Abstract
Peritoneal adhesions are serious complications of surgery, and can result in pain, infertility, and potentially lethal bowel obstruction. Pharmacotherapy and barrier devices have reduced adhesion formation to varying degrees in preclinical studies or clinical trials; however, complete prevention of adhesions remains to be accomplished. We and others have hypothesized that the limitations of the two approaches could be overcome by combining their strengths in the context of controlled drug delivery. Here we review the role of polymeric systems in the prevention of peritoneal adhesions, with an emphasis on our recent work in developing and applying polymeric drug delivery systems such as nano-or microparticles, hydrogels, and hybrid systems for peritoneal use.
Keywords: peritoneal adhesions, adhesion barrier, controlled drug delivery, particulate drug delivery system, hydrogels
1. Introduction
Peritoneal adhesions are bands of tissue that form between structures in the abdomen and pelvis following surgery, trauma, infection, and other harmful events. The incidence of adhesions following surgery is very high; some estimate an incidence as high as 80% [1]. The consequences can be severe. Compression of the viscera can cause severe pain or infertility, and obstruction of the bowel is potentially lethal. Quite apart from the toll in human suffering and mortality, the associate health care costs are in the billions of dollars in the United States alone [1]. Consequently, adhesion prevention has been an area of absorbing interest for many years among physicians, scientists, and corporations.
Research in this field has taken a number of forms. Possibly the single largest contribution to preventing adhesions came through the development and adoption of sound surgical practices, not the least of which was the use of powder-free gloves. The two other principal areas of research were directed toward the pharmacotherapy of adhesion formation, based on the understanding at the time of the pathophysiology of the condition, and the creation of biocompatible barrier devices to keep the injured surfaces separated during healing. The pathophysiology and more-or-less experimental pharmacotherapy of peritoneal adhesion formation have been reviewed extensively, as have the pros and cons of the plethora of biomaterials and barrier devices that have been employed [1-6].
2. Pharmacological approaches to preventing peritoneal adhesions
A wide variety of compounds which target different pathophysiological contributions to adhesion formation have been used in attempts to prevent adhesions [7]. Representative drugs are classified by their therapeutic targets in Table 1. Injury to the peritoneum produces an inflammatory exudate and fibrin matrix [1]. One major category of drugs attempted to mitigate the effects of inflammation in promoting adhesion formation. A variety of steroidal [8-11] and non-steroidal anti-inflammatory compounds [12-16] have been tested, as well as agents that interfere with specific cytokines [17] or vascular permeability [18], chemotherapeutic agents [19] and immunosuppressive drugs [20]. In a related vein, many agents that scavenge the free radicals generated by inflammation have been tested [21, 22].
Table 1.
Selected pharmacological agents for adhesion prevention
| Therapeutic targets | Drug category | Examples | References |
|---|---|---|---|
| Reduce inflammation | Steroidal anti-inflammatory drugs | Dexamethasone; progesterone; hydrocortisone; prednisone | [8-11] |
| Non-steroidal anti-inflammatory drugs | Ibuprofen; flurbiprofen; indomethacin; tolmetin; nimesulide | [12-16] | |
| Inhibitors of proinflammatory cytokines | Antibodies to transforming growth factor (TGF)-β1 | [17] | |
| Antihistamine | Diphenhydramine; promethazine | [18, 64] | |
| Free radical scavengers | Melatonin; vitamin E; superoxide dismutase | [22, 65, 66] | |
| Prevent fibrin clot formation | Anti-coagulants | Heparin; ancrod | [29, 42] |
| Stimulate fibrinolysis | Proteolytic agents | Tissue-type plasminogen activator; streptokinase; urokinase; pepsin; trypsin; Neurokinin 1 receptor antagonist | [30-39, 67] |
| Inhibit fibroblast proliferation | Antiproliferative agents | Mitomycin C | [19, 41] |
A separate approach targeted the apparent imbalance between the deposition of fibrin and its destruction (fibrinolysis) in the injured peritoneum. In the course of normal tissue repair, that matrix would be removed by fibrinolysis in 2-3 days post-surgery [23]. However, in the injured state, that balance is shifted to favor the formation of fibrinous strands, which eventually are infiltrated by cells and become organized, creating the nascent adhesions [5, 24-28]. Pharmacotherapy of this problem has employed anticoagulants, which prevent the formation of fibrin strands [29], and fibrinolytics, which destroy them [30-36]. Less specific proteolytic agents have also been used, to a similar end [37-39]. Beyond these, a bewildering variety of agents have been used. A much more limited number of authors have incorporated compounds within hydrogels [30, 40-42], microspheres [9] and similar devices. A substantial number of these investigators have reported success in using drugs to prevent adhesions in animal models.
3. Barrier devices
Ideally, a barrier device should be easy to use via both laparoscopic and open procedures, provide unrestricted coverage of the affected peritoneum, and remain effective throughout healing [3]. Barrier devices have been tested or commercialized in various forms including polymer solutions [43-45], solid membranes [46], pre-formed [47-49] or in situ cross-crosslinkable hydrogels [41, 50, 51]. Solutions, mostly consisting of viscous polysaccharides, are applied by a variety of methods (spraying, pouring, extrusion from a container) at the end of the surgical procedure. They may then cross-link in situ. Membranes or pre-formed gels are placed directly on potential sites of adhesions, but then must be fixed to the underlying tissues (e.g. by suturing). That fixation can act as a focus of adhesion formation. For laparoscopic applications or situations in which damaged areas are hard to access or predict, in situ cross-linkable hydrogels are attractive modalities as they can be applied as free-flowing liquids and subsequently gel in a manner that fits the topography of the injured site. Among the various devices, five were approved or cleared for sale by the Food and Drug Administration of the United States of America (U.S. FDA) and are currently in the market: regenerated cellulose (Interceed®) and expanded polytetrafluoroethylene (Preclude®), both of which are largely used in gynecological operations; hyaluronic acid-carboxymethycellulose (Seprafilm®) which is used for general and gynecological surgery in the U.S. and Europe; polylactide membrane (Surgiwrap®) and, most recently, 4% icodextrin solution (Adept®). Representative barrier devices that have come as far as eliciting corporate interest are summarized in Table 2; the total list of materials that have been tested experimentally is much longer.
Table 2.
