Abstract
Infection of neonates with Borna disease virus (BDV) induces severe meningoencephalitis and neurological disorder in wild-type but not in β2-microglobulin-deficient mice of strain MRL (H-2k). Temporary in vivo depletion of CD8+ T cells delayed BDV-induced disease for several weeks. Depletion of CD4+ T cells had a similar beneficial effect, indicating that the BDV-induced neurological disorder in mice is a CD4+ T cell-dependent immunopathological process that is mediated by CD8+ T cells. Lymphocytes prepared from brains of diseased mice were mainly from the CD8+ T cell subset. They showed up-regulation of activation markers and exerted strong MHC I-restricted cytotoxic activity against target cells expressing the BDV nucleoprotein p40. Infection of B10.BR (H-2k) or congenic C57BL/10 (H-2b) mice resulted in symptomless, lifelong persistence of BDV in the brain. Superinfection with a recombinant vaccinia virus expressing BDV p40 but not with other vaccinia viruses induced severe neurological disease and encephalitis in persistently infected B10.BR mice but not in persistently infected C57BL/10 mice, indicating that the disease-inducing T cell response is restricted to the nucleoprotein of BDV in H-2k mice. Our results demonstrate that the cellular arm of the immune system may ignore the presence of a replicating virus in the central nervous system until proper antigenic stimulation at a peripheral site triggers the antiviral response.
Borna disease virus (BDV) is the causative agent of a nonpurulent meningoencephalitis observed predominantly in horses and sheep (1, 2). It is an enveloped virus with a single-stranded RNA genome of negative polarity (3, 4), and it represents the prototype member of a new virus family designated Bornaviridae in the order Mononegavirales (5, 6). BDV has an extraordinarily broad host range in warm-blooded animals, and it can replicate in the central nervous system (CNS) of a large number of experimentally infected animal species (1). BDV infections of humans with psychiatric disorders were reported (7–10). However, the etiological role of BDV in human mental disease remains to be elucidated.
In naturally infected hosts and in experimentally infected adult rats, neurological disease and behavioral abnormalities seem to result mainly from immunopathological processes (11–13). Strong perivascular and parenchymal infiltrations of CD4+ and CD8+ T cells were observed, and their appearance in the brain correlated with onset of disease symptoms (11, 13–16). Initially, the role of CD4+ T cells for induction of Borna disease was emphasized (17, 18). However, recent studies in the rat model system indicated that immunopathology is, in fact, mediated by CD8+ T cells that require help from the CD4+ T cell subset (15, 16, 19, 20). Lymphocytes from brains of infected rats showed cytotoxic activity in vitro mainly against target cells expressing the viral nucleoprotein (21).
We recently established a mouse model for BDV-induced neurological disease (22). In this experimental system, animals of the MRL strain are infected intracerebrally with BDV. Under these conditions the virus replicates readily in brain neurons, where it reaches maximal titers by 3–4 weeks postinfection. After 4–6 weeks, most infected MRL mice suddenly start to show signs of strong neurological disorder, including ataxia, movement disorders, paraparesis, apathy, and death. Because histological examinations of brains from diseased mice showed massive lymphocytic infiltrations and because infection of β2-microglobulin-deficient MRL mice lacking CD8+ T cells did not result in disease, it was concluded that immunopathological mechanisms were at work (22). However, no data concerning the specificity of the T cells involved in the disease-inducing immune response have been available to date.
A unique feature of the mouse model system is that although the virus replicates to similar titers in brains of all mouse strains, susceptibility to BDV-induced neurological disease depends strongly on the genetic background of the animals (22). MRL mice are highly susceptible, whereas C57BL/6 and closely related strains of mice are resistant. Interestingly, susceptible and resistant mice produce antibodies to BDV antigens (22), indicating that the humoral arm of the immune system readily responds to the viral infection. To account for the phenotype of disease-resistant mice, we speculated that because BDV replication is restricted to immunoprivileged sites in the brain, the cellular arm of the immune system may fail to recognize the viral antigens. In agreement with this hypothesis, we report here that immunization of persistently infected healthy B10.BR mice with a recombinant vaccinia virus expressing the BDV nucleoprotein resulted in neurological disease.
MATERIALS AND METHODS
Mice.
CBA/J mice were from Charles River Breeding Laboratories, MRL/MpJ and B10.BR mice were from The Jackson Laboratory, and C57BL/10 mice were from the animal facility of the Max-Planck Institute for Immunology, Freiburg, Germany. All breedings were done in the local animal facility.
Viruses.
BDV-He/80 was used throughout this study. Stocks of rat-adapted BDV were used that represented the fourth or fifth consecutive passage in brains of newborn Lewis rats, or a stock of mouse-adapted virus prepared from the fourth passage in brains of newborn-infected BALB/c mice was used. Preparation of virus stocks and determination of virus titers were done as described (22). Titers were approximately 106 focus-forming units/ml for stocks of rat-adapted virus and 2 × 105 focus-forming units/ml for the stock of mouse-adapted BDV. Recombinant vaccinia viruses expressing the various BDV proteins were constructed and propagated according to standard procedures by using strain WR. Other vaccinia viruses were VV-NA, expressing influenza A virus neuraminidase (23), and VV-NP/VSV, expressing the nucleoprotein of vesicular stomatitis virus (24).
