a mechanism of action unrelated to gap junction inhibition (Harris et al. 2002). In rabbit aortic rings, which are relaxed by anandamide in a partially endothelium-dependent manner, the anandamide response was unaffected by gap junction inhibitors (Mukhopadhyay et al. 2002), and similar findings were reported for rat isolated coronary arteries (White et al. 2001).
Finally, Zygmunt et al. (1999) described an unusual indirect pathway. They demonstrated that anandamide induces vasorelaxation in rat mesenteric and hepatic arteries, and in guinea pig basilary artery through the activation of TRPV1 receptors on sensory neurons, causing the release of the vasodilatory peptide calcitonin gene-related peptide (CGRP) (see also Sect. 4 below, “Role of Vanilloid TRPV1 Receptors in the Cardiovascular Effects of Cannabinoids”).
3.2 Novel Endothelial Endocannabinoid Receptor
The possible existence of cannabinoid receptors distinct from CB1 or CB2 was first suggested by findings that potent synthetic cannabinoids as well as THC do not elicit vasodilation in the same rat mesenteric vascular bed preparations in which anandamide and methanandamide have strong vasodilator activity (Wagner et al. 1999). In these experiments, the effects of anandamide and methanandamide could be inhibited by SR141716, but the concentration required was somewhat higher (1–10 μM) than required for inhibition of CB1 receptors (Járai et al. 1999; Chaytor et al. 1999; Mukhopadhyay et al. 2002; Wagner et al. 2001; White et al. 2001). Also, the ability of SR141716 to inhibit anandamide-induced vasodilation was lost following endothelial denudation (Chaytor et al. 1999; Járai et al. 1999; Mukhopadhyay et al. 2002; Wagner et al. 2001). These findings led to the postulation of an endothelial site, somewhat sensitive to inhibition by SR141716 but distinct from CB1 or CB2 receptors, that contributes to anandamide-induced vasodilation in the mesenteric circulation (Járai et al. 1999) and, possibly, in other vascular beds, such as the rat coronary circulation (Ford et al. 2002). In this latter study, a nonCB1/nonCB2 mechanism mediating the negative inotropic effect of anandamide has been also identified.
More recently, a non-CB1/non-CB2 site was also postulated to exist on glutamatergic terminals in the mouse hippocampus, where its activation by cannabinoids inhibits glutamatergic transmission and excitatory postsynaptic potentials (EPSPs) (Hájos et al. 2001). Similar to the endothelial site, the site in the hippocampus is susceptible to inhibition by SR141716, but it can be activated by the synthetic cannabinoid WIN55,212-2 (Hájos et al. 2001). A similarly WIN55,212-2-sensitive, but SR141716-insensitive, non-CB1/non-CB2 site that can activate guanosine triphosphate (GTP)γS labeling in brain membranes has been identified in CB1 knockout mice (Breivogel et al. 2001) and in astrocytes, where its stimulation inhibits cyclic adenosine monophosphate (cAMP) production (Sagan et al. 1999). Since the endothelial site is insensitive to WIN55,212-2 in the rat mesentery (Wagner et al. 1999) or in the rabbit aorta (Mukhopadhyay et al. 2002), and abn-cbd does not inhibit glutamatergic EPSPs in rat hippocampus (M. Begg, D.M. Lovinger, G. Kunos, unpublished results), it is very likely distinct from the non-CB1 site described in the rat CNS.
Abn-cbd is a synthetic analog of the behaviorally inactive plant-derived cannabinoid, cannabidiol. Several years ago, abn-cbd was reported to be inactive in two, rather non-specific, behavioral paradigms used to screen cannabinoids in mice, but to cause profound hypotension in dogs (Adams et al. 1977). This prompted us to speculate that abn-cbd may be a selective agonist of the putative vascular endothelial cannabinoid receptor. A detailed study of the pharmacology of abn-cbd supported this possibility (Járai et al. 1999). Abn-cbd does not bind to CB1 receptors in the rat brain or to the human CB2 receptor at concentrations up to 100 μM (Offertáler et al. 2003), and is inactive in the Martin behavioral tetrad in mice at doses up to 60 mg/kg (Járai et al. 1999). Yet, abn-cbd (20 mg/kg i.v.) causes SR141716-sensitive hypotension in both wild-type and CB1 receptor knockout mice. Furthermore, abn-cbd causes endothelium-dependent vasodilation in the buffer-perfused mesenteric vascular bed isolated from rats or from wild-type as well as CB1 knockout mice, and these effects are also inhibited by 1–5 μM of SR141716 (Járai et al. 1999). The parent compound of abn-cbd, cannabidiol (10 μM), has no vasodilator activity in the rat isolated, perfused mesenteric vascular bed preparation, but is able to inhibit the vasodilation caused by abn-cbd or anandamide (Járai et al. 1999). This suggested that abn-cbd is an agonist and cannabidiol is an antagonist of a novel endothelial cannabinoid receptor mediating vasodilation. Additional experiments indicated that the vasodilator response to abn-cbd is not affected by NG-nitro-l-arginine methylester (L-NAME)+indomethacin, suggesting that endothelial NO and prostacyclin are not involved. However, a combination of apamin (100 nM) and charybdotoxin (100 nM), inhibitors of calcium-activated potassium channels, significantly attenuated the vasodilation caused by abn-cbd. As the same combination of potassium channel blockers inhibits EDHF-induced mesenteric vasodilation (Randall and Kendall 1998), these findings were compatible with the possible release of EDHF through activation of this novel endothelial site (Járai et al. 1999; also see above).
Capsazepine, an inhibitor of the vanilloid TRPV1 receptor, does not influence the mesenteric vasodilator response to abn-cbd at a concentration that blocks the vasodilator effect of capsaicin or the vasodilator response to anandamide in endothelial-intact preparations (Járai et al. 1999). These observations distinguish the endothelial cannabinoid receptor from TRPV1 receptors, but are compatible with the involvement of the latter in the endothelium-independent, SR141716-insensitive component of the effect of anandamide, as suggested earlier by Zygmunt et al. (1999).
Similar conclusions were reached by Howlett and coworkers, who investigated the vasodilator action of anandamide in isolated aortic rings from rabbits (Mukhopadhyay et al. 2002). In those experiments, the vasorelaxant effect of anandamide had a major (80%) endothelium-dependent and a minor endothelium-independent component, thus making it an attractive model for further exploration of the pharmacological properties of the endothelial site (Mukhopadhyay et al. 2002). The endothelium-dependent component was found to be SR141716-sensitive and also to involve pertussis toxin (PTX)-sensitive G proteins and NO production, whereas the endothelium-independent minor component appeared to be via a PTX-insensitive mechanism involving TRPV1 receptors, CGRP, and NO (Mukhopadhyay et al. 2002). These findings are in general good agreement with the earlier findings in the rat and suggest, in addition, that the non-CB1 endothelial receptor is coupled to Gi/Go.
The possibility that the endothelial cannabinoid receptor is a GPCR is supported by recent findings in rat-isolated mesenteric artery segments (representing small conduit vessels approximately 200 μm in diameter) set up in a wire myograph (Offertáler et al. 2003). The responses of these preparations were similar to those observed using the perfused mesenteric vascular bed, where resistance changes reflect the response of precapillary arterioles (20–30 μm in diameter), in that the vasorelaxant effect of abn-cbd was NO-independent (resistant to inhibition by L-NAME) but sensitive to inhibition by apamin plus charybdotoxin. The observed insensitivity to NO, also observed in the case of anandamide in the same preparation (Harris et al. 2002), is different from the situation in the rabbit aorta (see above) or in the rat renal artery (Deutsch et al. 1997), and may reflect species- and/or vascular region-specific differences. Importantly, the vasorelaxation by abn-cbd was not inhibited by the vanilloid TRPV1 receptor antagonist capsazepine, and the inhibitory effects of the toxin combination implicate calcium-activated potassium channels. However, in agreement with the observations of Mukhopadhyay et al. (2002) with anandamide, mesenteric vasorelaxation by abn-cbd could be inhibited by PTX in endothelium-intact but not in endothelium-denuded preparations, which is compatible with the endothelial cannabinoid receptor being a GPCR coupled to Gi/Go (Offertáler et al. 2003). Ho and Hiley (2003) reported, however, that the mesenteric vasodilator effect of abn-cbd was unaffected by PTX, even though its other properties, including its endothelium dependence and susceptibility to inhibition by the compound O-1918 (see below) were similar to those reported by Offertáler et al.