Barrier devices marketed or under development
| Product (company) | Chemical properties | Biological properties | Disadvantages | Development status in the U.S. | References |
|---|---|---|---|---|---|
| Interceed (Ethicon) | Oxidized regenerated cellulose membrane; hydrophilic | Absorbed within 3-10 days; Effective in two prospective randomized trials involving 137 patients. | Limited effectiveness in presence of blood and peritoneal fluids | Marketed | [68, 69] |
| Seprafilm (Genzyme) | Hyaluronic acid-carboxymethycellulose membrane; hydrophilic | Absorbed within 2 weeks. | Requires careful handling at open operation; Difficult to use laparoscopically | Marketed | [70-72] |
| Sepragel (Genzyme) | Hyaluronic acid-carboxymethycellulose gel; hydrophilic | Bioresorbable | Indicated for nasal/sinus surgery as a space-occupying gel | [49] | |
| Sepracoat (Genzyme) | Sodium hyaluronate solution; hydrophilic | Elimination half-life in the peritoneum: ~26 hours [73] | Insufficient evidence of clinical effectiveness | Not FDA approved. (Marketed in Europe) | [74] |
| Intergel (Lifecore) | Ferric hyaluronate gel; hydrophilic | Elimination half-life of 0.5% Ferric hyaluronate in the peritoneum: ~51 hours [73] | Withdrawn from clinical studies due to the high morbidity associated with postoperative peritonitis and anastomotic dehiscence. | Clinical study discontinued | [73, 75, 76] |
| Incert (Anika) | Chemically crosslinked hyaluronic acid; hydrophilic | Bioresorbable | Difficult to use laparoscopically | Pilot human trials in UK from 2004. | [77] |
| Adcon-P (Gliatech Inc.) | Gelatin/proteoglycan; hydrophilic | Bioresorbable | Note FDA recall of previous product Adcon-L. | Animal tests only as of 2003 | [78, 79] |
| Adept (Innovata) | Icodextrin solution; hydrophilic | A median of 40% of the instilled icodextrin was absorbed from the peritoneal solution during a 12-hour dwell. Peritoneal residence time of 4% icodextrin >4 days [80]. | Leakage of fluid from the surgical site; Abdominal distension and discomfort | FDA approved for use in gynecological laparoscopic procedures in Aug. 2006. (Marketed in Europe) | [81, 82] |
| Hyskon (Medisan pharmaceuticals) | Dextran 70; hydrophilic | Bioresorbable | Local and systemic side-effects due to osmotic and antigenic properties [83, 84]; Not sufficient evidence of clinical effectiveness | Indicated as hysteroscopy fluid | [83-85] |
| NOCC (Kytogenics) | N,O-carboxymethyl chitosan; hydrophilic | Bioresorbable | Pilot clinical trial did not demonstrate significant difference between treatment and control groups in anti-adhesion efficacy [86]. | Phase III clinical trial (2002) | [86-88] |
| FloGel (Alliance Pharm Co.) | Poloxamer 407; hydrophilic | Thermosensative; bioresorbable | Need refrigeration | Early clinical development | [89] |
| SprayGel (Confluent Surgical) | In situ crosslinkable polyethylene glycol gel; hydrophilic | Bioresorbable in 6 days; degrades into water-soluble polyethylene glycol molecules | Need a specialized applicator and air supply | Clinical investigation | [90, 91] |
| Surgiwrap sheet (MAST Biosurgery) | Polylactide membrane; hydrophobic | Bioresobable in 24 weeks; degrades into water and CO2. | Need fixation | Cleared for sale by the FDA in May 2005. (Marketed in Europe) | [92] |
| Repel (Life Medical Sciences) | Polyethylene glycol and polylactic acid block copolymer membrane | Bioresorbable | Need fixation; Difficult to use laparoscopically | Clinical studies on-going | [93, 94] |
| FocalGel (Focal) | Polyethylene glycol and polylactic acid | Photo-crosslinkable; bioresorbable | Need light source | [95] | |
| Preclude (W.L. Gore) | Expanded Polytetrafluoroethylene membrane; hydrophobic | Non-biodegradable | Need strict sterility; Need fixation; Difficult to use laparoscopically; Permanent presence of a foreign body in the peritoneum | Marketed | [96] |
Many devices are derived from polysaccharides such as hyaluronic acid, cellulose, dextran, or chitosan. Polysaccharides are particularly popular because they either are or resemble naturally occurring biological compounds, and they have been shown to be compatible with a variety of tissues in other biomedical applications. Synthetic polymers based on polyethylene glycol or polylactic acid have also been used. Advantages of synthetic polymers include the relative ease with which their properties can be controlled, and the lower cost compared to some (but not all) natural polysaccharides that have to undergo extraction and purification processes. In many respects the specific choice of materials is empirical, often driven in part by issues of intellectual property. At this time there is no clear set of rules relating biomaterial physicochemical properties to their biological effects and/or responses in the peritoneum, except in the broadest terms.
Barrier approaches have succeeded to varying degrees in preventing adhesion formation in animal models and/or clinical studies, although none of them are consistently effective in all surgical scenarios [6]. Some of the principal limitations of the existing devices include a brief residence time at the site of administration [52], difficulty in handling or fixation to tissues, and incompatibility with laparoscopic procedures [41]. Another disadvantage often related to membrane or pre-formed hydrogel forms is a relative difficulty in determining the size and shape of the area to be covered a priori. Therefore, the surgeon must, to some extent, predict the possible location, size and shape of potential sites of adhesion formation [5].
4. Polymers for controlled drug release in the peritoneal cavity
From the above, it will be apparent that dozens of investigators have cured adhesions in one animal model or another. So why is the clinical problem not solved? It is, unfortunately, not possible to know that which is not published, and so the reasons for which so many therapies that were successful in print did not enter clinical practice is not known. However, we have hypothesized that ultimately the failure of purely pharmacological therapies lay in the rapid clearance of drugs from the peritoneum. Conversely, the shortcomings of barrier devices – aside from physical design issues etc.-were that they did not directly address the basic biological problems. Controlled release technology could provide sustained drug levels, and if desirable, could also provide a barrier function.
In designing a drug delivery system for the peritoneum, at least two principal approaches were apparent (Fig. 1 A & B). One would be micro-or nanoparticulate, as such particles would be easy to disperse throughout the peritoneum if necessary. The other would be hydrogel-based, since they could be made out of materials that have been used in the peritoneum without significant adverse effects, such as hyaluronic acid or cellulose. In situ cross-linkability would make these materials easy to apply, and would make it easy to cover discreet areas of injury at the discretion of the surgeon. Alternatively, drugs could be loaded in pre-formed films of such materials. A third approach, that we will describe below, would be to combine particulate and hydrogel-based systems (Fig. 1 C).
Fig. 1.

Drug delivery systems for post-surgical adhesion prevention. Drugs can be delivered in a particulate system (A), a coating or barrier device such as a hydrogel (B), or a combination of a particulate system and a barrier device (‘hybrid’) (C). Scanning electron microscopic (SEM) images of nanoparticles (A, middle panel) and microparticles (A, right panel). Picture of chitosan gel cross-linked by UV irradiation (B, middle panel) and SEM of lyophilized cross-linked hyaluronan hydrogel (B, right panel). SEM images of lyophilized hybrid gel (C, middle panel) and magnified view of lyophilized hybrid gel (C, right panel). Note the roughness of the hybrid gel surface, indicating embedded nanoparticles.
One caveat in developing peritoneal drug delivery systems, which we have found to be a much greater issue in the peritoneum than in most other anatomic locations, is that drug delivery platforms must first be evaluated as to their intrinsic potential to elicit adhesions, as that could offset the positive therapeutic effects from the pharmacological agents being delivered.
