Abstract
To study molecular events involved in B lymphocyte development and V(D)J rearrangement, we have established an efficient system for the differentiation of embryonic stem (ES) cells into mature Ig-secreting B lymphocytes. Here, we show that B lineage cells generated in vitro from ES cells are functionally analogous to normal fetal liver-derived or bone marrow-derived B lineage cells at three important developmental stages: first, they respond to Flt-3 ligand during an early lymphopoietic progenitor stage; second, they become targets for Abelson murine leukemia virus (A-MuLV) infection at a pre-B cell stage; third, they secrete Ig upon stimulation with lipopolysaccharide at a mature mitogen-responsive stage. Moreover, the ES cell-derived A-MuLV-transformed pre-B (EAB) cells are phenotypically and functionally indistinguishable from standard A-MuLV-transformed pre-B cells derived from infection of mouse fetal liver or bone marrow. Notably, EAB cells possess functional V(D)J recombinase activity. In particular, the generation of A-MuLV transformants from ES cells will provide an advantageous system to investigate genetic modifications that will help to elucidate molecular mechanisms in V(D)J recombination and in A-MuLV-mediated transformation.
During development, B lymphocytes undergo a process of stage-specific differentiation (1, 2). CD117+ (c-kit) hematopoietic progenitors (3) with a restricted B cell lineage potential can be identified by surface expression of CD45R (B220), CD43, and AA4.1 molecules (1, 2). Recently, it has been shown that the cytokine Flt-3 ligand (Flt-3L) enhances B cell lineage commitment and differentiation from uncommitted bone marrow (BM) progenitors (4, 5). Flt-3L also synergizes with IL-7 to induce the proliferation of primitive CD43+ CD45Rlo CD24− (heat-stable antigen) B cell progenitors (6).
Following commitment to the B cell lineage, up-regulation of CD19 and CD24 surface expression characterizes differentiation to the pro-B cell stage. B cell progenitors begin to undergo DNA rearrangement of their V, D, and J loci to generate a diverse repertoire of antigen-specific surface Ig (1, 2, 7). Experiments with Abelson murine leukemia virus (A-MuLV)-transformed, pre-B cell lines have provided key insights into the regulation and mechanism of V(D)J rearrangement, and have been instrumental in establishing the phenotype of B cell precursors (8–10). Rearrangement at the Ig-heavy chain locus begins in pro-B cells, and productive rearrangements result in the expression of μ heavy chain during the pre-B cell stage (1, 2). The μ heavy chain pairs with a surrogate light chain to form a pre-B cell receptor complex, which signals these cells to proliferate and promotes their differentiation to the CD43− CD22+ late pre-B cell stage (CD45R+CD19+CD24+) (11). During this stage, productive rearrangement at the light chain loci (κ or λ) results in the generation of immature surface IgM+ B cells that undergo selection and give rise to mature IgM+ IgD+ B cells (11). Functionally, B cells that have differentiated to a mature stage are denoted by their ability to secrete Ig upon mitogen activation (12).
Several methods have been described for the generation of lymphohematopoietic progenitors from embryonic stem (ES) cells in vitro (13–15). In a method described by Nakano et al., undifferentiated ES cells were cocultured on the macrophage colony-stimulating factor-deficient BM stromal cell line OP9, resulting in the appearance of hematopoietic cell clusters 8 days later (15). The advantages of this system for the generation of ES-derived lymphocytes in vitro have been extensively reviewed by Nakano (16). In particular, a small fraction of ES cells eventually gave rise to IgM+ B cells after long-term culture (40 days) on the OP9 stromal cell line (15). In light of these findings, we sought to determine whether the differentiation of ES cells in vitro resulted in progenitor and mature B lymphocytes that are functionally equivalent to those generated in vivo. To this end, we chose to investigate representative events from early, middle, and late stages of B cell development; i.e., response of early progenitors to Flt-3L, susceptibility of pre-B cells to A-MuLV infection, and mitogenic activation of mature B cells by lipopolysaccharide (LPS).
Our findings show that the addition of Flt-3L dramatically enhanced B lymphopoiesis in the ES/OP9 cocultures and promoted the proliferation of a progenitor B cell subset. Furthermore, we were able to generate permanent ES cell-derived A-MuLV-transformed pre-B (EAB) cell lines. These EAB cell lines were phenotypically and functionally equivalent to standard mouse fetal liver-derived A-MuLV-transformed pre-B cell lines. Finally, our results show that ES/OP9 coculture-derived progenitor B cells develop into mature mitogen-responsive B cells, secreting Ig upon stimulation with LPS. These findings therefore demonstrate that the OP9 stromal cell line, with the addition of Flt-3L, is sufficient for the differentiation of ES cells into lymphohematopoietic progenitors and is efficient for the development of these progenitors into mature functional B cells. Collectively, our findings suggest that the developmental program of B cells derived in vitro from ES cells closely parallels the intrinsic development of B cells in vivo.
