Abstract
Multimeric peptide/class II MHC staining reagents were synthesized and shown to bind with appropriate specificity to T cell hybridomas. A small, expanded population of T cells detected with one of these reagents in peptide-immunized C57BL/10 mice persisted for several months. This population expanded further on secondary immunization. Equating the extent of binding of this reagent to T cell receptor affinity, we saw little correlation of immunizing peptide dose to T cell receptor affinity at the peak of the primary response. However, there was an inverse relation between peptide dose and the apparent receptor affinity of the T cells that were present several months after a primary response or after a secondary stimulation either in vivo or in vitro.
Tracking the cellular dynamics of antigen-specific T cell responses in vivo has been difficult, not only because the responding precursors occur at low frequency, but also because the peptide/MHC ligand for the T cell receptor (TCR) is cell bound and of low affinity. These latter problems have been overcome by methods for producing soluble MHC molecules bearing single peptides (1–4) and for producing fluorescent multimeric versions of these molecules (5–10). The cooperative binding achieved with these multivalent ligands produces an avidity high enough that antigen-specific T cells can be detected by flow cytometry. In examining the binding of these types of reagents to T cells with receptors of known affinity for monovalent peptide/MHC, we observed a direct correlation between the extent of multimer binding and receptor affinity (10).
The phenomenon of affinity maturation is well established in B cell responses. With multiple immunizations of T cell-dependent antigens, B cells bearing Ig receptors of steadily increasing affinity grow to dominate the B cell pool (11, 12). These cells appear to arise by selection of somatically mutated receptors as antigen becomes limiting (13–15). Recent studies have demonstrated that T cells having higher average affinity for peptide/MHC are selected after multiple exposures to antigen (16, 17). Whether this affinity maturation is primarily the result of the experience of repeated exposure to antigen or a shaping of the T cell repertoire during antigen waning (18–20) is unknown.
We examined these questions for CD4+ T cells responding to a peptide presented by the mouse class II MHC molecule, IAb. We tracked the frequency and relative affinities of peptide/MHC-binding T cells during both primary and secondary immune responses. Our results show that with repeated or prolonged exposure to antigen, limiting doses of antigen select for T cells with higher-affinity receptors.
MATERIALS AND METHODS
Mice and Immunizations.
C57BL/10 SnJ mice were purchased from The Jackson Laboratory and housed in the Animal Care Facility at the National Jewish Medical and Research Center. Synthetic 3K peptide (3Kp; ASFEAQKAKANKAVD, 15-mer), 2W peptide (2Wp; AWGALANWAVDS, 12-mer), and 4Sp (ASFEASGASANSAVDSA, 15-mer) peptides were prepared, purified by reverse-phase chromatography, and analyzed by MS by the Biological Resource Center at the National Jewish Center. Mice 8–14 weeks old were injected s.c. between the shoulder blades with 100 μl of emulsified complete Freund’s adjuvant (CFA) containing 50 μg or 0.5 μg synthetic peptide. For analysis of the secondary immune response, mice were injected s.c. with 50 μg or 0.5 μg peptide in incomplete Freund’s adjuvant (IFA) at two sites along the back 9 weeks after the primary immunization. Draining lymph nodes were harvested 8 days after the primary immunization and 5 days after the secondary immunization for further analyses.
In Vitro T Cell Stimulation and Hybridoma Production.
For the analysis of multimer-specific staining of T cells expanded in vitro, 4 × 106 lymph node cells from immunized mice were incubated with various concentrations of 3Kp or a control peptide that binds IAb avidly, 4Sp, in 1-ml cultures containing 1% mouse serum in Click’s medium for 4.5 days. Cells from 1-ml cultures were stained as described below.
For production of T cell hybridomas, lymph node cells harvested 8 days after primary immunization with either 50 μg or 0.5 μg peptide were stimulated in vitro for 5 days with 100 or 0.1 μg/ml 3Kp, respectively. Surviving viable T cells were expanded an additional 3 days with IL-2 before fusion with the BW5147 α–β– thymoma as described (21). Hybridomas were screened for antigen specificity by testing for IL-2 produced in response to C57BL/10 splenocytes with or without the immunizing peptide at 25 μg/ml as described (22).
Production of Soluble Peptide-IAb Molecules and Multimeric Staining Reagents.
Soluble IAb molecules were produced in High Five (Invitrogen) insect cells by using a derivative of the dual-promoter vector pAcUW51 (PharMingen) that has been described (3). For each construct, the β chain carried a leader sequence followed by an antigenic peptide sequence and a glycine-rich linker at its amino terminus end (4). The carboxyl terminus of the β chain carried a peptide tag allowing for biotinylation at a unique position by the birA enzyme (10, 23).
Soluble peptide-IAb heterodimers were purified from culture supernatants by affinity chromatography with the M5/114 mAb (24). The yield of MHC class II heterodimer in the 0.01- to 1-μg/ml range was determined by sandwich ELISA, capturing with plate-bound M5/114 mAb and detecting with biotinylated 17/227 mAb (25). Biotinylation efficiency was determined by ELISA relative to samples cleared of biotinylated protein by using avidin-agarose beads (Vector Laboratories).