Animal Infections.
Newborn MRL, CBA/J, B10.BR, and C57BL/10 mice (less than 24 h of age) were infected by the intracerebral route with 10 μl of undiluted BDV stock (2 × 103–1 × 104 focus forming units) into the thalamic region of the left hemisphere by using a Hamilton syringe. Vaccinia virus infections were carried out by i.v. injections of 5 × 106 plaque-forming units of virus in a volume of 200 μl into the tail veins of 6- to 8-week-old mice.
Analysis of Disease Symptoms.
Virus-infected mice were examined daily and weighed frequently during the critical stages of infection and disease. Disease symptoms were scored as follows: 0 = no symptoms; 1 = low degree of ataxia, torticollis, unphysiological and uncontrolled movements of extremities when animal held up by the tail, rough fur, or hunched posture; 2 = pronounced weight loss, severe ataxia and torticollis, paraparesis, apathy, characteristic position of hind limbs when animal lifted by the tail as described (22), or death.
Flow Cytometry.
Approximately 50 μl of blood was taken from the tail veins of antibody-treated mice and were resuspended in PBS containing 2% FCS/0.1% NaN3/10 units/ml heparin (Liquemin; Hoffmann–LaRoche). The cell suspension was incubated with R-phycoerythrin (R-PE)-labeled anti-CD4 and fluorescein-labeled anti-CD8 antibodies (1:200; Life Technologies, Gaithersburg, MD) for 20 min at room temperature, washed, and incubated with FACS lysing solution (Becton-Dickinson) as described by the manufacturer to eliminate red blood cells. For detection of activation markers, lymphocytes from brain or spleen were stained with fluorescein-labeled anti-CD8 or anti-CD4 (1:200; Life Technologies) and biotinylated anti-CD25, anti-CD62L, or anti-CD69 (PharMingen) followed by incubation with streptavidin-R-PE (Caltag, South San Francisco, CA). Analysis of cells was performed on a FACScan flow cytometer (Becton-Dickinson).
Histology and Immunohistochemical Staining.
Complete brain hemispheres from sacrificed animals were preserved in Zamboni’s fixative (4% paraformaldehyde and 15% picric acid in 0.25 M sodium phosphate, pH 7.5) and embedded in paraffin. Four-micrometer sagittal sections were stained with hematoxylin/eosin and viewed and photographed under a Leitz Dialux 20 EB microscope. The degree of encephalitis was scored on an arbitrary scale from 0 to 3: 0 = no infiltrates, 1 = up to three perivascular infiltrates per brain section with one or two layers of cells; 2 = up to six perivascular infiltrates per brain section with multilayer appearance and one or two parenchymal infiltrates; three = more than six perivascular infiltrates per brain section with multiple layers of cells and strong infiltration of parenchyma at multiple sites. Immunostaining of brain sections was performed with a monoclonal mouse antibody against BDV p40 (38/17C1) in a 1:200 dilution overnight at 4°C. After extensive washing, bound antibody was detected by using the peroxidase-based Vectastain-elite ABC kit (Vector Laboratories) according to the manufacturer’s instructions and counterstained with hematoxylin.
In Vitro Cytotoxicity Assay of Brain Lymphocytes.
Brain lymphocytes were isolated as described (21, 25), and their cytolytic activity was determined by a standard 51Cr release assay. Target cells were prepared as follows: 5 × 106 L929 (H-2k) or MC57G (H-2b) cells in 0.5 ml of DMEM/10% FCS were labeled in suspension with 250 μCi of 51Cr (Amersham Pharmacia) for 2 h at 37°C. After three washings, the cells were infected with the indicated recombinant vaccinia virus at a multiplicity of infection of 5 for 2–4 h. Target cells were incubated with effector cells for 6 h at 37°C, and the percentage of specific 51Cr release was calculated.
RESULTS
Temporary Depletion of CD8+ and CD4+T Cell Subsets in Vivo Delays Onset of BDV-Induced Neurological Disorder.
We demonstrated recently that β2-microglobulin knockout mice (β2m−) lacking functional CD8+ T cells do not develop CNS inflammation and neurological disorder after infection with BDV as newborns (22). Because β2m− mice have additional impairments of the immune system (26), we wanted to confirm the relevance of CD8+ T cells in the pathogenesis of BDV-induced disease by in vivo depletion experiments. Newborn-infected MRL mice were treated at 15 or 18 days of age with a mAb against CD8 followed by a second antibody administration 3–4 days later. Flow cytometry indicated that the percentage of CD8+ T cells had dropped below 1% of total peripheral blood lymphocytes by 5 days postadministration of the first dose of anti-CD8 antibody and remained below 1% for at least 16 days (data not shown). At later time points, levels of CD8+ T cells increased again but did not reach their normal level within 35 days. Of the nine anti-CD8-treated mice from three independent experiments, seven (78%) did not show detectable signs of neurological disease until 7 weeks of age, whereas all nine control animals developed severe neurological disorder within this period of time and had to be sacrificed (Table 1). Disease onset in the two antibody-treated animals that developed disease was delayed by about 12 days. These two cases occurred in experiment 2 in which treatment with anti-CD8 antibody was started at day 18 instead of day 15 as in the other two experiments, raising the possibility that correct timing of antibody treatment is of critical importance for disease prevention.