An endothelial site of action of abn-cbd is further documented by its ability to activate p42/44 MAP kinase and Akt phosphorylation in cultured HUVEC (Offertáler et al. 2003). As in the earlier studies using the perfused mesenteric vascular bed, in the myograph preparations abn-cbd is a full agonist, i.e., it completely reverses phenylephrine-induced contractions with an EC50 of 2–3 μM. Unexpectedly, cannabidiol and SR141716, both of which antagonize abn-cbd-induced vasodilation in the resistance vessels of the mesenteric arterial bed preparation, act as full agonists in the small conduit vessels (EC50 of 0.82 μM for cannabidiol and 6.4 μM for SR141716). Thus, these latter compounds are most likely partial agonists rather than pure antagonists at the endothelial cannabinoid receptor. This prompted us to develop structurally modified analogs of cannabidiol to search for a pure antagonist. The compound O-1918 does not relax mesenteric arterial segments at concentrations up to 30 μM, but competitively inhibits the vasodilator response to abn-cbd without affecting vasodilation to carbachol or CGRP (Offertáler et al. 2003). The endothelial site of action of O-1918 is further supported by its ability to antagonize the activation of p42/44 mitogen-activated protein (MAP) kinase and Akt phosphorylation in HUVEC (Offertáler et al. 2003). The finding that O-1918 also inhibits the mesenteric vasorelaxant effect of anandamide strongly suggest that the same endothelial receptor is the site of action of anandamide. Similar to abn-cbd, O-1918 does not bind to CB1 or CB2 receptors. Recent studies indicate that O-1918 inhibits the mesenteric vasorelaxant effect of two putative novel endocannabinoids, N-arachidonoyl dopamine (O’Sullivan et al. 2004) and virodhamine (C.R. Hiley, personal communication). These findings are important because the relatively low potency of anandamide in eliciting mesenteric vasorelaxation could suggest that the primary endogenous ligand at these receptors is not anandamide.
Inhibition of the vasodilator effect of abn-cbd by charybdotoxin (Ho and Hiley 2003; Offertáler et al. 2003; see also above) has suggested the involvement of a calcium-activated K+ channel in this effect. In a recent study using the whole cell patch-clamp technique, we have described a voltage-dependent outward current in HUVEC that is carried by K+ ions and is blocked by charybdotoxin and iberiotoxin, suggesting the involvement of the large conductance calcium-activated potassium (BKCa) channel (Begg et al. 2003). Although abn-cbd did not elicit a current on its own, it caused a concentration-dependent increase in the voltage-induced K+ current that was sensitive to PTX, suggesting the involvement of a Gi/Go-coupled receptor. The increase in K+ current by abn-cbd was unaffected by relevant concentrations of SR141716 or SR144528, which argues against the involvement of CB1 and CB2 receptors (Begg et al. 2003). This was further supported by the lack of effect on K+ currents of HU-210, a potent CB1/CB2 receptor agonist, which is devoid of mesenteric vasodilator activity. On the other hand, the abn-cbd-induced increase in K+ current was antagonized by O-1918, which produced no effect by itself (Begg et al. 2003). The finding that the iberiotoxin-sensitive current induced by the selective BKCa opener NS-1619 was unaffected by O-1918 indicates that blockade of the effect of abn-cbd by O-1918 occurs at a site proximal to the channel, most likely at the receptor (Begg et al. 2003). This compound as well as PTX similarly inhibited the endothelium-dependent vasorelaxing effect of abn-cbd in the rat isolated mesenteric artery (Offertáler et al. 2003). This raises the possibility that activation of BKCa channels is involved in the mesenteric vasodilation mediated by this novel endothelial receptor.
The endogenous cannabinoid anandamide also increased the K+ current evoked by a single voltage step; however, its effect was only observed at high concentrations. The effect of anandamide was partially inhibited by O-1918 or PTX; thus, part of the effect of anandamide may not be mediated by the same pathway as abn-cbd (Begg et al. 2003). Anandamide acted as a full agonist in the rat isolated mesenteric artery preparation with an EC50 comparable to that of abn-cbd (Offertáler et al. 2003), suggesting that there may be subtle differences between the rat and human receptors. The low apparent efficacy of anandamide for the human endothelial receptor could also suggest the existence of an endogenous ligand(s) other than anandamide (see also above).
In HUVEC, intracellular cyclic guanosine monophosphate (cGMP) increased the voltage-induced outward current, comparable with the effect of abn-cbd. A similar increase in outward current was also produced by YC-1, an activator of soluble guanylyl cyclase. The increases evoked by abn-cbd and cGMP were inhibited by a protein kinase G inhibitor KT-5823, and the effect of abn-cbd, but not of cGMP, was also blocked by the guanylyl cyclase inhibitor 1H-(1,2,4)oxadiazolo[4,3-a]quinozalin-1-one (ODQ). Furthermore, cGMP continued to increase the K+ current under Ca2+-clamped conditions, indicating that its action is not due to modulation of [Ca2+]i. The effects of abn-cbd, cGMP, and YC-1 on K+ currents were not additive, suggesting that these compounds utilize a common intracellular pathway (Begg et al. 2003). Finally, abn-cbd was found to increase cellular cGMP levels, and this effect could be inhibited by O-1918 (Begg et al. 2003). Together, these data suggest that the novel, Gi/Go-coupled receptor activated by abn-cbd is positively coupled to guanylyl cyclase to raise intracellular cGMP, which activates protein kinase G.
Recently, it has been proposed that TRPV4 Ca2+ entry channels in vascular endothelial cells contribute to the vasorelaxant effect of anandamide via its enzymatic degradation, yielding arachidonic acid and its subsequent P450 epoxygenase-dependent metabolism (Watanabe et al. 2003). TRPV4 channels are unlikely to be involved in the effects of abn-cbd on the outward current or on vascular tone, because these effects are sensitive to PTX, whereas TRPV4-mediated calcium entry is not. Furthermore, potentiation of the outward current by abn-cbd persisted in the presence of clamped intracellular calcium (Begg et al. 2003). Also, R-methanandamide, an anandamide analog resistant to enzymatic degradation and therefore unlikely to give rise to P450 metabolites, has a mesenteric vasodilator effect similar to that of anandamide (Wagner et al. 1999).
Extracellular calcium is known to have a potent vasodilator effect, particularly in the mesenteric circulation, where it is thought to contribute to the postprandial vasodilation associated with the intestinal absorption of nutrients. Bukoski and coworkers found that SR141716 can inhibit Ca2+-induced mesenteric vasodilation through a sensory nerve-dependent mechanism, which led them to suggest that anandamide may be a sensory nerve-derived vasodilator mediator (Ishioka and Bukoski 1999). Interestingly, O-1918 also inhibits calcium-induced mesenteric vasorelaxation, which is similar in wild-type and CB1 receptor knockout mice (Bukoski et al. 2002), suggesting that the vasodilation by extracellular calcium is most likely mediated by the endothelial abn-cbd-sensitive receptor.
Collectively, the above-mentioned results indicate that the synthetic cannabinoid ligands abn-cbd and O-1918 act as a selective agonist and silent antagonist, respectively, of a novel vascular endothelial receptor distinct from CB1 and CB2 that mediates mesenteric vasodilation, and is coupled to a phosphoinositide (PI)3-kinase/Akt-dependent pathway through Gi/Go.
3.3 Direct Cardiodepressant Effects of Cannabinoids
In contrast to the growing knowledge on the vascular effects of cannabinoids, little is known about cannabinoid-induced direct cardiac effects. The endocannabinoid anandamide (Felder et al. 1996), anandamide amidohydrolase (Bilfinger et al. 1998), and traces of the message for the CB1 receptor (Galiegue et al. 1995) have all been detected in the human heart. In a more recent study, the existence of CB1 receptors was confirmed in human atrial myocytes by immunoblotting and immunohistochemistry (Bonz et al. 2003). In the same study, it was demonstrated that anandamide, R-methanandamide, and HU-210 dose-dependently decrease contractile performance in isolated, electrically paced human atrial muscle. A selective and potent CB1 antagonist, AM251 (Gatley et al. 1997), blocked the negative inotropic effect of all three drugs, and the involvement of CB2 receptor activation, NO, or prostanoid release could all be excluded (Bonz et al. 2003). Consistently with these in vitro results, HU-210 decreases cardiac output in rats in vivo in a CB1 receptor-dependent manner (Wagner et al. 2001b). Previous studies have also demonstrated that anandamide caused SR141716-sensitive coronary vasorelaxation in isolated perfused rat hearts (Randall and Kendall 1997; Fulton and Quilley 1998), implicating cannabinoid receptors. These effects of anandamide were not mimicked by arachidonic acid, indicating that the vasodilator effect was not mediated by arachidonic acid metabolites.
In isolated, perfused, rat Langendorff heart preparations, anandamide and R-methanandamide, but not palmitoylethanolamide or the selective CB2 receptor agonist JWH015, significantly reduced both left ventricular developed and coronary perfusion pressures, indicating decreased myocardial contractile function and coronary vasodilation (Ford et al. 2002). Interestingly, anandamide-mediated vasodilatation and negative inotropy were both sensitive to inhibition by the CB1 antagonist SR141716 and the CB2 antagonist SR144528, but not to the TRPV1 antagonist capsazepine, which led the authors to propose a novel site distinct from classic CB1 and CB2 receptors (Ford et al. 2002).
In agreement with the observations on isolated cardiac preparations, our recent unpublished results using the Millar pressure-volume conductance system (see below and also Figs. 1 and 2) to directly measure cardiac performance in vivo strongly suggest the crucial importance of the cardiac component in the hemodynamic effects of cannabinoids. Taken together, the above-mentioned studies suggest that CB1 receptors are present in cardiomyocytes, and cannabinoids may decrease cardiac contractility through both CB1-dependent and CB1-independent mechanisms.
