Abstract
Lymphocyte circulation plays an important role in the generation of a specific immune response. Mature lymphocytes continuously circulate between blood and lymph, entering the lymphoid tissue via high endothelial venules. Trafficking across high endothelial venules of peripheral lymph nodes (PLN) depends on the expression of l-selectin. It has been shown that l-selectin is rapidly cleaved from the surface by a metalloproteinase after in vitro activation. Here, we show that ligation of CD4, without ligation of the T cell receptor for antigen, causes down-regulation of l-selectin on T helper cells. This down-regulation is caused by proteolytic cleavage by a metalloproteinase and is reversible by the addition of hydroxamic acid-based metalloproteinase inhibitors. We show that in vivo down-regulation of l-selectin in huCD4tg mice by mAb reduces the homing of lymphocytes to PLN in adoptive transfer experiments. Because CD4 is a coreceptor for HIV-1, the down-regulation of l-selectin induced by CD4 ligation could play a role in the pathogenesis of AIDS. We provide evidence that CD4 ligation by HIV-1 induces metalloproteinase-dependent l-selectin down-regulation. Reduced levels of l-selectin expression might contribute to immune deficiency in individuals infected with HIV by inhibiting T cell redistribution and decreasing the probability of an encounter between specific lymphocytes and viral antigens in PLN.
The majority of circulating lymphocytes are of the naive phenotype (CD45–RA+ l-selectin+) and migrate throughout the body. This trafficking of lymphocytes is often referred as “homing” and requires a sequence of critical adhesion events to allow the cell to leave the bloodstream and enter lymphoid tissue (1). Interactions between leukocytes and endothelial cells are mediated by members of the selectin, integrin, and Ig superfamilies (2). The first essential step in homing of naive lymphocytes to peripheral lymph nodes (PLN) is the interaction of l-selectin with its ligand on high endothelial venules, the peripheral node addressin (3). Binding of l-selectin to its ligand mediates tethering and rolling of lymphocytes in high endothelial venules. After this primary adhesion, up-regulation of the αLβ2-integrin LFA-1 triggers arrest and diapedesis of the cell into the PLN (1). The importance of l-selectin in this multistep process and its role in specific immune responses is best exemplified in l-selectin-deficient mice. In l-selectin-deficient mice, T cells do not home to PLN; primary T cell responses to antigen are impaired; and cutaneous delayed-type hypersensitivity responses do not occur (4, 5). In addition, injection of anti-l-selectin mAb into wild-type mice has been shown to result in impaired homing of naive lymphocytes to PLN (6).
Lymphocyte migration to the spleen differs from migration into lymph nodes, because the spleen is not supported by the lymphatic system. Thus, cells entering the spleen migrate directly back into the blood. Naive and memory T cells have been shown to home equally to the spleen, independently of their expression levels of l-selectin (7). Because homing to PLN depends on l-selectin expression, naive T cells (CD45RA+l-selectin+) enter PLN directly from the bloodstream across high endothelial venules, compared with most memory T cells (CD45RO+l-selectin−), which enter PLN via afferent lymphatics draining nonlymphoid tissue (8).
After cellular activation by phorbol esters, l-selectin is down-regulated by a metalloproteinase that can be inhibited by hydroxamic acid-based metalloproteinase inhibitors (9, 10). The recent cloning of tumor necrosis factor-α converting enzyme (TACE) and the generation of TACE-deficient mice suggest that TACE is also responsible for the cleavage of l-selectin (11–13). Shed soluble l-selectin (sl-selectin) retains its functional binding activity (14) and has been associated with disease (15).
Homing of naive CD4+ T cells to PLN and the generation of primary immune responses within that tissue depends on the expression of cell adhesion molecules. l-selectin is critical for homing of naive CD4+ T cells to PLN (16). Additional cell adhesion molecules are required for the generation of a specific immune response. T cells first must adhere to antigen-presenting cells, an interaction that is mediated primarily by the interactions between LFA and the intercellular adhesion molecule-1 (ICAM-1) and between CD2 and LFA-3. The CD4 receptor further stabilizes the binding of the T cell to the antigen-presenting cell through its association with the α2- or β2-domains of MHC class II molecules (17). Thus, expression of l-selectin and CD4 is essential for T helper (Th) cells to contribute efficiently to the elimination of foreign antigen.
During a normal immune response, engagement of the CD4 receptor and the T cell receptor for antigen (TCR) occurs simultaneously. It has been shown that crosslinking of the CD4 receptor in the absence of antigen inhibits TCR-dependent signaling (18) and prompts activation-induced cell death after subsequent crosslinking of the TCR (19). Thus, a negative signal given by CD4 ligation alone may help to prevent inappropriate activation of CD4+ T cells by MHC class II positive cells that do not express the T cell-specific antigen.
We tested whether this negative signal induced by CD4 ligation, in the absence of activation through the TCR, affects the expression of T cell adhesion molecules, and we establish a role of the CD4 receptor in the regulation of adhesion and recirculation of resting T cells. We show here that crosslinking of CD4 reduces expression levels of l-selectin and alters lymphocyte recirculation and homing to PLN. This down-regulation of l-selectin is caused by shedding, as determined by the fact that it is reversed by the addition of hydroxamic acid-based metalloproteinase inhibitors. In addition, we report that crosslinking CD4 by envelope glycoprotein (env) expressed on HIV-1-infected cells results in down-regulation of l-selectin on Th cells, in the absence of detectable activation, as measured by CD69 expression. It has been reported that HIV-1 and simian immunodeficiency virus modulate the homing pattern of T cells and induce preferential homing to the intestine in engrafted severe combined immunodeficiency mice (20). Thus, the down-regulation of l-selectin by HIV-1 may result in redistribution of T cells and interfere with the ability of T cells to mount an immune response in lymphoid organs, the preferred location and source of viral infection.