Table 1.
In vivo T cell depletion delays Borna disease in mice
| Experiment | Administration of mAb* (days p.i.) | No. of fatally diseased mice (score 2)†/no. of treated mice
|
||
|---|---|---|---|---|
| Not depleted | CD4-depleted | CD8-depleted | ||
| 1 | 15 and 18 | 4/4 | 0/3 | 0/3 |
| 2 | 18 and 22 | 2/2 | 1/3 | 2/3 |
| 3 | 15 and 18 | 3/3 | 0/3 | 0/3 |
MRL mice infected as newborns with a BDV stock passaged four times in newborn rats were injected i.p. with 170–200 μl of a 100-fold concentrated hybridoma supernatant containing either anti-CD4 (YTS 191.1) or anti-CD8 (YTS 169.4.2) mAb (27). p.i., postinfection.
Numbers refer to development of disease within 7 weeks after infection.
We also determined the contribution of CD4+ T cells to BDV-induced disease by in vivo depletion of this T cell subset. Interestingly, eight of nine mice depleted of CD4+ T cells did not develop disease within the normal time range of 4 to 7 weeks postinfection (Table 1), indicating an important role of CD4+ T cells in the immunopathological process. Again, start of depletion at day 18 resulted in incomplete protection. Thus, transient depletion of CD8+ or CD4+ T cells conferred temporary protection against fatal BDV-induced disease, indicating that both major T cell subsets are necessary for the development of Borna disease in MRL mice.
Lymphocytic Brain Infiltrates Contain a High Proportion of Activated CD8+T Cells.
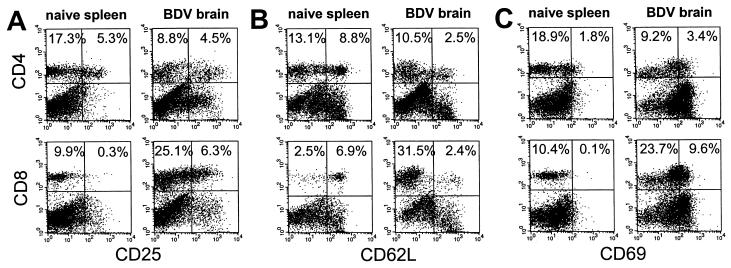
About 3 × 106 viable lymphocytes could be recovered from brains of MRL mice at the peak of disease. The CD8+ T cells in these preparations outnumbered CD4+ T cells by a factor of 2.3, whereas in peripheral blood and in spleen this ratio was about 0.5. Analysis of activation markers of CD8+ T cells from brains of diseased animals revealed up-regulation of CD25 (IL-2 receptor) and CD69 (very early activation marker) expression (Fig. 1 A and C) and down-regulation of CD62L (l-selectin) (Fig. 1B). Expression of CD49d (integrin α4) on brain-derived CD8+ T cells was only slightly higher than on CD8+ T cells from spleen (data not shown). This pattern of surface marker expression indicated an activated state of the recovered CD8+ T cells. Brain-derived CD4+ T cells also were activated as judged by significantly increased expression of CD69 (Fig. 1C), and decreased expression of CD62L (Fig. 1B).
Figure 1.
Expression of activation markers on brain-derived CD8+ T cells. Neonatally infected MRL mice were sacrificed at the peak of neurological disease, and brain lymphocytes were isolated. Uninfected age-matched MRL mice served as donors for spleen lymphocytes. Lymphocytes were stained with anti-CD8-FITC or anti-CD4-FITC and biotinylated mAb specific for CD25 (IL-2 receptor) (A), CD62L (l-selectin) (B), or CD69 (very early activation antigen) (C) followed by streptavidin-R-PE detection. Three independent experiments were performed and dot plots are shown from one representative experiment. (Left) Analysis of spleen lymphocytes from a naive mouse. (Right) Brain-derived lymphocytes. Percentages of CD4+ and CD8+ T cells positive for the indicated marker are given in the upper quadrants.
Brain Lymphocytes Show CD8+ T Cell-Dependent and MHC I-Restricted Cytotoxic Activity Mainly Against Target Cells Expressing the Viral Nucleoprotein p40.