4.1. Poly α-hydroxy acids
In designing a particulate drug delivery system, it seemed reasonable to begin with the poly α-hydroxy acids, specifically the poly(lactic-co-glycolic) acids (PLGAs). These polymers had an excellent record of biocompatibility and could control the release of a wide range of drugs very effectively. They were known to degrade to lactic and glycolic acids, then to be metabolized to carbon dioxide and water. Although they caused an inflammatory response, it is transient. Even if polymeric residue and its attendant indolent chronic inflammatory response last for months, eventually tissue reaction in most tissues can resolve completely [53]. Finally, there was a large body of experience with them, and they were approved for use in humans by U.S. FDA.
The experience in the peritoneum was quite different. We injected 10 to 100 mg of particles composed of a high molecular weight (MW) poly(lactic-co-glycolic) acid into the murine peritoneum, covering a thousand-fold range of sizes from 250 nm to 250 μm [54]. In the microparticle size range, all groups were capable of producing adhesions, although the incidence varied. Adhesions occurred most frequently on the anterior parietal peritoneum, which is the dependent portion of the peritoneum in the quadruped mouse. These findings raised the concern that in the bipedal human, even small quantities of particles would accumulate in the relatively confined lower abdomen and pelvis, causing adhesions. Nanoparticles of the same material caused almost no adhesions, but that was only because they left the peritoneum and migrated to the reticuloendothelial system, particularly the spleen. Microparticles made of lower MW PLGA caused less adhesions than those with higher MW PLGA. We speculated that the reason for the decrease in adhesions related to the more rapid degradation, with a resulting shorter tissue dwell time. Others have reduced peritoneal adhesions by using PLGA microspheres to release dexamethasone, a potent steroidal anti-inflammatory drug [9]. Interestingly, in that study, microspheres with a low loading of dexamethasone worsened adhesions, while microspheres with a high loading reduced adhesions. This suggests that the vehicle itself promoted adhesions formation, a tendency that was partly offset by the adhesion-preventing action of the drug. In furthering our work, we decided that it was best to select vehicles that had little intrinsic adhesion-forming tendency, and so decided not to use polymeric particles alone.
4.2. Polysaccharide-based matrices
Others had previously used hydrogels in the peritoneum to release low [41] and high MW compounds [30], with varying degrees of effectiveness. In particular, we were interested in developing a system that would be easy to use, in addition to being biocompatible and providing adequate control of drug release.
4.2.1. Chitosan
One initial approach was to use a UV cross-linkable chitosan hydrogel [55], based on the view that chitosan was generally considered to be biocompatible [56, 57]. We demonstrated in vitro that it had excellent properties in terms of cohesiveness and macromolecule release kinetics (data not shown). The formulation was also attractive in that it would be possible to apply in situ. In vitro cytotoxicity of cross-linked chitosan was minimal. Chitosan itself and the modified chitosan precursor to the cross-linked gel were minimally toxic to mesothelial cells. Chitosan itself, but not the modified chitosan was quite toxic to macrophages at a relatively high concentration (2 mg/ml). The cytotoxicity of some cationic polymers (e.g., polyamines) in phagocytic cells has been documented [58]. The greater toxicity of chitosan compared to the modified chitosan in macrophages may be related to the degree of deacetylation of chitosan (i.e. the number of primary amines) [55]. However, this did not seem worrisome since the cross-linked chitosan would contain no unmodified chitosan. The in vivo experience was quite different. Rabbits treated with the UV-cross-linked formulation developed exuberant adhesions, even in the absence of prior peritoneal injury. The causative factor was not the UV irradiation but the material itself. Both modified and unmodified chitosan could induce adhesions. In investigating the cause of the adhesions, we found that the modified chitosan and the cross-linked gel increased the expression of proinflammatory cytokines or chemokines such as TNF-α and MIP-2 (a murine IL-8 analogue). Whether this explained the formation of adhesions is unclear, given that unmodified chitosan caused adhesions but did not increase cytokine release in vitro - although the latter could be due its effect on cell viability.
4.2.2. Cross-linked hyaluronan matrices
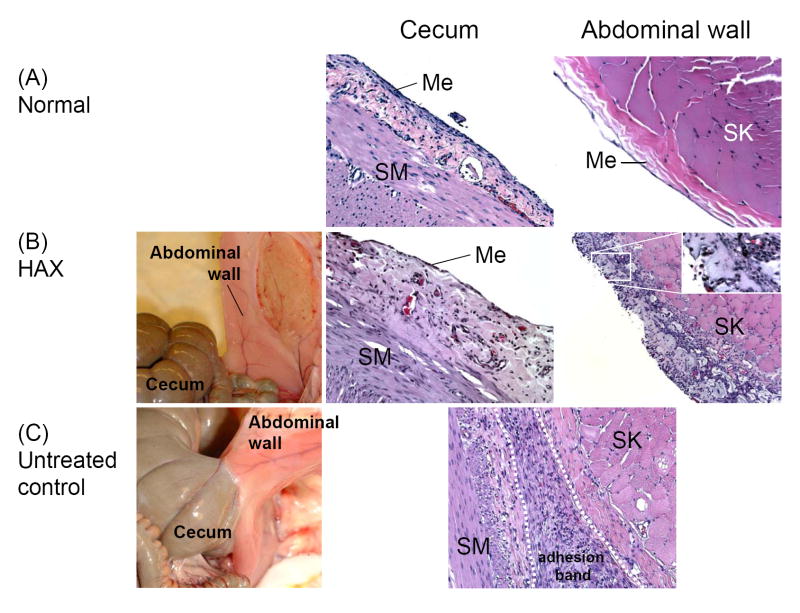
We therefore turned to materials with an established track record of biocompatibility in the peritoneum [59]. We chose hyaluronan (HA) as the base material for this reason; it had also been used extensively for intraperitoneal applications. We employed a previously described synthetic method [60] to produce a cross-linkable HA hydrogel. In brief, we produced one HA derivatized to have a hydrazide moiety, and another HA derivatized to have an aldehyde. These cross-linked upon mixing, forming a slowly hydrolysable hydrazone compound. This method had the advantage of allowing in situ cross-linking without the use of initiators, cross-linking chemicals, or extra equipment for cross-linking such as a light source. We had prior experience with this approach for drug delivery [61]. One early concern was whether the derivatized HAs, particularly the aldehyde derivative, would be reactive with biological components and cause toxic side effects. It was reassuring to find that the cross-linked HA gels (HAX) showed minimal or no cytotoxicity in mesothelial cells in vitro, and showed excellent intraperitoneal biocompatibility in a murine screening test. This formulation was very effective in preventing adhesions in a rabbit cecal abrasion-sidewall defect model [59] (Fig. 2). Interestingly, the cross-linked matrix itself caused a modest increase in the expression of tissue-type plasminogen activator, raising the possibility that its effectiveness in preventing adhesions might be due to an intrinsic biological effect in addition to the barrier effect.
Fig. 2.
Adhesion formation [59]. (A) Normal cecum (200x) and abdominal wall surface (400x). (B) Healed cecum surface (200x) and abdominal wall (100x) treated with cross-linked hyaluronan gel (HAX) one week post-surgery. Note the mesothelial layer on the treated cecum comparable to that of normal cecum, and bluish HAX residue on the treated abdominal wall (Inset in right panel shows higher magnification of gel in area denoted by rectangle.). (C) An adhesion (200x) found in an injured, untreated animal. SM: smooth muscle; Me: mesothelial layer; SK: skeletal muscle of abdominal wall.