Therefore, the coculture system alone or in combination with A-MuLV infection and the use of differentiated genetically manipulated ES cells in vitro should prove valuable in the elucidation of molecular mechanisms controlling differentiation and Ig gene rearrangement during B cell development.
MATERIALS AND METHODS
Cell Lines.
The BM stromal cell line, OP9 (15), was cultured as a monolayer in αMEM supplemented with 2.2 g/liter sodium bicarbonate and 20% FCS (ES grade and lot tested; HyClone, Logan, UT). OP9 media were also used for ES/OP9 cocultures. The ES cell line R1, obtained from G. Caruana (Mt. Sinai Hospital, Toronto), was cultured on a confluent monolayer of mitomycin C-treated embryonic fibroblasts with 1 ng/ml leukemia inhibitory factor (R & D Systems, Minneapolis, MN). ES and embryonic fibroblast cells were maintained in DMEM, supplemented with 15% FCS, 2 mM glutamine, 110 μg/ml sodium pyruvate, 50 μM 2-mercaptoethanol, and 10 mM Hepes (pH 7.4). The A-MuLV producer cell line, 54 clone-2, was obtained from G. Wu (Ontario Cancer Institute, Toronto). 54 clone-2 is an A-MuLV-P160-transformed NIH 3T3 nonproducer cell line superinfected with Moloney–MuLV–Clone-2, derived by Rosenberg and Witte (17). The 54 clone-2 cell line and all EAB cell lines were maintained in RPMI medium 1640, 10% FCS, and 50 μM 2-mercaptoethanol. All cocultures were incubated at 37°C in a humidified incubator containing 5% CO2 in air. Periodic testing indicated that all cell lines were maintained as mycoplasma-free cultures.
ES/OP9 Coculture, in Vitro Generation of B Cells, and A-MuLV Infection.
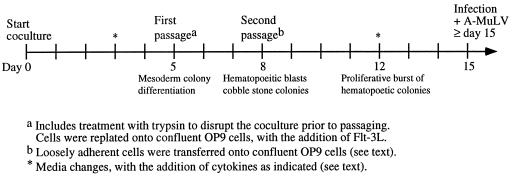
For hematopoietic induction, a single-cell suspension of 104 R1 ES cells was seeded onto a confluent OP9 monolayer in 6-well plates. The media were changed at day 3; by day 5, nearly 100% of the ES colonies differentiated into mesoderm-like colonies. The cocultures were trypsinized (0.25%; GIBCO/BRL) at day 5; the single-cell suspension was preplated for 30 min; and nonadherent cells (1 to 2 × 106) were reseeded onto new confluent OP9 layers in 10-cm dishes. At day 6 or day 7, small clusters of hematopoietic-like, smooth round cells began to appear. At day 8, loosely adherent cells were gently washed off and placed onto new OP9 layers (without trypsin). This treatment enriched cells with hematopoietic potential and left behind differentiated mesoderm and undifferentiated ES colonies. After this passage, hematopoietic colonies expanded with noticeable proliferation between days 10 and 12 and thereafter. By day 19, the total number of CD45+ cells that were recovered from the ES/OP9 cocultures was approximately 105 cells. Fig. 1 provides a schematic outline of the ES/OP9 coculture protocol for the generation of B lineage cells, including the addition of cytokines as indicated. Human Flt-3L was used at a final concentration of 5 ng/ml, or mouse Flt-3L was used at a final concentration of 20 ng/ml (R & D Systems). Cells were cultured in the presence of exogenous Flt-3L from day 5. The addition of Flt-3L at day 5 appeared to represent a critical temporal window for the enhancement of B lymphopoiesis, because the enhancement was not observed when Flt-3L was added at later times (on or after day 8). The media were changed and/or the cells were passaged without trypsin [i.e., they were made into single-cell suspension and filtered (70 μm)] between days 8 and 15.
Figure 1.
Outline of the standard protocol used for the differentiation of ES cells into B-lineage lymphocytes. ES cells were induced to differentiate into hematopoietic cells on the BM-derived stromal cell line OP9. To generate mature, functional B cells, hematopoietic cells were maintained on OP9 cells. To generate EAB cells, cocultures were infected with A-MuLV on or after day 15. Flt-3L was added for all media changes after day 5.
To generate sIgM+ B cells, the lymphohematopoietic cells were harvested at day 15, and replated onto a fresh OP9 monolayer. At day 28, cells were stimulated with LPS (10 μg/ml) for 4 days. The cells and culture supernatant were then harvested for flow cytometry and ELISA analysis, respectively. In a separate experiment, cells were stimulated with LPS (100 μg/ml) for 48 hours, and analyzed for the up-regulation of CD80 (B7–1).
