Abstract
Background
Interleukin-1 (IL-1) is a cytokine involved in the initiation and amplification of the defence response in infectious and inflammatory diseases. IL-1 receptor antagonist (IL-1ra) is an inactive member of the IL-1 family and represents one of the most potent mechanisms for controlling IL-1-dependent inflammation. IL-1ra has proven effective in the therapy of acute and chronic inflammatory diseases in experimental animal models and also in preliminary clinical trials. However, optimisation of therapeutic schedules is still needed. For instance, the use of drug delivery systems targeting specific mucosal sites may be useful to improve topical bioavailability and avoid side effects associated with systemic administration.
Results
In order to develop systems for the delivery of IL-1ra to mucosal target sites, a Streptococcus gordonii strain secreting human IL-1ra was constructed. The recombinant IL-1ra produced by S. gordonii was composed of the four amino acid residues RVFP of the fusion partner at the N-terminus, followed by the mature human IL-1ra protein. RFVP/IL-1ra displayed full biological activity in vitro in assays of inhibition of IL-1β-induced lymphocyte proliferation and was released by recombinant S. gordonii in vivo both at the vaginal and the gastrointestinal mucosa of mice. RFVP/IL-1ra appeared beneficial in the model of ulcerative colitis represented by IL-2-/- mice (knock-out for the interleukin-2 gene), as shown by the body weight increase of IL-2-/- mice locally treated with S. gordonii producing RFVP/IL-1ra.
Conclusions
These results indicate that recombinant S. gordonii can be successfully used as a delivery system for the selective targeting of mucosal surfaces with therapeutic proteins.
Background
In several inflammatory disorders, local delivery of therapeutic molecules is often preferred to systemic treatments. Therefore, the design and development of novel drug delivery systems able to reach specific tissues and organs is crucial to obtain high selectivity and efficacy with limited side effects. Drug delivery by gene therapy may represent a valid strategy for the treatment of immune-based inflammatory diseases, such as rheumatoid arthritis, multiple sclerosis, allergic asthma, diabetes, and inflammatory bowel disease (IBD) [1]. Patients suffering from IBD (e.g., Crohn's disease and ulcerative colitis) are currently treated with corticosteroid drugs, immunosuppressants, and antibiotics. Novel therapeutic approaches, including local inhibition of pro-inflammatory cytokines (e.g., TNF-α and IL-1β) and prebiotic/probiotic modulation of the enteric flora, are also under investigation [2-4].
The IL-1 family of cytokines includes two agonist proteins, IL-1α and IL-1β, and one antagonist protein, IL-1ra. IL-1β (and in certain cases also IL-1α) is a very potent immunostimulatory and inflammatory cytokine, responsible for initiating and amplifying the host response to invasion. However, if not properly controlled, IL-1 can cause fever, acute inflammation, tissue destruction, organ failure, and eventually shock and death (reviewed in [5]). IL-1ra is capable of inhibiting IL-1 both in vitro and in vivo, thus representing a natural powerful mechanism to control IL-1-dependent responses and avoid pathological derangements (reviewed in [6-8]). IL-1ra functions as a competitive receptor antagonist with no detectable agonist activity. In experimental animal models, IL-1ra has demonstrated excellent therapeutic potential against acute and chronic inflammatory pathologies [6-9], also being effective in prolonging survival during endotoxic shock [8,10-12]. Gene therapy with adenoviral vectors carrying the IL-1ra gene also yielded promising results in experimental models of type 1 diabetes and ischemic brain damage [13,14]. In human clinical trials, IL-1ra has been administered to patients with septic shock, rheumatoid arthritis, graft-versus-host disease, and multiple sclerosis (reviewed in [8]). While only a modest benefit was achieved in patients with septic shock [8,15], IL-1ra clearly reduced progression of joint destruction due to rheumatoid arthritis [8,16-18]. In IBD, progression and chronicisation of the disease appears to be linked to an imbalance between pro-inflammatory and anti-inflammatory cytokines [19,20]. In particular, the mucosal imbalance between inflammatory IL-1β and anti-inflammatory IL-1ra apparently plays a critical role [19,21-23], and the presence of the allele 2 of the IL-1ra gene is associated with increased incidence and severity of the disease [24-26]. So far, pre-clinical studies have demonstrated beneficial effects of anti-cytokine approaches, including IL-1ra, in experimental models of colitis in rats and rabbits (reviewed in [8,19]).
Streptococcus gordonii is a human oral commensal that has been proposed as a delivery vehicle for vaccine antigens and microbicides [27-30]. Using the M6 protein of Streptococcus pyogenes as a fusion partner, a host-vector system was developed in S. gordonii allowing either expression at the cell surface [31], or secretion of heterologous proteins into the culture medium [32]. Due to its capability of colonising certain host mucosae (reviewed in [27]), S. gordonii represents a promising candidate for delivering vaccine antigens, recombinant antibodies, and therapeutic drugs to mucosal surfaces.
In the present study, an S. gordonii strain producing and delivering human IL-1ra to target mucosal sites was constructed. The results obtained show that the recombinant IL-1ra secreted by S. gordonii is biologically active in vitro and, when administered in vivo in the gastrointestinal mucosa, alleviated the symptoms of IBD in an experimental animal model.
Results
Production of human IL-1ra by S. gordonii
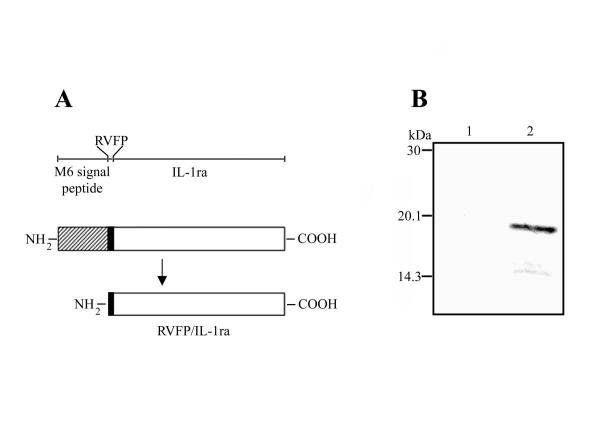
By using the streptococcal M6 protein as the fusion partner [31], an S. gordonii strain secreting human IL-1ra into the culture medium was constructed and denominated GP1300. The presence of the emm6 signal sequence at the N-terminus and of two stop codons at the C-terminus of the fusion allowed translocation and secretion of the protein, respectively. After cleavage of the M6 leader peptide (42 amino acids), the recombinant IL-1ra consisted in the first four amino acid residues of M6 (RVFP) followed by 152 amino acids of the mature human IL-1ra. The resulting fusion protein (156 amino acids) was thus denominated RVFP/IL-1ra (Figure 1A). Production of RVFP/IL-1ra by GP1300 was assessed by Western blot analysis of trichloroacetic acid (TCA)-precipitated proteins released into the culture medium using IL-1ra-specific polyclonal antibodies. A protein band of approximately 19 kDa (the expected molecular mass of the fusion protein is 17.2 kDa) was detected in the GP1300 sample, while no reactivity was observed in the control sample (Figure 1B). The amount of recombinant IL-1ra secreted by S. gordonii was 0.1 mg per litre, as estimated by ELISA. These results indicate that S. gordonii is a suitable system to produce recombinant human IL-1ra.