4 Role of Vanilloid TRPV1 Receptors in the Cardiovascular Effects of Cannabinoids
Structural similarities between anandamide and vanilloid compounds such as capsaicin (Di Marzo et al. 1998) raised the possibility of an interplay between these two systems. Indeed, Zygmunt et al. (1999) demonstrated that in rat mesenteric arteries, the endothelium-independent vasodilator effect of anandamide is inhibited by the TRPV1 receptor antagonist capsazepine or by a CGRP receptor antagonist. They further demonstrated that anandamide binds to the cloned TRPV1 receptor with micromolar affinity, and at nanomolar concentrations it releases immunoreactive CGRP from sensory nerve terminals located in the vascular adventitia (Zygmunt et al. 1999). A similar involvement of TRPV1 receptors in the mesenteric vasodilator action of methanandamide has also been suggested (Ralevic et al. 2000). These observations support the hypothesis that anandamide-induced vasodilation involves activation of TRPV1 receptors in sensory nerves and the subsequent release of the potent vasodilator peptide CGRP.
The above observations do not implicate the endothelium in the vasodilator response to anandamide. Other studies, which documented both endothelium-dependent and endothelium-independent components for the vasodilator effect of anandamide, confirmed the role of TRPV1 receptors but only for the endothelium-independent component (Járai et al. 1999; Mukhopadhyay et al. 2002). The endothelium-dependent vasodilator effect of anandamide in the rabbit aorta or the similar effect of abn-cbd in rat mesenteric arteries is unaffected by capsazepine (Mukhopadhyay et al. 2002; Járai et al. 1999; Offertáler et al. 2003; Ho and Hiley 2003). Interestingly, sensory nerve terminals also appear to have CB1 receptors, stimulation of which by very low doses of anandamide or by the synthetic cannabinoid HU-210, neither of which results in activation of TRPV1 receptors, inhibits sensory neurotransmission (reviewed in Ralevic et al. 2002). Furthermore, a recent study by Zygmunt et al. (2002) indicates that THC and cannabinol, but not other psychotropic cannabinoids, can elicit CGRP release from periarterial sensory nerves by a mechanism that is independent of not only CB1 and CB2 receptors, but also of vanilloid TRPV1 receptors. Thus, the sensory nerve-dependent effects of cannabinoids are complex, as interactions with CB1 and TRPV1 receptors appear to have opposite functional consequences, and there may be additional actions independent of both of these receptors. TRPV1 receptors are not involved in the dilation of isolated coronary arteries by anandamide either in the sheep, where the effect is endothelium dependent (Grainger and Boachie-Ansah 2001), or in the rat, where it is endothelium independent (White et al. 2001). Furthermore, in the rat mesenteric arterial bed, the role of sensory nerves and vanilloid receptors in the dilator effect of anandamide was found to be conditional on the presence of NO (Harris et al. 2002).
TRPV1-containing afferent nerve fibers are present on the epicardial surface of the heart and the activation of these receptors by epicardially injected capsaicin evokes a sympathoexcitatory response with a brief increase in blood pressure (Zahner et al. 2003). Capsaicin infusion also induces a moderate pressor effect in pigs (Kapoor et al. 2003). We have recently found (Pacher et al. 2004) that i.v. injection of 10 μg/kg capsaicin evokes only a brief pressor response in wild-type mice, while at the much higher dose of 100 μg/kg its effect has both a depressor and a pressor component. In contrast, capsaicin elicited no change in blood pressure in TRPV1−/− mice, suggesting that TRPV1 receptors mediate the cardiogenic sympathetic or Bezold-Jarisch reflex in mice, which is in agreement with recent reports in which the cardiovascular effects of capsaicin were inhibited by TRPV1 receptor antagonists (Smith and McQueen 2001; Zahner et al. 2003).
In the absence of anandamide-induced hypotension in CB1 knockout mice, the physiological relevance of the interaction of anandamide with TRPV1 receptors has been questioned (Szolcsányi 2000). We previously reported that anandamide causes a triphasic blood pressure response where the predominant hypotensive effect is preceded by a transient, vagally mediated drop in heart rate and blood pressure followed by a brief pressor response (Varga et al. 1995, see also Sect. 2 and Fig. 1). This initial component is missing in TRPV1−/− mice (Pacher et al. 2004) and is unaffected by the CB1 antagonist SR141716 in rats (Varga et al. 1995). Bolus intravenous injections of anandamide may reach high enough plasma concentrations for a few seconds to activate TRPV1 receptors, which may explain the above findings. Indeed, the phase I transient hypotension and bradycardia do not appear when anandamide is injected slowly to limit its peak plasma concentration (Z. Jarai, J.A. Wagner, G. Kunos, unpublished observations). In contrast, the prolonged hypotensive phase of the anandamide response is characterized by decreased cardiac contractility and total peripheral resistance (TPR), which are similar in TRPV1+/+ and TRPV1−/− mice and were completely antagonized by SR141716, implicating CB1 receptors (Pacher et al. 2004). In agreement with this observation, the sustained hypotensive and bradycardic effects of cannabinoids are totally absent in mice lacking the CB1 receptor (Ledent et al. 1999; Járai et al. 1999). Thus, TRPV1 receptors are not involved in the sustained cardiovascular response to anandamide, but may become transiently activated in response to pharmacological concentrations achieved after bolus i.v. injections. These findings are in agreement with a recent report by Malinowska et al (2001) who found that in rats the transient vagal activation to a bolus injection of anandamide was partially blocked by the TRPV1 antagonists capsazepine or ruthenium red, whereas the CB1-mediated prolonged hypotension remained unaffected.
Collectively, the above-mentioned studies show that the sustained hypotensive effect of anandamide involves a marked cardiodepressor component in addition to a decrease in TPR, and these effects are mediated by CB1 but not TRPV1 receptors. The role of TRPV1 receptors is limited to the transient activation of the Bezold-Jarisch reflex by very high plasma concentrations of anandamide.
5 Pathophysiological Role of the Endocannabinergic System in Cardiovascular Disorders
Recent studies indicate that the endogenous cannabinergic system plays an important role in cardiovascular regulation under various pathophysiological conditions, and pharmacological manipulation of this system may offer novel therapeutic approaches in a variety of cardiovascular disorders.
5.1 Role of the Endocannabinergic System in Hemorrhagic, Endotoxic, and Cardiogenic Shock and Liver Cirrhosis
The profound and long-lasting, yet reversible, hypotension elicited by potent synthetic cannabinoids (Lake et al. 1997a) suggested that endocannabinoids may be involved in pathological conditions associated with extreme hypotension such as various forms of shock. Observations over the last decade have provided evidence for a key role of endocannabinoids in the hypotension associated with hemorrhagic (Wagner et al. 1997), endotoxic (Varga et al. 1998; Liu et al. 2003; Bátkai et al. 2004a; Wang et al. 2001; Godlewski et al. 2004), and cardiogenic shock (Wagner et al. 2001a, 2003). The vasodilated state associated with advanced liver cirrhosis appears to be due to a similar mechanism (Bátkai et al. 2001; Ros et al. 2002), which is most likely secondary to the endotoxemia commonly found in patients with late-stage cirrhosis (Lumsden et al. 1988).
In many of these conditions, circulating macrophages and platelets were found to contain elevated levels of endocannabinoids and to elicit SR141716-sensitive hypotension when injected into normal rats, suggesting the involvement of CB1 receptors. In cirrhosis this was also suggested by the observed increase in CB1 receptor mRNA and binding sites in vascular endothelial cells from cirrhotic human livers (Bátkai et al. 2001). However, SR141716 can also inhibit a receptor(s) distinct from CB1 or CB2 (see above), so the relative role of such receptors versus CB1 receptors needed to be explored. In a recent study (Bátkai et al. 2004a), we reported that the acute hypotensive response of anesthetized rats to lipopolysaccharide (LPS) is inhibited by SR141716, but not by AM251, an antagonist equipotent with SR141716 at CB1 receptors (Gatley et al. 1997), but devoid of inhibitory potency at the SR141716-sensitive endothelial and myocardial receptors described above (Ford et al. 2002; Ho and Hiley 2003; O’Sullivan et al. 2004). Furthermore, LPS caused similar, SR141716-sensitive hypotension in wild-type mice and in mice deficient in CB1 or both CB1 and CB2 receptors. Detailed hemodynamic analysis of the effects of LPS also indicated that the hypotension is primarily due to decreased cardiac contractility rather than decreased peripheral vascular resistance. These findings therefore suggest that receptors distinct from CB1 or CB2 are primarily responsible for the acute, SR141716-sensitive hypotensive response to LPS (Bátkai et al. 2004a). Anandamide is a ligand for such receptors and LPS stimulates the synthesis of anandamide in macrophages (Liu et al. 2003). Whether LPS may induce the synthesis of additional endogenous ligands for such receptors remains to be determined. Increased target organ sensitivity to anandamide may also play a role in the hemodynamic effects of LPS, as suggested by the potentiation of the vasodilator effect of anandamide in mesenteric beds isolated from endotoxemic rats (Orliac et al. 2003).
