Abstract
TRAF5 [tumor necrosis factor (TNF) receptor-associated factor 5] is implicated in NF-κB and c-Jun NH2-terminal kinase/stress-activated protein kinase activation by members of the TNF receptor superfamily, including CD27, CD30, CD40, and lymphotoxin-β receptor. To investigate the functional role of TRAF5 in vivo, we generated TRAF5-deficient mice by gene targeting. Activation of either NF-κB or c-Jun NH2-terminal kinase/stress-activated protein kinase by tumor necrosis factor, CD27, and CD40 was not abrogated in traf5−/− mice. However, traf5−/− B cells showed defects in proliferation and up-regulation of various surface molecules, including CD23, CD54, CD80, CD86, and Fas in response to CD40 stimulation. Moreover, in vitro Ig production of traf5−/− B cells stimulated with anti-CD40 plus IL-4 was reduced substantially. CD27-mediated costimulatory signal also was impaired in traf5−/− T cells. Collectively, these results demonstrate that TRAF5 is involved in CD40- and CD27-mediated signaling.
Tumor necrosis factor receptor (TNFR) superfamily members transmit signals regulating proliferation, differentiation, and apoptosis in various types of cells (1, 2). TNFR-associated factors (TRAFs) emerged as a novel family of downstream mediators on the signal-transduction pathway of the TNFR superfamily (3). To date, six members of the TRAF family have been identified (4–12). All TRAFs, except for TRAF1, are composed of N-terminal zinc RING finger and multiple zinc fingers, coiled-coil, and C-terminal receptor-binding (TRAF) domains. With the exception of TRAF4, all the other TRAFs have been shown to interact directly with some members of the TNFR superfamily lacking death domains (3). TRAF2 also interacts indirectly with death domain receptors via the adapter molecules, TRADD and RIP (13). Overexpression of TRAF2, -5, and -6 activates NF-κB, and truncated TRAF2, -5, and –6, which lack Zn-binding domains, act as a dominant negative inhibitor in receptor-mediated NF-κB activation (9, 10, 14), suggesting that these TRAFs are common mediators for NF-κB activation by TNFR superfamily members. It has been shown that TRAF2, -5, and -6 recruit NF-κB-inducing kinase (NIK), which, in turn, activates IκB kinases (15–17). TRAF2, -5, and -6 also participate in the activation of c-Jun NH2-terminal kinase (JNK)/stress-activated protein kinase (SAPK), which is mediated by two members of the MAP kinase kinase kinase (MAPKKK) family, MAP kinase/ERK kinase kinase 1 (MEKK1) and apoptosis signal-regulating kinase 1 (ASK1) (16, 18). These results indicated that the two signaling pathways to activation of NF-κB and JNK/SAPK diverge downstream of TRAFs. Although these pathways have been investigated extensively, other signaling cascades potentially mediated by TRAFs have been largely unknown. Moreover, although both TRAF2 and TRAF5 are recruited to CD27, CD30, CD40, OX40, lymphotoxin-β receptor (LT-βR), herpesvirus entry mediator, and receptor activator of NF-κB, it remains to be determined whether TRAF2 and TRAF5 have individually specific functions or act redundantly in transmitting signals via these receptors (3).
Recent generation of TRAF2-deficient mice and transgenic mice expressing a dominant negative form of TRAF2 (TRAF2-DN) revealed that TRAF2 is requisite for JNK/SAPK activation but not for NF-κB activation by TNF (19, 20). Whereas TRAF2- and TRAF3-deficient mice die earlier (19, 21), TRAF3-deficient mice showed impaired T-dependent immune response (21). Collectively, these results suggested that each TRAF could act redundantly or specifically in particular signaling cascades.
To examine the functional role of TRAF5 in vivo, we generated TRAF5-deficient mice by gene targeting. Whereas NF-κB or JNK/SAPK activation by CD27 or CD40 was not abrogated, we found that CD27- and CD40-mediated lymphocyte activation was substantially impaired in traf5−/− lymphocytes.
MATERIALS AND METHODS
Cells.
Murine mastcytoma P815, murine CD70-transfected P815 (CD70-P815), and murine CD80-transfected P815 (CD80-P815) have been described (22) and were maintained in RPMI 1640 medium containing 10% FCS.
Generation of traf5−/− Mice by Gene Targeting.