Figure 1.
Structure and production of RVFP/IL-1ra by S. gordonii. A. Structure of RVFP/IL-1ra produced by S. gordonii. By using the streptococcal M6 protein as the fusion partner, a recombinant protein composed of the M6 signal peptide (42 amino acids; hatched), the first four amino acids of M6 (RVFP; black), and the mature human IL-1ra (152 amino acids; white) was produced. After cleavage of the M6 leader peptide, RVFP/IL-1ra (156 amino acids) is secreted into the culture medium. A schematic representation of the different parts of the fusion protein is shown in the upper part of the figure. B. Immunoblot analysis of total proteins present in the culture supernatant of S. gordonii. Culture supernatants of the control GP204 strain (lane 1) and of the RVFP/IL-1ra-producing GP1300 strain (lane 2) were precipitated with TCA, separated by 15% SDS-PAGE, and reacted with a polyclonal antibody to human IL-1ra. Positions of molecular mass standards are indicated to the left.
IL-1ra secreted by S. gordonii is biologically active
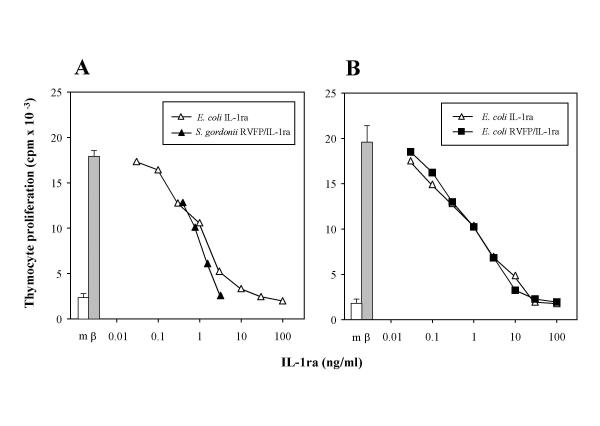
In order to verify that RVFP/IL-1ra produced by the GP1300 strain retains IL-1ra-like biological activity, its ability to inhibit IL-1-mediated proliferation of murine thymocytes was assessed. As a control, a reference standard of wild type human IL-1ra expressed in Escherichia coli was used. IL-1β-induced proliferation could be inhibited in a dose-dependent fashion by both wild type IL-1ra and the culture medium of S. gordonii GP1300 (Figure 2A). Specific inhibitory activities of the GP1300 culture medium (containing 0.1 mg/litre of RVFP/IL-1ra) and of wild type IL-1ra were 1.25 × 106 Antagonist Units (AU) per mg and 1.0 × 106 AU/mg, respectively. Culture supernatant of the control S. gordonii GP204 strain did not contain measurable levels of IL-1 inhibitory activity (data not shown). In order to verify that the presence of the RVFP residues did not affect the biological activity of IL-1ra produced by S. gordonii, RVFP/IL-1ra was also expressed in E. coli, purified, and assayed for IL-1 inhibitory activity as described above. Also in this case, the dose-dependent inhibition of IL-1β-induced thymocyte proliferation by RVFP/IL-1ra was fully comparable to that of the reference standard wild type IL-1ra, with a specific inhibitory activity of approximately 1.25 × 106 AU/mg in both cases (Figure 2B). These results show that RVFP/IL-1ra is capable of inhibiting IL-1 to an extent fully comparable to that of wild type IL-1ra, indicating that the presence of RVFP at the N-terminal does not affect the biological activity of IL-1ra produced by S. gordonii.
Figure 2.
Inhibition of IL-1-mediated thymocyte proliferation. A. Thymocyte proliferation induced by 0.3 ng/ml of IL-1β (β; grey bar) could be inhibited in a dose-dependent fashion by a standard preparation of wild type human IL-1ra expressed in E. coli (open triangles). The IL-1 inhibitory activity of the wild type IL-1ra reference standard was compared to that of the culture supernatant of recombinant S. gordonii GP1300 (solid triangles), which contained 0.1 mg/litre RVFP/IL-1ra (as assessed by ELISA). B. Thymocyte proliferation to 0.3 ng/ml IL-1β (β; grey bar) was inhibited in a dose-dependent fashion by both the wild type IL-1ra reference standard (open triangles) and the recombinant RVFP/IL-1ra expressed in E. coli (solid squares). Results are the mean ± SEM of 3–9 replicate determinations within single experiments, representative of five performed. SEM lower than 10 % are not shown. Background thymocyte proliferation in culture medium without IL-1β (m, open bar) is also reported.
IL-1ra is produced in vivo by recombinant S. gordonii
One of the potential uses of IL-1ra-producing S. gordonii is the local delivery of the anti-inflammatory molecule to a specific mucosal site. Therefore, the ability of S. gordonii to stably release IL-1ra at the mucosal level was tested. In order to improve recovery of bacteria from mucosal sites, a double-antibiotic-resistant strain was constructed by transforming S. gordonii GP1300 with the chromosomal DNA of S. gordonii GP204 [33], that carries a point mutation conferring resistance to streptomycin. The new erythromycin- and streptomycin-resistant strain was denominated GP1294. S. gordonii GP1294 was inoculated intravaginally into mice, and the presence of recombinant bacteria was evaluated in vaginal samples collected from mice once per week. In vivo production of IL-1ra by S. gordonii was assessed by both immunoblotting on erythromycin- and streptomycin-resistant bacterial colonies and immunofluorescence directly carried out on vaginal smears using anti-IL-1ra specific antibodies. In accordance with published data [34], it was possible to isolate recombinant bacteria producing RVFP/IL-1ra from vaginal samples for eight consecutive weeks (data not shown). In addition, to verify that S. gordonii was also capable of releasing IL-1ra in vivo in the gastrointestinal tract (GI), mice were inoculated via the intragastric route with the GP1294 strain. In this case, IL-1ra production was tested by ELISA on different parts of the GI tract of mice sacrificed 3, 11, and 28 days after bacterial administration. IL-1ra was detected in GI samples up to day 11 from the last inoculum with the highest amounts of antagonist found in the stomach and caecum (Table 1). It was not possible to detect IL-1ra in the GI tract of mice sacrificed four weeks after inoculum (Table 1). These data confirm that S. gordonii may represent a reliable system to deliver a drug molecule, such as IL-1ra, to target mucosal sites.
Table 1.