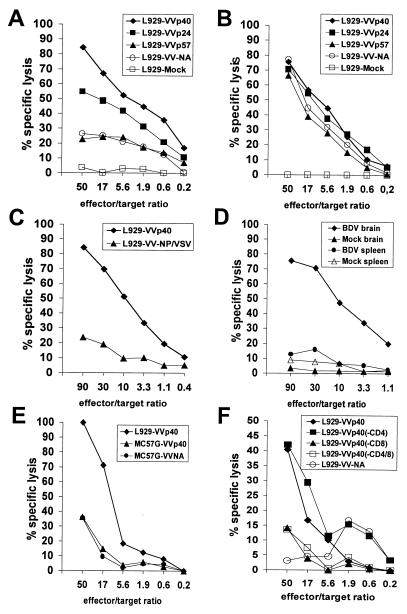
To determine the potential cytolytic activity of brain-derived CD8+ T cells, we performed in vitro cell-mediated cytotoxicity assays on haplotype-matched target cells expressing BDV antigens. Because BDV does not multiply readily in mouse cells, we employed recombinant vaccinia viruses that code for the BDV protein p40, p24, or gp94 (p57) to infect mouse L929 (H-2k) cells that then were used as cytotoxic T lymphocyte (CTL) targets. The phenotype of the various recombinant vaccinia viruses was confirmed by immunoprecipitation experiments using monospecific antisera to the various BDV proteins and lysates of infected CV-1 cells (data not shown). When freshly isolated brain lymphocyte preparations from heavily diseased MRL mice were used as effectors without prior in vitro restimulation, strong cytotoxic activity against p40-expressing L929 cells was observed (Fig. 2A). In contrast, L929 cells expressing gp94 (p57) were not lysed to significantly higher levels than L929 cells infected with a vaccinia virus expressing an irrelevant protein, whereas lysis of p24-expressing cells reached intermediate levels (Fig. 2A). Target cell preparations infected with the various recombinant vaccinia viruses were lysed to a similar extent by vaccinia virus-specific CTLs, whereas mock-infected L929 cells were not killed (Fig. 2B), indicating that the observed differences in target cell lysis by BDV-specific CTLs were not due to different degrees of vaccinia virus infection (Fig. 2B). Brain lymphocyte preparations from diseased CBA/J mice, another mouse strain with H-2k haplotype that develops neurological disorder after BDV infection with intermediate frequency (22), also showed high cytolytic activity against p40-expressing L929 cells (Fig. 2C). Brain lymphocyte preparations from naive MRL mice contained only very low numbers of lymphocytes. Mock preparations recovered from these brains showed no lytic effect on target cells (Fig. 2D). Unlike brain lymphocyte preparations from diseased animals that showed specific lysis of p40-expressing L929 cells, effector cells prepared from their spleens showed no such activity (Fig. 2D).
Figure 2.
Ex vivo CTL activity of brain lymphocytes from BDV-infected mice is directed mainly against the viral nucleoprotein p40. Brain lymphocytes from severely diseased MRL mice (A) or VV-specific CTLs (B) were used as effectors on VV-infected L929 target cells expressing the indicated recombinant BDV proteins or irrelevant control proteins. (C) CTL assay with lymphocytes from the brain of a severely diseased CBA/J (H-2k) mouse. (D) Spleen lymphocytes of MRL mice lack BDV-specific CTLs. Effector cells from brains or spleens of either severely diseased BDV-infected or healthy mock-infected MRL mice were incubated with L929 target cells expressing BDV p40. Brain lymphocyte preparations from uninfected MRL mice usually contained less than 105 lymphocytes. They were resuspended in the same volume of medium as lymphocytes from brains of diseased animals, and identical volumes of both cell preparations were used in the CTL assays. (E) MHC restriction of CTL activity. Target cells were either MC57G cells (H-2b) or L929 cells (H-2k) infected with the indicated vaccinia viruses. Effector cells were from brains of MRL (H-2k) mice with severe Borna disease. (F) Specific depletion of CD8+ T cells from brain lymphocyte preparations abolishes CTL activity. Brain lymphocyte preparations containing 2 × 106 lymphocytes/ml were incubated for 30 min at 4°C with 107 magnetic beads coated with anti-CD4 or anti-CD8 antibodies (Dynal, Great Neck, NY) to deplete the respective T cell subset. Lymphocytes complexed to magnetic beads were removed, and the remaining cells were used as effectors in standard cytotoxicity assays. Spontaneous lysis of target cells usually was less than 20% except in F, where it was 40%. Specific lysis is plotted against the indicated effector-to-target-cell ratios.
MC57G cells (H-2b) infected with VV-p40 were not lysed specifically by brain lymphocytes from diseased MRL mice, although the same preparation was able to lyse p40-expressing L929 cells (Fig. 2E). By contrast, MC57G target cells infected with a vaccinia virus expressing the lymphocytic choriomeningitis virus (LCMV) glycoprotein were killed efficiently by spleen cells prepared 8 days after infection of a C57BL/6 mouse (H-2b) with LCMV (data not shown). Thus, the observed in vitro cytolytic activity of mouse brain lymphocytes toward BDV p40-expressing target cells was MHC I-restricted. Consistent with this observation, in vitro depletion of CD8+ T cells from brain lymphocyte preparations abrogated cytotoxic activity, whereas CD4+ T cell-depleted preparations still were able to lyse p40-expressing target cells (Fig. 2F). Thus, the CD8+ T cell subset exerts the major cytotoxic activity in vitro and is probably the effector cell population responsible for the disease-inducing effects in vivo.
Peripheral Expression of p40 Induces Neurological Disorder in Persistently Infected B10.BR (H-2k) but Not in Congenic C57BL/10 (H-2b) Mice.