MATERIALS AND METHODS
Animals.
Transgenic 313/BL6 (huCD4tg) mice were generated as described (21). CD4 knockout mice expressing huCD4 only on Th cells (mCD4KO/huCD4tg) were a gift from D. Littman (Skirball Institute, New York; ref. 22). Mice were bred in our animal facility at the National Jewish Medical and Research Center. C57BL/6 mice were purchased from The Jackson Laboratory and maintained in our facility.
Mouse Cell Preparation.
Mouse lymphocytes from PLN (inguinal and axillary), mesenteric lymph nodes, and spleen were prepared as single cell suspensions. Spleen cells were treated with NH4Cl solution (0.83%) to lyse red blood cells. Cells were resuspended in Iscove’s modified Dulbecco’s medium supplemented with 10% (vol/vol) heat-inactivated FCS, 100 μg/ml penicillin/streptomycin, and 2 mM l-glutamine. CD4+ T cells were purified by negative selection. Lymphocytes were incubated with biotinylated anti-CD8 and anti-B220 mAbs. Cells with bound biotinylated mAbs were removed by streptavidin (SA)-conjugated magnetic beads (Dynal, Great Neck, NY).
Human Cell Preparation.
Human peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll/Hypaque (Amersham Pharmacia) density-gradient centrifugation of heparinized blood obtained from healthy donors. CD4+ T cells were purified by negative selection. PBMCs were incubated with 20 μg/ml anti-CD8 mAb (OKT8, American Type Culture Collection) and applied to a column of goat anti-mouse IgG-coated Immulan beads (Biotecx Laboratories, Houston). The purified cell population was analyzed by flow cytometry and 80–95% of the cells were CD4+. Cells were kept in supplemented RPMI medium 1640.
Antibodies.
The following hybridoma lines were used, and antibodies were purified from culture supernatant by affinity chromatography with protein G- or A-Sepharose (Amersham Pharmacia). Mel-14 (anti-ml-selectin mAb), GK1.5 (anti-mCD4 mAb), and 53-6.7 (anti-mCD8 mAb) were from the American Type Culture Collection. Leu-3a (anti-huCD4 mAb) was from the Memorial Sloan–Kettering Cancer Center (New York). B8-24-3.1 (anti-H2Kb mAb) was a gift from U. Staerz (National Jewish Medical and Research Center). M1/42.3.9.8 (anti-H2Kall mAb) and RA3-6B2 (anti-B220 mAb) were gifts from J. Cambier (National Jewish Medical and Research Center). H57-597 (anti-mTCRαβ mAb; ref. 23) and 500A2 (anti-mCD3 mAb) were also used. Biotinylated H1.2F3 (anti-mCD69 mAb) and 53-6.7-phycoerythrin (PE) were purchased from PharMingen. Leu-8–FITC or Leu-8–PE (anti-hul-selectin), peridinin chlorophyll protein (PerCP)-conjugated Leu-4 (anti-huCD3 mAb), Leu-2a–PE (anti-huCD8 mAb), and Leu-23–FITC or Leu-23–PE (anti-huCD69 mAb) were purchased from Becton Dickinson. To reveal biotinylated antibodies, cells were incubated with PerCP-conjugated SA or PE-conjugated SA (Becton Dickinson) as the second-step reagent.
Immunofluorescence Analysis.
Lymphocytes (n = 0.5 × 106 to 1 × 106) were incubated with saturating amounts of FITC-, PE-, or biotin-conjugated antibodies. After washing, cells were incubated with PerCP-conjugated SA, fixed in 1% paraformaldehyde, and analyzed on a fluorescence-activated cell sorter (FACSCalibur flow cytometer, Becton Dickinson). A total of 20,000 events were analyzed for each sample. l-selectin expression and CD69 expression on enriched murine CD4+ T cells were revealed by staining cells with biotinylated anti-l-selectin or anti-CD69 mAb and PE-conjugated SA. Human CD4+ T cells were stained with PE-conjugated anti-l-selectin or anti-CD69 mAb. To determine the expression of l-selectin and CD69 on Th cells within a whole lymphocyte population, murine lymphocytes were stained with FITC-conjugated anti-TCR mAb, PE-conjugated anti-CD8 mAb, and biotinylated anti-l-selectin or anti-CD69 mAb and PerCP-conjugated SA. Human PBMCs were stained with PerCP-conjugated anti-CD3 mAb, PE-conjugated anti-CD8 mAb, and FITC-conjugated anti-l-selectin mAb. Only cells within the live and TCR+ CD8− gate were analyzed.
In Vitro and in Vivo Crosslinking of the CD4 Receptor.
For in vitro down-regulation of l-selectin, lymphocytes of mCD4KO/huCD4tg or C57BL/6 mice were incubated with 20 μg/ml anti-huCD4, anti-mCD4, or anti-MHC class I (H2Kall) mAb. Human PBMCs were incubated with 20 μg/ml anti-huCD4 mAb. Cells (n = 2 × 106) were cultured in complete Iscove’s modified Dulbecco’s medium or RPMI medium 1640 on a goat anti-mouse/rat IgG-coated (50 μg/ml, Jackson ImmunoResearch) 24-well plate (Costar). After 4–16 h, cells were harvested and l-selectin expression and CD69 expression on Th cells were determined by flow cytometry. To inhibit l-selectin shedding, lymphocytes of mCD4KO/huCD4tg mice were treated with the indicated concentrations of the metalloproteinase inhibitors, BB3103 (British Biotechnology, Oxford, U.K.) or KD-IX-73-4 (a gift from T. K. Kishimoto, Boehringer Ingelheim), for 1 h before CD4 ligation. Cells were cultured for 4 h on a goat anti-mouse-coated plate in the presence of either inhibitor.
