Abstract
After initial seeding by thymic emigrants, homeostatic regulation of the T cell pool has been thought to occur entirely within the periphery. Here we report that the degree of thymic emigration directly affects the number and the CD4/CD8 ratio of peripheral T cells. We demonstrate that the increase in T cell pool size caused by the engraftment of 2, 6, or 9 thymic lobes correlates almost exactly with the number of emigrants exported from those grafts in the previous 3 weeks, regardless of how long the graft has been in place. The extent of the increase supports the concept of a 3-week period after thymic export in which emigrant T cells are exempt from peripheral T cell homeostasis. This apparent exclusion of recent thymic emigrants from the niche-based regulation of peripheral T cell numbers ensures repertoire turnover throughout adult life and provides the basis of a direct and previously unrecognized role for the thymus in the regulation of peripheral T cell homeostasis.
Keywords: thymus, thymus grafting, CD4, CD8 ratio
There is a consensus emerging that T cell numbers are regulated through competition for a finite number of essential survival factors (1–3). These factors, collectively termed niches, are thought to provide stability in the T cell pool through the removal of excess cells when all niches are occupied and the proliferation and/or increased longevity of peripheral T cells when niches are vacant. Although consistent with the stable pool size of normal young animals, this hypothesis is inconsistent with long-term changes to T cell numbers caused by age (4, 5), disease (6–8), and experimental manipulations (9–11). Many of these instances are also associated with changes to the rate of thymic export and support our earlier study (11) demonstrating a persistent role for the thymus in peripheral T cell regulation.
Studies involving the reconstitution of lymphocyte-depleted mice have shown that the number and CD4/CD8 ratio of T cells can be restored through the peripheral expansion of adoptively transferred cells, irrespective of the number or type of T cells injected (3, 12–15). These reports imply that the thymus has only a minor influence on the adult T cell pool, simply acting as a source of new T cells to be subsequently regulated within the periphery (16). However, it is not clear whether reconstitution of a lymphocyte-deficient environment is comparable to maintaining the diverse repertoire of normal mice. Recent studies have demonstrated that expansion triggered by lymphopenia is restricted to the activated/memory pool, whereas generation of the naive T cell pool depends on thymic export (13, 17, 18). This provided an explanation for the gross overrepresentation of memory T cells in the T cell pool after reconstitution (14, 19) and raised the important question of whether the thymus responds to feedback from the peripheral T cell pool to regulate naïve T cell numbers (14). We tested this and found that the number and CD4/CD8 ratio of recent thymic emigrants was independent of the number and CD4/CD8 ratio of peripheral T cells (20). Furthermore, increasing peripheral T cell levels with thymic transplants had no effect on thymic export (11). Thus, although export from the thymus is the only means by which naïve T cell levels can be increased, their release from the thymus is independent of the peripheral T cell pool.
Based on these findings, changes to thymic export would be expected to have a significant impact on the level of peripheral T cells. On the other hand, the effects of age-related thymic atrophy, thymectomy, or thymic grafting do not support an absolute correlation between thymic export and T cell numbers. Our recent data suggest that the impact of the thymus on T cell levels is caused and limited by a transient exemption of recent thymic emigrants (RTE) from the survival requirements of niche-based homeostasis in the first 3 weeks postexport (11). Thymic emigrants would accumulate independently of peripheral T cell levels, but become subject to normal regulatory pressures after 3 weeks, thereby limiting the effect of altered thymic export on cell numbers, but ensuring the ongoing turnover of T cell specificities. This is consistent with the magnitude of the increase in T cell pool size after thymic grafting and the fall in numbers after thymectomy, both of which are inconsistent with thymus-independent regulation of the peripheral pool (11, 21).
We now report on the nature of changes to the T cell pool caused by thymic grafting and contrast the changing composition of the thymic emigrant population to the relative stability of the overall pool. We confirm that the thymus influences the T cell pool throughout adult life and precisely define the means by which emigrants modulate the number, CD4/CD8 ratio, and longevity of peripheral T cells. Based on these data, a more complete theory of homeostatic regulation is proposed that incorporates an active role for thymic emigrants.
MATERIALS AND METHODS
Mice.
C57BL/6J and Ly5 congenic mice were obtained from Monash University and the Walter and Eliza Hall Institute Central Animal Houses and housed under conventional conditions.