IL-1ra in the gastro-intestinal tract of mice inoculated intragastrically with recombinant S. gordonii GP1294.
| IL-1ra (OD450) at different time points after bacterial inoculuma | |||
| Sample b | (day 3) | (day 11) | (day 28) |
| Stomach | 0.478 | 0.244 | 0.000 |
| Small intestine | 0.061 | 0.062 | 0.000 |
| Caecum | 0.051 | 0.029 | 0.000 |
| Colon | 0.028 | 0.055 | 0.000 |
| Caecum content | 0.026 | 0.283 | 0.000 |
| Faeces | 0.036 | 0.025 | 0.000 |
a The presence of IL-1ra was determined by ELISA and expressed as net OD readings at 450 nm. As reference, standard IL-1ra readings were the following: 50 pg/ml, 0.018; 100 pg/ml, 0.030; 500 pg/ml, 0.236; 1000 pg/ml, 0.575. b IL-2-/- mice inoculated with the IL-1ra-expressing S. gordonii GP1294 strain were sacrificed 3, 11, and 28 days after the last inoculum. Different parts of the GI tract were removed, homogenised, and subjected to ELISA. Culture supernatants of strains GP1294 and GP204 were used as positive and negative controls, respectively.
IL-1ra-producing S. gordonii has beneficial effects in murine IBD
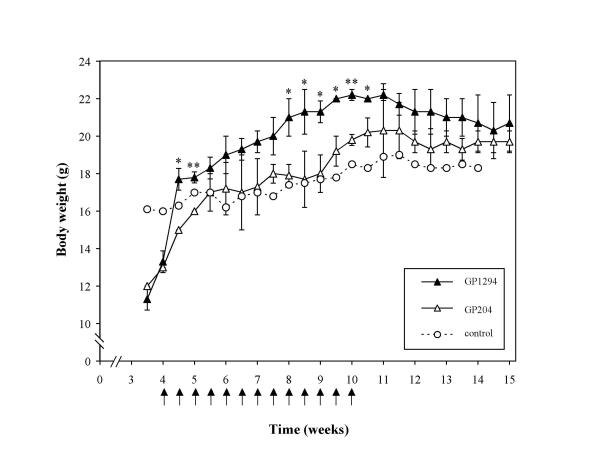
Mice deficient in the interleukin-2 gene (IL-2-/-) spontaneously develop a progressive IBD similar to human ulcerative colitis between 4 to 15 weeks of age [35,36]. The disease is associated with increased levels of IL-1 [37,38], and treatment with systemic IL-1ra diminishes colitis severity [37]. As we showed that S. gordonii GP1294 was able to release IL-1ra in the GI tract of mice (Table 1), it was thus examined whether production of IL-1ra by S. gordonii in the GI tract of IL-2-/- mice could be effective in decreasing the IBD-like pathology. Starting at 4 weeks of age, animals were treated for 7 consecutive weeks with either S. gordonii GP1294 or the GP204 control strain. As a measure of disease progression, body weight was recorded up to the 15th week of age. A significant weight gain was observed in the first week of treatment (week 4 to 5) in the animal group inoculated with the GP1294 strain (Figure 3). A more consistent weight increase was achieved from week 8 to week 10.5 in mice treated with S. gordonii GP1294, with statistically significant differences compared to animals inoculated with the control strain (Figure 3). After treatment interruption at week 10, differences in body weight between the two animal groups could no longer be detected. These results suggest that recombinant S. gordonii, that is able to release bioactive IL-1ra in the GI tract, can improve the symptoms of ulcerative colitis. It may be hypothesized that such improvement is due to the inhibition of IL-1-dependent inflammation by IL-1ra, although further investigation is needed to confirm this hypothesis.
Figure 3.
Body weight of IBD-affected IL-2-deficient mice treated with RVFP/IL-1ra producing S. gordonii. Two groups of six IL-2-/- mice were inoculated intragastrically with 1 × 1010 CFU of either S. gordonii strain GP1294 (RVFP/IL-1; solid triangles) or GP204 (control; open triangles). One untreated mouse (open circles) served as a control of disease progression. Starting at 4 weeks of age, bacteria were administered (indicated by arrows) to mice twice per week for a total of 7 consecutive weeks. Body weight (in grams) of individual mice was recorded twice per week up to 15 weeks of age, and expressed as mean ± SD. Statistically significant (t test) differences between mice treated with S. gordonii GP1294 and those receiving GP204 are represented by asterisks (*, P < 0.05; **, P < 0.01).
Discussion
IL-1 is a potent immunostimulating and inflammatory cytokine playing a major role in the onset and development of the defence response. IL-1 activity can be detrimental if not properly controlled, resulting in the development and chronicisation of several immune and inflammatory pathologies. Inhibition of IL-1 can be accomplished by several means, including the use of neutralising antibodies, soluble receptors, inhibitors of IL-1-activating enzymes, and most of all the receptor antagonist IL-1ra [5]. IL-1ra has been used in pre-clinical studies of autoimmune and immune-mediated diseases, septic shock, localised inflammatory diseases, neurodegenerative conditions, metabolic dysfunctions, and cancer (reviewed in [8]). Encouraging results were obtained in both osteoarthritis animal models and rheumatoid arthritis patients either by local/systemic administration of IL-1ra [8,18,39], or by gene therapy using ex vivo transfer of IL-1ra-transfected synovial fibroblasts directly into the joints [8,16-18,40-43]. Delivery of anti-inflammatory drugs to mucosal surfaces may also be crucial in patients suffering from localised inflammatory conditions, such as gingival inflammation, periodontal disease, sinusitis, allergic rhinitis, vaginal infections, otitis, conjunctivitis, Sjögren syndrome and IBD. However, gene therapy is sometimes not feasible for mucosal drug delivery, because of the intrinsic problems of the approach (that implies genetic manipulation of human cells and their re-introduction into the body) and of those related to the choice of the appropriate target cells.
In this work, a strategy for mucosal drug delivery is proposed, consisting in the in situ synthesis and release of the recombinant protein IL-1ra by a genetically engineered S. gordonii strain. Mucosal delivery of therapeutics by recombinant bacteria has been successfully attempted for the first time by Steidler et al. [44,45] and more recently also by other groups [29,46,47]. However, the use of anti-inflammatory IL-1ra is novel in the field. S. gordonii has already been described as a delivery vehicle for vaccine antigens and microbicides [27-30]. By using the previously developed host-vector system [31], the coding sequence of the mature human IL-1ra was cloned in-frame into the emm6 gene, encoding the M6 protein of S. pyogenes. After cleavage of the M6 leader peptide, the resulting mature fusion protein includes the RVFP residues of the M6 protein and the 152 amino acids of human IL-1ra. Production of recombinant RVFP/IL-1ra was confirmed by Western blotting of proteins secreted into the culture supernatant. In accordance with previous data [32], the presence of four amino acids of M6 was sufficient to ensure a correct translocation and release of the fusion protein into the medium to a concentration of approximately 0.1 mg per litre. Although the quantity of recombinant human IL-1ra produced by S. gordonii is not as large as that reported in other studies [48,49], high productivity is not a major issue here, since this work aims at developing a mucosal drug delivery system rather than a system for large-scale production of drugs. Indeed, a continuous and prolonged (8 weeks) production of IL-1ra was detected at the level of the vaginal mucosa of mice following topical delivery of a single inoculum of IL-1ra-secreting S. gordonii. In addition, S. gordonii was also shown to release recombinant RVFP/IL-1ra in vivo in the GI tract of mice for at least 11 days after bacterial administration. Therefore, this approach appears suitable for achieving the in vivo release of RVFP/IL-1ra at mucosal surfaces.