For in vivo down-regulation of l-selectin, huCD4tg mice were injected intravenously with 100 μg of anti-huCD4, anti-l-selectin, or anti-MHC class I (H2Kb) mAb. After 4–16 h, PLN were isolated, and l-selectin expression and CD69 expression on Th cells were determined by flow cytometry.
Confocal Microscopy.
Purified CD4+ T cells from mCD4KO/huCD4tg mice were treated with Leu-3a as described in Materials and Methods. Cells were placed on poly-l-lysine-coated (Sigma) glass coverslips, and fixed in 1% paraformaldehyde. For intracellular labeling, cells were made permeable with 0.1% saponin. Cells were labeled with anti-CD3 and anti-l-selectin mAb. Cells were labeled further with Cy3-tagged donkey anti-rat Ab to reveal l-selectin staining and biotinylated anti-Syrian hamster Ab (Jackson ImmunoResearch). Biotinylated mAb bound to 500A2 was visualized with SA-FITC (Jackson ImmunoResearch). Other cells were incubated with rhodamine-phalloidin (Molecular Probes). Cells were analyzed with epipolarized-xenon illumination by using a Peltier-cooled charge-coupled device (SpectraSource, Westlake Village, CA) mounted on a Zeiss Axiophot microscope and processed with slidebook software (Intelligent Imaging Innovations, Denver). Out-of-focus haze was removed from images by using the nearest-neighbor deconvolution module for slidebook.
Adoptive Transfer.
HuCD4tg mice were injected intravenously with 100 μg of anti-huCD4, anti-l-selectin, or anti-MHC class I (H2Kb) mAb. After 12 h, lymphocytes from PLN were isolated and labeled with 5-(and-6)-carboxyfluorescein diacetate succinimidyl ester (CFSE, 0.5 μM, Molecular Probes), and 1 × 107 labeled cells were injected intravenously in a volume of 200 μl into nonirradiated C57BL/6 recipients. After 1 h, numbers of CFSE-labeled cells in PLN and spleen were determined by flow cytometry. A total of 200,000 events were analyzed. Recovery of cells from anti-huCD4 and anti-l-selectin mAb-treated mice was compared with that from untreated mice by using the Wilcoxon signed rank test.
Coculture Assay with HIV-1.
Stocks of HIV-1 NL4-3 virus were made from acutely infected CEM cells at the time of peak virus replication and stored at −70°C. Infection of Jurkat cells was accomplished by incubating cells at a multiplicity of infection of 0.2. Control cells were mock infected with culture supernatants of uninfected CEM cells. Before coculture, mock and HIV-1 NL4-3-infected Jurkat cells were incubated with medium, 10 μg/ml soluble CD4 or 10 μg/ml BSA. Human CD4+ PBMCs were purified from peripheral blood and labeled with CFSE. For the coculture, 2 × 106 resting CD4+ human PBMCs were mixed with 2 × 106 mock or HIV-1 NL4-3-infected Jurkat cells. Cells were resuspended and cultured for 12 h with or without soluble CD4 or BSA in the culture medium. l-selectin expression and CD69 expression on CFSE+ cells were determined by flow cytometry.
RESULTS
In Vitro and in Vivo Down-Regulation of l-Selectin.
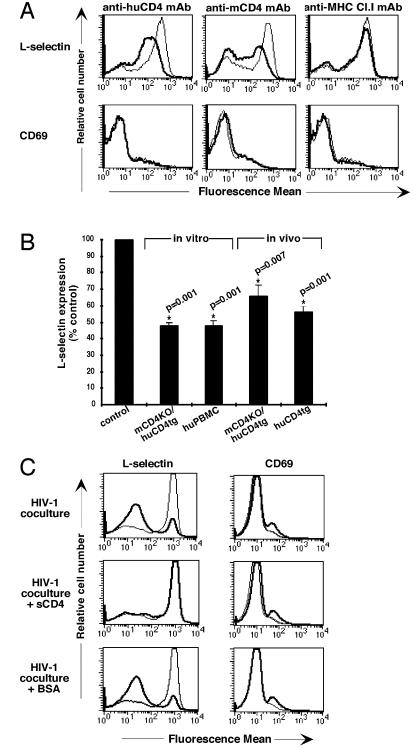
Ligation of the CD4 receptor has been shown to interfere with subsequent T cell activation (18, 19, 24, 25). To determine the effects of CD4 ligation on resting T cells in the absence of activation, lymphocytes from huCD4tg or C57BL/6 mice and human PBMCs were assayed for expression of the lymphocyte homing receptor l-selectin and the activation marker CD69 after crosslinking of the huCD4 receptor. As shown in Fig. 1A, in vitro ligation of CD4, but not MHC class I, with mAb reduced levels of l-selectin expression on Th cells of transgenic and nontransgenic mice. CD69 expression on the cell surface was unchanged in response to CD4 ligation. A highly significant reduction of l-selectin expression levels could also be observed after ligation of the CD4 receptor on human PBMCs in vitro and on Th cells of transgenic mice in vivo (Fig. 1B). Surface expression of l-selectin was reduced within 30 min of CD4 ligation, and increased l-selectin expression levels were not detected at any time point tested (data not shown). l-selectin was reexpressed within 16 h after removal of the anti-CD4 secondary crosslinking Ab (data not shown). Crosslinking of MHC class I with mAb in vitro and in vivo did not affect expression levels of l-selectin. In addition, the expression of the cell-activation markers CD44 and CD25 and of the integrin VLA-4 was unchanged at all time points tested (data not shown). Thus, l-selectin down-regulation is specific to ligation of the CD4 receptor and is not associated with altered surface expression of specific markers of cell activation.