Endocannabinoid-mediated cardiovascular effects appear to have survival value, as indicated by the increased mortality, despite the increase in blood pressure, following blockade of CB1 receptors in hemorrhagic (Wagner et al. 1997) and cardiogenic shock (Wagner et al. 2001a, 2003). In contrast, treatment with the cannabinoid agonists THC or HU-210 improved endothelial function and increased survival in cardiogenic (Wagner et al. 2001a, 2003) and endotoxic shock (Varga et al., 1998). Endocannabinoids may improve tissue oxygenation in these conditions by counteracting the excessive sympathetic vasoconstriction triggered by hemorrhage or myocardial infarction. In addition, endocannabinoids may mediate important protective mechanisms against hypoxic damage in the heart and vasculature and also exert potent anti-inflammatory effects (reviewed in Hiley and Ford 2003, 2004; Walter and Stella 2004, see also below).
5.2 Role of the Endocannabinergic System in Myocardial Reperfusion Damage
Interest in the investigation of potential cardioprotective effects of cannabinoids has recently been rekindled by a study of Lagneux and Lamontagne (2001) in which they compared the effects of a period of 90 min of low-flow ischaemia, followed by 60 min reperfusion at normal flow, in isolated hearts from rats pre-treated with bacterial endotoxin or saline. Endotoxin pretreatment reduced infarct size and enhanced functional recovery on reperfusion relative to the saline controls (preconditioning). The beneficial effects of endotoxin-induced preconditioning were blocked by the CB2 receptor antagonist SR144528 but not by the CB1 antagonist SR141716 (Lagneux and Lamontagne, 2001). Similarly, Joyeux et al. (2002) found that the infarct-size-reducing effect of heat stress preconditioning was also abolished by SR144528 but not by SR141716. In contrast, in an isolated perfused rat heart model in which preconditioning was induced by a brief period of ischemia (5 min), blockade of either CB1 receptors with SR141716 or CB2 receptors with SR1445278 abolished the protective effect of preconditioning. Preconditioning (5 min) also preserved the endothelium-dependent vasodilation evoked by serotonin (5-HT) in another study (Bouchard et al. 2003) in which both CB1 and CB2 receptors were implicated. Lepicier et al. (2003) have shown that the CB2 receptor-mediated cardioprotection by endocannabinoids and synthetic cannabinoid ligands involves p38/ERK1/2 and protein kinase C (PKC) activation.
In an anesthetized rat model of ischemia/reperfusion injury (I/R), induced by coronary occlusion/reocclusion, Krylatov et al. have demonstrated that HU-210 and anandamide reduced the infarct size and the incidence of ventricular arrhythmias through activation of CB2 but not CB1 receptors (Krylatov et al. 2001; 2002a,b,c; Ugdyzhenkova et al. 2001; 2002). More recently, in an anesthetized mouse model of myocardial I/R, the mixed CB1/CB2 receptor agonist WIN55,212-2 significantly reduced the extent of leukocyte-dependent myocardial damage. The protective effect of WIN55,212-2 was abolished by the selective CB2 receptor antagonist AM630 and not affected by the selective CB1 receptor antagonist AM251 (DiFilippo C et al. 2004).
5.3 Role of Endocannabinergic System in Hypertension
As early as in the 1970s, the potential use of cannabinoid ligands as antihypertensive agents had been considered (Archer 1974; Crawford and Merritt 1979; Varma et al. 1975; Zaugg and Kyncl 1983), in the hope that their neurobehavioral and cardiovascular effects could be separated. Although an early study in normotensive rats indicated rapidly developing tolerance to the hypotensive and bradycardic effects of THC (Adams et al. 1976), a subsequent study in spontaneously hypertensive rats (SHR) found no evidence for tolerance for the same effects during a similar, 10-day treatment period (Kosersky 1978).
Following the introduction of selective CB receptor antagonists in the mid 1990s, the finding that treatment of normotensive rats or mice with CB1 antagonists alone does not affect blood pressure (Lake et al. 1997a; Varga et al. 1995), and that baseline blood pressure is similar in CB1 knockout mice and their wild-type littermates (Járai et al. 1999; Ledent et al. 1999), indicated the absence of an endocannabinergic “tone” in the maintenance of normal levels of blood pressure. This is also suggested by the lack of significant hypotension following inhibition of anandamide transport (Calignano et al. 1997), in agreement with the relatively modest hypotensive effect of anandamide in normotensive animals (Lake et al. 1997a; Varga et al. 1995). However, SHR respond with greater and longer-lasting hypotension than normotensive rats to both THC (Kosersky 1978) and anandamide (Lake et al. 1997b; Bátkai et al. 2004b). THC inhalation evokes a greater and longer-lasting decrease of arterial blood pressure in hypertensive as compared to normotensive individuals (Crawford and Merritt 1979). Although the mechanism underlying this increased sensitivity has not been explored, it could suggest a role for the endocannabinoid system in regulating cardiovascular functions in hypertension. In a recent study we used three different models of experimental hypertension to explore this possibility (Bátkai et al. 2004b). The results document a significant endocannabinergic tone in hypertension that limits increases in blood pressure and cardiac contractile performance through tonic activation of cardiac and vascular CB1. They also indicate that upregulation of cardiac and vascular CB1 contributes to this tone, the potentiation of which, by inhibiting the inactivation of endogenous anandamide, can normalize blood pressure and cardiac contractile performance in hypertension. These findings raise the interesting possibility of the therapeutic use of inhibitors of fatty acid amide hydrolase in the treatment of hypertension.
6 Conclusions
Functional CB1 receptors are present in vascular tissue as well as the myocardium, and cannabinoid agonists and endocannabinoids exert major hypotensive and cardiodepressor effects in vivo through the stimulation of CB1 receptors. There is evidence for the existence of an as-yet-undefined endothelial and cardiac receptor or receptors that mediate certain endocannabinoid-induced cardiovascular effects. Vanilloid TRPV1 receptors can be activated by anandamide, but the role of these receptor in in vivo hemodynamic effects appears to be limited to the transient activation of the Bezold-Jarisch reflex by very high initial plasma concentrations of anandamide.
Endocannabinoids play important roles in a variety of pathophysiological conditions including hemorrhagic, endotoxic, and cardiogenic shock, and in the hemodynamic sequelae of advanced liver cirrhosis. Furthermore, pharmacological manipulation of the endocannabinoid system may offer novel therapeutic approaches in hypertension and ischemic heart disease.