4.2.3. The hybrid system
Although the cross-linked HA performed very well, there was still room for improvement. As stated above, one hypothesis underlying our work was that barrier devices would work better if supplemented by controlled release technology. We had found that the HA-based hydrogels controlled the rate of release of proteins and other drugs [61] only slightly. While that slight difference might yet prove to be important, the release kinetics could be improved by incorporating a more hydrophobic polymeric delivery system such as PLGA micro-or nanoparticles into the hydrogel system. Thus we would be able to marry the relative strengths and weaknesses of hydrogels (excellent peritoneal biocompatibility, poor drug release kinetics) and polymeric particles (poor peritoneal biocompatibility, excellent control of release kinetics) [62]. Polymeric particles can, depending on the formulation, release drugs for days to weeks. The exact duration will depend on the characteristics of the drug (hydrophilicity, hydrophobicity, charge, molecular weight, etc.), the polymer (type, molecular weight, etc.), and the specifics of the encapsulation method. Drug release for days to weeks has been achieved with both small molecules and macromolecules. The hybrid system is prepared simply by mixing the hydrogel precursors and nanoparticle suspensions then co-injecting them. The nanoparticles (Fig. 1A) were distributed homogeneously throughout the hydrogel matrix (Fig. 1C). We chose nanoparticles over microparticles for our initial work in this direction because they would leave the peritoneum without causing adhesions if they broke free of the gel, whereas microparticles might stay and cause adhesions [54]. These hybrid gels showed minimal cytotoxicity in mesothelial cells. Furthermore, they showed excellent biocompatibility in the murine peritoneum, with minimal adhesion formation even though nanoparticles entrapped within the hydrogel could not leave the peritoneal cavity. In a rabbit model of adhesion formation, the hybrid gels were at least as effective (and statistically significantly better) than HAX in preventing adhesions, even in the absence of any active payload (released drug). It remains to be seen whether this formulation is even more effective when loaded with active agents.
4.2.4. Cellulose derivatives
HA-based systems have two major drawbacks: they are costly, and they could be degraded too rapidly in vivo by endogenous hyaluronidase. We have hypothesized that hybridization of HA with other biocompatible polysaccharides that are not degraded enzymatically in humans could slow degradation while preserving HA’s excellent biocompatibility. We synthesized aldehyde derivatives of cellulose derivatives (hydroxypropylmethylcellulose, carboxymethylcellulose, and methylcellulose), and cross-linked them to HA-dihydrazide [63]. The aldehyde-cellulose derivatives were somewhat more cytotoxic in vitro to mesothelial cells and peritoneal macrophages than HA-aldehyde, but showed no toxicity or adhesion formation in a murine intraperitoneal screening test. It is possible that the lack of toxicity in vivo reflects the rapidity of in situ cross-linking, which may leave little free gel precursor. All three HA-cellulose derivative gels were effective in reducing adhesions. HA-methycellulose was slightly more effective than the others, perhaps because it degraded more slowly.
4.2.5. Effect of polymeric drug delivery systems in the peritoneum
To date, there are relatively few published reports where drugs were delivered via vehicles that clearly controlled the rate of drug release, and even fewer where that control was demonstrated. However, there are studies that give reason to be optimistic in this regard. As mentioned above, polymeric microspheres containing a high loading of steroids mitigated adhesion formation [9]. Some success has also been achieved with a number of anticoagulant and fibrinolytic compounds delivered via hydrogels [29, 30, 42]. In the latter cases, the hydrogels themselves had independent effectiveness as barrier devices, but less than the hydrogel and drug together.
5. Conclusions
Despite the number of animal studies and the human experience involving biomaterials in the peritoneum, and even though we have some sense of what does work, it is difficult to point to specific material properties as explaining why some materials are biocompatible and effective in preventing adhesions and others are not. Although most materials that prevent adhesions are hydrophilic, it does not follow that hydrophobic materials cannot be used (e.g. Teflon or polylactide). Perhaps, as with some other biological properties, there is a U-shaped relation between the propensity toward adhesions and hydrophobicity. Similarly, it is difficult to make general statements about what physical properties are best. The hydrogels used in our studies have significantly lower shear moduli than that of Teflon, but both hydrogels and Teflon can be used as adhesion barriers. Quite apart from the direct physicochemical effects of the materials on their environment, little is known about the tissue responses elicited by them and the potential indirect mechanisms of injuring or healing surrounding tissues. The design of ideal materials is complicated by the fact that our ignorance of the important parameters governing anti-adhesion devices extends to such basic questions as: How long should they persist in the peritoneum? How much of the peritoneal cavity should be covered? If controlled release of drugs is to be used, what drug or drug combination is best? Until these and other issues are resolved, a sub-optimal empiricism is going to drive research and practice.
Many materials have been tested within the peritoneum. While there can be little doubt that some materials control the rate of drug release more effectively than others, what little has been published on drug delivery in the peritoneum suggests that most materials that have been used as barriers have the potential to be used as drug delivery systems. As we have seen, some have been tried [29, 30, 42], although so far none have into translated clinical practice. Whether the converse is true, i.e. whether the more hydrophobic polymers used for conventional drug delivery are suitable as intraperitoneal barriers, remains to be proven.
Since the initial efforts to prevent post-surgical adhesions were made more than a century ago [6], a number of anti-adhesion devices have been developed and used, but their efficacy and scope of use still remain limited. Adhesion formation continues to be a challenge in peritoneal surgery. However, given the accumulated knowledge from research and clinical experience, and with recent advances in tissue engineering and drug delivery technology, there is reason to be optimistic that complete prevention of post-surgical adhesions is an achievable goal.