A genomic DNA clone for traf5 was isolated by screening a 129/Sv/J mouse genomic DNA library with cDNA encoding a RING finger domain of TRAF5 as a probe. The cDNA encoding the RING finger domain contained two separate exons, which were designated exon I and exon II. The targeting construct (T5-KO) was made by replacing the exon II encoding a C-terminal half of the RING finger domain and surrounding introns with a pMC1neo gene cassette (Stratagene) in the reverse orientation to the endogenous traf5 gene (Fig. 1A). Linealized T5-KO then was transfected into embryonic stem (ES) cells (R1 cells) by electroporation. Neomycin-resistant ES clones were selected by G418 and GANC as described (23). Homologous recombinants were identified by Southern blotting. Genomic DNA was isolated and digested with EcoRI or BamHI, and Southern blots were hybridized with the 3′ flanking probe (probe A) as shown in Fig. 1A. Germ-line chimeras were generated by the aggregation method as described (24). The resulting male chimeras were backcrossed with C57BL/6J females, and germ-line transmission in F1 traf5+/− mice was verified by Southern blot analysis. Genotyping of the F2 mice was performed by PCR analysis of tail genomic DNA. The PCR primers (P1, 5′-GGG TCA TGC CAC TTG TTC GA-3′, and P2, 5′-ACC CAC ACG AGG AAG GTC TGA-3′; see Fig. 1A) were designed to encompass the 0.7-kb fragment containing exon II and surrounding introns, which was replaced by pMC1neo, resulting in a 1.2-kb fragment in the targeted allele.
Figure 1.
Targeted disruption of the murine traf5 gene. (A) A portion of the traf5 gene containing 5 exons (solid boxes) is indicated (Top). The targeting construct (Middle) was designed to replace exon II, which encodes the C-terminus of the RING finger domain with a pMC1neo cassette in a reverse orientation. (Bottom) The mutant locus resulting from homologous recombination. An extra EcoRI restriction site introduced by the pMC1neo cassette was used for the detection of homologous recombination. (B) Southern blot analysis of genomic DNA from wild-type and traf5+/− (clone 46, 48) ES cells. DNA was digested with EcoRI and hybridized with probe A as described in A. (C) PCR analysis of genomic DNA from wild-type, traf5+/−, and traf5−/− animals. Tail DNA was amplified by PCR using P1 and P2 primers as described in A. (D) Western blot analysis of TRAF5 protein expression. Total lung lysates from wild-type, traf5+/−, and traf5−/− mice were subjected to affinity purification by using glutathione beads preadsorbed with GST-LT-βR fusion protein (L) or control beads preadsorbed with GST protein alone (G). The positive control lysate (P) was prepared from 293 cells transfected with TRAF5 expression vector. The bound materials were fractionated by SDS/PAGE, and the Western blot was probed with polyclonal antibodies raised against a peptide derived from TRAF5-N, TRAF5-C, or N-terminal TRAF2 (25).
Western Blot Analysis.
Total lung lysates were prepared from traf5+/+, traf5+/−, and traf5−/− littermates by homogenization in a lysis buffer containing 20 mM Hepes (pH 7.2), 150 mM NaCl, 0.5% Triton-X100, 2 mM EDTA, 1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 mM PMSF, and 1 μg/ml pepstatin. TRAF2 and TRAF5 were affinity-purified from the lung lysates by using glutathione-Sepharose (Amersham Pharmacia) preadsorbed with glutathione S-transferase (GST) or a fusion protein GST-LT-βR containing the cytoplasmic region of human LT-βR (9). After three washes in the lysis buffer, the bound proteins were eluted and fractionated by 10% SDS/PAGE and blotted onto polyvinylidene fluoride membrane (Millipore). The Western blot was probed with polyclonal antibodies against N-terminal peptide (TRAF5-N) or C-terminal peptide of TRAF5 (TRAF5-C) (Santa Cruz Biotechnology). The blot then was developed by using an enhanced chemiluminescence system according to the manufacturer’s instructions (Amersham Pharmacia). As a control, the same membrane was reprobed with a polyclonal antibody against N-terminal peptide of TRAF2 (a gift from D. V. Goeddel, Tularik, South San Francisco) (25).
Flow Cytometric Analysis.
Single-cell suspensions were prepared from the thymus, spleen, lymph nodes, and bone marrow. Cells were incubated with FITC- or phycoerythrin (PE)-conjugated mAbs and analyzed by using a FACScan (Becton Dickinson). The following mAbs from PharMingen were used: anti-B220, anti-CD4, anti-CD8, anti-CD3, anti-CD23, anti-CD54, anti-Fas, anti-CD80, and anti-CD86.
Proliferation of B and T Cells.
