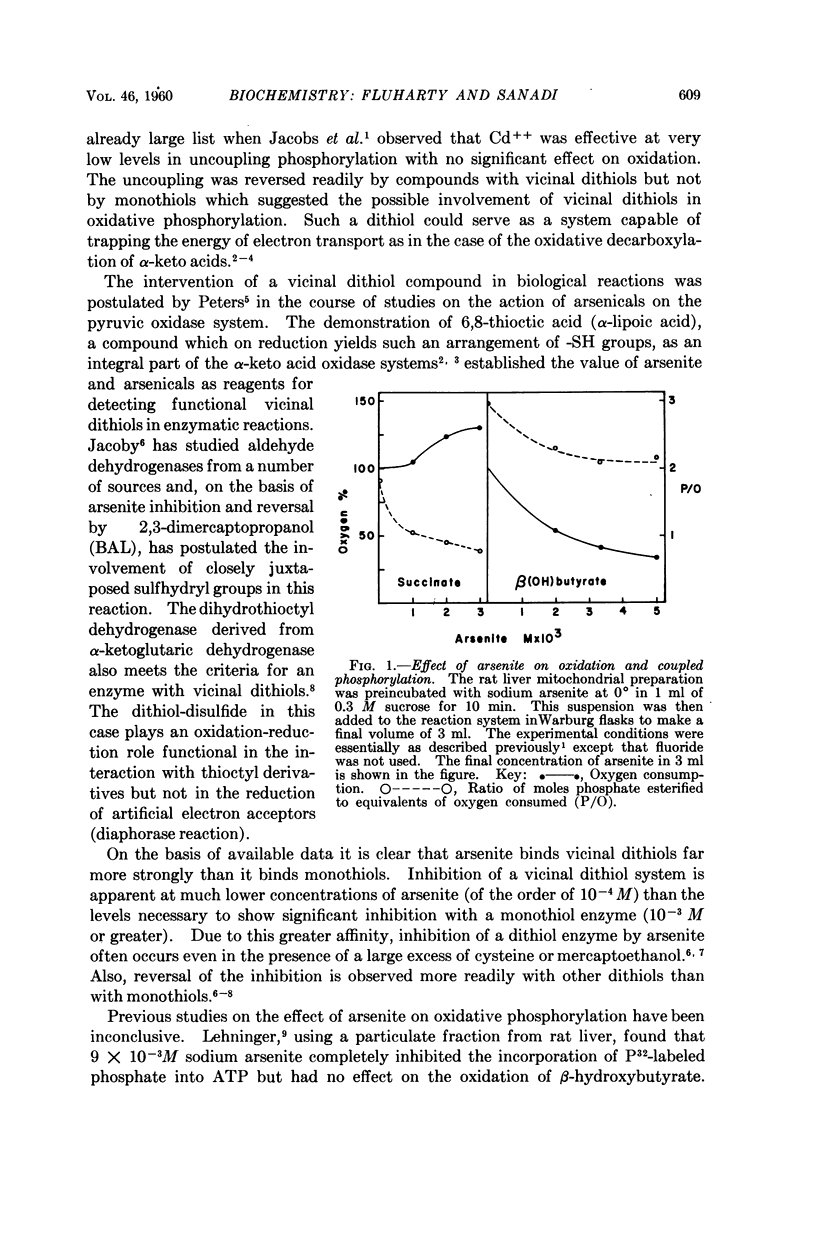
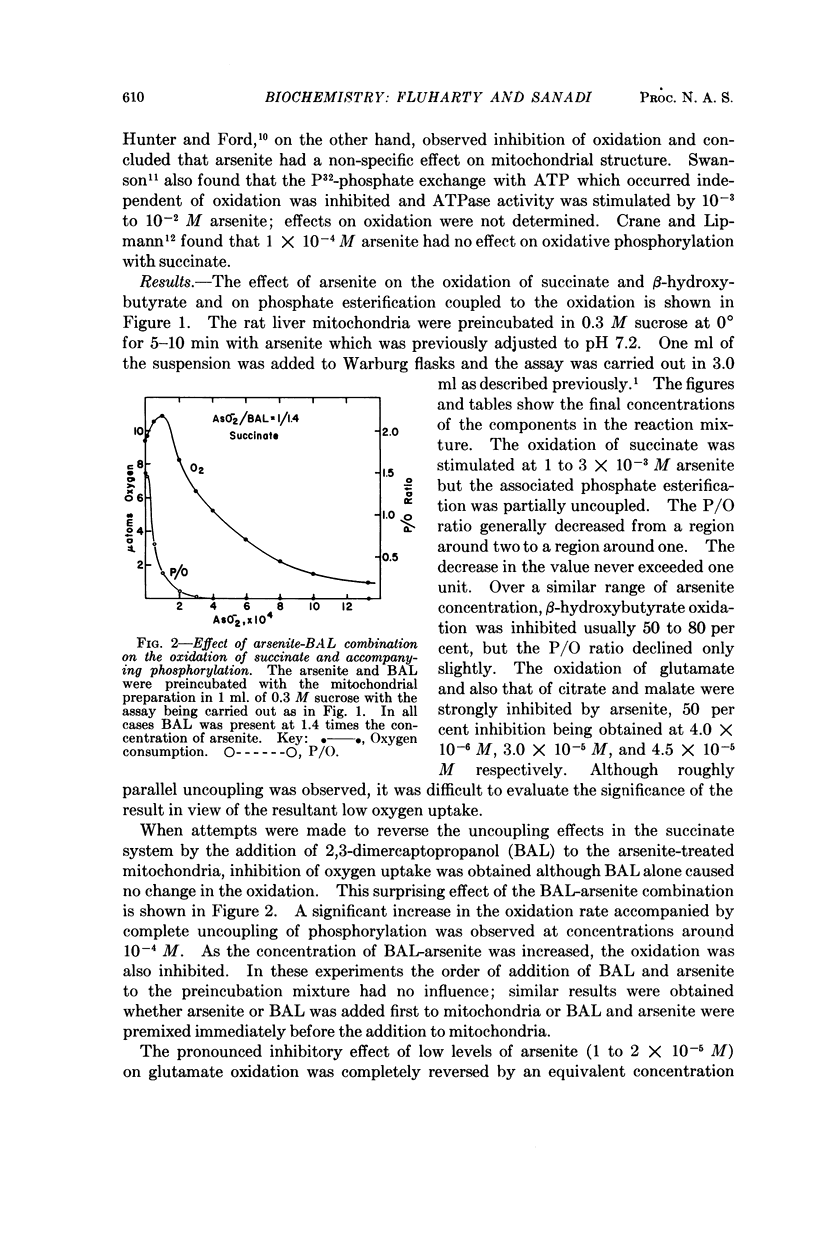
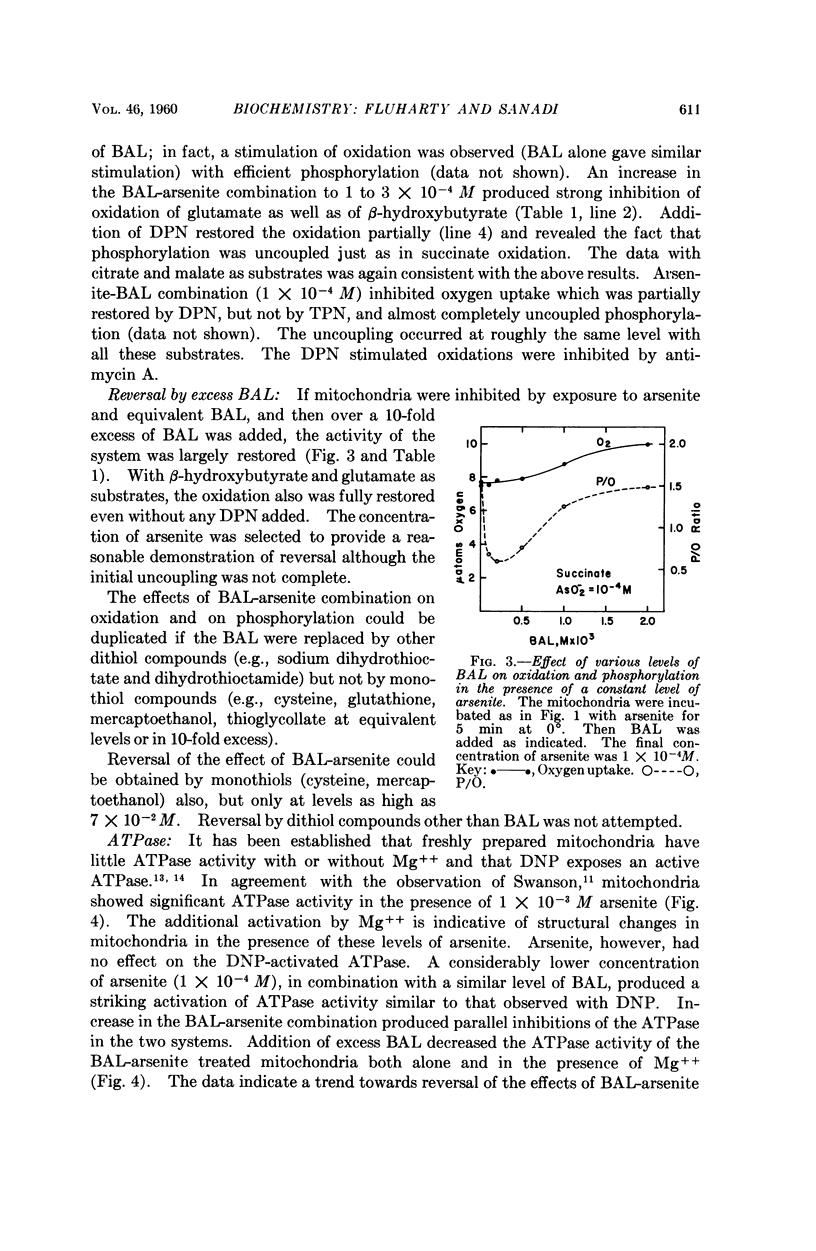
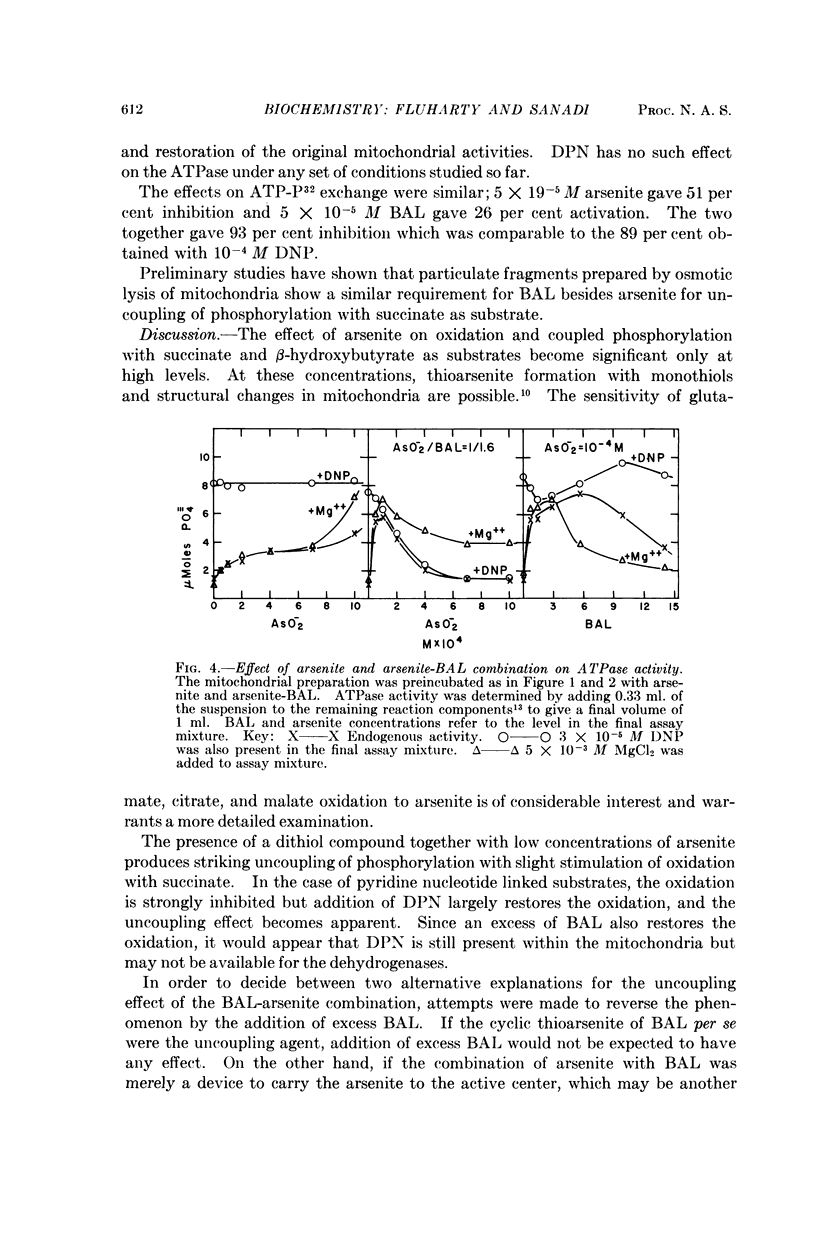
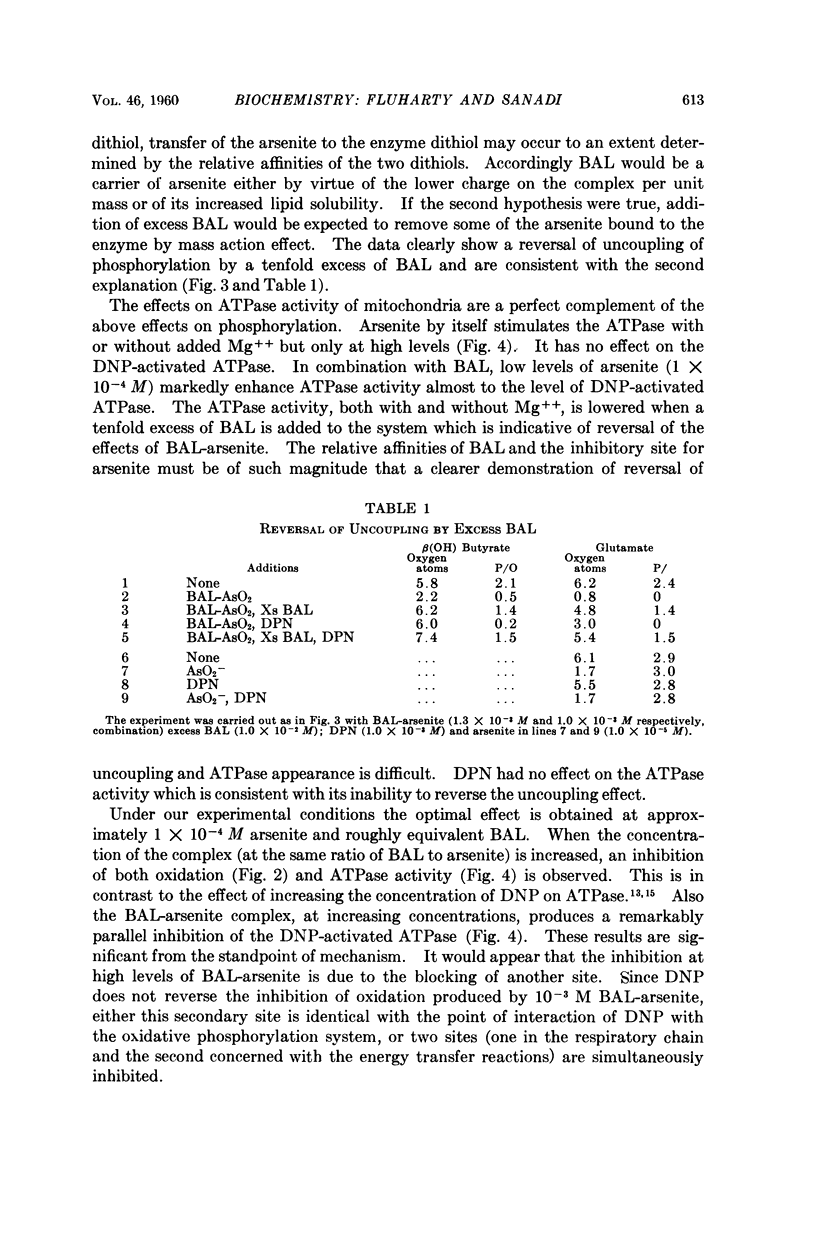
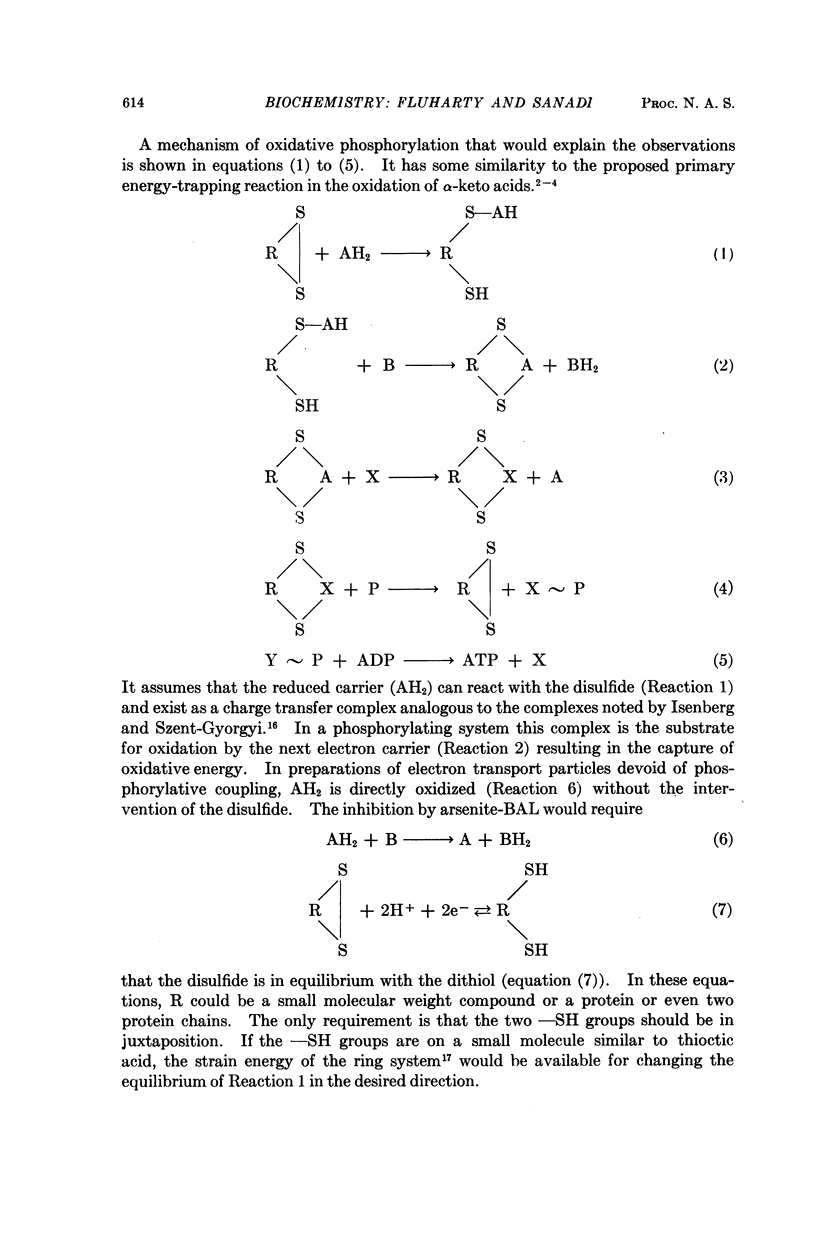
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRADLEY L. B., JACOB M., JACOBS E. E., SANADI D. R. Uncoupling of oxidative phosphorylation by cadmium ion. J Biol Chem. 1956 Nov;223(1):147–156. [PubMed] [Google Scholar]
- CALVIN M., MASSINI P. The path of carbon in photosynthesis. XX. The steady state. Experientia. 1952 Dec 15;8(12):445–457. doi: 10.1007/BF02139287. [DOI] [PubMed] [Google Scholar]