To generate EAB cell lines, IL-7 (5 ng/ml) (R & D Systems) was added at day 8 to Flt-3L-containing ES/OP9 cocultures to maintain immature pre-B cells. Cocultures were infected by adding an undiluted virus stock harvested from a 4-day confluent plate of the A-MuLV producer cell line 54 clone-2. Cocultures from a 10-cm dish were infected by replacing the medium with 3 ml of virus stock containing 4 μg/ml of polybrene (Sigma) and IL-7. The plate was rocked periodically at 37°C for 2 to 4 hours. After this period, 5 ml of fresh OP9 medium containing IL-7 was added to the plate. The medium was changed 5 days later to EAB medium with IL-7, but without Flt-3L. Subsequent media changes lacked IL-7. Three separate experiments gave rise to EAB cell lines. Infections were performed on day 15, 28, or 49. Flow cytometry analyses showed that all EAB lines displayed the same phenotype. Although the day of infection varied, in each experiment a significant population of CD45R+ CD24+ IgM− immature pre-B cells containing known A-MuLV targets were present (8). However, we noted that transformants arose 33 days after the day-15 infection and only 13 days after the day-49 infection. Infected cells were grown in bulk, and then cloned by limiting dilution. Abelson-transformed clones showed a doubling time of about 18 hours, and the presence of integrated copies of the viral genome was confirmed by Southern blot analysis.
Flow Cytometry.
Antibodies for flow cytometry were purchased from PharMingen, except for biotinylated anti-AA4.1 mAb, which was obtained from C. Paige (Ontario Cancer Institute). Staining of cells was performed as described (18).
Reverse Transcription (RT)–PCR.
Single-cell suspensions prepared from OP9 cells and total RNA was isolated with the use of the Trizol RNA isolation protocol (GIBCO/BRL). RNA was resuspended in 25 μl of diethyl pyrocarbonate (DEPC)-treated (0.1%) distilled water. cDNA was prepared from 1 μg of RNA by using random hexamer primers and the cDNA cycle kit (Invitrogen). Subsequent PCR analysis was performed with titrations of cDNA in a 1:5 dilution series in distilled water. Distilled water and RT reactions done in the absence of AMV reverse transcriptase were included as negative controls. All PCRs were performed by using the same cDNA batches as shown for β-actin, and all PCR products corresponded to the expected molecular sizes. The gene-specific primers used for PCR were (5′ → 3′): β-actin-5′, GAT GAC GAT ATC GCT GCG CTG; β-actin-3′, GTA CGA CCA GAG GCA TAC AGG; stem cell factor (SCF)-5′, TCT TCA ACT GCT CCT ATT T; SCF-3′, ACT GCT ACT GCT GTC ATT C; Flt-3L-5′, ACA CCT GAC TGT TAC TTC AGC; Flt-3L-3′, CCT GGG CCG AGG CTC TGG; IL-7–5′, ACT ACA CCC ACC TCC CGC A; IL-7–3′, TCT CAG TAG TCT CTT TAG G. Products were separated by agarose gel electrophoresis on a 1.6% gel, and visualized by ethidium bromide staining; reverse photo images are shown in Fig. 3.
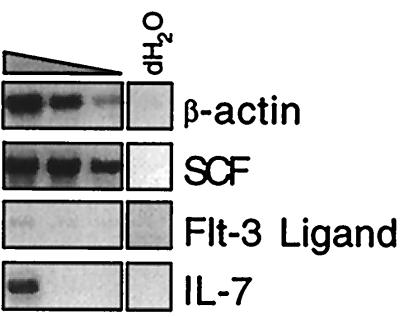
Figure 3.
Cytokine expression in the OP9 cell line. Total RNA from OP9 cells was analyzed for the expression of several cytokines by RT–PCR. cDNAs were prepared from 1 μg of total RNA. OP9 cDNAs were diluted in a 1:5 series and then amplified simultaneously by PCR with gene-specific primer pairs as indicated.
Extrachromosomal DNA Recombination Assay.
EAB cells or the Abelson line 204–1-8 were transfected with the recombination reporter plasmid (pWTSJΔ) as described (19–21).
RESULTS AND DISCUSSION
Induction of Hematopoiesis in ES/OP9 Cocultures.
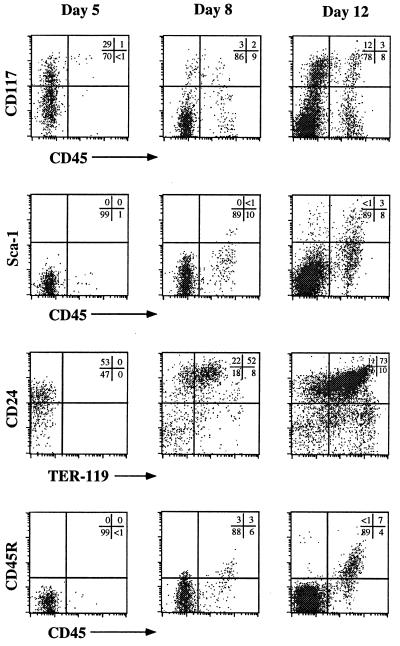
An in vitro system developed by Nakano et al. (15) allows for the differentiation of ES cells into lymphohematopoietic precursors on coculture with the macrophage colony-stimulating factor-deficient BM-derived stromal cell line, OP9. Fig. 1 provides a schematic outline of the ES/OP9 coculture system (see Materials and Methods for details). As shown in Fig. 2, flow cytometric analyses of cells harvested at different times after initiation of the ES/OP9 coculture revealed that CD45+ cells (22–24) were first observed by day 5 of coculture. By day 8, the CD45+ cells also expressed CD117 and Sca-1 on their surface, thus displaying a phenotype analogous to that of early hematopoietic stem cells (Fig. 2) (3, 25). A significant portion of early hematopoiesis occurring in the coculture system typically gave rise to cells of the erythroid lineage (26) as was evident by the large fraction of CD24+ cells staining positive for TER-119 (days 8 and 12; Fig. 2). Although the majority of cocultured day-12 cells belonged to the erythroid lineage (CD24hi CD45− TER-119+), Fig. 2 also shows that the CD45+ cells expressed low to high levels of CD45R. This phenotype indicates that B lineage cells emerged from the coculture between days 8 and 12 (1, 2). Although this B lineage phenotype was clearly apparent by day 12, long-term cultures (>20 days) seldom resulted in the generation of CD19+ IgM+ B cells (data not shown). This result is in keeping with that previously observed by Nakano et al. (15). To address one possible mechanism for the inefficient B lymphopoiesis observed, we characterized the cytokines expressed by OP9 cells.