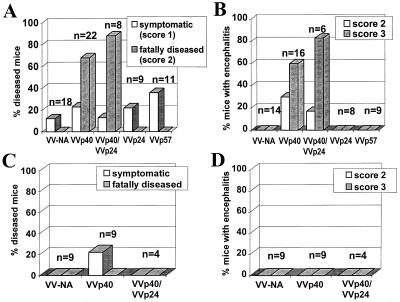
In contrast to the situation in MRL mice, infection of newborn B10.BR and C57BL/10 mice with BDV results in seroconversion and lifelong viral persistence in the CNS without overt disease. None of the approximately 100 B10.BR and congenic C57BL/10 mice with persistent BDV infections of the CNS that we have analyzed to date has ever developed neurological disease, and histological examinations of brains of such animals yielded no evidence of lymphocyte infiltrations. To challenge the hypothesis that immunological ignorance of T cells is the basis for the symptomless persistence of BDV in the CNS of disease-resistant mice, we tested whether induction of a strong BDV-specific immune response would trigger neurological disease in such animals. Superinfection of persistently infected B10.BR mice with VV-p40 induced severe neurological disease with frequent fatal outcome in more than 60% of the challenged animals (Fig. 3A). Symptoms that first were observed about 7–10 days post-vaccinia virus challenge included ataxia, paresis, apathy, and dramatic weight loss. Animals usually had to be sacrificed 2–3 days after onset of disease symptoms. Superinfection with a mixture of p40- and p24-expressing vaccinia viruses resulted in an even higher rate of fatal cases, whereas superinfection with VV-p24 alone did not result in clear neurological disease (Fig. 3A). Superinfection with VV-gp94 (p57) or with a vaccinia virus expressing influenza A virus neuraminidase did not induce disease (Fig. 3A). Furthermore, age-matched control mice (n = 13) that were not infected with BDV remained healthy after challenge with VV-p40 (data not shown), indicating that both BDV infection and antiviral immune response are necessary to induce neurological disease in B10.BR mice. These results suggest that resistance of certain mouse strains to neurological disease after intracerebral BDV infection is not due to central tolerance or peripheral deletion of BDV-specific T cells, but rather to immunological ignorance of the viral infection in the CNS. Interestingly, immunizations with VV-p40 or other recombinant vaccinia viruses did not trigger disease in persistently infected C57BL/10 mice (Fig. 3C), demonstrating that the H-2k haplotype is of critical importance for disease susceptibility.
Figure 3.
Infection with VV-p40 triggers disease in persistently infected B10.BR but not in C57BL/10 mice. Symptomless, persistently infected B10.BR (A and B) or C57BL/10 (C and D) mice were superinfected with various recombinant vaccinia viruses as indicated and observed for a maximum of 12 days after superinfection. (A and C) Health status. Mice were examined and weighed daily. Severity of disease was scored on an arbitrary scale as described in Materials and Methods. (B and D) Degree of CNS inflammation. Bars indicate the percentage of animals with symptoms. Scoring was on an arbitrary scale from 0 to 3 as described. The percentage of animals with severe encephalitis (scores 2 and 3) is depicted. Persistent BDV infection of all animals was confirmed by immunohistochemical staining of the p40 antigen in CNS cells on paraffin-embedded brain sections.
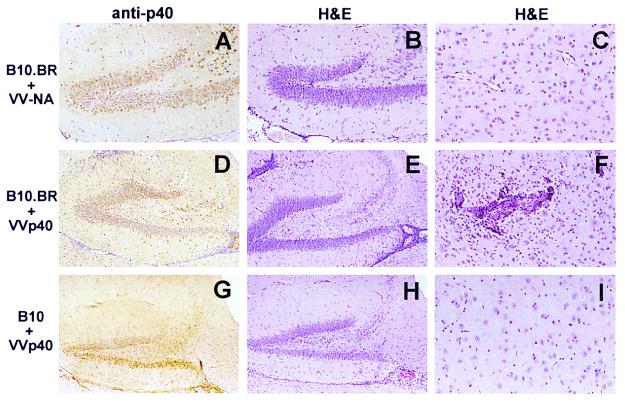
Brains of VV-p40-challenged B10.BR mice that developed disease showed strong encephalitis (Fig. 3B) with massive mononuclear cell infiltrates predominantly in the thalamus (Fig. 4F) and in the hippocampus formation (Fig. 4E). These histological hallmarks were not found in persistently infected B10.BR mice challenged with VV-NA (Figs. 4 B and C) or other control vaccinia viruses (Fig. 3B). In C57BL/10 mice in which superinfection with neither VV-p40 nor VV-NA induced disease, no prominent encephalitis could be observed (Fig. 3D). Encephalitic lesions were absent in the thalamus (Fig. 4I), cortex, and brain stem (data not shown). However, few perivascular mononuclear cells were observed frequently in the hippocampus formation (Fig. 4H). Such infiltrates often were present in brains of animals superinfected with VV-NA or other vaccinia virus recombinants (data not shown), indicating that activation of lymphocytes during the course of vaccinia virus infection contributes to this histological picture. Immunostaining of tissue sections with a mAb to the BDV p40 antigen demonstrated that all animals used for this study contained similar numbers of virus-infected cells in the brain (Fig. 4 A, D, and G and data not shown).
Figure 4.
Encephalitis after superinfection of symptomless BDV-infected B10.BR mice with VV-p40. Shown are sagittal brain sections from persistently infected B10.BR (A– F) or C57BL/10 (G– I) mice after superinfection with recombinant vaccinia viruses expressing influenza A virus neuraminidase (VV-NA, A– C) or BDV p40 (VV-p40, D– I). Sections were stained with hematoxylin/eosin or with a mAb directed against BDV p40 as indicated. [×100 (A, B, D, E, G, and H) and ×250 (C, F, and I).]