Biotinylated, peptide-IAb heterodimers were purified by size-exclusion chromatography on Superdex-200, and fluorescent multimeric complexes were prepared from peptide-IAb-bio molecules and phycoerythrin-conjugated streptavidin (PESA) as described (10).
Flow Cytometric Analysis.
Flow cytometry was performed on a FACSCaliber instrument, and analysis used cell quest Software (Becton Dickinson) and mkflow histogram analysis software (available on request). Hybridomas were stained and analyzed for TCR and CD4 content by using biotinylated H597 (26) and the APC-conjugated anti-CD4 antibody RM4–5 (PharMingen).
Lymph node cells, either freshly isolated or cultured, were washed and incubated with peptide-IAb multimers for 2 hr at 37°C in tissue culture medium containing 10% FCS. Typically, 5–8 × 106 cells were incubated with 40 μg/ml staining reagent in a final volume of 300 μl. The cells were incubated an additional 45 min with antibodies against CD4 (allophycocyanin-conjugated RM4–5), B220 (cy-chrome-conjugated RA3–6B2), and CD44 (fluorescein-conjugated IM7) (PharMingen). Approximately 106 events were analyzed by flow cytometry.
RESULTS
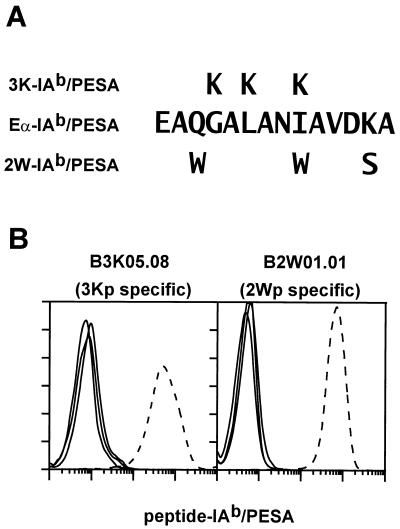
Multivalent cell-staining complexes of soluble IAb molecules with covalently associated antigenic peptides were prepared as described in Materials and Methods, using procedures that have been used previously to prepare staining reagents from soluble IAd and IEk molecules (10). Soluble IAb molecules harboring the 3K, 2W, or mouse Eα(52–68) (Eα) peptide sequences (Fig. 1A) were singly biotinylated in vitro through a sequence tag on the C terminus of the β chain and assembled into soluble multimers with PESA. The three IAb staining reagents, 3K-IAb/PESA, 2W-IAb/PESA, and Eα-IAb/PESA along with a reagent constructed with a different isotype of MHC class II, MCC-IEk/PESA (10), were used to stain the T cell hybridomas B3K05.06 and B2W01.01, which are specific for 3Kp and 2Wp bound to IAb, respectively (Fig. 1B). Each hybridoma stained strongly with the appropriate multimeric reagent. For each hybridoma, the three inappropriate reagents bound at a similar low level, although slight nonspecific binding was seen with the Eα-IAb/PESA reagent.
Figure 1.
Specific binding of peptide-IAb staining reagents to T cell hybridomas. (A) Sequences of the antigenic peptide portion of the staining reagents 3K-IAb/PESA, Eα-IAb/PESA, and 2W-IAb/PESA. (B) T cell hybridomas (3 × 105) specific for 3Kp/IAb (B3K05.06, Left) and 2Wp/IAb (B2W01.01, Right) were incubated separately with the three peptide-IAb/PESA-staining reagents or MCC-IEk/PESA at 40 μg/ml in 100 μl of complete tissue culture medium for 2 hr at 37°C. Washed cells were analyzed by flow cytometry for phycoerythrin fluorescence, and histograms are shown. In Left and Right the histogram of the stain performed with the cognate-staining reagent is the dotted line and all other histograms are solid lines.