The RVFP/IL-1ra produced by S. gordonii is biologically active. Both RVFP/IL-1ra-containing S. gordonii supernatant and purified RVFP/IL-1ra were able to inhibit IL-1-induced thymocyte proliferation in a dose-dependent manner, with an antagonist activity fully comparable to that of wild type IL-1ra. Thus, addition of four extra amino acids at the N-terminal of IL-1ra did not alter its IL-1-inhibitory activity, confirming that S. gordonii is a feasible system for producing biologically active human proteins. The preliminary evidence of the activity of RVFP/IL-1ra delivered in vivo by S. gordonii was obtained in the murine model of IBD in IL-2-/- mice. The IBD pathology was chosen because a significant alteration of the IL-1β/IL-1ra homeostatic equilibrium towards inflammation is present in IBD [21,23]. Few weeks after birth, IL-2-/- mice spontaneously develop IBD-like symptoms, including diarrhoea, intestinal bleeding, frequent rectal prolapse, and also weight loss [35]. In IL-2-/- mice colitis there are increased levels of IL-1 [37,38], and treatment with IL-1ra reduces disease onset and progression [37]. By carefully monitoring body weight fluctuations as an index of IBD progression, a significant weight gain was observed in animals inoculated with the RVFP/IL-1ra-producing S. gordonii GP1294 strain as compared to control animals. The beneficial effects of recombinant bacteria was observed for the whole period of treatment. The present results thus suggest that mucosal delivery of anti-inflammatory IL-1ra produced by recombinant S. gordonii can reduce disease severity in the IL-2-/- IBD-like pathology, representing the "proof-of-principle" of the suitability of S. gordonii for mucosal delivery of therapeutic proteins. This finding is in agreement with a recent report describing effective reduction and prevention of ulcerative colitis like symptoms in two different animal models of IBD by using genetically engineered Lactococcus lactis producing anti-inflammatory murine IL-10 [45]. In our case, to prove the actual therapeutic effectiveness of IL-1ra produced by S. gordonii, a more extensive study with a detailed analysis of inflammatory parameters in this and other animal models would be required.
S. gordonii can colonise the oral and vaginal mucosa of mice for up to eight weeks [27]. Although the bacterium persists in the murine intestinal mucosa for few weeks [28], it does not efficiently colonise the murine gut. This could explain why, differently from the long-lasting colonisation in the vagina, the beneficial effects of intragastrically delivered IL-1ra-producing S. gordonii on IBD progression were limited to the period of treatment. However, this fact may still be of great advantage in terms of future therapeutic applications, as it allows a thorough control of timing and dosage of drug delivery.
In conclusion, the drug delivery system here described is suitable for further developments for human use. However, in this case, additional modifications would be needed to avoid the escape of a genetically modified micro-organism into the external environment through faecal excretion. This containment could be achieved for instance by making the micro-organism auxotrofic for an essential molecule not available in the environment, as recently described by Steidler et al. [50].
Conclusions
The possibility of limiting and controlling the delivery of a cytokine inhibitor of high and selective activity such as IL-1ra may offer enormous advantages in terms of efficacy and safety in the treatment of certain inflammatory disorders, where the pathogenic role of IL-1 is demonstrated. In the present work, we propose the use of S. gordonii to deliver IL-1ra in a biologically active form to affected mucosal surfaces. The system is safe, since S. gordonii is a commensal micro-organism of the human oral cavity, and it can be easily controlled in terms of quantity of drug produced and duration of treatment. Moreover, by using the recently developed co-expression system [51], it is also feasible to construct a drug delivery system simultaneously producing IL-1ra together with other anti-inflammatory cytokines (e.g. IL-4, IL-10), neutralising antibodies, or soluble receptors. In conclusion, S. gordonii could represent a novel, cost-effective therapeutic approach, in alternative to gene therapy, for the selective delivery of drugs to diseased mucosal sites.
Methods
S. gordonii strains
Strain GP1221 [31] was the recipient for transformation experiments and the control in Western blotting and thymocyte proliferation assays. Strain GP204 [33] was used as a control in animal experiments. S. gordonii GP1300 and GP1294 secrete human IL-1ra into the culture medium. Strains were grown in Tryptic Soy Broth (TSB; Difco, Detroit, MI) at 37°C with 5% CO2. Antibiotics were added at the following concentrations: erythromycin at 1 μg/ml, kanamycin and streptomycin at 500 μg/ml.
Construction of S. gordonii strains producing human IL-1ra
An S. gordonii strain secreting human IL-1ra into the culture medium was constructed using the previously described host-vector system [31]. The cDNA encoding the mature human IL-1ra (accession no. M63099) was PCR-amplified using primers IL-1ra-Forward (5'-GGCTCTAGACCATCTGGTCGTAAGTCT-3', the XbaI restriction site is underlined) and IL-1ra-Reverse (5'-CATGAATTCCATGGTACCAGCTGCAGA-3', the EcoRI site is underlined), and cloned in-frame into the emm6 gene of plasmid pSMB55 [31] digested with AvrII and EcoRI. The new plasmid was named pSMB55/IL-1ra. In order to achieve protein translocation across the cell wall, the sequence encoding the first four amino acids (RVFP) of the M6 protein was added at the 5'-end of the IL-1ra coding sequence, whereas the presence of two transcriptional stop codons at the 3'-end of IL-1ra allowed secretion of the protein. As recombinant IL-1ra produced by S. gordonii is preceded by the RVFP residues, it will be referred to as RVFP/IL-1ra. Competent cells of S. gordonii GP1221 were transformed with plasmid pSMB55/IL-1ra to obtain the erythromycin-resistant strain GP1300. In order to construct an erythromycin- and streptomycin-resistant strain for animal experiments, GP1300 was transformed with the chromosomal DNA of S. gordonii GP204 [33], and the new strain was denominated GP1294. Procedures for S. gordonii transformation, screening and genetic analysis of transformants were as previously described [33].
Determination of IL-1ra production by recombinant S. gordonii
To verify that S. gordonii GP1300 released IL-1ra into the culture medium, strains GP1300 and GP1221 were grown in TSB to early stationary phase. Culture supernatant samples were precipitated with 5% TCA and subjected to 15% sodium dodecyl sulfate-polyacrylamide gel elecrophoresis (SDS-PAGE). After transferring onto nitrocellulose filters (Protran; Schleicher and Schuell, Dassel, Germany), immunoblotting was performed using a 1:100,000 dilution of rabbit polyclonal antibodies raised against human IL-1ra expressed and purified from E. coli (see below). Quantitative assessment of IL-1ra in the culture supernatant of S. gordonii GP1300 was performed by ELISA using the IL-1ra Biotrak ELISA kit (Amersham Biosciences, Little Chalfont, UK) in accordance to the manufacturer's instructions.