DISCUSSION
We used the mouse model of Borna disease virus infection (22) to characterize the immunopathological mechanisms of this disease. Our data demonstrate that the onset of BDV-induced disease in wild-type MRL mice was delayed by temporary antibody depletion of CD8+ T cells. We found further that brain inflammation and neurological symptoms were prevented by temporary depletion of CD4+ T cells in MRL mice, indicating that disease development also was strongly dependent on these cells. In BDV-infected Lewis rats, reduced encephalitis and improved clinical parameters also were achieved by application of CD4-specific antibodies, but antibody treatment had to be started before virus infection and had to be repeated frequently (19). CD4+ T cells have been reported to be critical for controlling persisting CNS infections of mice with the JHM strain of mouse hepatitis virus (28) or highly virulent strains of LCMV (29–31). CD4+ T cells in the CNS seem to prevent premature apoptosis of CD8+ T cells (28).
By measuring the expression of surface markers we demonstrated that highly activated CD4+ and CD8+ T cells were present in brains of diseased MRL mice. Interestingly, the very early activation marker CD69 was up-regulated on the majority of brain-derived lymphocytes, indicating that these cells had experienced continuous antigen stimulation in vivo, possibly in the CNS. These CD69hi CD8+ T cells were cytolytically active because they were able to kill target cells expressing the BDV nucleoprotein in vitro. Cytotoxic CD69hi CD8+ T cells were detected previously during persistent CNS inflammation caused by a neurotropic influenza A virus (32).
The in vitro cytotoxicity assays with lymphocytes from brains of diseased H-2k mice showed strong reactivity toward target cells expressing the BDV nucleoprotein, whereas target cells expressing the BDV glycoprotein or the phosphoprotein were not lysed effectively. Interestingly, in Lewis rats the CTL response also is directed almost exclusively against the BDV nucleoprotein p40 (21). Because no specific lysis of target cells expressing BDV proteins was observed with lymphocytes from spleens of diseased mice, it appears that only few BDV-specific CTLs were present in this organ.
A unique feature of the mouse model for Borna disease is that certain inbred strains do not spontaneously develop neurological disease, although they carry large amounts of virus in the brain (22). Here we extended this observation by showing that persistently infected B10.BR mice that, like MRL mice, carry the H-2k allele did not develop neurological disease. This constellation made it possible to test whether the peaceful coexistence of virus and host could be disturbed by triggering a strong antiviral immune response by recombinant vaccinia viruses expressing BDV antigens. We found that the majority of persistently infected B10.BR mice quickly developed neurological disease after immunization with the BDV nucleoprotein. Importantly, induction of neurological disease was not possible with vaccinia virus recombinants expressing either the large BDV glycoprotein or the BDV phosphoprotein. This finding correlates well with antigen specificity of the antiviral CTL response in H-2k animals that is directed mainly against the p40 antigen of BDV. It is important to note that immunization with VV-p40 did not induce disease in persistently infected congenic H-2b mice, conclusively demonstrating that Borna disease in mice is MHC-dependent.
The ease by which encephalitis could be induced in persistently infected B10.BR mice by a single round of peripheral immunization is remarkable. It indicates that BDV p40-specific cytotoxic T cells were neither deleted nor functionally inactivated in our mice. It seems rather that the potentially reactive T cells simply did not respond to the virus infection. Immunological ignorance, defined as a state during which potentially reactive T cells are not activated in spite of cognate antigen being present in the organism, is the most likely form of immunological tolerance operative in our mice before immunization. This form of immunological tolerance is expected to be overcome readily by peripheral immunization with the cognate antigen as shown for a number of autoimmune diseases, the best-studied example of which is experimental allergic encephalitis (EAE; for review, see ref. 33).
It seems unlikely that the nonresponsiveness of T cells toward BDV in the brains of our mice relates simply to the paradigm that the CNS is an immunoprivileged site. The concept of immune privilege of certain organs, including the CNS, originally was introduced on the basis of transplantation studies that identified sites with reduced graft-rejection rates (34, 35). Immunoprivilege of the CNS has been attributed to a number of factors including lack of lymphatic drainage from the brain, presence of the blood–brain barrier, and low expression levels of MHC class I and II antigen on neuroectodermal cells. However, this concept has been challenged by a number of recent findings that showed that the CNS is indeed subject to extensive immunological surveillance (36–38) and that only naive T cells are excluded from trafficking through the CNS (37, 38). Accordingly, grafts in the CNS can be rejected when a peripheral graft-specific immune reaction is initiated (39). Thus, decreased graft rejection in the CNS is probably a result of T cell ignorance. Our study is related to work of Stevenson et al. (40, 41), which showed that abortive infection of parenchymal brain cells with a nonneurovirulent strain of influenza A virus did not induce a detectable antibody response and that a neurovirulent strain failed to induce an immune response as long as virus replication was confined to the CNS parenchyma.