Small resting B cells were prepared from splenocytes as described (22). Small resting B cells (1 × 105) were stimulated with 10 μg/ml anti-CD40 mAb (HM40–3; PharMingen), 10% volume of culture supernatant of cells producing murine CD40L-CD8 chimeric molecule (26), 10 ng/ml IL-4 (R&D Systems), 10 μg/ml affinity-purified goat anti-mouse IgM antibody (Jackson ImmunoResearch Laboratories), or 5 μg/ml lipopolysaccharide (LPS; Sigma). Thymocytes (2 × 105/well) were cocultured with irradiated P815, CD70-P815, or CD80-P815 (2 × 104/well) in the presence of anti-CD3 mAb (1 μg/ml) as described (22). These cultures were pulsed for the last 8 hr of a 48-hr culture period with 0.5 μCi/well of [3H]thymidine (Amersham Pharmacia). Cells then were harvested onto glass-fiber filters, and the radioactivity was measured in a micro-β-counter (Micro Beta Plus; Wallac, Gaithersburg, MD).
Up-Regulation of Surface Molecules on B Cells.
Small resting B cells were cultured for 48 hr in the absence (unstimulated) or presence of anti-CD40 mAb (10 μg/ml) or LPS (5 μg/ml). The cells then were stained with FITC-conjugated anti-CD23, anti-CD54, anti-CD80, anti-CD86, or anti-Fas mAb and analyzed by flow cytometry.
Electrophoretic Mobility-Shift Assay (EMSA).
EMSA was performed essentially as described (9). Briefly, 3 × 107 thymocytes were incubated with agonistic anti-CD27 mAb (10 μg/ml; PharMingen) on ice for 30 min. Then, the cells were washed with ice-cold PBS and crosslinked with prewarmed goat anti-hamster Igs (100 μg/ml; Cappel) at 37°C for 15 min. For CD40 or LPS stimulation, 2 × 107 splenocytes were incubated with anti-CD40 mAb (10 μg/ml) at 37°C for 15 min or LPS (5 μg/ml) for 30 min. Then, the nuclear extracts were prepared. The nuclear extracts were subjected to EMSA. Reactions were subjected to 6% PAGE and analyzed on a Fuji BAS2000 image analyzer.
In Vitro Kinase Assay.
In vitro kinase assay was performed essentially as described (27). Briefly, 5 × 106 thymocytes were incubated with agonistic anti-CD27 mAb (10 μg/ml) on ice for 30 min. Then, the cells were washed with ice-cold PBS and crosslinked with prewarmed goat anti-hamster Igs (100 μg/ml) at 37°C for 15 min. For CD40 stimulation, purified B cells (5 × 106) were incubated with anti-CD40 (10 μg/ml) at 37°C for 15 min. The cells were lysed in the lysis buffer containing 1 mM NaF and 0.1 mM Na3VO4, incubated with rabbit polyclonal anti-JNK antibody (Santa Cruz Biotechnology), and precipitated with protein G-Sepharose beads (Amersham Pharmacia). The immunoprecipitates then were subjected to in vitro kinase assay by using GST-c-Jun (1–79) (a gift from E. Nishida, Kyoto University) as a substrate. The reaction was stopped by addition of Laemmli’s sample buffer, and phosphorylated proteins were subjected to 12% SDS/PAGE and analyzed with a Fuji BAS2000 image analyzer.
In Vitro Ig Production.
Small resting B cells (1 × 105) were stimulated with anti-CD40 (10 μg/ml) plus IL-4 (10 ng/ml) for 6 days. Concentrations of IgM and IgG1 in the culture supernatants were measured by ELISA. In brief, plates were coated with 5 μg/ml monoclonal rat anti-mouse IgM or IgG1 (PharMingen) overnight at 4°C. Plates were washed and blocked with 1% BSA-PBS. Diluted culture supernatants were incubated for 1 hr. Then, plates were washed and bound IgM and IgG1 were determined with biotin-conjugated rat anti-mouse IgM or anti-IgG1 followed by avidin-biotinylated peroxidase complex (Vector Laboratories).
Analysis of Humoral Immune Responses.