Thymic Grafting.
Thymic lobes were removed from newborn pups and engrafted beneath the kidney capsule of 5- to 6-week-old female mice according to a previously described technique (11).
Cell Suspensions.
Mice were killed by CO2 asphyxiation at various time points after thymic grafting. Separate cell suspensions were prepared from lymph nodes, spleen, thymus (both in situ and grafted), and blood by previously established techniques. (11).
Antibodies and Flow Cytometry.
For flow cytometric analysis, phycoerythrin (PE)-labeled anti-CD25, anti-CD69, and anti-CD4; biotinylated anti-Vβ 3, 4, 6, 8.1/8.2, 10, 11, and 12; anti-CD8α; anti-CD44; FITC-labeled CD45.2 (Ly5.2); and allophycocyanin (APC)-labeled anti-CD8 were purchased from PharMingen). Biotinylated anti-CD4 and streptavidin-tricolor conjugates were purchased from Caltag (South San Francisco, CA). FITC Isomer 1 (for intrathymic injection) was purchased from Becton Dickinson. Annexin V-Biotin (PharMingen) was used to detect cells undergoing apoptosis by binding to phospholipid phosphatidylserine on the cell surface (22). Cell suspensions were stained for analysis by flow cytometry in V-bottom tubes (3 × 106 cells per test) by using previously described techniques. Flow cytometry data were collected by using a FACScalibur machine (Becton Dickinson) and analyzed by using cell quest software (Becton Dickinson).
FITC Labeling of Thymocytes.
Details of this technique were identical to those described elsewhere (23). Briefly, animals were anesthetized and the chest was opened (or kidney was exposed, in the case of grafted mice) to reveal the thymic lobes. Each lobe was injected with approximately 10 μl of 350 μg/ml FITC (in PBS), which typically resulted in random labeling of 30–60% of the thymocyte population (70–80% for grafted thymuses). The wound was closed with a surgical staple, and the mouse was warmed until fully recovered from anesthesia. Mice were killed by CO2 asphyxiation ≈24hr postinjection, with graft and lymphoid organs taken for analysis. Instruments were washed between removal of each organ, and the FITC-injected thymus was always removed last to avoid cross-contamination of samples.
Quantification of Emigrant Populations in Host Mice.
Emigrant cells were detected in two ways. For detection of RTE, the lymphoid organs were removed approximately 24 hr after intrathymic injection of FITC. After cell counts, samples were stained with anti-CD4-PE and anti-CD8-Biotin (detected with streptavidin-tricolor) or anti-CD8-APC for flow cytometric analysis. Emigrant cells were identified as live-gated FITC+ cells that expressed either CD4 or CD8 (to omit autofluorescing cells and doublets). The percentages of FITC+ CD4 and CD8 cells among live-gated cells were summed to provide the total emigrant percentage for lymph nodes and spleen respectively. A similar procedure was used for quantifying donor-derived T cells exported from grafted congenic thymuses up to 16 weeks prior. In these cases, emigrant cells were identified by expression of the Ly5.2 antigen.
Calculation of Peripheral T Cell Pool Size.
The peripheral pool was considered equal to the total number of spleen cells plus twice the total of lymph node cells (pooled from mesenteric, inguinal, and axillary nodes) (15).
Statistical Analysis.
Student’s unpaired t tests were used to compare two groups of normally distributed data. ANOVA tests were used to compare three or more groups of normally distributed data.
RESULTS
Overall T Cell Numbers Increase in Proportion to the Number of Thymic Grafts.
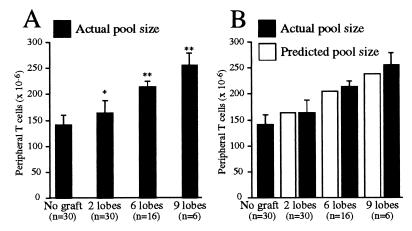
Mice grafted with two thymic lobes contained an average 21 × 106 more peripheral T cells than normal mice (30 mice tested; P ≤ 0.001, unpaired Student’s t test) (Fig. 1A). Further significant increases were observed for mice engrafted with six and nine lobes (P ≤ 0.0001, unpaired Student’s t test). The average increases for mice with six and nine thymic grafts above nongrafted controls were 71 × 106 and 111 × 106 cells, respectively. The 15, 50, and 77% increases to T cell pool size resulting from engraftment of two, six, and nine lobes, respectively, represent a direct correlation between the number of grafted thymic lobes and the resultant increase in T cell numbers. Mice typically were grafted for 4–8 weeks with no difference in pool size detected between mice in this time range. Increases were stable and did not alter with time (see Fig. 2), indicating there was no correlation between pool size and the time of engraftment beyond the initial increase. The increases were equivalent to those predicted by the modified niche theory of homeostasis (see Discussion; also see Fig. 1B).