Figure 1.
Down-regulation of l-selectin after in vitro and in vivo ligation of CD4 by mAb or HIV-1. (A) The mouse or human CD4 or MHC class I receptors on enriched CD4+ T cells of mCD4KO/huCD4tg or C57BL/6 mice were crosslinked in vitro as described. After 4 h, cells were harvested and analyzed by flow cytometry. Thin lines represent l-selectin expression or CD69 expression on untreated cells; thick lines represent l-selectin expression or CD69 expression on anti-CD4 or anti-MHC class I mAb-treated cells. Results are representative of at least five experiments. (B) The CD4 receptors on lymphocytes of mCD4KO/huCD4tg mice or human PBMCs were crosslinked in vitro as described in Materials and Methods. For in vivo studies, mCD4KO/huCD4tg and huCD4tg mice were injected with 100 μg of anti-huCD4 mAb. Cells were analyzed 4–16 h later for l-selectin expression by flow cytometry. Cell viability was unchanged after crosslinking of the CD4 receptor (data not shown). Fluorescence mean values of l-selectin expression on live Th cells are shown as percentage of the untreated control. P values and asterisks indicate significance and were obtained by applying the Wilcoxon signed rank test. (C) CFSE-labeled human CD4+ PBMCs were cocultured with HIV-1 NL4-3-infected (thick line) or mock-infected (thin line) Jurkat cells. Before coculture, Jurkat cells were incubated with medium, 10 μg/ml soluble CD4 or 10 μg/ml BSA. Cells were harvested after 12 h, and levels of l-selectin expression and CD69 expression on CFSE+ cells were determined by flow cytometry by using two-color staining. Results are representative of at least five experiments.
We tested next whether down-regulation of l-selectin is induced by binding of HIV-1 env to the CD4 receptor. Purified resting CD4+ human PBMCs were cocultured with HIV-1 NL4-3-infected Jurkat cells as described in Materials and Methods. To discriminate between l-selectin expressed on Jurkat cells and CD4+ human PBMCs, CD4+ human PBMCs were CFSE-labeled before mixing the two cell populations. CFSE is a green fluorescent dye that stains intracellular macromolecules of cells without interfering with the expression of surface markers (26). CFSE-labeled populations can be identified by flow cytometry. The level of l-selectin expression on CFSE+ CD4+ human PBMCs in coculture with NL4-3-infected cells was significantly lower than the level on cells cocultured with mock-infected cells, whereas CD69 expression was unchanged (Fig. 1C). Levels of CD3 expression were also unchanged (data not shown). Soluble CD4 blocks the binding of HIV-1 to the CD4 receptor and completely reversed down-regulation of l-selectin in the coculture, whereas BSA had no effect on the expression levels of l-selectin. Thus, down-regulation of l-selectin occurs after CD4 ligation by HIV-1 env in human PBMCs, thereby mimicking the effects seen with anti-CD4 mAbs in huCD4tg mice and human PBMCs. CD4 ligation by HIV-1 NL4-3 induced an almost complete loss of l-selectin expression on Th cells, compared with the reduced l-selectin levels seen in response to anti-CD4 mAb. In addition, l-selectin expression levels were unchanged when CD4+ human PBMCs were incubated with free virus or soluble gp120 (data not shown). These differences could be caused by high concentrations and continued expression of env on HIV-1-infected cells, generating a more potent, sustained signal than the mAb or gp120 (27).
In summary, l-selectin down-regulation occurs in human PBMCs after CD4 ligation by mAb or by HIV-1.
In Vitro Down-Regulation of l-Selectin Is Caused by Shedding and Is Inhibited by Hydroxamic Acid-Based Metalloproteinase Inhibitors.
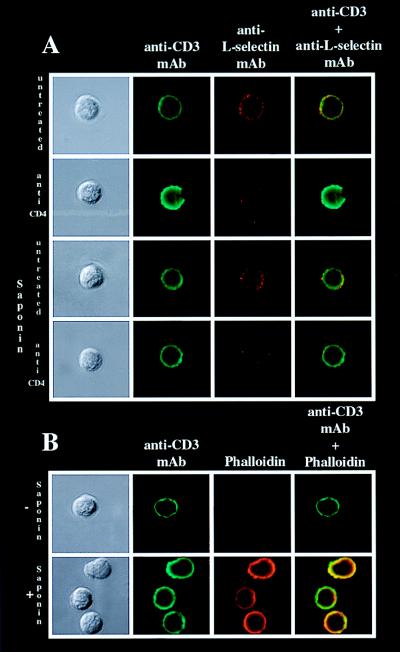
Activation-induced l-selectin down-regulation has been shown to be caused by proteolytic cleavage of l-selectin (9, 28). To test whether the observed down-regulation of l-selectin is caused by shedding and not by internalization, cells that had and had not been made permeable were analyzed by confocal microscopy for levels of intracellular and extracellular l-selectin expression after CD4 crosslinking. Extracellular l-selectin expression was reduced after CD4 ligation on treated and untreated cells. However, CD3 expression was unchanged, and no intracellular l-selectin was detectable (Fig. 2A). Cells were stained with phalloidin as a control for sufficient permeability (Fig. 2B). Thus, in vitro ligation of CD4 with mAb caused the release of l-selectin from the cell surface.