Figure 2.
Temporal kinetics of hematopoietic induction in ES/OP9 cocultures. Representative flow cytometric analysis of various surface markers are shown for days 5, 8, and 12 of coculture. These results represent cocultures without exogenous addition of Flt-3L.
RT–PCR Analysis of Cytokines Expressed by OP9 Cells.
Several groups have demonstrated a critical role for certain cytokines in early B cell development (5). SCF, Flt-3L, and IL-7 have been shown to promote and support the growth of early B cell progenitors (4–6, 27–29). To characterize the potential roles that these cytokines may play in the induction of ES cell differentiation on coculture with OP9 cells, we performed an RT–PCR analysis for cytokines expressed by OP9 cells. As shown in Fig. 3, OP9 cells expressed high levels of SCF and moderate levels of IL-7, but almost undetectable levels of Flt-3L. The low level of Flt-3L suggests a possible explanation for the inefficient B lymphopoiesis in ES/OP9 cocultures and is in accord with recent findings demonstrating that Flt-3L synergizes with IL-7 to enhance B cell differentiation from uncommitted BM progenitor cells (4). The importance of Flt-3L for the ES/OP9 in vitro system is underscored by the observation that, whereas Flt-3L + IL-7 selectively stimulate the production of pro-B cells, SCF + IL-7 predominantly support the production of mature granulocytes (4). Thus, the emerging ES cell-derived hematopoietic progenitors may be adversely affected by the low level of Flt-3L relative to SCF.
Flt-3L Enhances the Generation of B Lymphocytes from ES/OP9 Cocultures.
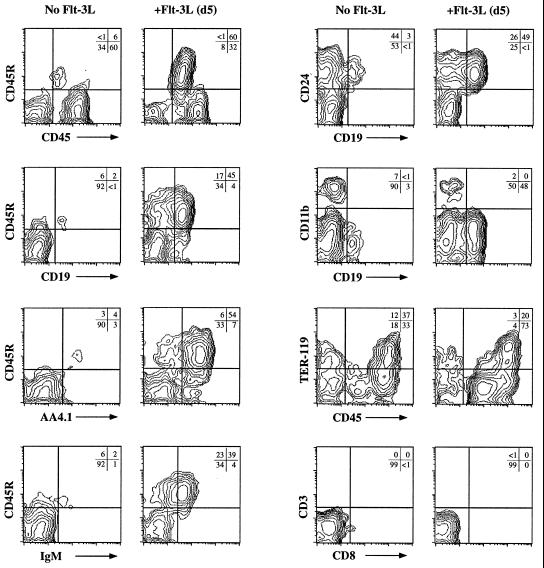
In light of the above findings, we examined whether hematopoietic progenitors generated from ES/OP9 cocultures would respond to the exogenous addition of Flt-3L and result in an enhanced induction of B lineage commitment. To this end, Flt-3L was added at day 5 of the ES/OP9 coculture, when hematopoietic cells were first observed (Fig. 2). Analysis of the day-19 cocultures revealed that the addition of Flt-3L dramatically enhanced the generation of B lymphocytes from the ES/OP9 cocultures (60% vs. 6% CD45R+ cells, with Flt-3L and without Flt-3L, respectively) (Fig. 4). Thus, the addition of Flt-3L to the ES/OP9 coculture at day 5 increased the recovery of B lineage cells at later times by ≥10-fold (Fig. 4). Significantly, the frequency of myeloid, CD11b+ (Mac-1), and erythroid, TER-119+ cells was diminished in the Flt-3L-treated cultures (Fig. 4). Evidence for T lymphocyte differentiation was not observed in these cultures (CD3 vs. CD8; Fig. 4). The phenotype of day-19 ES/OP9 coculture cells clearly shows that the addition of Flt-3L resulted in a specific increase in the generation of CD19+ CD45R+ AA4.1+ CD24+ IgM+ cells (Fig. 4), although we observed only a slight increase in the total number of cells (≈30%). With the addition of Flt-3L at day 5, B lymphopoiesis in the ES/OP9 coculture system occurred with high efficiency in all (now more than 25) of the independent trials.