Figure 1.
(A) Thymic grafting causes a significant increase in T cell pool size proportional to the number of grafted lobes. The T cell pool size of mice grafted with two, six, or nine thymic lobes was significantly higher than those of normal mice (two lobes, P < 0.001; six or nine lobes, P < 0.0001; unpaired Student’s t test) and significantly different from each other (P < .0001; ANOVA test). (B) The increase in T cell pool size caused by thymic grafting is consistent with separate regulation of thymic emigrants. Estimations of the extent of the increases caused by thymic grafting based on the number of grafted lobes were made by using a modified theory of peripheral T cell homeostasis. This theory proposes that emigrant T cells survive outside the restrictions of homeostatic regulation for up to 3 weeks postthymic export. The resultant estimates closely matched the pool sizes of mice engrafted with two, six, and nine thymic lobes.
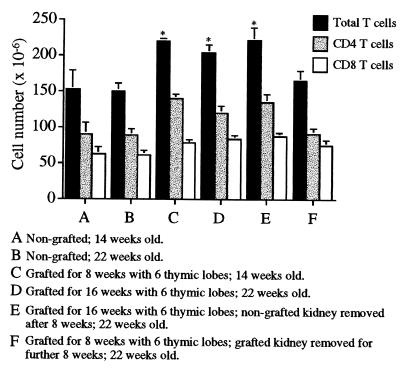
Figure 2.
Changes in T cell pool size are the direct result of changed thymic export rates. Six-week-old mice were grafted with six thymic lobes, and CD4, CD8, and overall T cell pool sizes were compared with those of nongrafted controls after a further 8 or 16 weeks. Although a significant difference was seen between grafted and nongrafted mice (P < .0001, unpaired Student’s t test), no difference was observed between mice grafted for 8 or 16 weeks. A minimum of six mice were used per group, with error bars representing 1 SD from the mean.
T Cell Numbers Stabilize at a Level Determined by Thymic Export.
A comparison of the T cell pool sizes of mice grafted with six thymic lobes for 8 or 16 weeks revealed a significant increase of approximately 50% above control levels in both groups (P ≤ 0.0001, unpaired Student’s t test). This indicated that an increase in pool size occurred within 8 weeks of thymic engraftment and was maintained without further increase. Removal of the grafted lobes after 8 weeks caused T cell numbers to fall by 16 weeks to the level of nongrafted mice. Removal of the contralateral (nongrafted) kidney alone caused no reduction in cell numbers, excluding the possibility that the decrease was due to surgical stress (Fig. 2).
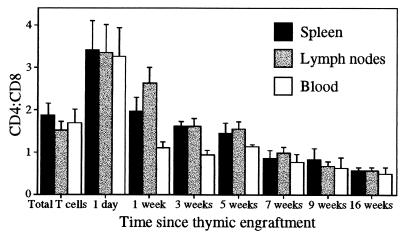
The CD4/CD8 Ratio of Thymic Emigrants Decreases with Time.
The emigrant population from Ly5 congenic thymic grafts was tracked for up to 16 weeks postthymic engraftment. The CD4/CD8 ratio of the emigrant population shifted progressively from ≈3.5:1 to below 1:1 16 weeks postengraftment (Fig. 3). The shift began within 1 week of export and reached similar levels by 5 weeks in lymphocytes from the spleen, lymph nodes, and blood, although initial levels fell most rapidly among blood lymphocytes. One-day-old emigrants were detected by using intrathymic FITC injection.
Figure 3.
The CD4/CD8 ratio of an emigrant T cell population falls with age. Donor-derived T cells from thymic grafts were detected in Ly5.1 recipient mice by using anti-Ly5.2, anti-CD4, and anti-CD8. Mean results from a minimum of five mice are indicated at each time point, with separate analysis performed for spleen, lymph nodes, and blood. Error bars represent 1 SD from the mean.