References
- Abadji V, Lin S, Taha G, Griffin G, Stevenson LA, Pertwee RG, Makriyannis A. (R)-Methanandamide: a chiral novel anandamide possessing higher potency and metabolic stability. J Med Chem. 1994;37:1889–1893. doi: 10.1021/jm00038a020. [DOI] [PubMed] [Google Scholar]
- Adams MD, Chait LD, Earnhardt JT. Tolerance to the cardiovascular effects of delta9-tetrahydrocannabinol in the rat. Br J Pharmacol. 1976;56:43–48. doi: 10.1111/j.1476-5381.1976.tb06957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams MD, Earnhardt JT, Martin BR, Harris LS, Dewey WL, Razdan RK. A cannabinoid with cardiovascular activity but no overt behavioral effects. Experientia. 1977;33:1204–1205. doi: 10.1007/BF01922330. [DOI] [PubMed] [Google Scholar]
- Archer RA. The cannabinoids: therapeutic potentials. Annu Rep Med Chem. 1974;9:253–259. doi: 10.1016/s0065-7743(08)61448-7. [DOI] [PubMed] [Google Scholar]
- Bátkai S, Járai Z, Wagner JA, Goparaju SK, Varga K, Liu J, Wang L, Mirshahi F, Khanolkar AD, Makriyannis A, Urbascheck R, Garcia N, Jr, Sanyal AJ, Kunos G. Endocannabinoids acting at vascular CB1 receptors mediate the vasodilated state in advanced liver cirrhosis. Nat Med. 2001;7:827–832. doi: 10.1038/89953. [DOI] [PubMed] [Google Scholar]
- Bátkai S, Pacher P, Osei-Hyiaman D, Radaeva S, Offertáler L, Bukoski RD, Kunos G. Endocannabinoids are involved in regulating cardiovascular function in spontaneously hypertensive rats. Hypertension. 2003;42:A263. [Google Scholar]
- Bátkai S, Pacher P, Járai Z, Wagner JA, Kunos G. Cannabinoid antagonist SR141716 inhibits endotoxic hypotension by a cardiac mechanism not involving CB1 or CB2 receptors. Am J Physiol Heart Circ Physiol. 2004a;287:H595–H600. doi: 10.1152/ajpheart.00184.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bátkai S, Pacher P, Osei-Hyiaman D, Radaeva S, Liu J, Harvey-White J, Offertaler L, Mackie K, Rudd MA, Bukoski RD, Kunos G. Endocannabinoids acting at CB1 receptors regulate cardiovascular function in hypertension. Circulation. 2004b;110(14):1996–2002. doi: 10.1161/01.CIR.0000143230.23252.D2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg M, Baydoun A, Parsons ME, Molleman A. Signal transduction of cannabinoid CB1 receptors in a smooth muscle cell line. J Physiol. 2001;531:95–104. doi: 10.1111/j.1469-7793.2001.0095j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg M, Mo FM, Offertaler L, Bátkai S, Pacher P, Razdan RK, Lovinger DM, Kunos G. G protein-coupled endothelial receptor for atypical cannabinoid ligands modulates a Ca2+-dependent K+ current. J Biol Chem. 2003;278:46188–46194. doi: 10.1074/jbc.M307258200. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Jones RT. Cardiovascular effects of prolonged delta-9-tetrahydro-cannabinol ingestion. Clin Pharmacol Ther. 1975;18:287–297. doi: 10.1002/cpt1975183287. [DOI] [PubMed] [Google Scholar]
- Bilfinger TV, Salzet M, Fimiani C, Deutsch DG, Tramu G, Stefano GB. Pharmacological evidence for anandamide amidase in human cardiac and vascular tissues. Int J Cardiol. 1998;64:S15–22. doi: 10.1016/s0167-5273(98)00031-x. [DOI] [PubMed] [Google Scholar]
- Birmingham MK. Reduction by 9-tetrahydrocannabinol in the blood pressure of hypertensive rats bearing regenerated adrenal glands. Br J Pharmacol. 1973;48:169–171. doi: 10.1111/j.1476-5381.1973.tb08236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, Hanus L, De Petrocellis L, Tchilibon S, Ponde DE, Brandi I, Moriello AS, Davis JB, Mechoulam R, Di Marzo V. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol. 2001;134:845–852. doi: 10.1038/sj.bjp.0704327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonz A, Laser M, Kullmer S, Kniesch S, Babin-Ebell J, Popp V, Ertl G, Wagner JA. Cannabinoids acting on CB1 receptors decrease contractile performance in human atrial muscle. J Cardiovasc Pharmacol. 2003;41:657–664. doi: 10.1097/00005344-200304000-00020. [DOI] [PubMed] [Google Scholar]
- Bouchard JF, Lepicier P, Lamontagne D. Contribution of endocannabinoids in the endothelial protection afforded by ischemic preconditioning in the isolated rat heart. Life Sci. 2003;72:1859–1870. doi: 10.1016/s0024-3205(02)02474-8. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Griffin G, Di Marzo V, Martin BR. Evidence for a new G protein-coupled receptor in mouse brain. Mol Pharmacol. 2001;60:155–163. [PubMed] [Google Scholar]
- Bukoski RD, Bátkai S, Járai Z, Wang Y, Offertáler L, Jackson WF, Kunos G. CB(1) receptor antagonist SR141716A inhibits Ca(2+)-induced relaxation in CB(1) receptor-deficient mice. Hypertension. 2002;39:251–257. doi: 10.1161/hy0202.102702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calignano A, La Rana G, Beltramo M, Makriyannis A, Piomelli D. Potentiation of anandamide hypotension by the transport inhibitor, AM404. Eur J Pharmacol. 1997;337:R1–R2. doi: 10.1016/s0014-2999(97)01297-1. [DOI] [PubMed] [Google Scholar]
- Carruba MO, Bondiolotti G, Picotti GB, Catteruccia N, Da Prada M. Effects of diethyl ether, halothane, ketamine and urethane on sympathetic activity in the rat. Eur J Pharmacol. 1987;134:15–24. doi: 10.1016/0014-2999(87)90126-9. [DOI] [PubMed] [Google Scholar]
- Chataigneau T, Feletou M, Thollon C, Villeneuve N, Vilaine J-P, Duhault J, Vanhoutte PM. Cannabinoid CB1 receptor and endothelium-dependent hyperpolarization in guinea-pig carotid, rat mesenteric and porcine coronary arteries. Br J Pharmacol. 1998;123:968–974. doi: 10.1038/sj.bjp.0701690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaytor AT, Martin PEM, Evans WH, Randall MD, Griffith TM. The endothelial component of cannabinoid-induced relaxation in rabbit mesenteric artery depends on gap junctional communication. J Physiol (Lond) 1999;520:539–550. doi: 10.1111/j.1469-7793.1999.00539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopoulos A, Coles P, Lay L, Lew MJ, Angus JA. Pharmacological analysis of cannabinoid receptor activity in the rat vas deferens. Br J Pharmacol. 2001;132:1281–1291. doi: 10.1038/sj.bjp.0703930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford WJ, Merritt JC. Effects of tetrahydrocannabinol on arterial and intraocular hypertension. Int J Clin Pharmacol Biopharm. 1979;17:191–196. [PubMed] [Google Scholar]
- Darker IT, Millns PJ, Selbie L, Randall MD, S-Baxter G, Kendall DA. Cannabinoid (CB1) receptor expression is associated with mesenteric resistance vessels but not thoracic aorta in the rat. Br J Pharmacol. 1988;125:95P. [Google Scholar]
- Deutsch DG, Goligorsky MS, Schmid PC, Krebsbach RJ, Schmid HH, Das SK, Dey SK, Arreaza G, Thorup C, Stefano G, Moore LC. Production and physiological actions of anandamide in the vasculature of the rat kidney. J Clin Invest. 1997;100:1538–1546. doi: 10.1172/JCI119677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Di Filippo C, Rossi F, Rossi S, DAmico M. Cannabinoid CB2 receptor activation reduces mouse myocardial ischemia-reperfusion injury: involvement of cytokine/chemokines and PMN. J Leukoc Biol. 2004;75:453–459. doi: 10.1189/jlb.0703303. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Bisogno T, Melck D, Ross R, Brockie H, Stevenson L, Pertwee R, De Petrocellis L. Interactions between synthetic vanilloids and the endogenous cannabinoids system. FEBS Lett. 1998;436:449–454. doi: 10.1016/s0014-5793(98)01175-2. [DOI] [PubMed] [Google Scholar]
- Ellis EF, Moore SF, Willoughby KA. Anandamide and delta 9-THC dilation of cerebral arterioles is blocked by indomethacin. Am J Physiol. 1995;269:H1859–H1864. doi: 10.1152/ajpheart.1995.269.6.H1859. [DOI] [PubMed] [Google Scholar]
- Felder CC, Nielsen A, Briley EM, Palkovits M, Priller J, Axelrod J, Nguyen DN, Richardson JM, Riggin RM, Koppel GA, Paul SM, Becker GW. Isolation and measurement of the endogenous cannabinoid receptor agonist, anandamide, in brain and peripheral tissues of human and rat. FEBS Lett. 1996;393:231–235. doi: 10.1016/0014-5793(96)00891-5. [DOI] [PubMed] [Google Scholar]
- Fimiani C, Mattocks D, Cavani F, Salzet M, Deutsch DG, Pryor S, Bilfinger TV, Stefano GB. Morphine and anandamide stimulate intracellular calcium transients in human arterial endothelial cells: coupling to nitric oxide release. Cell Signal. 1999;11:189–193. doi: 10.1016/s0898-6568(98)00060-6. [DOI] [PubMed] [Google Scholar]