Acknowledgments
Financial support was provided by the DuPont/MIT Alliance (to DSK) and GM073626 (to DSK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.diZerega GS. Peritoneum, peritoneal healing, and adhesion formation. In: diZerega GS, editor. Peritoneal Surgery. Springer; New York: 2000. pp. 3–37. [Google Scholar]
- 2.Saravelos HG, Li TC. Physical barriers in adhesion prevention. The Journal of Reproductive Medicine. 1996;41:42–51. [PubMed] [Google Scholar]
- 3.Al-Musawi D, Thompson JN. Adhesion prevention: state of art. Gynaecological Endoscopy. 2001;10:123–130. [Google Scholar]
- 4.Peck LS, Goldberg EP. Polymer solutions and films as tissue-protective and barrier adjuvants. In: diZerega GS, editor. Peritoneal Surgery. Springer; New York: 2000. pp. 499–520. [Google Scholar]
- 5.diZerega GS. Use of adhesion prevention barriers in pelvic reconstructive and gynecologic surgery. In: diZerega GS, editor. Peritoneal Surgery. Springer; New York: 2000. pp. 379–399. [Google Scholar]
- 6.Wiseman DM. Adhesion Prevention: Past the Future. In: diZerega GS, editor. Peritoneal Surgery. Springer; New York: 2000. pp. 401–417. [Google Scholar]
- 7.Rodgers KE, diZerega GS. Developing pharmacologic agents for adhesionprevention. In: diZerega GS, editor. Peritoneal Surgery. Springer; New York: 2000. pp. 441–457. [Google Scholar]
- 8.Kucukozkan T, Ersoy B, Uygur D, Gundogdu C. Prevention of adhesions by sodium chromoglycate, dexamethasone, saline and aprotinin after pelvic surgery. ANZ J Surg. 2004;74:1111–1115. doi: 10.1111/j.1445-1433.2004.03261.x. [DOI] [PubMed] [Google Scholar]
- 9.Hockel M, Ott S, Siemann U, Kissel T. Prevention Of Peritoneal Adhesions In The Rat With Sustained Intraperitoneal Dexamethasone Delivered By A Novel Therapeutic System. Annales Chirurgiae Et Gynaecologiae. 1987;76:306–313. [PubMed] [Google Scholar]
- 10.Buckenmaier CC, Pusateri AE, Harris RA, Hetz SP. Comparison of antiadhesive treatments using an objective rat model. American Surgeon. 1999;65:274–282. [PubMed] [Google Scholar]
- 11.Maurer J, Bonaventura L. The effect of aqueous progesterone on operative adhesion formation. Fertil Steril. 1983;39:485–489. doi: 10.1016/s0015-0282(16)46937-2. [DOI] [PubMed] [Google Scholar]
- 12.LeGrand EK, Rodgers KE, Girgis W, Struck K, Campeau JD, DiZerega GS, Kiorpes TC. Efficacy of tolmetin sodium for adhesion prevention in rabbit and rat models. J Surg Res. 1994;56:67–71. doi: 10.1006/jsre.1994.1011. [DOI] [PubMed] [Google Scholar]
- 13.Guvenal T, Cetin A, Ozdemir H, Yanar O, Kaya T. Prevention of postoperative adhesion formation in rat uterine horn model by nimesulide: a selective COX-2 inhibitor. Human Reproduction. 2001;16:1732–1735. doi: 10.1093/humrep/16.8.1732. [DOI] [PubMed] [Google Scholar]
- 14.Nishimura K, Nakamura R, diZerega G. Ibuprofen inhibition of postsurgical adhesion formation: a time and dose response biochemical evaluation in rabbits. J Surg Res. 1984;36:115–124. doi: 10.1016/0022-4804(84)90076-3. [DOI] [PubMed] [Google Scholar]
- 15.Holtz G. Failure of a nonsteroidal anti-inflammatory agent (ibuprofen) to inhibit peritoneal adhesion reformation after lysis. Fertil Steril. 1982;37:582–583. doi: 10.1016/s0015-0282(16)46171-6. [DOI] [PubMed] [Google Scholar]
- 16.De Leon F, Toledo A, Sanfilippo J, Yussman M. The prevention of adhesion formation by nonsteroidal antiinflammatory drugs: an animal study comparing ibuprofen and indomethacin. Fertil Steril. 1984;41:639–642. doi: 10.1016/s0015-0282(16)47792-7. [DOI] [PubMed] [Google Scholar]
- 17.Lucas P, Warejcka D, Young H, Lee B. Formation of abdominal adhesions is inhibited by antibodies to transforming growth factor-beta1. J Surg Res. 1996;65:135–138. doi: 10.1006/jsre.1996.0355. [DOI] [PubMed] [Google Scholar]
- 18.Avsar F, Sahin M, Aksoy F, Avsar A, Akoz M, Hengirmen S, Bilici S. Effects of diphenhydramine HCl and methylprednisolone in the prevention of abdominal adhesions. Am J Surg. 2001;181:512–515. doi: 10.1016/s0002-9610(01)00617-1. [DOI] [PubMed] [Google Scholar]
- 19.Cubukcu A, Alponat A, Gonullu N, Ozkan S, Ercin C. An experimental study evaluating the effect of Mitomycin C on the prevention of postoperative intraabdominal adhesions. J Surg Res. 2001;96:163–166. doi: 10.1006/jsre.2000.6059. [DOI] [PubMed] [Google Scholar]
- 20.Leondires M, Stubblefield P, Tarraza H, Jones M. A pilot study of cyclosporine for the prevention of postsurgical adhesion formation in rats. Am J Obstet Gynecol. 1995;172:1537–1539. doi: 10.1016/0002-9378(95)90492-1. [DOI] [PubMed] [Google Scholar]
- 21.Binda MM, Molinas CR, Koninckx PR. Reactive oxygen species and adhesion formation: clinical implications in adhesion prevention. Hum Reprod. 2003;18:2503–2507. doi: 10.1093/humrep/deg481. [DOI] [PubMed] [Google Scholar]
- 22.Ozcelik B, Serin IS, Basbug M, Uludag S, Narin F, Tayyar M. Effect of melatonin in the prevention of post-operative adhesion formation in a rat uterine horn adhesion model. Hum Reprod. 2003;18:1703–1706. doi: 10.1093/humrep/deg337. [DOI] [PubMed] [Google Scholar]
- 23.Thompson J. Peritoneal fibrinolysis and adhesion formation. In: diZerega GS, editor. Peritoneal Surgery. Springer; New York: 2000. pp. 133–142. [Google Scholar]
- 24.Rodgers KE. Peritoneal tissue repair cells. In: diZerega GS, editor. Peritoneal Surgery. Springer; New York: 2000. [Google Scholar]
- 25.Holmdahl L, Ivarsson ML. The role of cytokines, coagulation, and fibrinolyis in peritoneal tissue repair. Eur J Surg. 1999;165:1012–1019. doi: 10.1080/110241599750007810. [DOI] [PubMed] [Google Scholar]
- 26.Raftery A. Effect of peritoneal trauma on peritoneal fibrinolytic activity and intraperitoneal adhesion formation. An experimental study in the rat. Eur Surg Res. 1981;13:397–401. doi: 10.1159/000128208. [DOI] [PubMed] [Google Scholar]
- 27.Holmdahl L, Eriksson E, al-Jabreen M, Risberg B. Fibrinolysis in human peritoneum during operation. Surgery. 1996;119:701–705. doi: 10.1016/s0039-6060(96)80196-6. [DOI] [PubMed] [Google Scholar]