Expression and purification of human IL-1β, IL-1ra, and RVFP/IL-1ra in E. coli
Human IL-1β, IL-1ra, and the RVFP/IL-1ra construct were expressed and purified from E. coli. The cDNAs coding for the mature human IL-1β (GenBank accession number NM_000576), human IL-1ra, and RVFP/IL-1ra were cloned into the pRSET vector (Invitrogen, San Diego, CA), as previously described [52]. Expression of recombinant proteins was induced with 1 mM isopropyl β-D-thiogalactopyranoside (IPTG; Inalco, Milano, Italy), according to the manufacturer's instructions. Cells were harvested, disrupted by sonication, and the soluble fraction was collected by centrifugation. Purification was performed by chromatography on S-Sepharose Fast Flow (Pharmacia, Uppsala, Sweden), or Mono S 5/5 column (Pharmacia), followed by filtration using Centriplus 100 (Amicon, Beverly, MA). Purity was always higher than 95%, as evaluated by SDS-PAGE and densitometric analysis. Concentration of proteins in 6 M Guanidine-HCl was determined by calculating the extinction coefficients using the PC/Gene Software (Oxford Molecular, Cambridge, UK). Purified proteins were tested for biological activity using the assay of IL-1β-induced D10.G4.1 proliferation [53]. Binding to IL-1 receptors was analysed using both EL4-6.1 (expressing the IL-1RI receptor) and dexamethasone-treated 1H7 cells (expressing the IL-1RII receptor) [54]. The IL-1β activity was approximately 2.5 × 108 IU (International Units; [55]) per mg of protein, and KD were 0.12 nM and 0.61 nM for IL-1RI and IL-1RII, respectively. Inhibitory activities of the IL-1ra preparations were approximately 1.0 × 106 AU/mg, where one AU is the amount of IL-1ra that inhibits 50% of IL-1β-induced proliferation in the thymocytes proliferation assay (see below).
Thymocyte proliferation assay
Normal thymocytes (5 × 105 cells/well) from 4–8 week old C3H/HeJ mice (The Jackson Laboratory, Bar Harbor, ME) were incubated in microtitre plates (Cluster96, Costar, Cambridge, MA) in cell culture medium at 37°C with 5% CO2 for 72 hours. Culture medium was RPMI 1640 medium (Invitrogen, Life Technologies Inc., Carlsbad, CA), supplemented with 50 μg/ml of gentamycin (Sigma), 2 mM L-glutamine, 1.25 × 105 M 2-mercaptoethanol, 25 mM HEPES buffer, and 5% heat-inactivated fetal bovine serum (Hyclone, Sterile Systems, Logan, UT). Cells were incubated with a suboptimal concentration of phytohemoagglutinin (PHA, 1.5 μg/ml; GlaxoSmithKline, Brentford, UK) together with an optimal concentration of recombinant human IL-1β (typically 0.3 ng/ml). Serial dilutions of either S. gordonii GP1300 culture supernatant (containing 0.1 mg/litre of RVFP/IL-1ra) or purified E. coli RVFP/IL-1ra were tested for their capacity to inhibit IL-1β-induced proliferation, as compared to a standard curve of human recombinant IL-1ra expressed in E. coli. The latter reagent was repeatedly titrated for its IL-1β inhibitor activity in the thymocyte proliferation assay, yielding an average specific activity of 1.0 × 106 AU/mg. The same standardised preparation of wild type human recombinant IL-1ra was used as a reference standard in each assay [56]. Supernatant of the control GP1221 strain was used as a negative control. After 72 hours of incubation, each well received 25 μl of culture medium containing 0.5 μCi 3H-thymidine (Amersham), and plates were incubated for 18 hours. Cells were harvested on glass fiber filters and incorporated radioactivity was measured with a β-counter (Betaplate, Wallac, Turku, Finland). IL-1β inhibitory activities of IL-1ra-containing samples were calculated from inhibition curves, expressed in AU per mg of protein, and compared to the reference wild type IL-1ra standard.
Murine vaginal colonisation
Six week-old female BALB/c mice (Charles River Italia, Monza, Italy) were used. The RVFP/IL-1ra-producing GP1294 and the GP204 control strains were grown in TSB to late exponential phase, washed once, and resuspended in fresh medium. Each animal received a single intravaginal inoculum of 108 CFU in a total volume of 20 μl. Vaginal samples were collected once per week for 8 weeks using absorbent cylindrical wicks (Porvair Filtronics Ltd., Shepperton, UK), and plated onto blood agar plates containing streptomycin. Bacterial colonies were tested for erythromycin resistance and expression of IL-1ra by Western blot using an anti-IL-1ra rabbit serum (1:100,000). Vaginal samples were also subjected to immunofluorescence using an IL-1ra antiserum as described before [57]. All procedures for inoculation, collection and processing of samples, and colonisation assays were as previously described [57].
Assessment of IL-1ra in the GI tract of mice
Four weeks-old female IL-2 knock-out C57BL/6 mice (IL-2-/-), generated by gene targeting as previously described [58], were obtained by Jackson Laboratories (Bar Harbor, Maine, USA). Mice were inoculated intragastrically with the S. gordonii GP1294 strain (producing RVFP/IL-1ra). Details of bacterial preparation and administration to mice are below. In order to quantify production of RVFP/IL-1ra in the GI tract, three mice were sacrificed 3, 11, and 28 days after last bacterial inoculum. The stomach, small intestine, colon, and caecum were isolated, washed in cold phosphate-buffered saline pH 7.4 (PBS) and immediately homogenised in 2 ml of cold PBS. Homogenates were centrifuged (8800 × g,10 minutes, 4°C) and supernatants were stored at -20°C. Faeces and caecum were collected and treated as described [28]. Human IL-1ra was determined in GI tract samples by ELISA (R&D Systems, Minneapolis, MN, USA). Culture supernatants of S. gordonii GP1294 and GP204 were used as positive and negative controls, respectively. Net OD450 readings are reported, as qualitative indication of IL-1ra presence in the tissues. Precise quantitative determination of IL-1ra in organ homogenates is hampered by the presence of receptors, natural antibodies, and/or other non-specific inhibitors. As reference, OD450 readings for standard human recombinant IL-1ra are the following: 50 pg/ml, 0.018; 100 pg/ml, 0.030; 500 pg/ml, 0.236; 1000 pg/ml, 0.575.
Treatment of IL-2-/- mice with recombinant S. gordonii
The S. gordonii strains GP1294 (producing RVFP/IL-1ra) and GP204 (control) were grown in TSB to the beginning of stationary phase, washed once with PBS, and resuspended in PBS containing 3% skimmed milk to a final concentration of approximately 5 × 1010 CFU/ml. Two groups of 6 mice each were inoculated intragastrically twice per week for 7 consecutive weeks (week 4 to 10) with 200 μl of the bacterial suspension (approximately 1010 CFU/mouse) of either strain GP1294 or GP204. One untreated mouse served as a control to follow the course of disease progression. Mice were weighted twice per week for 15 weeks.