A lymphatic-like system allowing export of antigen recently has been shown to exist in the CNS (42). This antigen transport system probably also plays a critical role in the T cell activation process that precedes Borna disease in MRL mice. Because BDV-specific antibodies are induced in disease-susceptible and disease-resistant mice, it appears that antigen presenting cells of all tested mouse strains can process brain-derived viral antigens equally well for presentation on MHC class II molecules. However, it seems that only MRL mice can mount a vigorous CTL response toward such antigens at a high frequency.
Other models of autoimmunity resembling the situation in B10.BR mice persistently infected with BDV were established by creating transgenic mice that express LCMV antigens specifically in β cells of the pancreas (43, 44) or oligodendroglia in brain (45). Because transgene expression in these animals is limited strictly to pancreas and brain, respectively, the LCMV antigens are not presented to maturing T cells in the thymus and no central tolerance to the transgene product is induced. The transgenic animals are healthy because T cells of naive animals ignore the foreign antigen. However, they develop diabetes or neurological disease, respectively, after infection with LCMV because the antiviral T cell response not only eliminates virus-infected cells but also destroys noninfected cells that express the viral transgene. Because BDV is highly neurotropic in mice, an analogous situation may be operative in our system. Our experiments thus demonstrate that the peaceful coexistence of a replicating virus in the CNS and the ignorant immune system can be disturbed by immunization with suitable antigen.
Acknowledgments
We thank Rosita Frank for expert technical assistance, Friedrich Grässer (Homburg, Germany) for providing a sample of rabbit of antiserum to BDV gp94, and Lothar Stitz, BFAV Tübingen, Germany, for supplying mAb 38/17C1 against BDV p40. This work was supported by grants from Zentrum für Klinische Forschung I of the Universitätsklinikum Freiburg and from the Deutsche Forschungsgemeinschaft.
ABBREVIATIONS
- BDV
Borna disease virus
- CNS
central nervous system
- CTL
cytotoxic T lymphocyte
- LCMV
lymphocytic choriomeningitis virus
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Rott R, Becht H. Curr Top Microbiol Immunol. 1995;190:17–30. doi: 10.1007/978-3-642-78618-1_2. [DOI] [PubMed] [Google Scholar]
- 2.Ludwig H, Bode L, Gosztonyi G. Prog Med Virol. 1988;35:107–151. [PubMed] [Google Scholar]
- 3.Briese T, Schneemann A, Lewis A J, Park Y S, Kim S, Ludwig H, Lipkin W I. Proc Natl Acad Sci USA. 1994;91:4362–4366. doi: 10.1073/pnas.91.10.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cubitt B, Oldstone C, De la Torre J C. J Virol. 1994;68:1382–1396. doi: 10.1128/jvi.68.3.1382-1396.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schneemann A, Schneider P A, Lamb R A, Lipkin W I. Virology. 1995;210:1–8. doi: 10.1006/viro.1995.1311. [DOI] [PubMed] [Google Scholar]
- 6.De la Torre J C. J Virol. 1994;68:7669–7675. doi: 10.1128/jvi.68.12.7669-7675.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bode L, Zimmermann W, Ferszt R, Steinbach F, Ludwig H. Nat Med. 1995;1:232–236. doi: 10.1038/nm0395-232. [DOI] [PubMed] [Google Scholar]
- 8.De la Torre J C, Gonzalez-Dunia D, Cubitt B, Mallory M, Mueller-Lantzsch N, Grässer F A, Hansen L A, Masliah E. Virology. 1996;223:272–282. doi: 10.1006/viro.1996.0479. [DOI] [PubMed] [Google Scholar]
- 9.Salvatore M, Morzunow S, Schwemmle M, Lipkin W I Bornavirus Study Group. Lancet. 1997;349:1813–1814. doi: 10.1016/s0140-6736(05)61693-5. [DOI] [PubMed] [Google Scholar]
- 10.Iwata Y, Takahashi K, Peng X, Fukuda K, Ohno K, Ogawa T, Gonda K, Mori N, Niwa S, Shigeta S. J Virol. 1998;72:10044–10049. doi: 10.1128/jvi.72.12.10044-10049.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bilzer T, Planz O, Lipkin W I, Stitz L. Brain Pathol. 1995;5:223–230. doi: 10.1111/j.1750-3639.1995.tb00598.x. [DOI] [PubMed] [Google Scholar]
- 12.Narayan O, Herzog S, Frese K, Scheefers H, Rott R. Science. 1983;220:1401–1403. doi: 10.1126/science.6602380. [DOI] [PubMed] [Google Scholar]
- 13.Narayan O, Herzog S, Frese K, Scheefers H, Rott R. J Infect Dis. 1983;148:305–315. doi: 10.1093/infdis/148.2.305. [DOI] [PubMed] [Google Scholar]
- 14.Stitz L, Soeder D, Deschl U, Frese K, Rott R. J Immunol. 1989;143:4250–4256. [PubMed] [Google Scholar]