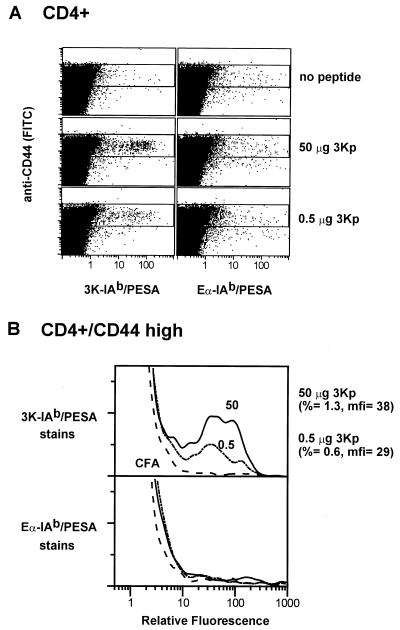
With these specific binding reagents we developed conditions for staining antigen-specific T cells freshly isolated from the lymph nodes of immunized mice. C57BL/10 mice were immunized with a high dose (50 μg) or a low dose (0.5 μg) of synthetic 3Kp in CFA. Eight days after immunization, lymphocytes in the draining lymph nodes were analyzed for T cells that bound the 3K-IAb/PESA staining reagent. Mice immunized with CFA alone were used as negative controls. The staining reagent Eα-IAb/PESA served as a control for nonspecific staining. The cells were counterstained with anti-CD4 to gate on CD4+ T cells, anti-B220 to gate out B cells, and anti-CD44 to identify activated/memory T cells. The results are shown in Fig. 2. Fig. 2a shows the relation between CD44 expression and the staining with the peptide-IAb/PESA reagents among CD4+ T cells. Very few CD4+ T cells from mice immunized with CFA alone stained with either peptide-IAb/PESA reagent. However, in mice immunized with either 50 μg or 0.5 μg 3Kp, a clear population of CD4+ T cells binding the 3K-IAb/PESA, but not the Eα-IAb/PESA reagent, was seen. These cells were confined to the CD44high population of CD4+ T cells.
Figure 2.
Direct detection of antigen-specific CD4+ T cells from normal C57BL/10 mice after primary immunization with 3K peptide. (A) C57BL/10 mice were immunized with CFA alone (row 1) or with 50 μg (row 2) or 0.5 μg (row 3) of 3Kp in CFA. After 8 days, a sampling of cells pooled from the draining nodes of 6–10 mice was stained with antibodies against CD4, B220, and CD44 and with the multimeric staining reagents 3K-IAb/PESA (Left) or Eα-IAb/PESA (Right). Peptide-IAb/PESA vs. FITC-CD44 plots are shown for CD4+, B220– cells. (B) Histograms of the events that fall in the boxed portions of the dot plots in A are shown. Histograms from mice immunized with 50 μg 3Kp, 0.5 μg 3Kp, or CFA alone are shown in solid, dotted, and dashed lines, respectively. (Upper) The percentages of CD4+CD44high cells (%) from 3Kp-immunized mice that stain with 3K-IAb/PESA are shown, as well as the mean fluorescence intensities (mfi) of the specifically staining cells. Control Eα-IAb/PESA histograms were matched and subtracted from the 3K-IAb/PESA histograms to calculate the percentage of specifically staining cells and their mean fluorescence intensities.
These T cells are better visualized in Fig. 2b, which shows histograms of the binding of the peptide-IAb/PESA reagents to CD4+CD44high T cells. When compared with the T cells from mice immunized with CFA alone, there was a small, but well-defined, expanded population of CD4+CD44high T cells from the 3Kp-immunized mice that bound the 3K-IAb/PESA, but not Eα-IAb/PESA, reagent. In mice immunized with 50 μg antigen, these cells represented 1.3% of the CD4+CD44high T cells. Not surprisingly, fewer cells from mice given the lower, 0.5-μg immunizing dose stained, such that 0.6% of the CD4+CD44high T cells stained with the 3K-IAb/PESA reagent.
We have shown previously that the intensity of staining of CD4+ T cells with MHC class II/PESA reagents is related directly to the affinity of the TCRs for the peptide–MHC construction used (10). We next tested whether limiting doses of antigen could encourage the outgrowth of brightly staining cells. The 0.5-μg dose of 3Kp used in the experiment described above apparently was limiting, as judged by the fact that it generated fewer antigen-specific T cells. However, we did not see any evidence that this low dose selected T cells with higher-affinity receptors within the 8 days of this primary response, as judged by the extent of binding of the 3K-IAb/PESA reagent. The average fluorescence of the 3K-IAb/PESA-binding T cells was very similar for both the 50-μg and 0.5-μg dose (Fig. 2b).
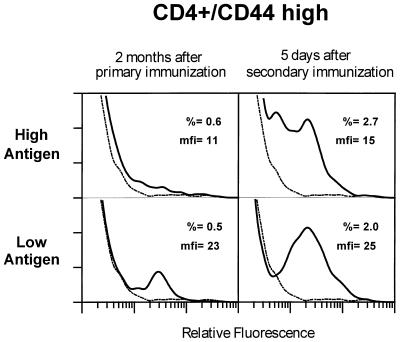
One explanation for the failure to see any influence of antigen dose on the apparent affinity of the responding T cells was that the single, short round of antigen selection was not enough to allow for the overgrowth of the higher-affinity cells. Therefore, we examined T cells from mice before and after they were boosted with antigen in IFA about 2 months after the initial immunization. Mice that received CFA alone in the first immunization received IFA alone in the second. Mice that received 50 μg or 0.5 μg of 3Kp initially received twice that dose again in the secondary immunization. The results are shown in Fig. 3.
Figure 3.