Statistical analysis
Differences in body weights between IL-2-/- mice receiving S. gordonii GP1294 and GP204 were analysed by using the unpaired Student's t test. Statistical significance was set at P < 0.05.
Authors' contributions
SR constructed the recombinant S. gordonii strains and wrote the manuscript.
GM prepared and expressed the constructs in E. coli, and made bacterial preparations.
PR extracted, purified and biochemically characterised the recombinant cytokines.
TM participated in the study on IL-2-/- mice.
PB performed the work of in vitro cytokine functional characterisation.
LX performed the experiments on IL-2-/- mice.
DM performed the experiments of vaginal colonisation.
AT conceived the study, and participated in its design and coordination.
LH coordinated the study on IL-2-/- mice.
GP coordinated the work of cytokine expression in S. gordonii and studies of in vivo delivery.
DB designed the work, coordinated the work of cytokine production in E. coli, purification, and functional characterisation, and supervised manuscript preparation.
All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
This work was supported by the Istituto Superiore di Sanità (grant no. 40C.74 and 45D1.04, within the PNR AIDS), the Commission of the European Union (contract no. QLK2-2001-01216), and the Consiglio Nazionale delle Ricerche (P.F. Biotecnologie). Diana Boraschi was supported by a grant from AIRC (Associazione Italiana Ricerca sul Cancro), Milano, Italy.
Contributor Information
Susanna Ricci, Email: riccisus@unisi.it.
Giovanni Macchia, Email: gmacchia@epo.nl.
Paolo Ruggiero, Email: paolo_ruggiero@chiron.it.
Tiziana Maggi, Email: tiziana_maggi@chiron.it.
Paola Bossù, Email: pbossu@unitus.it.
Li Xu, Email: li.xu@csb.ki.se.
Donata Medaglini, Email: medaglini@unisi.it.
Aldo Tagliabue, Email: atagliabue@ivi.int.
Lennart Hammarström, Email: lennart.hammarstrom@csb.ki.se.
Gianni Pozzi, Email: pozzi@unisi.it.
Diana Boraschi, Email: borasc@tin.it.
References
- Hedley ML. Gene therapy of chronic inflammatory disease. Adv Drug Deliv Rev. 2000;44:195–207. doi: 10.1016/S0169-409X(00)00095-8. [DOI] [PubMed] [Google Scholar]
- Holtmann MH, Galle PR, Neurath MF. Immunotherapeutic approaches to inflammatory bowel diseases. Expert Opin Biol Ther. 2001;1:455–466. doi: 10.1517/14712598.1.3.455. [DOI] [PubMed] [Google Scholar]
- Shanahan F. Inflammatory bowel disease: immunodiagnostics, immunotherapeutics, and ecotherapeutics. Gastroenterology. 2001;120:622–635. doi: 10.1053/gast.2001.22122. [DOI] [PubMed] [Google Scholar]
- Schultz M, Sartor RB. Probiotics and inflammatory bowel diseases. Am J Gastroenterol. 2000;95:19–21. doi: 10.1016/S0002-9270(99)00812-6. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Proinflammatory cytokines. Chest. 2000;118:503–508. doi: 10.1378/chest.118.2.503. [DOI] [PubMed] [Google Scholar]
- Arend WP, Malyak M, Guthridge CJ, Gabay C. Interleukin-1 receptor antagonist: role in biology. Ann Rev Immunol. 1998;16:27–55. doi: 10.1146/annurev.immunol.16.1.27. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Interleukin-1, interleukin-1 receptors and interleukin-1 receptor antagonist. Int Rev Immunol. 1998;16:457–499. doi: 10.3109/08830189809043005. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Dinarello CA, Ghezzi P. Interleukin-1 receptor antagonist. In: Mantovani A, Dinarello CA, Ghezzi P, editor. In Pharmacology of cytokines. Oxford: Oxford University Press; 2000. pp. 91–119. [Google Scholar]
- Dinarello CA, Moldawer LL. Proinflammatory and anti-inflammatory cytokines in rheumatoid arthritis. A primer for clinicians. Thousand Oaks: Amgen Inc. 2001.
- Ohlsson K, Bjork P, Bergenfeldt M, Hageman R, Thompson RC. Interleukin-1 receptor antagonist reduces mortality from endotoxin shock. Nature. 1990;348:550–552. doi: 10.1038/348550a0. [DOI] [PubMed] [Google Scholar]
- Wakabayashi G, Gelfand JA, Burke JF, Thompson RC, Dinarello CA. A specific receptor antagonist for interleukin 1 prevents Escherichia coli-induced shock in rabbits. FASEB J. 1991;5:338–343. doi: 10.1096/fasebj.5.3.1825816. [DOI] [PubMed] [Google Scholar]
- Hirsch E, Irikura VM, Paul SM, Hirsh D. Functions of interleukin 1 receptor antagonist in gene knockout and overproducing mice. Proc Natl Acad Sci USA. 1996;93:11008–11013. doi: 10.1073/pnas.93.20.11008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannoukakis N, Rudert WA, Ghivazzani SC, Gambotto A, Ricordi C, Trucco M, Robbins PD. Adenoviral gene transfer of the interleukin-1 receptor antagonist protein to human islets prevents IL-1 beta-induced beta-cell impairment and activation of islet cell apoptosis in vitro. Diabetes. 1999;48:1730–1736. doi: 10.2337/diabetes.48.9.1730. [DOI] [PubMed] [Google Scholar]
- Yang GY, Davidson BL, Betz AL. Overexpression of interleukin-1 receptor antagonist in the mouse brain reduces ischemic brain injury. Brain Res. 1997;751:181–188. doi: 10.1016/S0006-8993(96)01277-2. [DOI] [PubMed] [Google Scholar]
- Fisher CJ, Dhainaut JF, Opal SM, Pribble JP, Balk RA, Slotman GJ, Iberti TJ, Rackow EC, Shapiro MJ, Greenman RL, Reines HD, Shelly MP, Thompson BW, La Brecque JF, Catalano MA, Knaua WA, Sadoff JC. Recombinant human interleukin-1 receptor antagonist in the treatment of patients with sepsis syndrome. Results from a randomized, double-blind, placebo-controlled trial. Phase III rhIL-1ra Sepsis Syndrome Study Group. JAMA. 1994;271:1836–1843. doi: 10.1001/jama.271.23.1836. [DOI] [PubMed] [Google Scholar]
- Krishnan BR. Interleukin-1 receptor antagonist gene therapy for arthritis. Curr Opin Mol Ther. 1999;4:454–457. [PubMed] [Google Scholar]