Figure 2.
l-selectin is shed after in vitro CD4 ligation. Enriched huCD4+ T cells from mCD4KO/huCD4tg mice were incubated in vitro with or without anti-huCD4 mAb as described in Materials and Methods. After 4 h cells were harvested and analyzed for l-selectin expression by confocal microscopy. Samples were double stained for CD3 (shown in green) and l-selectin (shown in red). (A) CD3 expression and l-selectin expression on untreated and anti-huCD4 mAb-treated cells that had and had not been made permeable with saponin (0.1%). (B) To control for sufficient permeability, cells were stained with rhodamine-conjugated phalloidin. Results are representative of at least three experiments.
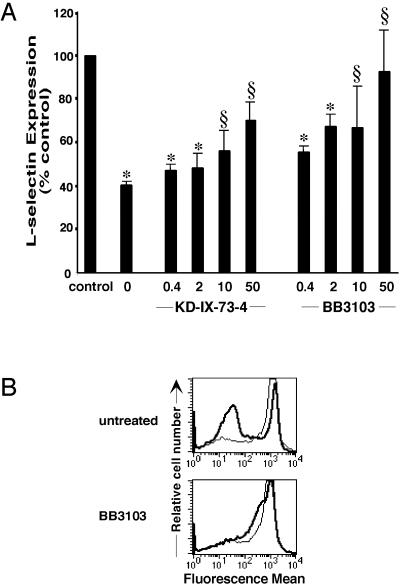
The proteolytic cleavage of l-selectin after cellular activation by phorbol esters is mediated by a metalloproteinase. Hydroxamic acid-based inhibitors of matrix metalloproteinases specifically inhibit activation-induced shedding of l-selectin in human PBMCs and neutrophils (10, 29). In our system, the matrix metalloproteinase inhibitors BB3103 and KD-IX-73-4 reversed l-selectin down-regulation induced by anti-CD4 mAb in mCD4KO/huCD4tg mice in a dose-dependent manner (Fig. 3A). Pretreatment of cells with metalloproteinase inhibitors had no effect on expression levels of CD4 or the amount of Leu-3a binding to the CD4 receptor (data not shown).
Figure 3.
l-selectin shedding is inhibited by metalloproteinase inhibitors. (A) Enriched CD4+ T cells from huCD4tg mice were treated in vitro with a metalloproteinase inhibitor, KD-IX-73-4 or BB3103, before crosslinking of the huCD4 receptor. After 4 h, cells were stained for l-selectin expression as described in Fig. 1. Fluorescent mean values of l-selectin expression on live Th cells are shown as percentage of the untreated control. ∗, High significance (P < 0.01); § no significance (P > 0.05). Results are representative of at least five experiments. (B) CFSE-labeled human CD4+ PBMCs were pretreated with 50 μg/ml BB3103 before exposure to NL4-3-infected (thick line) or mock-infected (thin line) Jurkat cells. After 12 h, l-selectin expression levels were determined as described in Materials and Methods.
HIV-1-mediated down-regulation of l-selectin also depends on the activity of a metalloproteinase. The metalloproteinase inhibitor BB3103 blocked the down-regulation of l-selectin on human PBMCs after crosslinking CD4 with HIV-1 (Fig. 3B). Thus, crosslinking of the CD4 receptor by HIV-1, as well as mAb, results in reduced expression of the lymphocyte homing receptor l-selectin because of proteolytic cleavage by a metalloproteinase.
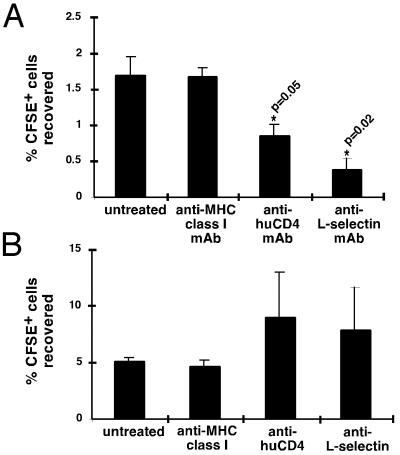
Loss of l-Selectin Reduces Homing to PLN in Adoptive Transfer Experiments.
As discussed, l-selectin is critical for lymphocytes to enter PLN but is not necessary for cells to enter the spleen (30). To assess the homing capability of lymphocytes after CD4 ligation, lymphocytes from mice injected with anti-huCD4 mAb, anti-l-selectin mAb, or anti-MHC class I (H-2Kb) mAb were CFSE labeled and adoptively transferred into nontransgenic recipients. The recovery of CFSE-labeled cells in PLN and spleen was assessed 1 h later by flow cytometry. Cells expressing reduced levels of l-selectin because of CD4 ligation and cells with bound anti-l-selectin mAbs homed less to PLN (Fig. 4A), whereas the number of recovered cells in the spleen tended to increase (Fig. 4B). Thus, the amount of l-selectin down-regulation seen in response to in vivo CD4 ligation (Fig. 1B) is enough to interfere with the ability of the cells to enter PLN. The effects of anti-l-selectin mAb in vivo have been described (6, 30). Treatment with anti-l-selectin mAb has been shown to cause shedding of l-selectin and to inhibit homing of lymphocytes to PLN and leukocyte migration to sites of inflammation. Our data confirm the results obtained with anti-l-selectin mAb and suggest that CD4 ligation has similar inhibitory effects on T cell recirculation.