Figure 4.
Flt-3L enhances the in vitro generation of ES cell-derived B lymphocytes. Flow cytometric analysis for various cell surface markers expressed on ES cell-derived B lineage cells from day-19 ES/OP9 cocultures. The addition of Flt-3L to the cocultures on day 5 resulted in a dramatic increase in the generation of B lymphocytes. Flt-3L was added each time the culture medium was replaced, as indicated in Fig. 1.
To assess directly which particular stage of differentiation was predominantly affected by the addition of exogenous Flt-3L, we analyzed the cell cycle status of different subsets of ES/OP9 coculture cells by flow cytometry. Table 1 shows that the AA4.1+ CD45R+ CD19+ population of early pro-B cells is most dramatically affected by the addition of exogenous Flt-3L, with an almost 2-fold increase in the percentage of cells in the S-G2/M phase of the cell cycle. Our analysis also revealed a similar increase in the percentage of the hematopoietic progenitor cells CD45lo CD34lo that proliferated in the presence of exogenous Flt-3L (Table 1). Taken together, these results are consistent with previous findings showing that Flt-3L enhances B lineage commitment and differentiation from early lymphoid progenitors (4, 5). Thus, the Flt-3L response of early B cell progenitors derived from ES/OP9 cocultures is akin to the response of in vivo B cell progenitors.
Table 1.
Cell cycle analysis in ES/OP9 cocultures
| ES/OP9 coculture | − Flt-3L, % | + Flt-3L, % |
|---|---|---|
| CD19+AA4.1+ | 38 | 65 |
| CD19+AA4.1− | 22 | 22 |
| CD19−AA4.1+ | 3 | 2 |
| CD45loCD34lo | 22 | 36 |
| CD45+CD34− | 9 | 6 |
| CD45−CD34+ | 58 | 66 |
Cells from day-19 ES/OP9 cocultures, with and without exogenous addition of Flt-3L, were analyzed for surface phenotype and DNA content by flow cytometry. Percentages of cells in S + G2/M phase of cell cycle within CD19 by AA4.1 and CD45 by CD34 subpopulations are shown.
Direct Generation of EAB Cells in Vitro.
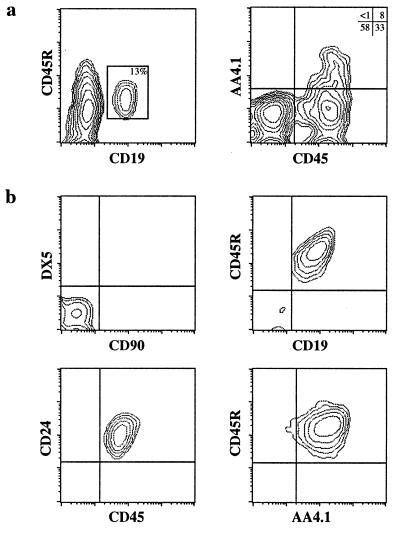
During the course of ES cell differentiation, first into lymphoid progenitors and then into B cells (Fig. 4), cells should transit through a developmental stage corresponding to that of fetal liver-derived pre-B cells. Because fetal liver is a rich source of targets for A-MuLV transformation (30), A-MuLV pre-B transformants may be directly obtainable from this in vitro system. As outlined in Fig. 1, differentiating ES cells were infected after day 15, at which time a significant population displayed a phenotype consistent with B-lineage commitment, i.e., CD19+ CD45R+ AA4.1+ (Fig. 5a). After the addition of virus-containing supernatant, the cells were cultured for an additional 2- to 4-week period, until transformed cells appeared (see Materials and Methods). EAB cell cultures were then cloned by limiting dilution.
Figure 5.
Characterization of EAB cells. (a) Flow cytometric analysis of day-15 ES/OP9 coculture for surface expression of CD19 vs. CD45R, and CD45 vs. AA4.1. The generation of ES cell-derived B lineage cells is clearly evident after 15 days of coculture. (b) Flow cytometric analysis of a representative EAB cell line shows nearly 100% homogeneity of pre-B cell-associated phenotype. Control staining with T and NK lineage mAbs is shown (Upper Left). The other panels show a composite phenotype, CD45R+ CD19+ CD24+ AA4.1+, consistent with the EAB cells designation as transformed pre-B cells.
Flow-cytometric analysis of a representative EAB cell clone is shown in Fig. 5b. The phenotype of these cells is virtually identical to that of the Abelson lines derived by transformation of fetal liver or adult BM cells in vitro (8, 9). In particular, EAB cells displayed a CD45R+ CD19+ CD24+ AA4.1+ sIgM−/lo Lin− phenotype (Fig. 5b), corresponding to that of pre-B cells (1, 2). Moreover, PCR analysis of the DNA (31) extracted from these cells revealed that they had undergone DJ and VDJ rearrangement of their Ig-heavy chain DNA loci (data not shown).