- Evans CH, Robbins PD, Ghivizzani SC, Herndon JH, Kang R, Bahnson AB, Barranger JA, Elders EM, Gay S, Tomaino MM, Wasko MC, Watkins SC, Whiteside TL, Glorioso JC, Lotze MT, Wright TM. Clinical trial to assess the safety, feasibility, and efficacy of transferring a potentially anti-arthritic cytokine gene to human joints with rheumatoid arthritis. Hum Gene Ther. 1996;7:1261–1280. doi: 10.1089/hum.1996.7.10-1261. [DOI] [PubMed] [Google Scholar]
- Gabay C, Arend WP. Treatment of rheumatoid arthritis with IL-1 inhibitors. Springer Semin Immunopathol. 1998;20:229–246. doi: 10.1007/s002810050032. [DOI] [PubMed] [Google Scholar]
- Rogler G, Andus T. Cytokines in inflammatory bowel disease. World J Surg. 1998;22:382–389. doi: 10.1007/s002689900401. [DOI] [PubMed] [Google Scholar]
- Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- Casini-Raggi V, Kam L, Chong YJT, Fiocchi C, Pizarro TT, Cominelli F. Mucosal imbalance of IL-1 and IL-1 receptor antagonist in inflammatory bowel disease. A novel mechanism of chronic intestinal inflammation. J Immunol. 1995;154:2434–2440. [PubMed] [Google Scholar]
- Ferretti M, Casini-Raggi V, Pizarro TT, Eisenberg SP, Nast CC, Cominelli F. Neutralization of endogenous IL-1 receptor antagonist exacerbates and prolongs inflammation in rabbit immune colitis. J Clin Invest. 1994;94:449–453. doi: 10.1172/JCI117345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne S, D'Agata ID, Hiscott J, Vanounou T, Seidman EG. Colonic explant production of IL-1 and its receptor antagonist is unbalanced in inflammatory bowel disease (IBD) Clin Exp Immunol. 1998;112:435–442. doi: 10.1046/j.1365-2249.1998.00595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield JC, Holden H, Tarlow JK, Di Giovine FS, McDowell TL, Wilson AG, Holdsworth CD, Duff GW. Novel genetic association between ulcerative colitis and the anti-inflammatory cytokine interleukin-1 receptor antagonist. Gastroenterology. 1994;106:637–642. doi: 10.1016/0016-5085(94)90696-3. [DOI] [PubMed] [Google Scholar]
- Tountas NA, Casini-Raggi V, Yang H, Di Giovine FS, Vecchi M, Kam L, Melani L, Pizarro TT, Rotter JI, Cominelli F. Functional and ethnic association of allele 2 of the interleukin-1 receptor antagonist gene in ulcerative colitis. Gastroenterology. 1999;117:806–813. doi: 10.1016/s0016-5085(99)70338-0. [DOI] [PubMed] [Google Scholar]
- Witkin SS, Gerber AS, Ledger WJ. Influence of interleukin-1 receptor antagonist gene polymorphism on disease. Clin Infect Dis. 2002;34:204–209. doi: 10.1086/338261. [DOI] [PubMed] [Google Scholar]
- Pozzi G, Oggioni MR, Medaglini D. Recombinant Streptococcus gordonii as live vehicle for vaccine antigens. In: Pozzi G, Wells JM, editor. In Gram-positive bacteria Vaccine vehicles for mucosal immunization. Springer-Verlag (Berlin) and Landes Bioscience (Georgetown, TX); 1997. pp. 35–60. [Google Scholar]
- Ricci S, Medaglini D, Rush CM, Marcello A, Manganelli R, Palù G, Pozzi G. Immunogenicity of the B monomer of the Escherichia coli heat-labile toxin expressed on the surface of Streptococcus gordonii. Infect Immun. 2000;68:760–766. doi: 10.1128/IAI.68.2.760-766.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beninati C, Oggioni MR, Boccanera M, Spinosa MR, Maggi T, Conti S, Magliani W, De Bernardis F, Teti G, Cassone A, Pozzi G, Polonelli L. Therapy of mucosal candidiasis by expression of an anti-idiotype in human commensal bacteria. Nat Biotechnol. 2000;18:1060–1064. doi: 10.1038/80250. [DOI] [PubMed] [Google Scholar]
- Giomarelli B, Provvedi R, Meacci F, Maggi T, Medaglini D, Pozzi G, Mori T, McMahon JB, Gardella RS, Boyd MR. The microbicide cyanovirin-N expressed on the surface of commensal bacterium Streptococcus gordonii captures HIV-1. AIDS. 2002;16:1351–1356. doi: 10.1097/00002030-200207050-00006. [DOI] [PubMed] [Google Scholar]
- Oggioni MR, Pozzi G. A host-vector system for heterologous gene expression in Streptococcus gordonii. Gene. 1996;169:85–90. doi: 10.1016/0378-1119(95)00775-X. [DOI] [PubMed] [Google Scholar]
- Medaglini D, Oggioni MR, Contorni M, Cavalieri F, Pozzi G. Secretion of heterologous proteins in Streptococcus gordonii (Streptococcus sanguis) Challis. In: Balla E, Berencsi G, Szentirmai A, editor. In DNA Transfer and Gene Expression in Microorganisms. Andover: Intercept Ltd; 1993. pp. 263–268. [Google Scholar]
- Pozzi G, Musmanno RA, Lievens PMJ, Oggioni MR, Plevani P, Manganelli R. Methods and parameters for genetic transformation of Streptococcus sanguis Challis. Res Microbiol. 1990;141:659–670. doi: 10.1016/0923-2508(90)90060-4. [DOI] [PubMed] [Google Scholar]
- Medaglini D, Oggioni MR, Pozzi G. Vaginal immunization with recombinant Gram positive bacteria. Am J Reprod Immunol. 1998;39:199–208. doi: 10.1111/j.1600-0897.1998.tb00354.x. [DOI] [PubMed] [Google Scholar]
- Sadlack B, Hartmut M, Schorle H, Schimpl A, Feller AC, Horak I. Ulcerative colitis-like disease in mice with disrupted interleukin-2 gene. Cell. 1993;75:253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- Horak I, Lohler J, Ma A, Smith KA. Interleukin-2 deficient mice: a new model to study autoimmunity and self-tolerance. Immunol Rev. 1995;148:35–44. doi: 10.1111/j.1600-065x.1995.tb00092.x. [DOI] [PubMed] [Google Scholar]
- de Villiers WJ, Varilek GW, de Beer FC, Guo JT, Kindy MS. Increased serum amyloid A levels reflect colitis severity and precede amyloid formation in IL-2 knockout mice. Cytokine. 2000;12:1337–1347. doi: 10.1006/cyto.2000.0716. [DOI] [PubMed] [Google Scholar]
- Autenrieth IB, Bucheler N, Bohn E, Heinze G, Horak I. Cytokine mRNA expression in intestinal tissue of interleukin-2 deficient mice with inflammatory bowel disease. Gut. 1997;41:793–800. doi: 10.1136/gut.41.6.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron JP, Fernandes JC, Martel-Pelletier J, Tardif G, Mineau F, Geng C, Pelletier J-P. Chondroprotective effect of intraarticular injections of interleukin-1 receptor antagonist in experimental osteoarthritis. Suppression of collagenase-1 expression. Arthritis Rheum. 1996;39:1535–1544. doi: 10.1002/art.1780390914. [DOI] [PubMed] [Google Scholar]