Humoral response to a T-dependent antigen was analyzed essentially as described (28). Briefly, traf5+/+ and traf5−/− mice (8-12 weeks old) were immunized with (4-hydroxy-3-nitrophenyl-acetyl)-chicken gamma globulin (molar ratio, 22:1; NP22-CG) adsorbed to alum. Immunization was performed by i.p. injection of 5 μg of NP22-CG/alum on day 0 and an i.p. boost with 5 μg of NP22-CG/alumn on day 21. Sera were collected on days 7, 14, 21, and 28. Total and high-affinity, NP-specific antibodies were determined by an ELISA against NP22-BSA- or NP2-BSA-coated ELISA plates (Immulon), respectively. NP-specific antibodies of IgM or IgG1 type were detected as described above. Arbitrary units of NP-specific antibodies were calculated by using an NP-specific mAb C6 (for IgG1) and standard immune sera (for IgM) as the standards. Statistical analysis was performed by Student’s t test, Welch’s t test, or nonparametric Mann–Whitney test.
RESULTS
Normal Development of TRAF5-Deficient Mice.
To elucidate the function of TRAF5 in vivo, we disrupted the murine traf5 gene in ES cells. A targeting vector was designed to replace the exon II encoding the C-terminal portion of the RING finger domain of TRAF5 with a neomycin-resistant gene (Fig. 1A). Heterozygous ES cell lines containing a mutated traf5 allele were used to generate germ-line chimeras by the aggregation method (24), and traf5+/− animals were obtained. Heterozygous animals were interbred to obtain homozygotes. Homologous recombination and the genotypes were confirmed by Southern blot analysis (Fig. 1B) or PCR (Fig. 1C). TRAF5 protein expression in tissue was determined by affinity purification by using GST-LT-βR (9) and subsequent Western blot analysis by using polyclonal antibodies against TRAF5-N, TRAF5-C, or N-terminal TRAF2 peptides. This analysis demonstrated the absence of intact or truncated TRAF5 protein in traf5−/− animals and also showed that deletion of traf5 gene did not affect the expression of TRAF2 (Fig. 1D). TRAF5-deficient mice were born at the expected Mendelian ratios (+/+:+/−:−/− = 118:203:121). These mice were healthy and showed no obvious abnormalities through 24 weeks of age. There was no developmental defect of lymph nodes and Peyer’s patches, indicating that LT-βR signaling during embryogenesis of secondary lymphoid organs is competent (28). Flow cytometric analysis of the expression of CD3, CD4, CD8, and B220 on thymocytes, splenocytes, or lymph node cells showed that lymphocyte composition was not altered in traf5−/− mice when compared with traf5+/+ mice (data not shown).
CD27-Mediated Costimulation Is Impaired in traf5−/− T Cells.
Engagement of CD27 by its ligand CD70 or agonistic anti-CD27 mAb provides a costimulatory signal for T cell proliferation, and both TRAF2 and TRAF5 are implicated in CD27-mediated activation of NF-κB and JNK/SAPK (27). Because CD27 is expressed on most thymocytes, we asked whether CD27-mediated costimulation was impaired in traf5−/− thymocytes. Our previous study showed that murine CD70 or CD80 transfectants (CD70-P815 or CD80-P815) enhanced proliferation of anti-CD3-stimulated T cells (22). Traf5+/+ and traf5−/− thymocytes then were cultured with P815, CD70-P815, or CD80-P815 in the presence of a suboptimal dose of anti-CD3 mAb as described (22). Although CD80-P815 enhanced proliferation of traf5+/+ and traf5−/− thymocytes comparably, traf5−/− thymocytes showed consistently lower responses than traf5+/+ thymocytes when costimulated by CD70-P815 (Fig. 2A). This indicated that CD27-mediated costimulation was impaired specifically in traf5−/− thymocytes.
Figure 2.
Impairment of CD27-mediated T cell costimulation but not in NF-κB or JNK/SAPK activation. (A) Thymocytes isolated from traf5+/+ (solid bars) and traf5−/−(hatched bars) mice were stimulated with anti-CD3 mAb in the presence of irradiated P815, P815-CD70, or P815-CD80 for 48 hr. Incorporation of [3H]thymidine was measured during the last 8 hr. Data are shown as mean cpm ± SEM from the triplicate samples and represent one of five independent experiments with similar results. (B) Thymocytes isolated from traf5+/+ and traf5−/− mice were stimulated with anti-CD27 followed by crosslinking with goat anti-hamster Igs for 15 min. EMSAs for NF-κB activation (Upper) and in vitro kinase assay for JNK/SAPK activation (Lower) were performed as described in Materials and Methods. B, oligonucleotide probe bound to NF-κB complexes.