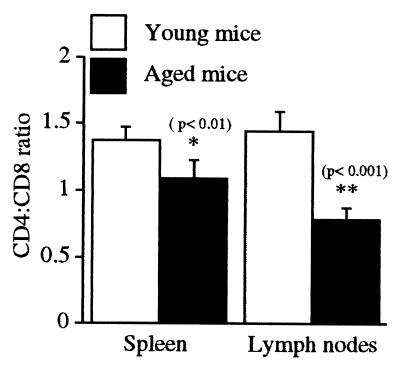
The CD4/CD8 Ratio of Peripheral T Cells Decreases with Age.
We compared the CD4/CD8 ratio of 14-week- and 14-month-old mice and found an age-associated difference that was consistent with data recently published by Barrat et al. (4). The shift was significant for both the spleen and lymph nodes but greatest in the latter, where a 1.5:1 ratio was seen in 14-week-old mice compared with less than 1:1 in 14-month-old mice (Fig. 4).
Figure 4.
The CD4/CD8 ratio of the overall T cell pool of young mice (14 weeks) is significantly higher than that of aged mice (14 months). Peripheral T cells from the spleen and lymph nodes of young and old, nongrafted mice were labeled with anti-CD4 and anti-CD8, and the CD4/CD8 ratios were compared. Five mice were analyzed in each group. Error bars represent 1 SD from the mean.
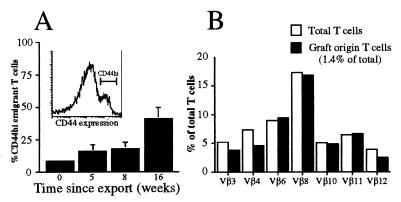
CD44 Expression Increases Slowly on Emigrant T Cell Population.
High CD44 levels were used in conjunction with markers for CD4, CD8, and Ly5.2 to determine the memory status of T cells. As reported previously (17, 24), RTE were predominantly CD44lo within 1 day of export. Progressively more emigrant T cells expressed high levels of CD44 with time, such that by week 16, approximately 35% were CD44hi (Fig. 5A). Expression levels in the overall T cell population were similar to those reported previously for young mice (4) (20% for lymph node T cells and 25% for splenic T cells; data not shown). Similar proportions of CD44hi cells were observed within CD4 and CD8 compartments at each time point (data not shown).
Figure 5.
(A) The increase in CD44hi cells among emigrant T cell populations postexport is gradual and does not indicate repertoire skewing. Donor-derived T cells from congenic thymic grafts were detected in the spleen, lymph nodes, and blood of Ly5.1 recipient mice by using anti-Ly5.2 in conjunction with anti-CD44, anti-CD4, and anti-CD8. A represents the mean proportion of CD44hi-expressing emigrant cells derived from recipient lymph nodes. Between 3 and 10 mice were assessed per group, with error bars representing 1 SD from the mean. B shows the Vβ profile of T lymphocytes (a similar distribution was observed for CD4 and CD8 subsets) derived from the lymph nodes of a mouse grafted for 9 weeks. This profile is representative of profiles from the spleen and lymph nodes from four different mice grafted with two thymic lobes for between 3 and 9 weeks.
T Cell Antigen Receptor β (TcRβ) Usage by Surviving Emigrants Is Similar to the Overall T Cell Pool.
To establish whether changes within the emigrant T cell population might be antigen-driven, the distribution of seven common TcR Vβ chains (Vβ 3, 4, 6, 8.1/8.2, 10, 11, and 12) among emigrant T cells was examined 3, 6, and 9 weeks postgrafting. At each time point, the TcR Vβ heterogeneity of the surviving emigrants was virtually identical to that of the overall T cell pool (Fig. 5B).
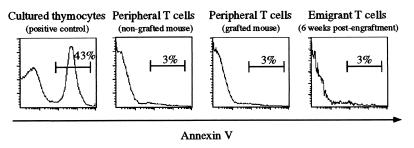
Absence of Overt Apoptosis Among T Cells of Thymic-Grafted Mice.