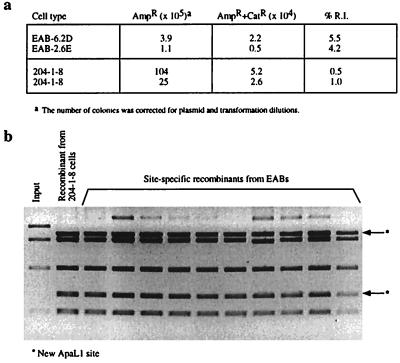
A signature feature of A-MuLV pre-B transformants is their ability to exhibit continued V(D)J recombination activity in culture (10). This feature can be assessed by using extrachromosomal V(D)J recombination substrates (19). Recombination activity is scored by the ability of the cell to site-specifically recombine a plasmid containing V(D)J recombination signals, after which the plasmid confers chloramphenicol resistance when introduced into Escherichia coli (19). All of the EAB clones tested positive with this assay. In comparison to a standard A-MuLV pre-B cell line, 204–1-8 (21), EAB clones showed an equal or higher level of recombination activity (Fig. 6a). The site-specific nature of the recombinants was confirmed by testing plasmid DNA recovered from chloramphenicol-resistant bacterial colonies for the presence of a diagnostic ApaLI site (19, 21). As shown in Fig. 6b, most plasmids possessed the expected ApaLI site, indicating that an authentic “signal joint” had been generated. Exceptional cases were caused by imprecise joining, which is typically observed at low levels for A-MuLV lines tested with the V(D)J recombination assay (10, 32, 33). In addition, alternative recombination products (in which a cryptic joining signal in the plasmid was targeted) (21) occurred at normal low frequencies. [One hundred recombinants were screened by colony filter hybridization (data not shown).] The quantity and quality of the recombinant products that were scored by the extrachromosomal assay was therefore in agreement with previous observations made on typical A-MuLV-transformed pre-B cell lines.
Figure 6.
Recombination analysis in EAB cells. Site-specific recombination of the transfected V(D)J-containing plasmid pWTSJΔ results in transcription of the chloramphenicol-resistant gene and creates a new ApaLI restriction endonuclease site. (a) Recombination index (R.I.) of two EAB cell lines vs. a standard Abelson line, 204-1-8. %R.I. is the ratio of double-resistant AmpR+CatR colonies over AmpR colonies. (b) Inverted EtBr gel image of site-specific recombination products in the extrachromosomal V(D)J recombination assay, showing the two bands generated by a new ApaLI site.
Generation of Mature Mitogen-Responsive Ig-Secreting B Lymphocytes.
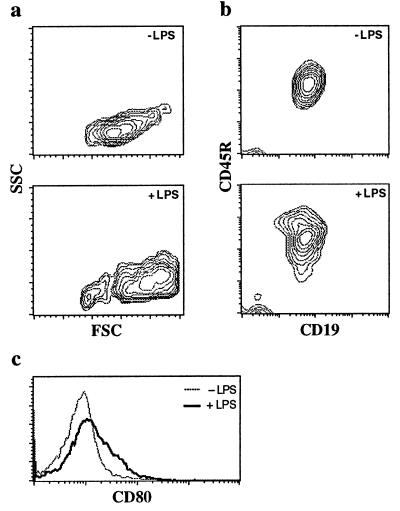
The analysis of cells of ES/OP9 cocultures with Flt-3L that were harvested later showed a large increase in the percentage of cells positive for B-lineage markers. After a 4-week culture period, nearly all (>90%) of the cells in the coculture were B lineage CD45R+ CD19+ lymphocytes (Fig. 7b). These ES-derived B lymphocytes displayed a CD11b−/lo phenotype (Fig. 4) and a small subset (2 to 3%) of the CD5+ B cells (data not shown), suggesting that CD5+ B cells (34) were not generated readily in the ES/OP9 cocultures.
Figure 7.
In vitro generation of ES-derived mature functional B cells. (a) Flow cytometric analysis for forward-scatter (FSC) by side-scatter (SSC). (b) CD19 by CD45R of ES/OP9 coculture with and without LPS treatment for 4 days. ELISA analysis of the culture supernatant collected 4 days after LPS stimulation showed high titer for secreted IgM (≈16 μg/ml). (c) Flow cytometric analysis for CD80 (B7-1) with and without LPS treatment for 48 hours. Mean fluorescence intensities: 10.25 for −LPS, and 21.81 for +LPS.
To demonstrate further the functional capabilities of the in vitro-generated B cells, day-28 cocultures were treated with LPS (12), after which the mature surface IgM+ CD19+ B cells increased in size (Fig. 7a) and proliferated extensively (data not shown). After mitogen activation, we looked for the expression of CD80 (B7–1), a costimulatory molecule that normally up-regulates on mature B cells after activation (35). Fig. 7c shows the increased surface expression of CD80 upon LPS stimulation, indicating that ES-derived B cells behave in a manner similar to normal B cells. Furthermore, culture supernatant from LPS-stimulated cells tested positive (by ELISA analysis, 16.4 ± 1.4 μg/ml) for the presence of IgM, revealing that these cells are capable of robust levels of Ig secretion. In contrast to previous reports (13–16), our findings provide evidence for the differentiation of ES cells into mature mitogen-responsive Ig-secreting B cells in vitro.