We next examined whether CD27-mediated activation of NF-κB and JNK/SAPK was impaired in traf5−/− thymocytes. Thymocytes from traf5+/+ and traf5−/− mice were stimulated with agonistic anti-CD27 mAb followed by goat anti-hamster Igs to aggregate CD27. The cells then were assayed for activation of NF-κB and JNK/SAPK by EMSA and in vitro kinase reactions. Unexpectedly, the activation of either NF-κB or JNK/SAPK by CD27 was not altered significantly in traf5−/− thymocytes (Fig. 2B).
CD40-Mediated Activation Is Impaired in traf5−/− B Cells.
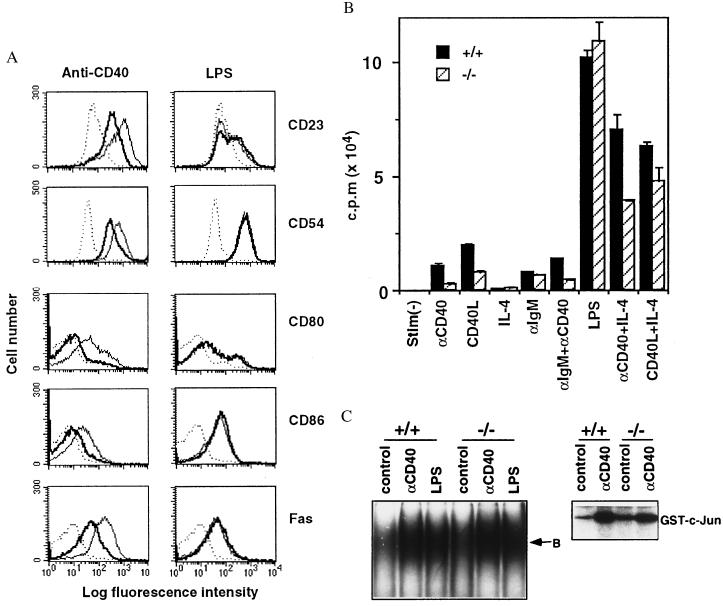
TRAF5 has been shown to interact with CD40 and truncated TRAF5-inhibited surface up-regulation of CD23 by CD40 (12). Small resting B cells from traf5+/+ and traf5−/− mice were stimulated with agonistic anti-CD40 mAb or LPS as a control. Both of these treatments enhanced the expression of CD23, CD54, CD80, CD86, and Fas on traf5+/+ B cells. However, the up-regulation of these molecules on anti-CD40-stimulated traf5−/− B cells was reduced substantially when compared with traf5+/+ B cells (Fig. 3A). On the other hand, the up-regulation of these molecules on LPS-stimulated traf5−/− B cells was comparable to traf5+/+ B cells. We next examined proliferation of B cells in response to various stimuli. Proliferative responses of traf5−/−B cells to anti-CD40, CD40 ligand, and anti-CD40 plus anti-IgM were reduced substantially when compared with those of traf5+/+ B cells (Fig. 3B). Moreover, addition of IL-4 partially restored the response. In contrast, proliferative responses of traf5−/− B cells to IL-4, anti-IgM, or LPS were comparable to those of traf5+/+ B cells. Collectively, these results indicated a specific defect in CD40-mediated activation of traf5−/− B cells.
Figure 3.
CD40-mediated B cell activation in vitro. (A) Up-regulation of CD23, CD54, CD80, CD86, and Fas by anti-CD40 or LPS stimulation. Purified B cells from traf5+/+ (thin line) and traf5−/− (thick line) were stimulated with anti-CD40 mAb or LPS or were unstimulated (dotted line). After 48 hr, the cells were stained with FITC-labeled anti-CD23, anti-CD54, anti-CD80, anti-CD86, or anti-Fas mAb. Data represent one of three independent experiments with similar results. (B) Proliferative responses. Purified B cells isolated from traf5+/+ (solid bars) and traf5−/− (hatched bars) mice were stimulated for 48 hr with anti-CD40 mAb, CD40L-CD8 chimeric protein (CD40L), IL-4, anti-IgM Ab, or LPS. Proliferation was assessed by [3H]thymidine incorporation during the last 8 hr. Data are shown as the mean cpm ± SEM from triplicate samples and represent one of three independent experiments with similar results. (C) Splenocytes (for EMSA) or purified B cells (for in vitro kinase assay) isolated from traf5+/+ and traf5−/− mice were stimulated with anti-CD40 mAb for 15 min or LPS for 30 min. EMSA for NF-κB activation (Left) and in vitro kinase assay for JNK/SAPK activation (Right) were performed as described in Materials and Methods. B, oligonucleotide probe bound to NF-κB complexes.