Expansion in the number of antigen-specific CD4 T cells detected with 3K-IAb/PESA upon secondary challenge with 3K peptide. C57BL/10 mice were immunized with 50 μg (Upper) or 0.5 μg (Lower) 3Kp in CFA and then left alone (Left) or restimulated 9 weeks later with 100 μg (Upper Right) or 1 μg (Lower Right) 3Kp in IFA. Control mice were treated with IFA only (dotted line). Five days after treatment with IFA and peptide, cells from draining lymph nodes were pooled and aliquots were stained as described in the legend to Fig. 2 and in Materials and Methods. Staining data are shown as described in the legend to Fig. 2.
Expanded, 3K-IAb/PESA-binding T cells were still detectable in the peptide-immunized mice 2 months after the primary immunization, although their frequency had dropped to ≈0.5% of CD4+CD44high cells in mice immunized with either dose of peptide. However, the relative staining intensities had changed. The T cells from mice immunized with the high dose of antigen still showed a broad pattern of staining, suggesting T cells with a broad range of receptor affinities. In contrast, the T cells from mice immunized with the low dose of antigen now were skewed toward a more intensely staining population, indicating higher-affinity receptors. This result suggests that with a low antigen dose, T cells with higher-affinity receptors tended to overgrow during the waning primary response.
Secondary immunization caused the rapid expansion of 3K-IAb-specific T cells. Five days after secondary immunization, 2–3% of CD4+CD44high T cells from mice given either peptide dose bound the 3K-IAb/PESA reagent. The difference in the level of staining between mice that received high or low doses of antigen was maintained. Whereas T cells from mice immunized twice with the high dose of antigen stained broadly, T cells from mice immunized twice with the low dose of antigen clearly were skewed toward brighter staining. Together these results suggested that T cells with higher-affinity receptors eventually blossomed out in these mice under the influence of limiting antigen.
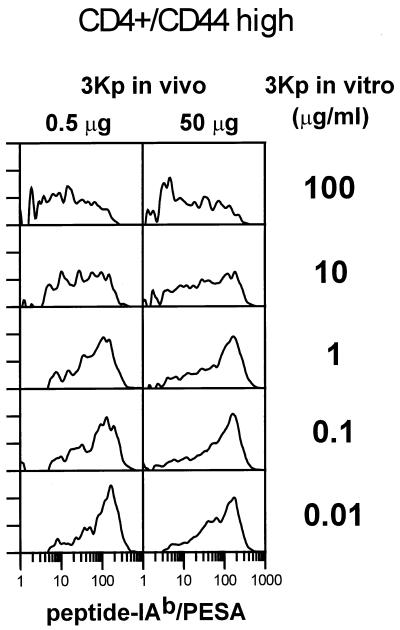
To test further the idea that, given enough expansion, lower doses of antigen will select for T cells with higher-affinity receptors, we looked at T cells from immunized mice expanded further in vitro with various doses of antigen. Fig. 4 shows the data with T cells taken from mice 8 days after immunization with 50 μg or 0.5 μg 3Kp and expanded further in vitro with various doses of 3Kp. The number of cells staining with the 3K-IAb/PESA reagent rose dramatically in these cultures. With either in vivo peptide dose, in vitro restimulation with high doses (10–100 μg) of 3Kp resulted in a broad pattern of staining with 3K-IAb/PESA, indicating a wide range of receptor affinities. As the dose of peptide fell to ≤1 μg/ml, the staining pattern clearly skewed toward more brightly staining cells, indicating the outgrowth of high-affinity T cells. This was true for T cells from mice primed originally with either dose of the peptide. Qualitatively similar results were obtained with T cells taken from mice 5 days after the secondary immunization (data not shown).
Figure 4.
T cells from immunized mice that were expanded in vitro on low doses of 3K peptide stained more intensely and at higher frequency with 3K-IAb/PESA than cells expanded on high doses of peptide. Eight days after mice were immunized with 50 or 0.5 μg 3Kp, lymph nodes were harvested and cells were cultured for 4.5 days in vitro at five different starting concentrations of 3Kp. Control Eα-IAb/PESA histograms were matched to and subtracted from the 3K-IAb/PESA histograms, and a normalized number of the specific, 3K-IAb/PESA-staining events are depicted in each histogram.
A surprising aspect of the results in Fig. 4 was the substantial portion of the T cells that expanded in vitro with 3Kp that failed to bind the 3K-IAb/PESA reagent at all. This was particularly noticeable with the T cells expanded in high doses of the peptide. For example, although 36% of the CD4+CD44high T cells from mice immunized with 50 μg and then expanded in vitro in 0.01 μg/ml 3Kp stained specifically with 3K-IAb/PESA, only 16% of the analogous cells that were expanded in vitro in 100 μg/ml 3Kp stained with 3K-IAb/PESA. It is possible that these T cells that fail to stain have extremely low affinity for 3Kp presented by IAb or that these blasting T cells responded to a trace contaminant present when synthetic 3Kp was presented by IAb. Alternatively, 3Kp may be able to bind to IAb in more than one frame, whereas the major frame is fixed by the linker on the 3K-IAb/PESA probe.