- 28.Holmdahl L, Falkenberg M, Ivarsson ML, Risberg B. Plasminogen activators and inhibitors in peritoneal tissue. APMIS: acta pathologica, microbiologica, et immunologica Scandinavica. 1997;105:25–30. doi: 10.1111/j.1699-0463.1997.tb00535.x. [DOI] [PubMed] [Google Scholar]
- 29.Reid RL, Lie K, Spence JE, Tulandi T, Yuzpe A. Clinical evaluation of the efficacy of heparin-saturated interceed for prevention of adhesion reformation in the pelvic sidewall of the human. In: Diamond MP, diZerega GS, Linsky CB, Reid RL, editors. Gynecologic Surgery and Adhesion Prevention. Wiley-Liss, Inc; New York: 1993. pp. 261–264. [PubMed] [Google Scholar]
- 30.Hill-West JL, Dunn RC, Hubbell JA. Local release of fibrinolytic agents for adhesion prevention. J Surg Res. 1995;59:759–763. doi: 10.1006/jsre.1995.1236. [DOI] [PubMed] [Google Scholar]
- 31.Doody KJ, Dunn RC, Buttram VC. Recombinant tissue plasminogen activator reduces adhesion formation in a rabbit uterine horn model. Fertil Steril. 1989;51:509–512. doi: 10.1016/s0015-0282(16)60563-0. [DOI] [PubMed] [Google Scholar]
- 32.Dunn RC, Mohler M. Effect of Varying Days of Tissue Plasminogen Activator Therapy on the Prevention of Postsurgical Adhesions in a Rabbit Model. J Surg Res. 1993;54:242–245. doi: 10.1006/jsre.1993.1038. [DOI] [PubMed] [Google Scholar]
- 33.Orita H, Fukasawa M, Girgis W, diZerega GS. Inhibition of postsurgical adhesions in a standardized rabbit model: intraperitoneal treatment with tissue plasminogen activator. Int J Fertil. 1991;36:172–177. [PubMed] [Google Scholar]
- 34.Menzies D, Ellis H. The role of plasminogen activator in adhesion prevention. Surgery, Gynecology & Obstetrics. 1991;172:362–366. [PubMed] [Google Scholar]
- 35.Dunn RC, Mohler M. Formation of adhesions after surgical injury and their prevention with tissue-type plasminogen activator in a rabbit pelvic model. Infertility. 1990;13:103–111. [Google Scholar]
- 36.Holmdahl LE, Al-Jabreen M, Risberg B. Role of fibrinolysis in the formation of postoperative adhesions. Wound Rep Reg. 1994;2:171–176. doi: 10.1046/j.1524-475X.1994.20306.x. [DOI] [PubMed] [Google Scholar]
- 37.Ochsner A, Garside E. Peritoneal adhesions: their prevention by the use of digestive ferments. Surg Gynecol Obstet. 1932;54:338–361. [Google Scholar]
- 38.Yardumian K, Cooper D. Pepsin in the prevention of abdominal adhesions. Arch Surg. 1934;29:264–276. [Google Scholar]
- 39.Walton R. Trypsin preparations suitable for the prevention of adhesions. J Pharmacol Exp Ther. 1930;40:403–411. [Google Scholar]
- 40.Jackson JK, Skinner KC, Burgess L, Sun T, Hunter WL, Burt HM. Paclitaxel-loaded crosslinked hyaluronic acid films for the prevention of postsurgical adhesions. Pharmaceutical Research. 2002;19:411–417. doi: 10.1023/a:1015175108183. [DOI] [PubMed] [Google Scholar]
- 41.Liu Y, Li H, Shu XZ, Gray SD, Prestwich GD. Crosslinked hyaluronan hydrogels containing mitomycin C reduce postoperative abdominal adhesions. Fertil Steril. 2005;83:1275–1283. doi: 10.1016/j.fertnstert.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 42.Chowdhury SM, Hubbell JA. Adhesion prevention with ancrod release via a tissue-adherent hydrogel. J Surg Res. 1996;61:58–64. doi: 10.1006/jsre.1996.0081. [DOI] [PubMed] [Google Scholar]
- 43.Burns JW, Skinner K, Colt J, Sheidlin A, Bronson R, Yaacobi Y, Goldberg EP. Prevention of tissue injury and postsurgical adhesions by precoating tissues with hyaluronic acid solutions. J Surg Res. 1995;59:644–652. doi: 10.1006/jsre.1995.1218. [DOI] [PubMed] [Google Scholar]
- 44.Reijnen MMPJ, Skrabut EM, Postma VA, Burns JW, Goor Hv. Polyanionic polysaccharides reduce intro-abdominal adhesion and abscess formation in a rat peritonitis model. J Surg Res. 2001;101:248–253. doi: 10.1006/jsre.2001.6288. [DOI] [PubMed] [Google Scholar]
- 45.Rodgers KE, Campeau J, Johns DB, diZerega GS, Girgis W. Reproduction of adhesion formation with hyaluronic acid after peritoneal surgery in rabbits. Fertil Steril. 1997;67:553–558. doi: 10.1016/s0015-0282(97)80085-4. [DOI] [PubMed] [Google Scholar]
- 46.Pressato D, Bigon E, Dona M, Pavesio A, Renier D, Bonafini L, Iaco PAD, Lise M. In: Kennedy JF, Phillips GO, Williams PA, editors. Hyaluronan derivatives in postsurgical adhesion prevention; Hyaluronan: Proceedings of an International Meeting; September 2000; North East Wales Institute UK. Cambridge, England: Woodhead Publishing; 2002. pp. 491–499. [Google Scholar]
- 47.Laco PAD, Stefanetti M, Pressato D, Piana S, Dona M, Pavesio A, Bovicelli L. A novel hyaluronan-based gel in laparoscopic adhesion prevention: preclinical evaluation in an animal model. Fertil Steril. 1998;69:318–323. doi: 10.1016/s0015-0282(98)00496-8. [DOI] [PubMed] [Google Scholar]
- 48.Burns JW, Skinner K, Colt MJ, Burgess L, Rose R, Diamond MP. A hyaluronate based gel for the prevention of postsurgical adhesions: evaluation in two animal species. Fertil Steril. 1996;56:814–821. [PubMed] [Google Scholar]
- 49.Leach RE, Burns JW, Dawe EJ, SmithBarbour MD, Diamond MP. Reduction of postsurgical adhesion formation in the rabbit uterine horn model with use of hyaluronate/carboxymethylcellulose gel. Fertil Steril. 1998;69:415–418. doi: 10.1016/s0015-0282(97)00573-6. [DOI] [PubMed] [Google Scholar]
- 50.Bennett SL, Melanson DA, Torchiana DF, Wiseman DM, Sawhney AS. Next-generation hydrogel films as tissue sealants and adhesion barriers. J Card Surg. 2003;18:494–499. doi: 10.1046/j.0886-0440.2003.00303.x. [DOI] [PubMed] [Google Scholar]
- 51.Sawhney AS, Pathak CP, Rensburg JJv, Dunn RC, Hubbell JA. Optimization of photopolymerized bioerodible hydrogel properties for adhesion prevention. J Biomed Mater Res. 1994;28:831–838. doi: 10.1002/jbm.820280710. [DOI] [PubMed] [Google Scholar]
- 52.Grainger D, Meyer W, Decherney A, Diamond M. The use of hyaluronic-acid polymers to reduce postoperative adhesions. J Gynecol Surg. 1991;7:97–101. [Google Scholar]
- 53.Kohane DS, Lipp M, Kinney RC, Anthony DC, Louis DN, Lotan N, Langer R. Biocompatibility of lipid-protein-sugar particles containing bupivacaine in the epineurium. J Biomed Mater Res. 2002;59:450–459. doi: 10.1002/jbm.1261. [DOI] [PubMed] [Google Scholar]