Upon stimulation with anti-CD40 plus IL-4 in vitro, B cells differentiate and produce IgG1 as well as IgM. To examine whether the defect in CD40-mediated signals of traf5−/− B cells could affect the differentiation, traf5+/+ or traf5−/− B cells were stimulated with anti-CD40 plus IL-4. Then, Ig production was assessed by isotype-specific ELISA. Production of IgM as well as IgG1 by traf5−/− B cells was reduced substantially when compared with traf5+/+ B cells (Table 1), suggesting that TRAF5 is involved in CD40-mediated Ig production and class switching.
Table 1.
In vitro Ig production of traf5+/+ and traf5−/− B cells
| IgM, ng/ml
|
IgG1, ng/ml
|
|||
|---|---|---|---|---|
| +/+ | −/− | +/+ | −/− | |
| Experiment 1 | 271.1 ± 40.0 | 66.7 ± 4.9 | 404.7 ± 21.6 | 130.0 ± 13.9 |
| Experiment 2 | 327.0 ± 34.1 | 137.3 ± 7.2 | 319.8 ± 19.3 | 222.5 ± 2.9 |
| Experiment 3 | 746.0 ± 217.6 | 104.7 ± 18.0 | 273.3 ± 28.0 | 80.7 ± 13.3 |
Purified B cells (1 × 105) were stimulated with anti-CD40 (10 μg/ml) plus IL-4 (10 ng/ml). After 6 days, IgM and IgG1 concentrations in the culture supernatants were measured by ELISA. IgM and IgG1 from unstimulated cultures were undetectable. Three independent experiments are shown, and the differences between traf5+/+ and traf5−/− are statistically significant (P < 0.001. Student’s t test).
It has been shown that CD40-mediated activation of JNK/SAPK, but not NF-κB, was impaired in B cells from TRAF2-DN transgenic mice (20). We then examined whether activation of NF-κB and JNK/SAPK by CD40 was impaired in traf5−/− B cells. Splenocytes were stimulated with anti-CD40 mAb or LPS for the indicated time for EMSA, and purified B cells were stimulated with anti-CD40 mAb for 15 min for in vitro kinase assay. As shown in Fig. 3C, CD40-mediated activation of NF-κB or JNK/SAPK apparently was not impaired in traf5−/− B cells.
Humoral Responses in traf5−/− Mice.
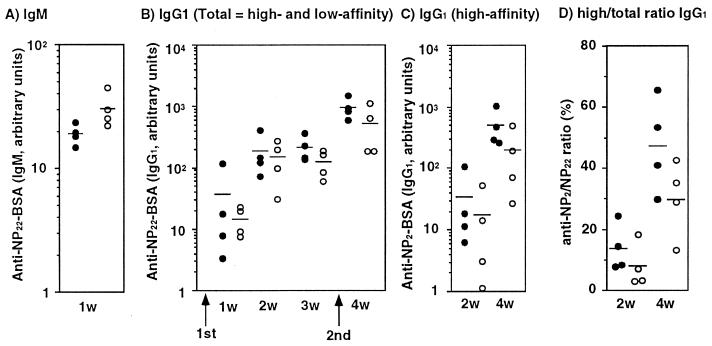
CD40/CD40L interaction plays a crucial role in humoral responses to T-dependent antigens, especially for class switching and germinal center formation (29, 30). The defect in CD40-mediated activation in traf5−/− B cells in vitro prompted us to explore whether humoral responses were impaired in traf5−/− mice. We first examined the antibody production against a T-dependent antigen, NP22-CG (molar ratio, 22:1). Eight- to 12-week-old traf5+/+ and traf5−/− mice were injected i.p. with 50 μg (high-dose protocol) or 5 μg (low-dose protocol) of NP22-CG adsorbed to alum. Each mouse was boosted with 5 μg of NP22-CG 21 days later, and the sera were collected at the indicated time points in Fig. 4. Total IgM and IgG1 anti-NP-specific antibodies or high-affinity IgG1 anti-NP antibody was determined by binding to densely (NP22-BSA) or sparsely (NP2-BSA) NP-haptenated BSA, respectively. Ratios of high-affinity IgG1 antibody to total IgG1 antibody also were calculated. Because we did not observe any significant difference in total IgM and IgG1 titers or high-affinity IgG1 titers between traf5+/+ and traf5−/− mice (data not shown) in a high-dose protocol, we next checked humoral response of traf5−/− mice to NP22-CG in a low-dose protocol. Traf5−/− mice consistently produced more anti-NP IgM than traf5+/+ mice after primary immunization (Fig. 4A). Although there is a trend of lower antibody production of both total and high-affinity IgG1 in traf5−/− mice compared with wild-type mice (Fig. 4B), the differences are not statistically significant based on Student’s t test, Welch’s t test, or nonparametric Mann–Whitney test (data not shown). However, there does appear to be a reduction in affinity maturation as evidenced by high/total ratio IgG1 at 4 weeks (P = 0.0537 by Student’s t test and P = 0.0584 by Welch’s t test). These data are similar to those observed in LT-β-deficient mice, in which there was no difference in total anti-NP titer but a reduction in affinity maturation (31). This decrease in affinity maturation also was observed in LT-βR-deficient animals at 21 days postimmunization (28). On the other hand, humoral response to a T-independent antigen, NP-Ficoll, in traf5−/− mice was almost comparable to that in traf5+/+ mice (data not shown). Collectively, these results demonstrated that traf5−/− mice showed a mild defect in humoral responses to a T-dependent antigen.