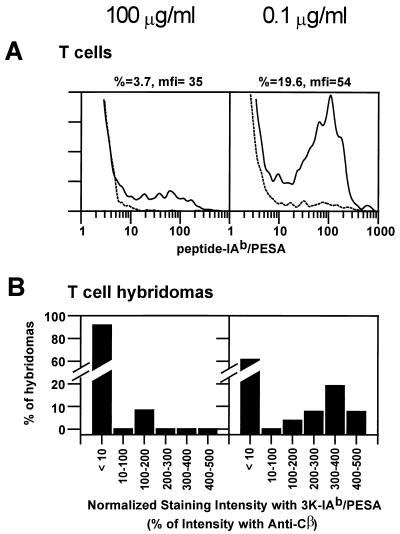
To take a closer look at these in vitro expanded T cells, we used them to produce T cell hybridomas. T cells were stimulated with 3Kp, using either 50 μg in vivo/100 μg in vitro or 0.5 μg in vivo/0.1 μg in vitro. After 5 days in vitro, surviving T cells were expanded further in IL-2 in the absence of antigen. Fig. 5A shows the pattern of staining of these T cell blasts before fusion. These results are similar to those seen in Fig. 4. Among the cells repeatedly exposed to high doses of peptide, a low percentage bound 3K-IAb/PESA (≈4% of CD4+CD44high cells), and those that did showed a wide range of affinities. In contrast, a higher percentage of cells expanded with a low dose of 3Kp stained with 3K-IAb/PESA (≈20% of CD4+CD44high cells), and they were skewed toward higher affinities. These T cell blasts were fused by standard techniques to produce T cell hybridomas.
Figure 5.
T cell hybridomas generated on low doses of 3K peptide were far more likely to bind 3K-IAb/PESA, and bind it strongly, than hybridomas raised on high doses of peptide. Lymphocytes from mice immunized once with 50 or 0.5 μg 3Kp in CFA were cultured for 4.5 days in vitro with 100 (Left) or 0.1 (Right) μg/ml 3Kp, respectively, and live cells were expanded in the presence of IL-2 for an additional 3 days. (A) Histograms of CD4+CD44high lymphoblasts stained with 3K-IAb/PESA (solid lines) or Eα-IAb/PESA (dotted lines) reagents are shown. Hybridomas made from these lymphoblasts that produced IL-2 in response to C57BL/10 splenocytes and the addition of 3Kp at 25 μg/ml were scored as 3Kp/IAb specific. (B) Collections of 3Kp/IAb-specific T cell hybridomas raised on high doses (Left) or low doses (Right) of 3Kp were stained separately with 3K-IAb/PESA and anti-Cβ antibody. The results are plotted as the percentage of hybridomas vs. the relative mean fluorescence intensity obtained with 3K-IAb/PESA as a percentage of the mean fluorescence intensity obtained with anti-Cβ.
Individual T cell hybridomas were analyzed for binding of 3K-IAb/PESA and for the level of surface T cell receptor. The results are summarized in Fig. 5B, which shows the frequency of T cell hybridomas that stained at various intensity levels for both T cell fusions. The intensity data are normalized to the level of surface T cell receptor on each hybridoma. Only two of 25 (8%) of the hybridomas produced with high-dose 3Kp expanded T cells stained with 3K-IAb/PESA. The normalized staining intensity of these two was among the lowest seen. On the other hand, 10 of 26 (38%) of the hybridomas produced with T cells expanded with a low dose of 3Kp-bound 3K-IAb/PESA, and these were skewed toward higher-intensity staining, i.e., higher-affinity binding to the peptide-IAb/PESA complex. These results confirmed that repeated exposure to low doses of the peptide selected for T cells with higher-affinity receptors. They also again reveal the presence of T cells that failed to bind 3K-IAb/PESA after expansion with synthetic 3Kp.
DISCUSSION
We have produced a set of fluorescent, multimeric class II MHC reagents of the IAb haplotype. These reagents bind strongly and with appropriate specificity to T cell hybridomas (Fig. 1). With these reagents we have detected antigen-specific CD4+ T cells in normal mice immunized with a peptide antigen. The frequency of antigen-specific CD4+ T cells detected 8 days after a primary immunization in CFA was ≈1% of CD4+CD44high T cells. Although accurate measurements of the frequency of antigen-specific precursor T cells in unimmunized mice have not been possible, frequencies of about 1 in 105 have been estimated. Thus, our results indicate a very large expansion during the primary response, on the order of 103-fold. Previously estimated frequencies of antigen-specific CD4+ T cells in immunized mice have varied considerably, from values similar to those reported here to some much lower (27–31). Recent experiments with multimeric class I reagents have, in several cases, found extraordinarily high frequencies of antigen-specific CD8+ T cells in virally infected mice, again much higher than had been predicted by other methods (9, 32–35). The application of methods such as the limiting dilution analysis and companion ELISPOT analysis generally has underestimated the frequency of antigen-specific T cells, because they can be less sensitive than direct staining and because not all antigen/MHC-binding T cells may have the function scored in the assay (production of a particular cytokine, cytotoxic T lymphocyte activity, help for B cell responses, etc.).