- 54.Kohane DS, Tse JY, Yeo Y, Padera R, Shubina M, Langer R. Biodegradable polymeric microspheres and nanospheres for drug delivery in the peritoneum. J Biomed Mater Res. 2006;77A:351–361. doi: 10.1002/jbm.a.30654. [DOI] [PubMed] [Google Scholar]
- 55.Yeo Y, Burdick JA, Highley CB, Marini R, Langer R, Kohane DS. Peritoneal application of chitosan and UV-cross-linkable chitosan. J Biomed Mater Res. 2006;78A:668–675. doi: 10.1002/jbm.a.30740. [DOI] [PubMed] [Google Scholar]
- 56.Risbud M, Bhonde M, Bhonde R. Chitosan-polyvinyl pyrrolidone hydrogel does not activate macrophages: potentials for transplantation applications. Cell transplantation. 2001;10:195–202. [PubMed] [Google Scholar]
- 57.Prasitsilp M, Jenwithisuk R, Kongsuwan K, Damrongchai N, Watts P. Cellular responses to chitosan in vitro: The importance of deacetylation. Journal of Materials Science: Materials in Medicine. 2000;11:773–778. doi: 10.1023/a:1008997311364. [DOI] [PubMed] [Google Scholar]
- 58.Haining WN, Anderson DG, Little SR, Berwelt-Baildon MSv, Cardoso AA, Alves P, Kosmatopoulos K, Nadler LM, Langer R, Kohane DS. pH-triggered microparticles for peptide vaccination. Journal of Immunology. 2004;173:2578–2585. doi: 10.4049/jimmunol.173.4.2578. [DOI] [PubMed] [Google Scholar]
- 59.Yeo Y, Highley CB, Bellas E, Ito T, Marini R, Langer R, Kohane DS. In situ cross-linkable hyaluronic acid hydrogels prevent post-operative abdominal adhesions in a rabbit model. Biomaterials. 2006;27:4698–4705. doi: 10.1016/j.biomaterials.2006.04.043. [DOI] [PubMed] [Google Scholar]
- 60.Bulpitt P, Aeschlimann D. New strategy for chemical modification of hyaluronic acid: preparation of functionalized derivatives and their use in the formation of novel biocompatible hydrogels. J Biomed Mater Res. 1999;47:152–169. doi: 10.1002/(sici)1097-4636(199911)47:2<152::aid-jbm5>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 61.Jia X, Colombo G, Padera R, Langer R, Kohane DS. Prolongation of sciatic nerve blockade by in situ cross-linked hyaluronic acid. Biomaterials. 2004;25:4797–4804. doi: 10.1016/j.biomaterials.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 62.Yeo Y, Ito T, Bellas E, Highley CB, Marini R, Kohane DS. In situ cross-linkable hyaluronic acid hydrogels containing polymeric nanoparticles for preventing post-operative abdominal adhesions. Ann Surg. 2006 doi: 10.1097/01.sla.0000251519.49405.55. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ito T, Yeo Y, Highley CB, Bellas E, Benitez C, Kohane DS. The prevention of peritoneal adhesions by in-situ cross-linking hydrogels of hyaluronic acid and cellulose derivatives. Biomaterials. doi: 10.1016/j.biomaterials.2006.10.02. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Replogle RL, Johnson R, Gross RE. Prevention of postoperative intestinal adhesions with combined promethazine and dexamethasone therapy: experimental and clinical studies. Ann Surg FIELD Full Journal Title:Annals of surgery. 1966;163:580–588. doi: 10.1097/00000658-196604000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Portilla Fdl, Ynfante I, Bejarano D, Conde J, Fernández A, Ortega JM, Carranza G. Prevention of peritoneal adhesions by intraperitoneal administration of vitamin E: An experimental study in rats. Dis Colon Rectum. 2005;47:2157–2161. doi: 10.1007/s10350-004-0741-6. [DOI] [PubMed] [Google Scholar]
- 66.Portz D, Elkins T, White R, Warren J, Adedevoh S, Randolph J. Oxygen free radicals and pelvic adhesions formation: I. Blocking oxygen free radical toxicity to prevent adhesion formation in an endometriosis model. Int J Fertil. 1991;36:39–42. [PubMed] [Google Scholar]
- 67.Reed KL, Fruin AB, Gower AC, Stucchi AF, Leeman SE, Becker JM. A neurokinin 1 receptor antagonist decreases postoperative peritoneal adhesion formation and increases peritoneal fibrinolytic activity. Proc Nat Acad Sci USA. 2004;101:9115–9120. doi: 10.1073/pnas.0403210101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sekiba K. Use of Interceed(TC7) absorbable adhesion barrier to reduce postoperative adhesion reformation in infertility and endometriosis surgery. The Obstetrics and Gynecology Adhesion Prevention Committee. Obstet Gynecol. 1992;79:518–522. [PubMed] [Google Scholar]
- 69.Prevention of postsurgical adhesions by INTERCEED(TC7), an absorbable adhesion barrier: a prospective randomized multicenter clinical study. INTERCEED(TC7) Adhesion Barrier Study Group. Fertil Steril. 1989;51:933–938. [PubMed] [Google Scholar]
- 70.Becker J, Dayton M, Fazio V, Beck D, Stryker S, Wexner S, Wolff B, Roberts P, Smith L, Sweeney S, Moore M. Prevention of postoperative abdominal adhesions by a sodium hyaluronate-based bioresorbable membrane: a prospective, randomized, double-blind multicenter study. J Am Coll Surg. 1996;183:297–306. [PubMed] [Google Scholar]
- 71.Diamond M. Reduction of adhesions after uterine myomectomy by Seprafilm membrane (HAL-F): a blinded, prospective, randomized, multicenter clinical study. Seprafilm Adhesion Study Group. Fertil Steril. 1996;66:904–910. [PubMed] [Google Scholar]
- 72.Vrijland W, Tseng L, Eijkman H, Hop W, Jakimowicz J, Leguit P, Stassen L, Swank D, Haverlag R, Bonjer H, Jeekel H. Fewer intraperitoneal adhesions with use of hyaluronic acid-carboxymethylcellulose membrane: a randomized clinical trial. Ann Surg. 2002;235:193–199. doi: 10.1097/00000658-200202000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thornton M, Johns D, Campeau J, Hoehler F, DiZerega G. Clinical evaluation of 0.5% ferric hyaluronate adhesion prevention gel for the reduction of adhesions following peritoneal cavity surgery: open-label pilot study. Human Reproduction. 1998;13:1480–1485. doi: 10.1093/humrep/13.6.1480. [DOI] [PubMed] [Google Scholar]
- 74.Diamond M. Fertil Steril. Vol. 69. 1998. Reduction of de novo postsurgical adhesions by intraoperative precoating with Sepracoat (HAL-C) solution: a prospective, randomized, blinded, placebo-controlled multicenter study. The Sepracoat Adhesion Study Group; pp. 1067–1074. [DOI] [PubMed] [Google Scholar]