Figure 4.
Specific humoral responses and affinity maturation in traf5−/− mice. Traf5+/+ (●) and traf5−/− (○) mice were immunized with 5 μg of NP22-CG/alum on day 0 and boosted with the same dose on day 21. NP-specific IgM (A), total IgG1 (B), and high-affinity IgG1 (C) antibodies were measured at the indicated time points. IgM antibodies were determined by ELISA against densely haptenated BSA (NP22-BSA). Total and high-affinity IgG1 antibodies were determined by ELISA against NP22-BSA and NP2-BSA, respectively. The levels of anti-NP IgG1 are indicated as arbitrary units by using an NP-specific IgG1 mAb of known affinity (C6; Kd = 3.3 × 10−7 M) as a standard. The levels of anti-NP IgM are indicated as arbitrary units determined by using an immune sera as a standard. In D, high-affinity/total IgG1 ratios were calculated from the value in B and C for each individual. Bars indicate the means of four mice in each experimental group. Data represent one of two independent experiments with similar results.
DISCUSSION
Recently, generated knock-out mice of various molecules that mediate signaling via the TNFR superfamily members revealed redundant or unique functions of each molecule in particular signaling cascades. TRAF5 and TRAF2 bind overlapping members of the TNFR superfamily (3). When overexpressed, both activate NF-κB- and JNK/SAPK-signaling pathways (16, 27), yet TRAF5- and TRAF2-deficient mice exhibit distinct phenotypes (19). Although CD40-mediated activation of NF-κB or JNK/SAPK was not affected in traf5−/− B cells, up-regulation of surface molecules and proliferation induced by CD40 were impaired. The defect of CD40-mediated signaling of traf5−/− B cells also was substantiated by the reduction of IgM and IgG1 production in vitro upon stimulation with anti-CD40 plus IL-4. A previous study showed that up-regulation of CD23, CD80, and Fas induced by CD40 was not correlated with the ability of CD40 to activate NF-κB (32). Although truncated TRAF3 inhibited up-regulation of CD23 by CD40 (5, 12), CD40-mediated signaling including up-regulation of CD23 or proliferation was not impaired in traf3−/− B cells (21). Given that TRAF2, -3, and -5 bind to the overlapping region of CD40 (12), truncated TRAF3 could displace endogenous TRAF2 or TRAF5, resulting in impairment of CD23 up-regulation. Collectively, these results indicated that TRAF5 is actually involved in these signaling pathways. CD27-mediated costimulatory signal also was impaired in traf5−/− T cells despite no significant impairment in CD27-mediated NF-κB and JNK/SAPK activation. Collectively, these results suggest that TRAF5 is involved in CD40- and CD27-mediated signaling independently of NF-κB and JNK/SAPK activation.
CD40/CD40L interaction is required for class switching and germinal center formation (29, 30). The mild defect in affinity maturation of IgG1 antibodies to T-dependent antigen observed in traf5−/− mice can directly reflect the impairment of CD40-mediated B cell activation observed in vitro. It has been shown that the up-regulation of CD80 and CD86 by CD40-mediated stimulation increases the activity of B cells as antigen-presenting cells. Thus, the partial defect in up-regulation of CD80 and CD86 on traf5−/− B cells also may be relevant to the mild defect of humoral response to T-dependent antigen. Alternatively, because CD27 also has been implicated in the regulation of humoral responses in vitro (33), the impairment of CD27-mediated signaling also may be involved. Impairment of T-dependent immune response also was observed in traf3−/− mice (30). Comparing the substantial reduction of in vitro Ig production of traf5−/− B cells with the only mild defect of humoral response to T-dependent antigen, other pathways could compensate the defect of CD40-mediated signals in traf5−/− mice in vivo.