- Makarov SS, Olsen JC, Johnston WN, Anderle SK, Brown RR, Baldwin AS, Haskill JS, Schwab JH. Suppression of experimental arthritis by gene transfer of interleukin 1 receptor antagonist cDNA. Proc Natl Acad Sci USA. 1996;93:402–406. doi: 10.1073/pnas.93.1.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Ladner U, Roberts CRFBN, Gay RE, Robbins PD, Evans CH, Gay S. Human IL-1ra gene transfer into human synovial fibroblast is chondroprotective. J Immunol. 1997;158:3492–3498. [PubMed] [Google Scholar]
- Pelletier JP, Caron JP, Evans C, Robbins PD, Georgescu HI, Jovanovic D, Fernandes JC, Martel-Pelletier J. In vivo suppression of early experimental osteoarthritis by interleukin-1 receptor antagonist using gene therapy. Arthritis Rheum. 1997;40:1012–1019. doi: 10.1002/art.1780400604. [DOI] [PubMed] [Google Scholar]
- Evans CH, Robbins PD. Potential treatment of osteoarthritis by gene therapy. Rheum Dis Clin North Am. 1999;25:333–344. doi: 10.1016/s0889-857x(05)70071-5. [DOI] [PubMed] [Google Scholar]
- Steidler L, Robinson K, Chamberlain LM, Schofield KM, Remaut E, Le Page RWF, Wells JM. Mucosal delivery of murine interleukin-2 (IL-2) and IL-6 by recombinant strains of Lactococcus lactis coexpressing antigen and cytokine. Infect Immun. 1998;66:3183–3189. doi: 10.1128/iai.66.7.3183-3189.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steidler L, Hans W, Schotte L, Neirynck S, Obermeier F, Falk W, Fiers W, Remaut E. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science. 2000;289:1352–1355. doi: 10.1126/science.289.5483.1352. [DOI] [PubMed] [Google Scholar]
- Kruger C, Hu Y, Pan Q, Marcotte H, Hultberg A, Delwar D, Van Dalen PJ, Pouwels PH, Leer RJ, Kelly CG, Van Dollenweerd C, Ma JKC, Hammarstrom L. In situ delivery of passive immunity by lactobacilli producing single-chain antibodies. Nat Biotechnol. 2002;20:702–706. doi: 10.1038/nbt0702-702. [DOI] [PubMed] [Google Scholar]
- Bermudez-Humaran LG, Langella P, Cortes-Perez NG, Gruss A, Tamez-Guerra RS, Oliveira SC, Saucedo-Cardenas O, Montes de Oca-Luna R, Le Loir Y. Intranasal immunization with recombinant Lactococcus lactis secreting murine interleukin-12 enhances antigen-specific Th1 cytokine production. Infect Immun. 2003;71:1887–1896. doi: 10.1128/IAI.71.4.1887-1896.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takii T, Honda H, Sasayama S, Kobayashi T, Ikezawa H, Udaka S, Oomoto Y, Onozaki K. Human interleukin-1 receptor antagonist: large-scale expression in Bacillus brevis 47-5Q. J Interferon Citokine Res. 1999;19:1325–1331. doi: 10.1089/107999099313000. [DOI] [PubMed] [Google Scholar]
- Zanette D, Dundon W, Soffientini A, Sottani C, Marinelli F, Akeson A, Sarubbi E. Human IL-1 receptor antagonist from Escherichia coli : large-scale microbial growth and protein purification. J Biotechnol. 1998;64:187–196. doi: 10.1016/S0168-1656(98)00111-4. [DOI] [PubMed] [Google Scholar]
- Steidler L, Neirynck S, Huyghebaert N, Snoeck V, Vermeire A, Goddeeris B, Cox E, Remon JP, Remaut E. Biological containment of genetically modified Lactococcus lactis for intestinal delivery of human interleukin 10. Nat Biotechnol. 2003;21:785–789. doi: 10.1038/nbt840. [DOI] [PubMed] [Google Scholar]
- Maggi T, Spinosa MR, Ricci S, Medaglini D, Pozzi G, Oggioni MR. Genetic engineering of Streptococcus gordonii for the simultaneous display of two heterologous proteins at the bacterial surface. FEMS Microbiol Lett. 2002;210:135–141. doi: 10.1016/S0378-1097(02)00610-9. [DOI] [PubMed] [Google Scholar]
- Bossù P, Visconti U, Ruggiero P, Macchia G, Muda M, Bertini R, Bizzarri C, Colagrande A, Sabbatini V, Maurizi G, Del Grosso E, Tagliabue A, Boraschi D. Transfected type II interleukin-1 receptor impairs responsiveness of human keratinocytes to interleukin-1. Am J Pathol. 1995;147:1852–1861. [PMC free article] [PubMed] [Google Scholar]
- Boraschi D, Bossù P, Ruggiero P, Tagliabue A, Bertini R, Macchia G, Gasbarro G, Pellegrini L, Melillo G, Ulisse E, Visconti U, Bizzarri C, Del Grosso E, Mackay AR, Frascotti G, Frigerio F, Grifantini R, Grandi G. Mapping of receptor binding sites on IL-1 beta by reconstruction of IL-1ra like domains. J Immunol. 1995;155:4719–4725. [PubMed] [Google Scholar]
- Stoppacciaro A, Bossù P, Ghiara P, Ruco LP, Censini S, Scapigliati G, Nuti S, Tagliabue A, Baroni CD, Boraschi D. Binding of IL-1 beta to IL-1R type II at single cell level. J Immunol. 1991;147:1561–1566. [PubMed] [Google Scholar]
- Poole S, Gaines Das RE. The international standards for interleukin-1 alpha and interleukin-1 beta. Evaluation in an international collaborative study. J Immunol Methods. 1991;142:1–13. doi: 10.1016/0022-1759(91)90286-O. [DOI] [PubMed] [Google Scholar]
- Ruggiero P, Bossù P, Macchia G, Del Grosso E, Sabbatini V, Bertini R, Colagrande A, Bizzarri C, Maurizi G, Di Cioccio V, D'Andrea G, Di Giulio A, Frigerio F, Grifantini R, Grandi G, Tagliabue A, Boraschi D. Inhibitory activity of IL-1 receptor antagonist depends on the balance between binding capacity for IL-1 receptor type 1 and IL-1 receptor type II. J Immunol. 1997;158:3881–3887. [PubMed] [Google Scholar]
- Medaglini D, Rush CM, Sestini P, Pozzi G. Commensal bacteria as vehicles for mucosal vaccines against sexually transmitted diseases: vaginal colonization with recombinant streptococci includes local and systemic antibodies in mice. Vaccine. 1997;15:1330–1337. doi: 10.1016/S0264-410X(97)00026-1. [DOI] [PubMed] [Google Scholar]
- Schorle H, Holtschke T, Hunig T, Schimpl A, Horak I. Development and function of T cells in mice rendered interleukin-2 deficient by gene targeting. Nature. 1991;352:621–624. doi: 10.1038/352621a0. [DOI] [PubMed] [Google Scholar]