- CHIGA M., PLAUT G. W. An enzyme system from mitochondria catalyzing adenosine diphosphate-adenosine triphosphate and orthophosphate-adenosine triphosphate exchange reactions. J Biol Chem. 1959 Nov;234:3059–3066. [PubMed] [Google Scholar]
- CRANE R. K., LIPMANN F. The effect of arsenate on aerobic phosphorylation. J Biol Chem. 1953 Mar;201(1):235–243. [PubMed] [Google Scholar]
- HUNTER F. E., Jr, FORD L. Inactivation of oxidative and phosphorylative systems in mitochondria by preincubation with phosphate and other ions. J Biol Chem. 1955 Sep;216(1):357–369. [PubMed] [Google Scholar]
- Isenberg I., Szent-Györgyi A. FREE RADICAL FORMATION IN RIBOFLAVIN COMPLEXES. Proc Natl Acad Sci U S A. 1958 Sep 15;44(9):857–862. doi: 10.1073/pnas.44.9.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg I., Szent-Györgyi A. ON CHARGE TRANSFER COMPLEXES BETWEEN SUBSTANCES OF BIOCHEMICAL INTEREST. Proc Natl Acad Sci U S A. 1959 Aug;45(8):1229–1231. doi: 10.1073/pnas.45.8.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARDY H. A., WELLMAN H. Oxidative phosphorylations; rôle