The most intriguing observation in this study was the inverse relation between antigen dose and the intensity of staining of the responding T cells with the specific multimer reagent. Although not evident at the peak of the primary response, the skewing to brighter-staining T cells by exposure to low-antigen doses was evident 2 months after the primary response (Fig. 3) in mice responding to a secondary immunization (Fig. 3) and among T cells expanded in vitro (Fig. 4). CD4 levels were similar among the differentially staining populations (data not shown). In vitro experiments with T cell hybridomas bearing receptors of known affinity have established a direct correlation between this affinity and the extent of binding of the class II multimeric reagents (10). Therefore, the most straightforward interpretation of our results is that, given enough time, limiting antigen favors the outgrowth of T cells with high-affinity receptors. This shaping of the TCR repertoire toward higher affinities in T cell responses to secondary antigenic challenge relative to naïve T cell responses recently has been demonstrated in both a bacterial infection model (16) and a classical peptide-immunization model (17) by using related, multimeric MHC-staining tools.
Studies with human T cell clones have correlated TCR down-regulation with the extent of stimulation by antigen (36, 37). Although receptor down-regulation may have decreased the multimer-staining intensity of T cells from mice immunized with high-antigen doses, it is unable to account entirely for the differential staining observed for several reasons. The broad staining pattern seen with high-antigen doses was stable in mice several months after the primary immunization (Fig. 3). T cells expanded in vitro with a high-antigen dose and then cultured in IL-2 in the absence of antigen maintained the proportion of dully staining cells (Fig. 5A). Additionally, the staining pattern was maintained in T cell hybridomas derived from the expanded T cells (Fig. 5B). A representative set of hybridomas that did or did not stain with 3K-IAb/PESA also was tested for IL-2 production in the presence of IAb bearing antigen-presenting cells and various doses of 3Kp. Whereas all hybridomas tested produced IL-2 in response to IAb-presented synthetic 3Kp, the hybridomas that failed to bind 3K-IAb/PESA required ≈100-fold-higher concentrations of peptide than did those that bound 3K-IAb/PESA detectably (data not shown). Lastly, only those hybridomas that stained detectably with 3K-IAb/PESA produced IL-2 in response to saturating concentrations of biotinylated 3K-IAb bound to streptavidin-coated plates (data not shown). Consistent with our results using T cell hybridomas, it recently has been shown that ex vivo CD8+ T cells that bind peptide–class I MHC complexes more avidly have greater sensitivity to peptide in cytotoxic T lymphocyte assays (16, 38).
T cells cannot take advantage of the mechanisms of somatic mutation to create cells with higher-affinity receptors during the course of an immune response. Therefore, the magnitude of the effect of antigen dose on the average affinity of the responding T cell population will depend on the breadth of T cell receptor affinities available in the naïve precursor pool. In our experiments using a single peptide antigen, the overall range of this effect was about 10- to 100-fold. Multiepitope antigens, such as large proteins or whole organisms, could be expected to stimulate a much more heterogeneous precursor pool. In these cases, the effect of antigen dose on T cell receptor affinity might be more dramatic. Although limiting doses of antigen increase TCR affinity, the range of affinities realized for any given antigen likely will be limited by a relatively low upper limit. A variety of receptor association constants measured in vitro has ranged from about 106 M−1 to 5 × 104 M−1 (39–45). It is possible that higher-affinity receptors will not be found in vivo at any antigen dose, because of the limitations imposed by the need for self-tolerance. A T cell bearing a receptor with very high affinity to a foreign peptide plus self-MHC may be almost certain to find a self-peptide/self-MHC ligand during development that will have an affinity high enough to cause its elimination.
Interestingly, although exposure to several rounds of high-antigen concentration maintained low-affinity T cells in the responding population, we saw no evidence for the loss or inactivation of high-affinity T cells in this population by mechanisms such as clonal deletion or anergy. These higher-affinity cells were still present in the staining patterns, and, indeed, reexposure to low-antigen concentrations led to their rapid outgrowth. These results suggest that, although antigen dose may determine the proportion of expanded T cells with a particular affinity, other factors, such as the adjuvant-induced cytokine milieu, may be more important in determining T cell survival.
In trying to understand CD4+ T cell memory, a thorny issue has been to what extent the phenomenon of memory reflects an expanded precursor pool vs. precursors with accelerated response kinetics. Our results suggest that T cell memory involves at least a substantial expansion of the precursor pool detectable at several months after primary immunization to the extent that peptide/CFA is a representative antigen/adjuvant. A similar conclusion has been reached by using these methods with CD8+ T cells (9, 46).
Finally, one might expect that T cells with higher-affinity receptors would be more effective in vivo in functions such as eliminating pathogens, inducing inflammation, or helping B cell antigen production. Our experiments have produced useful tools for the generation, identification, and isolation of T cells of different affinities that could be used to test this idea.