- 75.Wiseman DM. Possible Intergel Reaction Syndrome (pIRS) Annals of Surgery. 2006;244:630–632. doi: 10.1097/01.sla.0000239619.93579.ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tang CL, Jayne DG, Seow-Choen F, Ng YY, Eu KW, Mustapha N. A Randomized Controlled Trial of 0.5% Ferric Hyaluronate Gel (Intergel) in the Prevention of Adhesions Following Abdominal Surgery. Annals of Surgery. 2006;243:449–455. doi: 10.1097/01.sla.0000207837.71831.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Haney AF, Doty E. A barrier composed of chemically cross-linked hyaluronic acid (Incert) reduces postoperative adhesion formation. Fertility and Sterility. 1998;70:145–151. doi: 10.1016/s0015-0282(98)00116-2. [DOI] [PubMed] [Google Scholar]
- 78.Oncel M, Remzi FH, Senagore AJ, Connor JT, Fazio VW. Liquid antiadhesive product (Adcon-p) prevents post-operative adhesions within the intra-abdominal organs in a rat model. Int J Colorectal Dis. 2003;18:514–517. doi: 10.1007/s00384-003-0489-9. [DOI] [PubMed] [Google Scholar]
- 79.Oncel M, Remzi FH, Senagore AJ, Connor JT, Fazio VW. Comparison of a Novel Liquid (Adcon-P®) and a Sodium Hyaluronate and Carboxymethylcellulose Membrane (Seprafilm™) in Postsurgical Adhesion Formation in a Murine Model. Dis Colon Rectum. 2003;46 doi: 10.1007/s10350-004-6523-3. [DOI] [PubMed] [Google Scholar]
- 80.Hosie K, Gilbert JA, Kerr D, Brown CB, EM Peers. Fluid dynamics in man of an intraperitoneal drug delivery solution: 4% icodextrin. Drug Deliv. 2001;8:9–12. doi: 10.1080/107175401300002694. [DOI] [PubMed] [Google Scholar]
- 81.di Zerega GS, Verco SJS, Young P, Kettel M, Kobak W, Martin D, Sanfilippo J, Peers EM, Scrimgeour A, Brown CB. A randomized, controlled pilot study of the safety and efficacy of 4% icodextrin solution in the reduction of adhesions following laparoscopic gynaecological surgery. Hum Reprod. 2002;17:1031–1038. doi: 10.1093/humrep/17.4.1031. [DOI] [PubMed] [Google Scholar]
- 82.Menzies D, Pascual MH, Walz MK, Duron JJ, Tonelli F, Crowe A, Knight A. Use of icodextrin 4% solution in the prevention of adhesion formation following general surgery: from the multicentre ARIEL Registry. Ann R Coll Surg Engl. 2006;88:375–382. doi: 10.1308/003588406X114730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sites CK, Jensen BA, Glock JL, Blackman JA, Badger GJ, Johnson JV, Brumsted JR. Transvaginal ultrasonographic assessment of Hyskon or lactated Ringer’s solution instillation after laparoscopy: randomized, controlled study. J Ultrasound Med. 1997;16:195–199. doi: 10.7863/jum.1997.16.3.195. [DOI] [PubMed] [Google Scholar]
- 84.Gauwerky JF, Heinrich D, Kubli F. Complications of intraperitoneal dextran application for prevention of adhesions. Biol Res Pregnancy Perinatol. 1986;7:93–97. [PubMed] [Google Scholar]
- 85.Larsson B, Lalos O, Marsk L, Tronstad SE, Bygdeman M, Pehrson S, Joelsson I. Effect of intraperitoneal instillation of 32% dextran 70 on postoperative adhesion formation after tubal surgery. Acta Obstet Gynecol Scand. 1985;64:437–441. doi: 10.3109/00016348509155163. [DOI] [PubMed] [Google Scholar]
- 86.Diamond MP, Luciano A, Johns A, Dunn R, Young P, Bieber E. Reduction of postoperative adhesions by N,O-carboxymethylchitosan: a pilot study. Fertil Steril. 2003;80:631–636. doi: 10.1016/s0015-0282(03)00759-3. [DOI] [PubMed] [Google Scholar]
- 87.Zhou J, Elson C, Lee TDG. Reduction in postoperative adhesion formation and re-formation after an abdominal operation with the use of N, O-carboxymethyl chitosan. Surgery. 2004;135:307–312. doi: 10.1016/j.surg.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 88.Kennedy R, Costain DJ, McAlister VC, Lee TD. Prevention of experimental postoperative peritoneal adhesions by N,O-carboxymethyl chitosan. Surgery. 1996;120:866–870. doi: 10.1016/s0039-6060(96)80096-1. [DOI] [PubMed] [Google Scholar]
- 89.Leach R, Henry R. Reduction of postoperative adhesions in the rat uterine horn model with poloxamer 407. Am J Obstet Gynecol. 1990;162:1317–1319. doi: 10.1016/0002-9378(90)90044-8. [DOI] [PubMed] [Google Scholar]
- 90.Ferland R, Mulani D, Campbell PK. Evaluation of a sprayable polyethylene glycol adhesion barrier in a porcine efficacy model. Hum Reprod. 2001;16:2718–2723. doi: 10.1093/humrep/16.12.2718. [DOI] [PubMed] [Google Scholar]
- 91.Dunn R, Lyman MD, Edelman PG, Campbell PK. Evaluation of the SprayGel(TM) adhesion barrier in the rat cecum abrasion and rabbit uterine horn adhesion models. Fertility and Sterility. 2001;75:411. doi: 10.1016/s0015-0282(00)01677-0. [DOI] [PubMed] [Google Scholar]
- 92.Lamoutte H, Chatterji R. SurgiWrap(R) Mast bioresorbable sheet use for the prevention of soft tissue attachment; a two year experience. Biomaterials / Bioresorbable Technology, Mast Biosurgery. 2005 http://www.mastbio.com/pdf/mast_wp_LIT_303_A.pdf.
- 93.Okuyama N, Rodgers K, Wang C, Girgis W, Oz M, St Amand K, Pines E, DeCherney A, Rose E, Cohn D, diZerega G. Prevention of retrosternal adhesion formation in a rabbit model using bioresorbable films of polyethylene glycol and polylactic acid. J Surg Res. 1998;78:118–122. doi: 10.1006/jsre.1998.5317. [DOI] [PubMed] [Google Scholar]
- 94.Rodgers K, Cohn D, Hotovely A, Pine E, Diamond M, diZerega G. Evaluation of polyethylene glycol/polylactic acid films in the prevention of adhesions in the rabbit adhesion formation and reformation sidewall models. Fertil Steril. 1998;69:403–408. doi: 10.1016/s0015-0282(97)00574-8. [DOI] [PubMed] [Google Scholar]
- 95.Hill-West JL, Chowdhury SM, Sawhney AS, Pathak CP, Dunn RC, Hubbell JA. Prevention of post operative adhesions in the rat by in situ photopholymerization of bioresorbable hydrogel barriers. Obstetrics & Gynecology. 1994;83:59–64. [PubMed] [Google Scholar]
- 96.An expanded polytetrafluoroethylene barrier (Gore-Tex Surgical Membrane) reduces post-myomectomy adhesion formation. The Myomectomy Adhesion Multicenter Study Group. Fertil Steril. 1995;63:491–493. [PubMed] [Google Scholar]