CONCLUSIONS
The addition of exogenous Flt-3L to the ES/OP9 coculture system was found to be a key element in the development of an efficient and practical model system for the generation of mature, functional B lymphocytes from ES cells in vitro. The present findings support the notion that Flt-3L is an important factor in early B lymphopoiesis in vitro (4). Moreover, these results elucidate the manner in which the addition of Flt-3L to the ES/OP9 cocultures facilitates the generation of B lymphocytes. Flow cytometric analyses of ES/OP9 cocultures revealed that the differentiating ES-derived precursors approximate the temporal kinetics and phenotypic progression of known developmental stages that occur during B cell differentiation in vivo (Figs. 2, 4, 5a, and 7, and data not shown). The fact that ES-derived B cells follow a normal developmental pathway and are functionally analogous to progenitor and mature B cells in vivo provides strong evidence that this system will prove to be significant in the dissection of the molecular events that govern B cell differentiation.
The ability to obtain A-MuLV-transformed differentiated stable cell lines from a genetically modified ES cell entirely in vitro will generate additional applications. Because A-MuLV transformants are simple to produce and maintain and have a rapid doubling time, the derivation of EAB cell lines will add to the armory of possible approaches in studying lineage-specific gene-targeted mutations. For example, null mutations in certain genes involved in V(D)J recombination can be embryonic lethal (36); one may still be able, however, to assess the role of null mutations in the V(D)J recombination program by examining EAB cells derived directly from homozygous mutant ES cells (37).
The recent characterization of human ES cells (38, 39) together with our findings make it possible to envisage a system for the generation of human B cell progenitors and/or B lymphocytes directly from ES cells in vitro. Such a system would provide a limitless source of genetically defined ES cell-derived B cells with potentially therapeutic applications for individuals suffering from agammaglobulinemias or specific B cell dysfunctions.
Acknowledgments
We thank A. M. Michie for critical reading of the manuscript and C. Furlonger and N. Rosenberg for reagents and advice regarding A-MuLV infection. We also thank A. Labbé for assistance with the ELISA analysis, S. Trop for help with the cell cycle analysis, and G. Caruana for help in setting up the OP9 coculture system. S.M.L. is a Research Scientist of the National Cancer Institute of Canada, which, with funds from the Terry Fox Run (J.C.Z.-P. and S.M.L.), supported this work. Additional support came from the Medical Research Council of Canada (J.C.Z.-P. and J.R.C.). S.K.C. is supported by an award from the Lady Tata Memorial Trust.
ABBREVIATIONS
- A-MuLV
Abelson murine leukemia virus
- BM
bone marrow
- EAB
ES-derived Abelson-transformed pre-B cells
- ES
embryonic stem
- Flt-3L
Flt-3 ligand
- LPS
lipopolysaccharide
- RT–PCR
reverse transcription–PCR
- SCF
stem cell factor
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Hardy R R, Carmack C E, Shinton S A, Kemp J D, Hayakawa K. J Exp Med. 1991;173:1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Y S, Wasserman R, Hayakawa K, Hardy R R. Immunity. 1996;5:527–535. doi: 10.1016/s1074-7613(00)80268-x. [DOI] [PubMed] [Google Scholar]
- 3.Ikuta K, Uchida N, Friedman J, Weissman I L. Annu Rev Immunol. 1992;10:759–783. doi: 10.1146/annurev.iy.10.040192.003551. [DOI] [PubMed] [Google Scholar]
- 4.Veiby O P, Lyman S D, Jacobsen S E W. Blood. 1996;88:1256–1265. [PubMed] [Google Scholar]
- 5.Lyman S D, Jacobsen S E. Blood. 1998;91:1101–1134. [PubMed] [Google Scholar]
- 6.Hunte B E, Hudak S, Campbell D, Xu Y, Rennick D. J Immunol. 1996;156:489–496. [PubMed] [Google Scholar]
- 7.Hunte B E, Capone M, Zlotnik A, Rennick D, Moore T A. Eur J Immunol. 1998;28:3850–3856. doi: 10.1002/(SICI)1521-4141(199811)28:11<3850::AID-IMMU3850>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg N. Semin Cancer Biol. 1994;5:95–102. [PubMed] [Google Scholar]
- 9.Rosenberg N, Kincade P W. Curr Opin Immunol. 1994;6:203–211. doi: 10.1016/0952-7915(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 10.Lewis S M. Adv Immunol. 1994;56:27–150. doi: 10.1016/s0065-2776(08)60450-2. [DOI] [PubMed] [Google Scholar]