Acknowledgments
We thank Drs. Leszek Ignatowicz and Mark Scherer for cloning the original Eα-IAb and 3K-IAb expression vectors. We are grateful to Lisa Calavetta and colleagues in the Kappler–Marrack laboratory for many helpful and stimulating discussions. This work was supported in part by U.S. Public Health Service Grants AI-17134, AI-18785, and AI-22295.
ABBREVIATIONS
- TCR
T cell antigen receptor
- CFA
complete Freund’s adjuvant
- IFA
incomplete Freund’s adjuvant
- PESA
phycoerythrin-conjugated streptavidin
- 3Kp
3K peptide
- 2Wp
2W peptide
References
- 1.Scott C A, Garcia K C, Stura E A, Peterson P A, Wilson I A, Teyton L. Protein Sci. 1998;7:413–418. doi: 10.1002/pro.5560070222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garboczi D N, Utz U, Ghosh P, Seth A, Kim J, VanTienhoven E A, Biddison W E, Wiley D C. J Immunol. 1996;157:5403–5410. [PubMed] [Google Scholar]
- 3.Kozono H, White J, Clements J, Marrack P, Kappler J. Nature (London) 1994;369:151–154. doi: 10.1038/369151a0. [DOI] [PubMed] [Google Scholar]
- 4.Ignatowicz L, Winslow G, Bill J, Kappler J, Marrack P. J Immunol. 1995;154:3852–3862. [PubMed] [Google Scholar]
- 5.Altman J D, Moss P A H, Goulder P J R, Barouch D H, McHeyzer-Williams M G, Bell J I, McMichael A J, Davis M M. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 6.Callan M F, Tan L, Annels N, Ogg G S, Wilson J D, O’Callaghan C A, Steven N, McMichael A J, Rickinson A B. J Exp Med. 1998;187:1395–1402. doi: 10.1084/jem.187.9.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gallimore A, Glithero A, Godkin A, Tissot A C, Pluckthun A, Elliott T, Hengartner H, Zinkernagel R. J Exp Med. 1998;187:1383–1393. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuroda M J, Schmitz J E, Barouch D H, Craiu A, Allen T M, Sette A, Watkins D I, Forman M A, Letvin N L. J Exp Med. 1998;187:1373–1381. doi: 10.1084/jem.187.9.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murali-Krishna K, Altman J D, Suresh M, Sourdive D J, Zajac A J, Miller J D, Slansky J, Ahmed R. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 10.Crawford F, Kozono H, White J, Marrack P, Kappler J. Immunity. 1998;8:675–682. doi: 10.1016/s1074-7613(00)80572-5. [DOI] [PubMed] [Google Scholar]
- 11.Siskind G W, Benacerraf B. Adv Immunol. 1969;10:1–50. doi: 10.1016/s0065-2776(08)60414-9. [DOI] [PubMed] [Google Scholar]
- 12.Berek C, Milstein C. Immunol Rev. 1987;96:23–41. doi: 10.1111/j.1600-065x.1987.tb00507.x. [DOI] [PubMed] [Google Scholar]
- 13.Bernard O, Hozumi N, Tonegawa S. Cell. 1978;15:1133–1144. doi: 10.1016/0092-8674(78)90041-7. [DOI] [PubMed] [Google Scholar]
- 14.Han S, Zheng B, Schatz D G, Spanopoulou E, Kelsoe G. Science. 1996;274:2094–2097. doi: 10.1126/science.274.5295.2094. [DOI] [PubMed] [Google Scholar]
- 15.Melamed D, Benschop R J, Cambier J C, Nemazee D. Cell. 1998;92:173–182. doi: 10.1016/s0092-8674(00)80912-5. [DOI] [PubMed] [Google Scholar]
- 16.Busch D H, Pamer E G. J Exp Med. 1999;189:701–710. doi: 10.1084/jem.189.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Savage P A, Boniface J J, Davis M M. Immunity. 1999;10:485–492. doi: 10.1016/s1074-7613(00)80048-5. [DOI] [PubMed] [Google Scholar]
- 18.Alexander-Miller M A, Leggatt G R, Berzofsky J A. Proc Natl Acad Sci USA. 1996;93:4102–4107. doi: 10.1073/pnas.93.9.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alexander-Miller M A, Parker K C, Tsukui T, Pendleton C D, Coligan J E, Berzofsky J A. Int Immunol. 1996;8:641–649. doi: 10.1093/intimm/8.5.641. [DOI] [PubMed] [Google Scholar]
- 20.Speiser D E, Kyburz D, Stubi U, Hengartner H, Zinkernagel R M. J Immunol. 1992;149:972–980. [PubMed] [Google Scholar]
- 21.White J, Blackman M, Bill J, Kappler J, Marrack P, Gold D P, Born W. J Immunol. 1989;143:1822–1825. [PubMed] [Google Scholar]