- Fleming I, Schermer B, Popp R, Busse R. Inhibition of the production of endothelium-derived hyperpolarizing factor by cannabinoid receptor agonists. Br J Pharmacol. 1999;126:949–960. doi: 10.1038/sj.bjp.0702381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford WR, Honan SA, White R, Hiley CR. Evidence of a novel site mediating anandamide-induced negative inotropic and coronary vasodilator responses in rat isolated hearts. Br J Pharmacol. 2002;135:1191–1198. doi: 10.1038/sj.bjp.0704565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton DJ, Quilley J. Evidence against anandamide as the hyperpolarizing factor mediating the nitric oxide-independent coronary vasodilator effect of bradykinin in the rat. J Pharmacol Exp Ther. 1998;286:1146–1151. [PubMed] [Google Scholar]
- Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, Bouaboula M, Shire D, Le Fur G, Casellas P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- Gatley SJ, Lan R, Pyatt B, Gifford AN, Volkow ND, Makriyannis A. Binding of the non-classical cannabinoid CP 55,940, and the diarylpyrazole AM251 to rodent brain cannabinoid receptors. Life Sci. 1997;61:PL191–197. doi: 10.1016/s0024-3205(97)00690-5. [DOI] [PubMed] [Google Scholar]
- Gebremedhin D, Lange AR, Campbell WB, Hillard CJ, Harder DR. Cannabinoid CB1 receptor of cat cerebral arterial muscle functions to inhibit L-type Ca2+ channel current. Am J Physiol. 1999;266:H2085–H2093. doi: 10.1152/ajpheart.1999.276.6.H2085. [DOI] [PubMed] [Google Scholar]
- Godlewski G, Malinowska B, Schlicker E. Presynaptic cannabinoid CB1 receptors are involved in the inhibition of the neurogenic vasopressor response during septic shock in pithed rats. Br J Pharmacol. 2004;142:701–708. doi: 10.1038/sj.bjp.0705839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger J, Boachie-Ansah G. Anandamide-induced relaxation of sheep coronary arteries: the role of the vascular endothelium, arachidonic acid metabolites and potassium channels. Br J Pharmacol. 2001;134:1003–1012. doi: 10.1038/sj.bjp.0704340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajos N, Ledent C, Freund TF. Novel cannabinoid-sensitive receptor mediates inhibition of glutamatergic synaptic transmission in the hippocampus. Neuroscience. 2001;106:1–4. doi: 10.1016/s0306-4522(01)00287-1. [DOI] [PubMed] [Google Scholar]
- Hanus L, Abu-Lafi S, Fride E, Breuer A, Vogel Z, Shalev DE, Kustanovich I, Mechoulam R. 2-Arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor. Proc Natl Acad Sci U S A. 2001;98:3662–3665. doi: 10.1073/pnas.061029898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D, McCulloch AI, Kendall DA, Randall MD. Characterization of vasorelaxant responses to anandamide in the rat mesenteric arterial bed. J Physiol. 2002;539:893–902. doi: 10.1113/jphysiol.2001.013489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiley CR, Ford WR. Endocannabinoids as mediators in the heart: a potential target for therapy of remodelling after myocardial infarction? Br J Pharmacol. 2003;138:1183–1184. doi: 10.1038/sj.bjp.0705155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiley CR, Ford WR. Cannabinoid pharmacology in the cardiovascular system: potential protective mechanisms through lipid signalling. Biol Rev Camb Philos Soc. 2004;79:187–205. doi: 10.1017/s1464793103006201. [DOI] [PubMed] [Google Scholar]
- Hillard CJ. Endocannabinoids and vascular function. J Pharmacol Exp Ther. 2000;294:27–32. [PubMed] [Google Scholar]
- Ho WS, Hiley CR. Vasodilator actions of abnormal-cannabidiol in rat isolated small mesenteric artery. Br J Pharmacol. 2003;138:1320–1332. doi: 10.1038/sj.bjp.0705160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland M, John Challiss RA, Standen NB, Boyle JP. Cannabinoid CB1 receptors fail to cause relaxation, but couple via Gi/Go to the inhibition of adenylyl cyclase in carotid artery smooth muscle. Br J Pharmacol. 1999;128:597–604. doi: 10.1038/sj.bjp.0702842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC, Bidaut-Russell M, Devane WA, Melvin LS, Johnson MR, Herkenham M. The cannabinoid receptor: biochemical, anatomical and behavioral characterization. Trends Neurosci. 1990;13:420–423. doi: 10.1016/0166-2236(90)90124-s. [DOI] [PubMed] [Google Scholar]
- Huestis MA, Gorelick DA, Heishman SJ, Preston KL, Nelson RA, Moolchan ET, Frank RA. Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716. Arch Gen Psychiatry. 2001;58:322–328. doi: 10.1001/archpsyc.58.4.322. [DOI] [PubMed] [Google Scholar]
- Ishac EJ, Jiang L, Lake KD, Varga K, Abood ME, Kunos G. Inhibition of exocytotic noradrenaline release by presynaptic cannabinoid CB1 receptors on peripheral sympathetic nerves. Br J Pharmacol. 1996;118:2023–2028. doi: 10.1111/j.1476-5381.1996.tb15639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishioka N, Bukoski RD. A role for N-arachidonylethanolamine (anandamide) as the mediator of sensory nerve-dependent Ca2+-induced relaxation. J Pharmacol Exp Ther. 1999;289:245–250. [PubMed] [Google Scholar]
- Járai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BR, Zimmer AM, Bonner TI, Buckley NE, Mezey E, Razdan RK, Zimmer A, Kunos G. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc Natl Acad Sci U S A. 1999;96:14136–14141. doi: 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Járai Z, Wagner JA, Goparaju SK, Wang L, Razdan RK, Sugiura T, Zimmer AM, Bonner TI, Zimmer A, Kunos G. Cardiovascular effects of 2-arachidonoyl glycerol in anesthetized mice. Hypertension. 2000;35:679–684. doi: 10.1161/01.hyp.35.2.679. [DOI] [PubMed] [Google Scholar]
- Joyeux M, Arnaud C, Godin-Ribuot D, Demenge P, Lamontagne D, Ribuot C. Endo-cannabinoids are implicated in the infarct size-reducing effect conferred by heat stress preconditioning in isolated rat hearts. Cardiovasc Res. 2002;55:619–625. doi: 10.1016/s0008-6363(02)00268-7. [DOI] [PubMed] [Google Scholar]
- Kanakis C, Pouget JM, Rosen KM. The effects of Δ9-THC (cannabis) on cardiac performance with or without beta blockade. Circulation. 1976;53:703–709. doi: 10.1161/01.cir.53.4.703. [DOI] [PubMed] [Google Scholar]
- Kapoor K, Arulmani U, Heiligers JP, Garrelds IM, Willems EW, Doods H, Villalon CM, Saxena PR. Effects of the CGRP receptor antagonist BIBN4096BS on capsaicin-induced carotid haemodynamic changes in anaesthetised pigs. Br J Pharmacol. 2003;140:329–338. doi: 10.1038/sj.bjp.0705451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosersky DS. Antihypertensive effects of delta9-tetrahydrocannabinol. Arch Int Pharmacodyn Ther. 1978;233:76–81. [PubMed] [Google Scholar]
- Krylatov AV, Ugdyzhekova DS, Bernatskaya NA, Maslov LN, Mechoulam R, Pertwee RG, Stephano GB. Activation of type II cannabinoid receptors improves myocardial tolerance to arrhythmogenic effects of coronary occlusion and reperfusion. Bull Exp Biol Med. 2001;131:523–525. doi: 10.1023/a:1012381914518. [DOI] [PubMed] [Google Scholar]
- Krylatov AV, Bernatskaia NA, Maslov LN, Pertwee RG, Mechoulam R, Stefano GB, Sharaevskii MA, Sal’nikova OM. Increase of the heart arrhythmogenic resistance and decrease of the myocardial necrosis zone during activation of cannabinoid receptors. Ross Fiziol Zh Im I M Sechenova. 2002a;88:560–567. [PubMed] [Google Scholar]
- Krylatov AV, Uzhachenko RV, Maslov LN, Bernatskaya NA, Makriyannis A, Mechoulam R, Pertwee RG, Sal’nikova OM, Stefano JB, Lishmanov Y. Endogenous cannabinoids improve myocardial resistance to arrhythmogenic effects of coronary occlusion and reperfusion: a possible mechanism. Bull Exp Biol Med. 2002b;133:122–124. doi: 10.1023/a:1015574100494. [DOI] [PubMed] [Google Scholar]
- Krylatov AV, Uzhachenko RV, Maslov LN, Ugdyzhekova DS, Bernatskaia NA, Pertwee R, Stefano GB, Makriyannis A. Anandamide and R-(+)-methanandamide prevent development of ischemic and reperfusion arrhythmia in rats by stimulation of CB2-receptors. Eksp Klin Farmakol. 2002c;65:6–9. [PubMed] [Google Scholar]
- Kunos G, Járai Z, Bátkai S, Goparaju SK, Ishac EJ, Liu J, Wang L, Wagner JA. Endocannabinoids as cardiovascular modulators. Chem Phys Lipids. 2000;108:159–168. doi: 10.1016/s0009-3084(00)00194-8. [DOI] [PubMed] [Google Scholar]
- Kunos G, Bátkai S, Offertáler L, Mo F, Liu J, Karcher J, Harvey-White J. The quest for a vascular endothelial cannabinoids receptor. Chem Phys Lipids. 2002;121:45–56. doi: 10.1016/s0009-3084(02)00145-7. [DOI] [PubMed] [Google Scholar]