Figure 4.
l-selectin-dependent homing to PLN and l-selectin-independent homing to spleen. HuCD4tg mice were injected with 100 μg/ml of the indicated antibodies. After 12 h, lymphocytes from these mice were CFSE-labeled and adoptively transferred into C57BL/6 mice. After 1 h, recovery of CFSE+ cells in PLN (A) and spleen (B) was assessed by flow cytometry. Results are expressed as means ± SEM of three experiments, with two mice per condition in each experiment. The asterisks mark values that are significantly different from the control.
DISCUSSION
In this study, we establish a mechanism of regulation of l-selectin expression involving the CD4 receptor. Crosslinking of CD4 on resting Th cells of huCD4tg or nontransgenic mice or on human PBMCs reduced expression levels of l-selectin, the lymphocyte homing receptor. In addition, we show that HIV-1 has a down-regulatory effect on l-selectin expression on human PBMCs. Inhibition of this down-regulation by soluble CD4 suggests that viral env, rather than other molecules expressed on the surface of infected T cells, induces l-selectin shedding.
It has been shown in vitro that activation of lymphocytes by phorbol esters induces cleavage of l-selectin from the cell surface by a metalloproteinase, which can be blocked by hydroxamic acid-based metalloproteinase inhibitors (10, 31). The enzyme responsible for l-selectin cleavage is likely to be constitutively active, and cellular activation enhances its proteolytic activity (32). The data presented here show hydroxamate-sensitive shedding of l-selectin induced by CD4 ligation in the absence of cellular activation, as evidenced by unchanged expression levels of the activation markers CD69, CD25, and CD44.
How does CD4 ligation lead to metalloproteinase-dependent cleavage of l-selectin? While these studies were ongoing in T lymphocytes, Kahn et al. (33) showed that, in neutrophils, calmodulin is bound to the cytoplasmic tail of l-selectin and that inhibitors of calmodulin cause l-selectin shedding in the absence of cellular activation. Kahn et al. postulate that bound calmodulin protects l-selectin from cleavage by the metalloproteinase. Dissociation of calmodulin from the cytoplasmic tail could cause a conformational change in l-selectin, leading to accessibility of the extracellular cleavage site to the metalloproteinase and subsequent cleavage of l-selectin. Ligation of the CD4 receptor induces an increase in the concentration of intracellular calcium (data not shown). This calcium rise could cause the dissociation of calmodulin from l-selectin, with consequent shedding of l-selectin.
Cell-surface expression of l-selectin is critical for lymphocytes to enter PLN; homing to spleen and intestine is l-selectin-independent and uses alternate pathways (34, 35). Here, we show that ligation of CD4 interferes with the homing pattern of Th cells. Lymphocytes from anti-huCD4 mAb-treated mice home less to PLN and more to the spleen. Mice treated with anti-l-selectin mAb showed similar impairments in their homing to PLN (Fig. 4). The fact that treatment of mice with anti-huCD4 or anti-l-selectin mAb has similar effects suggests that high expression levels of l-selectin are critical for the recruitment of T cells to PLN. Supporting data that small changes in surface expression of l-selectin are sufficient to alter lymphocyte recirculation come from Tang et al. (36). They show that preferential migration of T cells into PLN and of B cells into spleen is regulated by different levels of l-selectin expression (36). In addition, a recent report by Stockton et al. (37) shows that lymphocyte migration is a finely tuned system. The expression of CD43, a sialoglycoprotein expressed on leukocytes, negatively regulates T cell homing at sites where l-selectin ligand expression on high endothelial venules is low. High densities of peripheral node addressin overcome this inhibitory effect and mediate homing of T cells into lymphoid tissue (37). These data suggest that small changes in l-selectin expression levels could interfere with this equilibrium and may explain why the level of l-selectin down-regulation induced by CD4 ligation in vivo (Fig. 1B) has such a profound effect on CD4+ T cell homing to PLN (Fig. 3).
The interaction between CD4 and MHC class II has been shown to influence numerous functions of the T cell (38). Doyle and Strominger (39) showed that CD4 interacts with MHC class II molecules even in the absence of TCR. In addition, ligation of the CD4 receptor in the absence of appropriate antigen has been shown to have a negative regulatory effect on T cell activation (18, 19, 24, 25). The data presented here suggest that CD4 ligation in vivo, e.g., by MHC class II or gp120 in individuals infected by HIV-1, could result in l-selectin down-regulation without engagement of TCR. It is intriguing to speculate that the immune system may have two independent mechanisms for assuring that irrelevant interactions between T cells and antigen-presenting cells do not lead to nonspecific immune responses: first, via inhibition of TCR-mediated responses after independent CD4 ligation; and second, via trafficking of T cells away from antigen-rich PLN.
Although we do not have direct evidence that HIV-1 causes l-selectin shedding in AIDS, a study analyzing adhesion molecules in HIV infection showed that the number of T cells expressing l-selectin is reduced in parallel with the stages of disease (40). In addition, individuals infected with HIV have a 3-fold increase of sl-selectin in serum (41). Part of this 3-fold elevation of sl-selectin in individuals positive for HIV-1 could be caused by in vivo activation. sl-selectin retains its functional binding activity and, in some individuals with AIDS, reaches levels that are above those shown to block leukocyte attachment to endothelium in vitro (14, 15). Thus, increased levels of sl-selectin in individuals positive for HIV-1 could also play a role in immune deficiency.