of inorganic phosphate and acceptor systems in control of metabolic rates. J Biol Chem. 1952 Mar;195(1):215–224. [PubMed] [Google Scholar]
- LARDY H. A., WELLMAN H. The catalytic effect of 2,4-dinitrophenol on adenosinetriphosphate hydrolysis by cell particles and soluble enzymes. J Biol Chem. 1953 Mar;201(1):357–370. [PubMed] [Google Scholar]
- MACKLER B., MAHLER H. R., GREEN D. E. Studies on metalloflavoproteins. I. Xanthine oxidase, a molybdoflavoprotein. J Biol Chem. 1954 Sep;210(1):149–164. [PubMed] [Google Scholar]
- MAHLER H. R., MACKLER B., GREEN D. E. Studies on metalloflavoproteins. III. Aldehyde oxidase: a molybdoflavoprotein. J Biol Chem. 1954 Sep;210(1):465–480. [PubMed] [Google Scholar]
- PLAUT G. W. A soluble enzyme from mitochondria catalyzing an exchange between inorganic phosphate and adenosine triphosphate. Arch Biochem Biophys. 1957 Jul;69:320–333. doi: 10.1016/0003-9861(57)90498-8. [DOI] [PubMed] [Google Scholar]
- REED L. J. Metabolic functions of thiamine and lipoic acid. Physiol Rev. 1953 Oct;33(4):544–559. doi: 10.1152/physrev.1953.33.4.544. [DOI] [PubMed] [Google Scholar]
- SANADI D. R., LANGLEY M., WHITE F. alpha-Ketoglutaric dehydrogenase. VII. The role of thioctic acid. J Biol Chem. 1959 Jan;234(1):183–187. [PubMed] [Google Scholar]
- SWANSON M. A. Studies on the non-oxidative exchange between inorganic phosphate and ATP, as catalyzed by intact mitochondria. Biochim Biophys Acta. 1956 Apr;20(1):85–91. doi: 10.1016/0006-3002(56)90266-9. [DOI] [PubMed] [Google Scholar]
- WADKINS C. L., LEHNINGER A. L. The adenosine triphosphate-adenosine diphosphate exchange reaction of oxidative phosphorylation. J Biol Chem. 1958 Dec;233(6):1589–1597. [PubMed] [Google Scholar]