- 22.Kappler J W, Skidmore B, White J, Marrack P. J Exp Med. 1981;153:1198–1214. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schatz P J. Bio/Technology. 1993;11:1138–1143. doi: 10.1038/nbt1093-1138. [DOI] [PubMed] [Google Scholar]
- 24.Bhattacharya A, Dorf M E, Springer T A. J Immunol. 1981;127:2488–2495. [PubMed] [Google Scholar]
- 25.Koch N, Hammerling G J, Tada N, Kimura S, Hammerling U. Eur J Immunol. 1982;12:909–914. doi: 10.1002/eji.1830121103. [DOI] [PubMed] [Google Scholar]
- 26.Kubo R T, Born W, Kappler J W, Marrack P, Pigeon M. J Immunol. 1989;142:2736–2742. [PubMed] [Google Scholar]
- 27.Marrack P, Kappler J, Kettman J. J Immunol. 1974;113:830–834. [PubMed] [Google Scholar]
- 28.Ewing C, Topham D J, Doherty P C. Virology. 1995;210:179–185. doi: 10.1006/viro.1995.1329. [DOI] [PubMed] [Google Scholar]
- 29.Hildeman D, Yanez D, Pederson K, Havighurst T, Muller D. J Virol. 1997;71:9672–9678. doi: 10.1128/jvi.71.12.9672-9678.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varga S M, Welsh R M. J Immunol. 1998;161:3215–3218. [PubMed] [Google Scholar]
- 31.Gutgemann I, Fahrer A M, Altman J D, Davis M M, Chien Y H. Immunity. 1998;8:667–673. doi: 10.1016/s1074-7613(00)80571-3. [DOI] [PubMed] [Google Scholar]
- 32.Tripp R A, Sarawar S R, Doherty P C. J Immunol. 1995;155:2955–2959. [PubMed] [Google Scholar]
- 33.Doherty P C. Science. 1998;280:227. doi: 10.1126/science.280.5361.227. [DOI] [PubMed] [Google Scholar]
- 34.Doherty P C, Allan W, Eichelberger M, Carding S R. Annu Rev Immunol. 1992;10:123–151. doi: 10.1146/annurev.iy.10.040192.001011. [DOI] [PubMed] [Google Scholar]
- 35.Zinkernagel R M, Bachmann M F, Kundig T M, Oehen S, Pirchet H, Hengartner H. Annu Rev Immunol. 1996;14:333–367. doi: 10.1146/annurev.immunol.14.1.333. [DOI] [PubMed] [Google Scholar]
- 36.Valitutti S, Muller S, Cella M, Padovan E, Lanzavecchia A. Nature (London) 1995;375:148–151. doi: 10.1038/375148a0. [DOI] [PubMed] [Google Scholar]
- 37.Valitutti S, Lanzavecchia A. Immunol Today. 1997;18:299–304. [PubMed] [Google Scholar]
- 38.Yee C, Savage P A, Lee P P, Davis M M, Greenberg P D. J Immunol. 1999;162:2227–2234. [PubMed] [Google Scholar]
- 39.Fremont D H, Rees W A, Kozono H. Curr Opin Immunol. 1996;8:93–100. doi: 10.1016/s0952-7915(96)80111-7. [DOI] [PubMed] [Google Scholar]
- 40.Lyons D S, Lieberman S A, Hampl J, Boniface J J, Chien Y, Berg L J, Davis M M. Immunity. 1996;5:53–61. doi: 10.1016/s1074-7613(00)80309-x. [DOI] [PubMed] [Google Scholar]
- 41.Matsui K, Boniface J J, Steffner P, Reay P A, Davis M M. Proc Natl Acad Sci USA. 1994;91:12862–12866. doi: 10.1073/pnas.91.26.12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garcia K C, Tallquist M D, Pease L R, Brunmark A, Scott C A, Degano M, Stura E A, Peterson P A, Wilson I A, Teyton L. Proc Natl Acad Sci USA. 1997;94:13838–13843. doi: 10.1073/pnas.94.25.13838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corr M, Slanetz A, Boyd L, Jelonek M, Khilko S, Al-Ramadi B, Kim Y, Maher S, Bothwell A, Margulies D. Science. 1994;265:946–949. doi: 10.1126/science.8052850. [DOI] [PubMed] [Google Scholar]
- 44.Liu C P, Crawford F, Marrack P, Kappler J. Proc Natl Acad Sci USA. 1998;95:4522–4526. doi: 10.1073/pnas.95.8.4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khandekar S S, Brauer P P, Naylor J W, Chang H C, Kern P, Newcomb J R, Leclair K P, Stump H S, Bettencourt B M, Kawasaki E, et al. Mol Immunol. 1997;34:493–503. doi: 10.1016/s0161-5890(97)00044-8. [DOI] [PubMed] [Google Scholar]
- 46.Flynn K J, Belz G T, Altman J D, Ahmed R, Woodland D L, Doherty P C. Immunity. 1998;8:683–691. doi: 10.1016/s1074-7613(00)80573-7. [DOI] [PubMed] [Google Scholar]