Prior work has analyzed the effect of HIV-1 on lymphocyte homing, with controversial results. Wang et al. (42) showed that exposure of resting CD4+ T cells to HIV-1 caused transient l-selectin up-regulation and increased homing of these cells to lymph nodes. In contrast, recent work by Marodon et al. (43) showed inhibition of l-selectin expression on T cells productively infected with HIV-1, although neither the mechanism nor in vivo effects of this inhibition were investigated. Donze et al. (20) found preferential homing of HIV-1-infected lymphocytes to the intestine, rather than to lymph nodes, in engrafted SCID mice. Finally, in recent work by Zhang et al. (44), who analyzed CD4+ T lymphocyte populations in peripheral lymphoid tissue from individuals infected with HIV-1, a significant depletion of naive CD45RA+ CD4+ T cells was seen, out of proportion with the depletion of total CD4+ T cells. At steady state, there are a number of possible explanations for this relative depletion, including differentiation, inhibition of production, and/or inhibition of homing. However, the rapid increases in both CD45RA+ and RA− subsets in tonsils and lymph nodes in response to antiretroviral drug therapy (44) suggest that redistribution is involved in the initial increases of CD4+ T cells in peripheral lymphoid tissues. These in vivo observations are consistent with our data showing that CD4 ligation by mAb or gp120-expressing cells in vitro induces l-selectin shedding and that CD4 ligation in vivo inhibits lymphocyte homing to PLN. Stable isotope-labeling techniques to allow tracking of cells in individuals infected with HIV will facilitate analysis of the role of l-selectin down-regulation in T cell redistribution in HIV disease.
Our data also have implications for treatment of inflammatory and autoimmune disorders. The specific induction of l-selectin down-regulation on lymphocytes by anti-CD4 mAb may provide an approach to alteration of l-selectin-mediated adhesion. We speculate that CD4 ligation in the absence of appropriate antigen inhibits autoimmune responses, in part, by altering T cell redistribution. In HIV-1 infection, this effect of CD4 ligation on T cell recirculation may have been subverted by the virus.
Acknowledgments
We thank R. Flavell and D. Littman for the huCD4tg mice, A. Dunlap for technical assistance with all mice matters, W. Townsend and S. Sobus for help with flow cytometry, and T. Kishimoto at Boehringer Ingelheim and British Biotechnology for providing the metalloproteinase inhibitors. This work was supported by National Institutes of Health Grants RO1 AI 23764D (to A.K.), RO1 AI 35513, and RO1 AI 40003; the Cancer Center of the University of Colorado Health Sciences Center; the Bender Foundation; and the Eleanore and Michael Stobin Trust (to T.H.F.). S.M. was funded by the German Academic Exchange Program (Germany), and T.H. was funded by Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie (Germany) Grant 01K/9762/5/TP V2.
ABBREVIATIONS
- PLN
peripheral lymph node
- CFSE
5-(and-6)-carboxyfluorescein diacetate succinimidyl ester
- TCR
T cell receptor for antigen
- sl-selectin
soluble l-selectin
- Th cell
T helper cell
- SA
streptavidin
- PBMC
peripheral blood mononuclear cell
- PE
phycoerythrin
- m-
mouse
- hu-
human
- -tg
transgenic
References
- 1.Butcher E C, Picker L J. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 2.Stoolman L M. Cell. 1989;56:907–910. doi: 10.1016/0092-8674(89)90620-x. [DOI] [PubMed] [Google Scholar]
- 3.Berg E L, Robinson M K, Warnock R A, Butcher E C. J Cell Biol. 1991;114:343–349. doi: 10.1083/jcb.114.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu J, Grewal I S, Geba G P, Flavell R A. J Exp Med. 1996;183:589–598. doi: 10.1084/jem.183.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steeber D A, Green N E, Sato S, Tedder T F. J Immunol. 1996;157:4899–4907. [PubMed] [Google Scholar]
- 6.Gallatin W M, Weissman I L, Butcher E C. Nature (London) 1983;304:30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- 7.Williams M B, Butcher E C. J Immunol. 1997;159:1746–1752. [PubMed] [Google Scholar]
- 8.Mackay C R, Marston W L, Dudler L. J Exp Med. 1990;171:801–817. doi: 10.1084/jem.171.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Preece G, Murphy G, Ager A. J Biol Chem. 1996;271:11634–11640. doi: 10.1074/jbc.271.20.11634. [DOI] [PubMed] [Google Scholar]
- 10.Feehan C, Darlak K, Kahn J, Walcheck B, Spatola A F, Kishimoto T K. J Biol Chem. 1996;271:7019–7024. doi: 10.1074/jbc.271.12.7019. [DOI] [PubMed] [Google Scholar]
- 11.Black R A, Rauch C T, Kozlosky C J, Peschon J J, Slack J L, Wolfson M F, Castner B J, Stocking K L, Reddy P, Srinivasan S, et al. Nature (London) 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 12.Moss M L, Jin S-L C, Milla M E, Burkhart W, Carter H L, Chen W-J, Clay W C, Didsbury J R, Hassler D, Hoffmann C R, et al. Nature (London) 1997;385:733–736. doi: 10.1038/385733a0. [DOI] [PubMed] [Google Scholar]