Annexin V binding (22) was used to determine whether the increased number of T cells in grafted mice resulted in increased levels of apoptosis. In the spleen, lymph nodes, and blood of mice grafted for 6 weeks [when pool size has increased and rate of emigrant cell loss appears to be greatest (11)], the level of T cell apoptosis measured by annexin V binding was similar for grafted and nongrafted mice (Fig. 6). Furthermore, although overnight culture of thymocytes (positive control) produced extensive apoptosis (annexin V binding, ≈43%), only low levels (<3%) were detected among emigrant T cells and the total T cell population, irrespective of their location and phenotype or derivation from thymic-grafted or normal mice.
Figure 6.
T cells from thymic-grafted mice are not enriched for apoptotic cells. Lymphocytes from mice grafted with nine thymic lobes for 6 weeks were isolated from spleen, lymph nodes, and blood and stained with anti-Ly5.2, anti-CD4, and anti-CD8 mAbs and biotinylated annexin V as an indicator of pending apoptosis. Thymocytes cultured overnight were used as a positive control for annexin V binding. Data are representative of profiles from four grafted and four nongrafted mice.
DISCUSSION
Although there is broad agreement that the numbers of T lymphocytes in the peripheral pool are regulated, current theories of homeostasis fail to account for changes in the T cell subsets of thymic-grafted (11, 21), thymectomized [both adult (25, 26) and neonatal (27)], and aged mice (4). These changes suggest an important role for thymic emigrants in T cell homeostasis, because the T cell pool of healthy, euthymic mice is otherwise remarkably resistant to change. In this regard, we have reported recently that thymic emigrants may be incorporated preferentially into the T cell pool for up to 3 weeks postexport (11), which suggests a functional as well as phenotypic (19, 28, 29) distinction between resident T cell subsets and RTE. The present study examines the role of thymic emigrants in determining the number and CD4/CD8 ratio of peripheral T cells and provides an explanation for many instances in which peripheral T cell homeostasis appears to have failed.
We previously reported a large increase in peripheral T cell numbers after engraftment of nine thymic lobes (11). Although it was possible that the sheer number of T cells exported from these additional thymuses simply exceeded the capacity of an otherwise effective homeostatic mechanism, the present study clearly shows a significant increase in T cell numbers occurring with two grafted thymic lobes. In this case, the daily increase in T cell export represents less than 1% of the total T cell pool and therefore is most unlikely to overwhelm homeostasis, confirming that thymic export can influence T cell numbers.
Despite the influence of the thymus, it is important to stress that the size of the T cell pool in adult thymectomized and thymic-grafted mice is not grossly abnormal. This supports the existence of peripheral homeostasis (2, 3, 12, 30–32), because neither the rate nor the composition of thymic-exported cells varies in response to changes in the peripheral T cell pool (11, 20). Theoretically, T cell numbers could be restricted by the finite availability of signals similar to those required for naïve CD4 (33–35), CD8 (18, 36), and memory (see refs. 37 and 38 for reviews) T cell survival. The increased T cell levels in thymic-grafted mice, however, demonstrate that niche availability cannot be the only factor determining pool size.
One interpretation consistent with the data in this study is that T cell numbers are regulated, not at a fixed level, but at a level influenced by thymic export. We propose that the “niche” theory of homeostasis applies to the vast majority of peripheral T cells, but that newly exported T cells are not immediately subject to the same homeostatic restrictions. This would allow variations in thymic export to cause changes in overall cell numbers, but, more importantly, to influence the composition of the T cell pool. In normal circumstances, variations in export would be minor, causing almost undetectable changes to T cell numbers, but the effect on pool size can be demonstrated by using thymic grafts to increase the number of RTE.
A clear indication of a functional distinction between “young” thymic emigrants and older resident T cells is that mice grafted with six thymic lobes for 16 weeks had T cell levels identical to those of mice grafted for only 8 weeks. The cell numbers were significantly (≈50%) higher than in nongrafted mice, but the increase did not equate to a simple accumulation of additional exported cells. Based on previously established rates of thymic emigration, each pair of grafted thymic lobes exports ≈1 × 106 cells to the periphery daily (11). Therefore, mice grafted with six lobes receive ≈168 × 106 additional cells (3 × 106 per day) after 8 weeks and 336 × 106 cells after 16 weeks. Yet at both 8 and 16 weeks, the actual increase in T cell numbers was only ≈71 × 106, equivalent to 3 weeks of exported cells. When grafted thymuses were removed after 8 weeks, the number of peripheral T cells returned to that of nongrafted controls. This indicated that the number of T cells responsible for the increase remained constant in mice grafted for 8 and 16 weeks, but were lost after graft removal.