- Lagneux C, Lamontagne D. Involvement of cannabinoids in the cardioprotection induced by lipopolysaccharide. Br J Pharmacol. 2001;132:793–796. doi: 10.1038/sj.bjp.0703902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake KD, Compton DR, Varga K, Martin BR, Kunos G. Cannabinoid-induced hypotension and bradycardia in rats mediated by CB1-like cannabinoid receptors. J Pharmacol Exp Ther. 1997a;281:1030–1037. [PubMed] [Google Scholar]
- Lake KD, Martin BR, Kunos G, Varga K. Cardiovascular effects of anandamide in anesthetized and conscious normotensive and hypertensive rats. Hypertension. 1997b;29:1204–1210. doi: 10.1161/01.hyp.29.5.1204. [DOI] [PubMed] [Google Scholar]
- Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, Bohme GA, Imperato A, Pedrazzini T, Roques BP, Vassart G, Fratta W, Parmentier M. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- Lepicier P, Bouchard JF, Lagneux C, Lamontagne D. Endocannabinoids protect the rat isolated heart against ischaemia. Br J Pharmacol. 2003;139:805–815. doi: 10.1038/sj.bjp.0705313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Kaminski NE, Wang DH. Anandamide-induced depressor effect in spontaneously hypertensive rats: role of the vanilloid receptor. Hypertension. 2003;41:757–762. doi: 10.1161/01.HYP.0000051641.58674.F7. [DOI] [PubMed] [Google Scholar]
- Liu J, Gao B, Mirshahi F, Sanyal AJ, Khanolkar AD, Makriyannis A, Kunos G. Functional CB1 cannabinoid receptors in human vascular endothelial cells. Biochem J. 2000;346:835–840. [PMC free article] [PubMed] [Google Scholar]
- Liu J, Bátkai S, Pacher P, Harvey-White J, Wagner JA, Cravatt BF, Gao B, Kunos G. LPS induces anandamide synthesis in macrophages via CD14/MAPK/PI3 K/NF-κB independently of platelet activating factor. J Biol Chem. 2003;278:45034–45039. doi: 10.1074/jbc.M306062200. [DOI] [PubMed] [Google Scholar]
- Lumsden AB, Henderson JM, Kutner MH. Endotoxin levels measured by a chromatographic assay in portal, hepatic and peripheral blood in patients with cirrhosis. Hepatology. 1988;8:232–236. doi: 10.1002/hep.1840080207. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Bari M, Lorenzon T, Bisogno T, Di Marzo V, Finazzi-Agro A. Anandamide uptake by human endothelial cells and its regulation by nitric oxide. Biol Chem. 2000;275:13484–13492. doi: 10.1074/jbc.275.18.13484. [DOI] [PubMed] [Google Scholar]
- Malinowska B, Godlewski G, Bucher B, Schlicker E. Cannabinoid CB1 receptor-mediated inhibition of the neurogenic vasopressor response in the pithed rat. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:197–202. doi: 10.1007/pl00005041. [DOI] [PubMed] [Google Scholar]
- Malinowska B, Kwolek G, Gothert M. Anandamide and methanandamide induce both vanilloid VR1- and cannabinoids CB1 receptor-mediated changes in heart rate and blood pressure in anaesthetized rats. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:562–569. doi: 10.1007/s00210-001-0498-6. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young CA, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Fride E, Di Marzo V. Endocannabinoids. Eur J Pharmacol. 1998a;359:1–18. doi: 10.1016/s0014-2999(98)00649-9. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Fride E, Ben-Shabat S, Meiri U, Horowitz M. Carbachol, an acetylcholine receptor agonist, enhances production in rat aorta of 2-arachidonoyl glycerol, a hypotensive endocannabinoid. Eur J Pharmacol. 1998b;362:R1–R3. doi: 10.1016/s0014-2999(98)00777-8. [DOI] [PubMed] [Google Scholar]
- Mombouli JV, Schaeffer G, Holzmann S, Kostner GM, Graier WF. Anandamide-induced mobilization of cytosolic Ca2+ in endothelial cells. Br J Pharmacol. 1999;126:1593–1600. doi: 10.1038/sj.bjp.0702483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay S, Chapnick BM, Howlett AC. Anandamide-induced vasorelaxation in rabbit aortic rings has two components: G protein dependent and independent. Am J Physiol. 2002;282:H2046–H2054. doi: 10.1152/ajpheart.00497.2001. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Nahas G, Trouve R. Effects and interactions of natural cannabinoids on the isolated heart. Proc Soc Exp Biol Med. 1985;180:312–316. doi: 10.3181/00379727-180-42181. [DOI] [PubMed] [Google Scholar]
- Niederhoffer N, Szabo B. Cannabinoids cause central sympathoexcitation and bradycardia in rabbits. J Pharmacol Exp Ther. 2000;294:707–713. [PubMed] [Google Scholar]
- Niederhoffer NB, Szabo B. Involvement of CB1 cannabinoid receptors in the EDHF-dependent vasorelaxation in rabbits. Br J Pharmacol. 1999;126:1383–1386. doi: 10.1038/sj.bjp.0702452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan SE, Kendall DA, Randall MD. Characterisation of the vasorelaxant properties of the novel endocannabinoid N-arachidonoyl-dopamine (NADA) Br J Pharmacol. 2004;141:803–812. doi: 10.1038/sj.bjp.0705643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offertáler L, Mo FM, Bátkai S, Liu J, Begg M, Razdan RK, Martin BR, Bukoski RD, Kunos G. Selective ligands and cellular effectors of a G protein-coupled endothelial cannabinoid receptor. Mol Pharmacol. 2003;63:699–705. doi: 10.1124/mol.63.3.699. [DOI] [PubMed] [Google Scholar]
- Orliac ML, Peroni R, Celuch SM, Adler-Graschinsky E. Potentiation of anandamide effects in mesenteric beds isolated from endotoxemic rats. J Pharmacol Exp Ther. 2003;304:179–184. doi: 10.1124/jpet.102.041095. [DOI] [PubMed] [Google Scholar]
- Pacher P, Liaudet L, Bai P, Mabley JG, Kaminski PM, Virág L, Deb A, Szabo E, Ungvári Z, Wolin MS, Groves JT, Szabo C. Potent metalloporphyrin peroxynitrite decomposition catalyst protects against the development of doxorubicin-induced cardiac dysfunction. Circulation. 2003;107:896–904. doi: 10.1161/01.cir.0000048192.52098.dd. [DOI] [PubMed] [Google Scholar]
- Pacher P, Bátkai S, Kunos G. Haemodynamic profile and responsiveness to anandamide of TRPV1 receptor knock-out mice. J Physiol (Lond) 2004;558:647–657. doi: 10.1113/jphysiol.2004.064824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plane F, Garland CJ. Influence of contractile agonists on the mechanism of endothelium-dependent relaxation in rat isolated mesenteric artery. Br J Pharmacol. 1996;119:191–193. doi: 10.1111/j.1476-5381.1996.tb15970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt PF, Hillard CJ, Edgemond WS, Campbell WB. N-Arachidonylethanolamide relaxation of bovine coronary artery is not mediated by CB1 cannabinoid receptor. Am J Physiol. 1998;43:H375–H381. doi: 10.1152/ajpheart.1998.274.1.H375. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Kendall DA, Randall MD, Zygmunt PM, Movahed P, Hogestatt ED. Vanilloid receptors on capsaicin-sensitive sensory nerves mediate relaxation to methanandamide in the rat isolated mesenteric arterial bed and small mesenteric arteries. Br J Pharmacol. 2000;130:1483–1488. doi: 10.1038/sj.bjp.0703456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic V, Kendall DA, Randall MD, Smart D. Cannabinoid modulation of sensory neurotransmission via cannabinoid and vanilloid receptors: Roles in regulation of cardiovascular function. Life Sci. 2002;71:2577–2594. doi: 10.1016/s0024-3205(02)02086-6. [DOI] [PubMed] [Google Scholar]
- Randall MD, Kendall DA. Involvement of a cannabinoid in endothelium-derived hyperpolarizing factor-mediated coronary vasorelaxation. Eur J Pharmacol. 1997;335:205–209. doi: 10.1016/s0014-2999(97)01237-5. [DOI] [PubMed] [Google Scholar]
- Randall MD, Kendall DA. Anandamide and endothelium-derived hyperpolarizing factor act via a common vasorelaxant mechanism in rat mesentery. Eur J Pharmacol. 1998;346:51–53. doi: 10.1016/s0014-2999(98)00003-x. [DOI] [PubMed] [Google Scholar]
- Randall MD, Alexander SP, Bennett T, Boyd EA, Fry JR, Gardiner SM, Kemp PA, McCulloch AI, Kendall DA. An endogenous cannabinoid as an endothelium-derived vasorelaxant. Biochem Biophys Res Commun. 1996;229:114–120. doi: 10.1006/bbrc.1996.1766. [DOI] [PubMed] [Google Scholar]
- Randall MD, Harris D, Kendall DA, Ralevic V. Cardiovascular effects of cannabinoids. Pharmacol Ther. 2002;95:191–202. doi: 10.1016/s0163-7258(02)00258-9. [DOI] [PubMed] [Google Scholar]