- 15.Bilzer T, Stitz L. J Immunol. 1994;153:818–823. [PubMed] [Google Scholar]
- 16.Planz O, Bilzer T, Stitz L. J Virol. 1995;69:896–903. doi: 10.1128/jvi.69.2.896-903.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richt J A, Stitz L, Wekerle H, Rott R. J Exp Med. 1989;170:1045–1050. doi: 10.1084/jem.170.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richt J A, Stitz L, Deschl U, Frese K, Rott R. J Gen Virol. 1990;71:2565–2573. doi: 10.1099/0022-1317-71-11-2565. [DOI] [PubMed] [Google Scholar]
- 19.Stitz L, Sobbe M, Bilzer T. J Virol. 1992;66:3316–3323. doi: 10.1128/jvi.66.6.3316-3323.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nöske K, Bilzer T, Planz O, Stitz L. J Virol. 1998;72:4387–4395. doi: 10.1128/jvi.72.5.4387-4395.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Planz O, Stitz L. J Virol. 1999;73:1715–1718. doi: 10.1128/jvi.73.2.1715-1718.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hallensleben W, Schwemmle M, Hausmann J, Stitz L, Volk B, Pagenstecher A, Staeheli P. J Virol. 1998;72:4379–4386. doi: 10.1128/jvi.72.5.4379-4386.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hausmann J, Kretzschmar E, Garten W, Klenk H-D. J Gen Virol. 1995;76:1719–1728. doi: 10.1099/0022-1317-76-7-1719. [DOI] [PubMed] [Google Scholar]
- 24.Yewdell J W, Bennink J R, Mackett M, Lefrancois L, Lyles D S M B. J Exp Med. 1986;163:1529–1538. doi: 10.1084/jem.163.6.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Irani D N, Griffin D E. J Immunol Methods. 1991;139:223–231. doi: 10.1016/0022-1759(91)90192-i. [DOI] [PubMed] [Google Scholar]
- 26.Raulet D H. Adv Immunol. 1994;55:381–421. doi: 10.1016/s0065-2776(08)60514-3. [DOI] [PubMed] [Google Scholar]
- 27.Cobbold S P, Jayasuriya A, Nash A, Prospero T D, Waldmann H. Nature (London) 1984;312:548–550. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- 28.Stohlman S A, Bergmann C C, Cua D J, Lin M T, Ho S, Wei W, Hinton D R. Adv Exp Med Biol. 1998;440:425–430. doi: 10.1007/978-1-4615-5331-1_53. [DOI] [PubMed] [Google Scholar]
- 29.Battegay M, Moskophidis D, Rahemtulla A, Hengartner H, Mak T W, Zinkernagel R M. J Virol. 1994;68:4700–4704. doi: 10.1128/jvi.68.7.4700-4704.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matloubian M, Concepcion R J, Ahmed R. J Virol. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zajac A J, Blattman J N, Murali-Krishna K, Sourdive D J D, Suresh M, Altman J D, Ahmed R. J Exp Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hawke S, Stevenson P G, Freeman S, Bangham C R. J Exp Med. 1998;187:1575–1582. doi: 10.1084/jem.187.10.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steinman L. Cell. 1996;85:299–302. doi: 10.1016/s0092-8674(00)81107-1. [DOI] [PubMed] [Google Scholar]
- 34.Medawar P B. Brit J Exp Pathol. 1948;29:58. [PMC free article] [PubMed] [Google Scholar]
- 35.Medawar P B. Proc R Soc London Ser B. 1958;148:145–153. doi: 10.1098/rspb.1958.0058. [DOI] [PubMed] [Google Scholar]
- 36.Wekerle H, Linington C, Lassmann H, Meyermann R. Trends Neurosci. 1986;9:271–277. [Google Scholar]
- 37.Hickey W F, Hsu B L, Kimura H. J Neurosci Res. 1991;28:254–260. doi: 10.1002/jnr.490280213. [DOI] [PubMed] [Google Scholar]
- 38.Bauer J, Bradl M, Hickley W F, Forss-Petter S, Breitschopf H, Linington C, Wekerle H, Lassmann H. Am J Pathol. 1998;153:715–724. doi: 10.1016/s0002-9440(10)65615-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mason D W, Charlton H M, Jones A J, Lavy C B, Puklavec M, Simmonds S J. Neuroscience. 1986;19:685–694. doi: 10.1016/0306-4522(86)90292-7. [DOI] [PubMed] [Google Scholar]
- 40.Stevenson P G, Hawke S, Sloan D J, Bangham C R. J Virol. 1997;71:145–151. doi: 10.1128/jvi.71.1.145-151.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stevenson P G, Freeman S, Bangham C R, Hawke S. J Immunol. 1997;159:1876–1884. [PubMed] [Google Scholar]
- 42.Cserr H F, Knopf P M. Immunol Today. 1992;13:507–512. doi: 10.1016/0167-5699(92)90027-5. [DOI] [PubMed] [Google Scholar]
- 43.Ohashi P S, Oehen S, Buerki K, Pircher H, Ohashi C T, Odermatt B, Malissen B, Zinkernagel R M, Hengartner H. Cell. 1991;65:305–317. doi: 10.1016/0092-8674(91)90164-t. [DOI] [PubMed] [Google Scholar]
- 44.Oldstone M B, Nerenberg M, Southern P, Price J, Lewicki H. Cell. 1991;65:319–331. doi: 10.1016/0092-8674(91)90165-u. [DOI] [PubMed] [Google Scholar]
- 45.Evans C F, Horwitz M S, Hobbs M V, Oldstone M B. J Exp Med. 1996;184:2371–2384. doi: 10.1084/jem.184.6.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]