Furthermore, assuming that the incorporation of thymic emigrants for a minimum of 3 weeks (11) is responsible for the increase in overall T cell numbers, the pool size of grafted mice should increase only by the number of T cells exported from the grafted thymus(es) in 3 weeks, irrespective of how long the graft is in place. Consistent with this, the mean peripheral T cell pool size of normal mice was 142 × 106 cells, whereas mice with two grafted lobes had a mean pool size of 163 × 106 cells, equal to the predicted increase of 21 × 106 cells (1 × 106 cells exported daily × 21 days). Similarly, the T cell pools of mice grafted with six or nine thymic lobes increased by an average of 71 × 106 and 111 × 106 cells, respectively, correlating closely with the predicted increases of 63 × 106 and 95 × 106 cells (Fig. 1B). In each case, the observed increase in T cell numbers was equivalent to the number of emigrants exported in the previous 3 weeks, irrespective of the age of the graft or the total number of cells exported.
The exemption of emigrant cells from niche-based homeostasis, however, is not incongruous with the stable peripheral T cell numbers of normal, euthymic mice. These mice are unlikely to experience the dramatic changes in thymic export induced by thymic grafting, whereas T cell loss associated with age-related thymic atrophy (5, 32, 39) is limited by the well established capacity of memory T cells to proliferate in lymphopenic environments (3, 14, 15, 30). Therefore, the major benefit of incorporation of thymic emigrants to the host is not the maintenance of overall cellularity, but in ensuring the ongoing replenishment of the T cell repertoire through replacement of older clones likely to be redundant or autoreactive (1).
In addition to T cell numbers, the CD4/CD8 ratio also has been described as independently regulated from cell input (8, 15, 40), although age-associated falls in the ratio and a failure to reestablish the normal ratio after selective depletion of either subset have cast doubt on this conclusion (8, 9). Our data indicate that the ratio is likely to reflect previously unidentified changes occurring within the emigrant population. Long-term tracking revealed that the CD4/CD8 ratio of emigrant T cells fell from greater than 3:1 at the time of export to less than 1:1, 16 weeks later. This shift appears to be due to a preferential loss of CD4 cells rather than to the relative expansion of CD8 cells. Consistent with previous reports (11, 18, 19, 30, 41), most emigrants remained CD44lo (indicative of a naive phenotype) and the small proportion of CD44hi cells was distributed equally between the CD4 and CD8 subsets. The broad T cell antigen receptor diversity of RTE also was maintained among surviving CD4 and CD8 emigrants. Despite this, no evidence of increased apoptosis was observed among CD4 T cells or within thymic-grafted mice in general. We cannot discount the possibility of migration to nonlymphoid tissue, but preliminary examinations of peripheral tissue (e.g., liver) showed no evidence of CD4 T cell accumulation (data not shown). Nevertheless, our observations provide an explanation for age-associated shifts in the CD4/CD8 ratio of normal mice. In aged mice, the reduction in T cell export caused by age-associated thymic atrophy reduces the displacement of resident T cells by emigrants, thereby increasing the average life span of peripheral T cells. Based on our evidence from emigrant T cells, this would result in the observed fall in the CD4/CD8 ratio that we and others have observed (4, 39, 42, 43).
Collectively, our results demonstrate that although emigrant T cells may not be required to maintain a T cell pool of relatively normal size, the thymus does exert a considerable, ongoing influence over the composition of the repertoire. With the increasing clinical significance of lymphocyte depletion caused by disease (e.g., AIDS) or disease intervention (e.g., chemotherapy), it is important to understand precisely how such a process may influence T cell pool reconstitution. This study suggests, for instance, that increasing thymic mass during the reconstitution of a lymphocyte-depleted T cell pool may preserve repertoire diversity and reduce the potentially redundant expansion of residual T cell clones to fill “space.” Similarly, the retention or regeneration of thymic function may reduce the homeostatic requirement to maintain cell numbers in the elderly by T cell expansion that is often blamed for the emergence of autoimmunity (1) and other forms of immune dysfunction (5, 32). Although thymic grafting is unlikely to be adopted as a therapy, many studies (5, 32, 44, 45) highlight the potential for reversing age-related thymic atrophy by the inhibition of sex steroids. The effects of this treatment during T cell pool reconstitution presently are under investigation in our laboratory.