- Ros J, Claria J, To-Figueras J, Planaguma A, Cejudo-Martin P, Fernandez-Varo G, Martin-Ruiz R, Arroyo V, Rivera F, Rodes J, Jimenez W. Endogenous cannabinoids: a new system involved in the homeostasis of arterial pressure in experimental cirrhosis in the rat. Gastroenterology. 2002;122:85–93. doi: 10.1053/gast.2002.30305. [DOI] [PubMed] [Google Scholar]
- Rosenkrantz H, Braude M. Acute, subacute and 23-day chronic marihuana inhalation toxicities in the rat. Toxicol Appl Pharmacol. 1974;28:428–441. doi: 10.1016/0041-008x(74)90228-2. [DOI] [PubMed] [Google Scholar]
- Sagan S, Venance L, Torrens Y, Cordier J, Glowinski J, Giaume C. Anandamide and WIN55,212–2 inhibit cyclic AMP-formation through G-protein-coupled receptors distinct from CB1 cannabinoid receptors in cultured astrocytes. Eur J Neurosci. 1999;11:691–699. doi: 10.1046/j.1460-9568.1999.00480.x. [DOI] [PubMed] [Google Scholar]
- Schlicker E, Timm J, Zentner J, Gothert M. Cannabinoid CB1 receptor-mediated inhibition of noradrenaline release in the human and guinea-pig hippocampus. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:583–589. doi: 10.1007/pl00005093. [DOI] [PubMed] [Google Scholar]
- Siqueira SW, Lapa AJ, Ribeiro do Valle J. The triple effect induced by delta 9-tetra-hydrocannabinol on the rat blood pressure. Eur J Pharmacol. 1979;58:351–357. doi: 10.1016/0014-2999(79)90305-4. [DOI] [PubMed] [Google Scholar]
- Smith PJ, McQueen DS. Anandamide induces cardiovascular and respiratory reflexes via vasosensory nerves in the anaesthetized rat. Br J Pharmacol. 2001;134:655–663. doi: 10.1038/sj.bjp.0704296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein EA, Fuller SA, Edgemond WS, Campbell WB. Physiological and behavioural effects of the endogenous cannabinoid, arachidonylethanolamide (anandamide), in the rat. Br J Pharmacol. 1996;119:107–114. doi: 10.1111/j.1476-5381.1996.tb15683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo B, Nordheim U, Niederhoffer N. Effects of cannabinoids on sympathetic and parasympathetic neuroeffector transmission in the rabbit heart. J Pharmacol Exp Ther. 2001;297:819–826. [PubMed] [Google Scholar]
- Szolcsányi J. Are cannabinoids endogenous ligands for the VR1 capsaicin receptor? Trends Pharmacol Sci. 2000;21:41–42. doi: 10.1016/s0165-6147(99)01436-4. [DOI] [PubMed] [Google Scholar]
- Ugdyzhekova DS, Bernatskaya NA, Stefano JB, Graier VF, Tam SW, Mekhoulam R. Endogenous cannabinoid anandamide increases heart resistance to arrhythmogenic effects of epinephrine: role of CB(1) and CB(2) receptors. Bull Exp Biol Med. 2001;131:251–253. doi: 10.1023/a:1017651432193. [DOI] [PubMed] [Google Scholar]
- Ugdyzhekova DS, Krylatov AV, Bernatskaya NA, Maslov LN, Mechoulam R, Pertwee RG. Activation of cannabinoid receptors decreases the area of ischemic myocardial necrosis. Bull Exp Biol Med. 2002;133:125–126. doi: 10.1023/a:1015526217332. [DOI] [PubMed] [Google Scholar]
- Valk PJ, Delwel R. The peripheral cannabinoid receptor, Cb2, in retrovirally-induced leukemic transformation and normal hematopoiesis. Leuk Lymphoma. 1998;32:29–43. doi: 10.3109/10428199809059244. [DOI] [PubMed] [Google Scholar]
- Varga K, Lake K, Martin BR, Kunos G Novel antagonist implicates the CB1 cannabinoid receptor in the hypotensive action of anandamide. Eur J Pharmacol. 1995;278:279–283. doi: 10.1016/0014-2999(95)00181-j. [DOI] [PubMed] [Google Scholar]
- Varga K, Lake KD, Huangfu D, Guyenet PG, Kunos G. Mechanism of the hypotensive action of anandamide in anesthetized rats. Hypertension. 1996;28:682–686. doi: 10.1161/01.hyp.28.4.682. [DOI] [PubMed] [Google Scholar]
- Varga K, Wagner JA, Bridgen DT, Kunos G. Platelet- and macrophage-derived endogenous cannabinoids are involved in endotoxin-induced hypotension. FASEB J. 1998;12:1035–1044. doi: 10.1096/fasebj.12.11.1035. [DOI] [PubMed] [Google Scholar]
- Varma DR, Goldbaum D. Effect of delta9-tetrahydrocannabinol on experimental hypertension in rats. J Pharm Pharmacol. 1975;27:790–791. doi: 10.1111/j.2042-7158.1975.tb09406.x. [DOI] [PubMed] [Google Scholar]
- Vidrio H, Sanchez-Salvatori MA, Medina M. Cardiovascular effects of (–)-11-OH-delta 8-tetrahydrocannabinol-dimethylheptyl in rats. J Cardiovasc Pharmacol. 1996;28:332–336. doi: 10.1097/00005344-199608000-00022. [DOI] [PubMed] [Google Scholar]
- Vizi ES, Katona I, Freund TF. Evidence for presynaptic cannabinoid CB(1) receptor-mediated inhibition of noradrenaline release in the guinea pig lung. Eur J Pharmacol. 2001;431:237–244. doi: 10.1016/s0014-2999(01)01413-3. [DOI] [PubMed] [Google Scholar]
- Vollmer RR, Cavero I, Ertel RJ, Solomon TA, Buckley JP. Role of the central autonomic nervous system in the hypotension and bradycardia induced by (–)-delta 9-trans-tetra-hydrocannabinol. J Pharm Pharmacol. 1974;26:186–192. doi: 10.1111/j.2042-7158.1974.tb09252.x. [DOI] [PubMed] [Google Scholar]
- Wagner JA, Varga K, Ellis EF, Rzigalinski BA, Martin BR, Kunos G. Activation of peripheral CB1 cannabinoid receptors in haemorrhagic shock. Nature. 1997;390:518–521. doi: 10.1038/37371. [DOI] [PubMed] [Google Scholar]
- Wagner JA, Varga K, Járai Z, Kunos G. Mesenteric vasodilation mediated by endothelial anandamide receptors. Hypertension. 1999;33:429–434. doi: 10.1161/01.hyp.33.1.429. [DOI] [PubMed] [Google Scholar]
- Wagner JA, Hu K, Bauersachs J, Karcher J, Wiesler M, Goparaju SK, Kunos G, Ertl G. Endogenous cannabinoids mediate hypotension after experimental myocardial infarction. J Am Coll Cardiol. 2001a;38:2048–2054. doi: 10.1016/s0735-1097(01)01671-0. [DOI] [PubMed] [Google Scholar]
- Wagner JA, Járai Z, Bátkai S, Kunos G. Hemodynamic effects of cannabinoids: coronary and cerebral vasodilation mediated by cannabinoid CB(1) receptors. Eur J Pharmacol. 2001b;423:203–210. doi: 10.1016/s0014-2999(01)01112-8. [DOI] [PubMed] [Google Scholar]
- Wagner JA, Hu K, Karcher J, Bauersachs J, Schafer A, Laser M, Han H, Ertl G. CB(1) cannabinoid receptor antagonism promotes remodeling and cannabinoid treatment prevents endothelial dysfunction and hypotension in rats with myocardial infarction. Br J Pharmacol. 2003;138:1251–1258. doi: 10.1038/sj.bjp.0705156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter L, Stella N. Cannabinoids and neuroinflammation. Br J Pharmacol. 2004;141:775–785. doi: 10.1038/sj.bjp.0705667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Liu Y, Ito Y, Hashiguchi T, Kitajima I, Yamakuchi M, Shimizu H, Matsuo S, Imaizumi H, Maruyama I. Simultaneous measurement of anandamide and 2-arachidonoylglycerol by polymyxin B-selective adsorption and subsequent high-performance liquid chromatography analysis: increase in endogenous cannabinoids in the sera of patients with endotoxic shock. Anal Biochem. 2001;294:73–82. doi: 10.1006/abio.2001.5015. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424:434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- White R, Hiley CR. A comparison of EDHF-mediated and anandamide-induced relaxations in the rat isolated mesenteric artery. Br J Pharmacol. 1997;122:1573–1584. doi: 10.1038/sj.bjp.0701546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R, Ho WS, Bottrill FE, Ford WR, Hiley CR. Mechanisms of anandamide-induced vasorelaxation in rat isolated coronary arteries. Br J Pharmacol. 2001;134:921–929. doi: 10.1038/sj.bjp.0704333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahner MR, Li DP, Chen SR, Pan HL. Cardiac vanilloid receptor 1-expressing afferent nerves and their role in the cardiogenic sympathetic reflex in rats. J Physiol. 2003;551:515–523. doi: 10.1113/jphysiol.2003.048207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaugg HE, Kyncl J. New antihypertensive cannabinoids. J Med Chem. 1983;26:214–217. doi: 10.1021/jm00356a017. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Högestätt ED, Waldeck K, Edwards G, Kirkup AJ, Weston AH. Studies on the effects of anandamide in rat hepatic artery. Br J Pharmacol. 1997;122:1679–1686. doi: 10.1038/sj.bjp.0701601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H-H, Sorgard M, Di Marzo V, Julius D, Högestätt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Andersson DA, Högestätt ED. Delta 9-tetrahydrocannabinol and cannabinol activate capsaicin-sensitive sensory nerves via a CB1 and CB2 cannabinoid receptor-independent mechanism. J Neurosci. 2002;22:4720–4727. doi: 10.1523/JNEUROSCI.22-11-04720.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]