- 13.Peschon J J, Slack J L, Reddy P, Stocking K L, Sunnarborg S W, Lee D C, Russell W E, Castner B J, Johnson R S, Fitzner J N, et al. Science. 1998;282:1281–1284. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- 14.Schleiffenbaum B, Spertini O, Tedder T F. J Cell Biol. 1992;119:229–238. doi: 10.1083/jcb.119.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gearing A J H, Newman W. Immunol Today. 1993;14:506–512. doi: 10.1016/0167-5699(93)90267-O. [DOI] [PubMed] [Google Scholar]
- 16.Steeber D A, Green N E, Sato S, Tedder T F. J Immunol. 1996;157:1096–1106. [PubMed] [Google Scholar]
- 17.Konig R, Germain R N. J Exp Med. 1995;182:779–787. doi: 10.1084/jem.182.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haughn L, Gratton S, Caron L, Sekaly R-P, Veillette A, Julius M. Nature (London) 1992;358:328–331. doi: 10.1038/358328a0. [DOI] [PubMed] [Google Scholar]
- 19.Newell M K, Haughn L J, Maroun C R, Julius M H. Nature (London) 1990;347:286–289. doi: 10.1038/347286a0. [DOI] [PubMed] [Google Scholar]
- 20.Donze H H, Cummins J E, Schwiebert R S, Kantele A, Han Y, Fultz P N, Jackson S, Mestecky J. J Immunol. 1998;160:2506–2513. [PubMed] [Google Scholar]
- 21.Paterson R K, Burkly L C, Kurahara D K, Dunlap A, Flavell R A, Finkel T H. J Immunol. 1994;153:3491–3503. [PubMed] [Google Scholar]
- 22.Killeen N, Sawada S, Littman D R. EMBO J. 1993;12:1547–1553. doi: 10.1002/j.1460-2075.1993.tb05798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kubo R T, Born W, Kappler J W, Marrack P, Pigeon M. J Immunol. 1989;142:2736–2742. [PubMed] [Google Scholar]
- 24.Goldman F, Crabtree J, Hollenback C, Koretzky G. J Immunol. 1997;158:2017–2024. [PubMed] [Google Scholar]
- 25.Dianzani U, Shaw A, Fernandez-Cabezudo M, Janeway C A., Jr Int Immunol. 1992;4:995–1001. doi: 10.1093/intimm/4.9.995. [DOI] [PubMed] [Google Scholar]
- 26.Weston S A, Parish C R. J Immunol Methods. 1990;133:87–97. doi: 10.1016/0022-1759(90)90322-m. [DOI] [PubMed] [Google Scholar]
- 27.Maldarelli F, Sato H, Berthold E, Orenstein J, Martin M A. J Virol. 1995;69:6457–6465. doi: 10.1128/jvi.69.10.6457-6465.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kahn J, Ingraham R H, Shirley F, Migaki G I, Kishimoto T K. J Cell Biol. 1994;125:461–470. doi: 10.1083/jcb.125.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walcheck B, Kahn J, Fisher J M, Wang B B, Fisk R S, Payan D G, Feehan C, Betageri R, Darlak K, Spatola A F, et al. Nature (London) 1996;380:720–723. doi: 10.1038/380720a0. [DOI] [PubMed] [Google Scholar]
- 30.Hamann A, Jablonski-Westrich D, Jonas P, Thiele H-G. Eur J Immunol. 1991;21:2925–2929. doi: 10.1002/eji.1830211205. [DOI] [PubMed] [Google Scholar]
- 31.Bennett T A, Lyam E B, Sklar L A, Rogelj S. J Immunol. 1996;156:3093–3097. [PubMed] [Google Scholar]
- 32.Chen A, Engel P, Tedder T F. J Exp Med. 1995;182:519–530. doi: 10.1084/jem.182.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kahn J, Walcheck B, Migaki G I, Jutila M A, Kishimoto T K. Cell. 1998;92:809–818. doi: 10.1016/s0092-8674(00)81408-7. [DOI] [PubMed] [Google Scholar]
- 34.Arbones M L, Ord D C, Ley K, Ratech H, Maynard-Curry C, Otten G, Capon D J, Tedder T F. Immunity. 1994;1:247–260. doi: 10.1016/1074-7613(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 35.Steeber D A, Tang M L K, Zhang X-Q, Müller W, Wagner N, Tedder T F. J Immunol. 1998;161:6638–6647. [PubMed] [Google Scholar]
- 36.Tang M L K, Steeber D A, Zhang X-Q, Tedder T F. J Immunol. 1998;160:5113–5121. [PubMed] [Google Scholar]
- 37.Stockton B M, Cheng G, Manjunath N, Ardman B, von Adrian U H. Immunity. 1998;8:373–381. doi: 10.1016/s1074-7613(00)80542-7. [DOI] [PubMed] [Google Scholar]
- 38.Kinch M S, Strominger J L, Doyle C. J Immunol. 1993;151:4552–4561. [PubMed] [Google Scholar]
- 39.Doyle C, Strominger J L. Nature (London) 1987;330:256–259. doi: 10.1038/330256a0. [DOI] [PubMed] [Google Scholar]
- 40.Park S W, Royal W, III, Semba R D, Wiegand G W, Griffin D E. Clin Diagn Lab Immunol. 1998;5:583–587. doi: 10.1128/cdli.5.4.583-587.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spertini O, Schleiffenbaum B, White-Owen C, Ruiz J P, Tedder T F. J Immunol Methods. 1992;156:115–123. doi: 10.1016/0022-1759(92)90017-n. [DOI] [PubMed] [Google Scholar]
- 42.Wang L, Robb C W, Cloyd M W. Virology. 1997;228:141–152. doi: 10.1006/viro.1996.8397. [DOI] [PubMed] [Google Scholar]
- 43.Marodon G, Landau N R, Posnett D N. AIDS Res Hum Retroviruses. 1999;15:161–171. doi: 10.1089/088922299311583. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Z-Q, Notermans D W, Sedgewick G, Cavert W, Wietgrefe S, Zupancic M, Gebhard K, Henry K, Boies L, Chen Z, et al. Proc Natl Acad Sci USA. 1998;95:1154–1159. doi: 10.1073/pnas.95.3.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]