- 11.Melchers F, Rolink A, Grawunder U, Winkler T H, Karasuyama H, Ghia P, Andersson J. Curr Opin Immunol. 1995;7:214–227. doi: 10.1016/0952-7915(95)80006-9. [DOI] [PubMed] [Google Scholar]
- 12.Cumano A, Dorshkind K, Gillis S, Paige C J. Eur J Immunol. 1990;20:2183–2189. doi: 10.1002/eji.1830201006. [DOI] [PubMed] [Google Scholar]
- 13.Gutierrez-Ramos J C, Palacios R. Proc Natl Acad Sci USA. 1992;89:9171–9175. doi: 10.1073/pnas.89.19.9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Potocnik A J, Nielsen P J, Eichmann K. EMBO J. 1994;13:5274–5283. doi: 10.1002/j.1460-2075.1994.tb06861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakano T, Kodama H, Honjo T. Science. 1994;265:1098–1101. doi: 10.1126/science.8066449. [DOI] [PubMed] [Google Scholar]
- 16.Nakano T. Semin Immunol. 1995;7:197–203. doi: 10.1016/1044-5323(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 17.Rosenberg N, Witte O N. J Virol. 1980;33:340–348. doi: 10.1128/jvi.33.1.340-348.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlyle J R, Michie A M, Furlonger C, Nakano T, Lenardo M J, Paige C J, Zúñiga-Pflücker J C. J Exp Med. 1997;186:173–182. doi: 10.1084/jem.186.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hesse J E, Lieber M R, Gellert M, Mizuuchi K. Cell. 1987;49:775–783. doi: 10.1016/0092-8674(87)90615-5. [DOI] [PubMed] [Google Scholar]
- 20.Lieber M R, Hesse J E, Mizuuchi K, Gellert M. Genes Dev. 1987;1:751–761. doi: 10.1101/gad.1.8.751. [DOI] [PubMed] [Google Scholar]
- 21.Lewis S M, Agard E, Suh S, Czyzyk L. Mol Cell Biol. 1997;17:3125–3136. doi: 10.1128/mcb.17.6.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ledbetter J A, Herzenberg L A. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- 23.Miller B A, Antognetti G, Springer T A. J Immunol. 1985;134:3286–3290. [PubMed] [Google Scholar]
- 24.Huang H, Auerbach R. Proc Natl Acad Sci USA. 1993;90:10110–10114. doi: 10.1073/pnas.90.21.10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spangrude G J, Heimfeld S, Weissman I L. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 26.Nakano T, Kodama H, Honjo T. Science. 1996;272:722–724. doi: 10.1126/science.272.5262.722. [DOI] [PubMed] [Google Scholar]
- 27.Hirayama F, Lyman S D, Clark S C, Ogawa M. Blood. 1995;85:1762–1768. [PubMed] [Google Scholar]
- 28.Hudak S, Hunte B, Culpepper J, Menon S, Hannum C, Thompson-Snipes L, Rennick D. Blood. 1995;85:2747–2755. [PubMed] [Google Scholar]
- 29.Jacobsen S E, Okkenhaug C, Myklebust J, Veiby O P, Lyman S D. J Exp Med. 1995;181:1357–1363. doi: 10.1084/jem.181.4.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenberg N, Baltimore D. J Exp Med. 1976;143:1453–1463. doi: 10.1084/jem.143.6.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pennycook J L, Marshall A J, Wu G E. In: Immunology Methods Manual. Lefkovits I, editor. New York: Academic; 1997. pp. 237–257. [Google Scholar]
- 32.Lieber M R, Hesse J E, Mizuuchi K, Gellert M. Proc Natl Acad Sci USA. 1988;85:8588–8592. doi: 10.1073/pnas.85.22.8588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meier J T, Lewis S M. Mol Cell Biol. 1993;13:1078–1092. doi: 10.1128/mcb.13.2.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hardy R R, Li Y S, Hayakawa K. Semin Immunol. 1996;8:37–44. doi: 10.1006/smim.1996.0006. [DOI] [PubMed] [Google Scholar]
- 35.Hathcock K S, Laszlo G, Pucillo C, Linsley P, Hodes R J. J Exp Med. 1994;180:631–640. doi: 10.1084/jem.180.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frank K M, Sekiguchi J M, Seidl K J, Swat W, Rathbun G A, Cheng H L, Davidson L, Kangaloo L, Alt F W. Nature (London) 1998;396:173–177. doi: 10.1038/24172. [DOI] [PubMed] [Google Scholar]
- 37.Mortensen R M, Conner D A, Chao S, Geisterfer-Lowrance A A T, Seidman J G. Mol Cell Biol. 1992;12:2391–2395. doi: 10.1128/mcb.12.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shamblott M J, Axelman J, Wang S, Bugg E M, Littlefield J W, Donovan P J, Blumenthal P D, Huggins G R, Gearhart J D. Proc Natl Acad Sci USA. 1998;95:13726–13731. doi: 10.1073/pnas.95.23.13726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomson J A, Itskovitz-Eldor J, Shapiro S S, Waknitz M A, Swiergiel J J, Marshall V S, Jones J M. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]