Acknowledgments
This work was supported by grants from the Australian National Health and Medical Research Council.
ABBREVIATION
- RTE
recent thymic emigrant(s)
References
- 1.Tanchot C, Rocha B. J Exp Med. 1997;186:1099–1106. doi: 10.1084/jem.186.7.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parijs L, Abbas A. Science. 1998;280:243–248. doi: 10.1126/science.280.5361.243. [DOI] [PubMed] [Google Scholar]
- 3.Butcher E, Picker L. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 4.Barrat F, Lesourd B, Louise A, Boulouis H-J, Vincent-Naulleau S, Thibault D, Sanaa M, Neway T, Pilet C. Clin Exp Immunol. 1997;107:593–600. doi: 10.1046/j.1365-2249.1997.3021199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirokawa K. In: Principles and Practice of Geriatric Medicine. Pathy M S J, editor. New York: Wiley; 1998. pp. 35–47. [Google Scholar]
- 6.Doherty P, Hamilton-Easton A, Topham D, Riberdy J, Brooks J, Cardin R. Semin Immunol. 1997;9:365–373. doi: 10.1006/smim.1997.0094. [DOI] [PubMed] [Google Scholar]
- 7.Adleman L, Wofsy D. J Acquired Immune Defic Syndr. 1993;6:144–152. [PubMed] [Google Scholar]
- 8.Amadori A, Zamarchi R, Chieco-Bianchi L. Immunol Today. 1996;17:414–417. doi: 10.1016/0167-5699(96)10049-9. [DOI] [PubMed] [Google Scholar]
- 9.Adleman L, Wofsy D. J Acquired Immune Defic Syndr Hum Retrovirol. 1996;11:334–340. doi: 10.1097/00042560-199604010-00003. [DOI] [PubMed] [Google Scholar]
- 10.Bell E, McDonagh M. Immunology. 1995;84:514–520. [PMC free article] [PubMed] [Google Scholar]
- 11.Berzins S, Boyd R, Miller J. J Exp Med. 1998;187:1839–1848. doi: 10.1084/jem.187.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanchot C, Rosado M, Agenes F, Freitas A, Rocha B. Semin Immunol. 1997;9:331–337. doi: 10.1006/smim.1997.0090. [DOI] [PubMed] [Google Scholar]
- 13.Tanchot C, Rocha B. Eur J Immunol. 1995;25:2127–2136. doi: 10.1002/eji.1830250802. [DOI] [PubMed] [Google Scholar]
- 14.Mackall C, Hakim F, Gress R. Semin Immunol. 1997;9:339–346. doi: 10.1006/smim.1997.0091. [DOI] [PubMed] [Google Scholar]
- 15.Rocha B, Dautigny N, Pereira P. Eur J Immunol. 1989;19:905–911. doi: 10.1002/eji.1830190518. [DOI] [PubMed] [Google Scholar]
- 16.George A, Ritter M. Immunol Today. 1996;17:267–272. doi: 10.1016/0167-5699(96)80543-3. [DOI] [PubMed] [Google Scholar]
- 17.Tough D, Sprent J. J Exp Med. 1994;179:1127–1135. doi: 10.1084/jem.179.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanchot C, Lemonnier F, Perarnau B, Freitas A, Rocha B. Science. 1997;276:2057–2062. doi: 10.1126/science.276.5321.2057. [DOI] [PubMed] [Google Scholar]
- 19.Sprent J, Tough D. Science. 1994;265:1395–1400. doi: 10.1126/science.8073282. [DOI] [PubMed] [Google Scholar]
- 20.Gabor M, Scollay R, Godfrey D. Eur J Immunol. 1997;27:2986–2993. doi: 10.1002/eji.1830271135. [DOI] [PubMed] [Google Scholar]
- 21.Wallis V, Leuchars E, Davies A. Immunology. 1978;35:1037–1043. [PMC free article] [PubMed] [Google Scholar]
- 22.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 23.Scollay R, Butcher E, Weissman I. Eur J Immunol. 1980;10:210–218. doi: 10.1002/eji.1830100310. [DOI] [PubMed] [Google Scholar]
- 24.Budd R, Cerottini J-C, MacDonald H. J Immunol. 1987;138:1009–1018. [PubMed] [Google Scholar]
- 25.Miller J, Mitchell G. Transplant Rev. 1969;1:3–42. doi: 10.1111/j.1600-065x.1969.tb00135.x. [DOI] [PubMed] [Google Scholar]
- 26.Swain S, Bradley L, Croft M, Tonkonogy S, Atkins G, Weinberg A, Duncan D, Hedrich S, Dutton R, Huston G. Immunol Rev. 1991;123:115–144. doi: 10.1111/j.1600-065x.1991.tb00608.x. [DOI] [PubMed] [Google Scholar]
- 27.Miller J. Proc R Soc London Ser B. 1962;156:415–428. [Google Scholar]
- 28.Berzins S, Davey G, Randle-Barret E, Malin M, Classon B, Fraser S, Boyd R. J Immunol. 1999;162:5119–5126. [PubMed] [Google Scholar]
- 29.Imanishi K, Seo K, Kato H, Miyoshi-Akiyama T, Zhang R, Takanashi Y, Imai Y, Uchiyama T. J Immunol. 1998;160:112–119. [PubMed] [Google Scholar]
- 30.Bell E, Sharpshott S. Semin Immunol. 1997;9:347–353. doi: 10.1006/smim.1997.0092. [DOI] [PubMed] [Google Scholar]
- 31.Freitas A, Agenes F, Coutinho G. Eur J Immunol. 1996;26:2640–2649. doi: 10.1002/eji.1830261115. [DOI] [PubMed] [Google Scholar]
- 32.Mackall C, Gress R. Immunol Rev. 1997;160:91–102. doi: 10.1111/j.1600-065x.1997.tb01030.x. [DOI] [PubMed] [Google Scholar]
- 33.Kirberg J, Berns A, von Boehmer H. J Exp Med. 1997;186:1269–1275. doi: 10.1084/jem.186.8.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takeda S, Rodewald H-R, Arakawa H, Bluethmann H, Shimuzu T. Immunity. 1996;5:217–228. doi: 10.1016/s1074-7613(00)80317-9. [DOI] [PubMed] [Google Scholar]
- 35.Brocker T. J Exp Med. 1997;186:1223–1232. doi: 10.1084/jem.186.8.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nesic V, Vukmanovic S. J Immunol. 1998;160:3705–3712. [PubMed] [Google Scholar]
- 37.Sprent J. Curr Opin Immunol. 1997;9:371–379. doi: 10.1016/s0952-7915(97)80084-2. [DOI] [PubMed] [Google Scholar]
- 38.Ahmed R. Science. 1996;272:1904. doi: 10.1126/science.272.5270.1904. [DOI] [PubMed] [Google Scholar]
- 39.Rice J, Bucy R. J Immunol. 1995;154:6644–6654. [PubMed] [Google Scholar]
- 40.Kraal G, Weissman I, Butcher E. Immunogenetics. 1983;18:585–592. doi: 10.1007/BF00345966. [DOI] [PubMed] [Google Scholar]
- 41.Gabor M, Godfrey D, Scollay R. Eur J Immunol. 1997;27:2986–2993. doi: 10.1002/eji.1830271135. [DOI] [PubMed] [Google Scholar]
- 42.Boersma W, Steinmeier F, Haaijman J. Cell Immunol. 1985;93:417–430. doi: 10.1016/0008-8749(85)90146-7. [DOI] [PubMed] [Google Scholar]
- 43.Callahan J, Kappler J, Marrack P. J Immunol. 1993;151:6657–6669. [PubMed] [Google Scholar]
- 44.Mackall C, Punt J, Morgan P, Farr A, Gress R. Eur J Immunol. 1998;28:1886–1893. doi: 10.1002/(SICI)1521-4141(199806)28:06<1886::AID-IMMU1886>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 45.Utsuyama M, Hirokawa K, Mancini C, Brunelli R, Leter G, Doria G. Mech Aging Dev. 1995;81:107–117. doi: 10.1016/0047-6374(95)01589-r. [DOI] [PubMed] [Google Scholar]