Previous studies demonstrated that TRAF2 is not requisite for NF-κB activation by TNF (19, 20). Our preliminary results based on NF-κB-dependent reporter gene assay by using 293 cells showed that truncated TRAF5 inhibited NF-κB activation by TNF (data not shown), suggesting that TRAF5 could be involved in NF-κB activation by TNF. We then asked whether TNF-mediated NF-κB activation was impaired in traf5−/− thymocytes or embryonic fibroblast (EF) cells. Unexpectedly, TNF-mediated NF-κB activation was not impaired in traf5−/− thymocytes or EF cells (data not shown), suggesting that TRAF5 is not requisite for TNF-mediated NF-κB activation. Alternatively, TRAF2 and TRAF5 might be functionally redundant in TNF-mediated NF-κB activation. Formally, the possibility cannot be excluded that a molecule other than TRAF2 or TRAF5 is involved in NF-κB activation by the TNFR superfamily to account for these results. Generation of TRAF2 and TRAF5 double-deficient mice will be required to address this issue.
As far as we examined, we could not detect any defect in LT-βR-mediated signaling, such as vascular cell adhesion molecule 1 induction and chemokine production, in traf5−/− EF cells stimulated by agonistic anti-LT-βR mAb or recombinant soluble LTα1β2 (data not shown). There was no defect of lymph node development and germinal center formation in TRAF5-deficient mice (data not shown), suggesting that TRAF5 might not be involved in LT-βR-mediated signalings. However, considering that TRAF2 and TRAF5 are implicated in LT-βR-mediated NF-κB activation (9, 34) and lymph node development was not impaired in TRAF2-deficient mice (19), TRAF2 and TRAF5 could be redundant in LT-βR-mediated signaling. In addition, both TRAF2 and TRAF5 have been implicated in the signaling via CD30, HVEM, OX40, and RANK (3). The traf5−/− mice will be useful for investigating further the signaling pathways via these receptors.
Acknowledgments
We thank Masahisa Shindo, Kazunori Hanaoka, Michiko Hanaoka, Kasuyoshi Takeda, Machiko Atsuta, Atsushi Tajima, Masaaki Abe, Yoshimasa Takahashi, Akio Nakane, and Hidehito Kuroyanagi for technical assistance and helpful discussions. We also thank Hidechika Azuma, Hideo Oshima, Noboru Motoyama, David V. Goeddel, Jeffrey L. Browning, Peter Lane, and Eisuke Nishida for reagents. This work was supported by grants from the Ministry of Education, Science, and Culture, the Ministry of Health, Japan.
ABBREVIATIONS
- TNFR
tumor necrosis factor receptor
- TRAF
TNFR-associated factor
- JNK
c-Jun NH2-terminal kinase
- SAPK
stress-activated protein kinase
- LT-βR
lymphotoxin-β receptor
- ES
embryonic stem
- LPS
lipopolysaccharide
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Beutler B, van Huffel C. Science. 1994;264:667–668. doi: 10.1126/science.8171316. [DOI] [PubMed] [Google Scholar]
- 2.Tracey K J, Cerami A. Annu Rev Med. 1994;45:491–503. doi: 10.1146/annurev.med.45.1.491. [DOI] [PubMed] [Google Scholar]
- 3.Arch R H, Gedrich R W, Thompson C B. Genes Dev. 1998;12:2821–2830. doi: 10.1101/gad.12.18.2821. [DOI] [PubMed] [Google Scholar]
- 4.Rothe M, Wong S C, Henzel W J, Goeddel D V. Cell. 1994;78:681–692. doi: 10.1016/0092-8674(94)90532-0. [DOI] [PubMed] [Google Scholar]
- 5.Cheng G, Cleary A M, Ye Z-s, Hong D I, Lederman S, Baltimore D. Science. 1995;267:1494–1498. doi: 10.1126/science.7533327. [DOI] [PubMed] [Google Scholar]
- 6.Hu H M, O’Rourke K, Boguski M S, Dixit V M. J Biol Chem. 1995;269:30069–30072. [PubMed] [Google Scholar]
- 7.Mosialos G, Birkenbach M, Yalamanchili R, VanArsdale T, Ware C F, Kieff E. Cell. 1995;80:389–399. doi: 10.1016/0092-8674(95)90489-1. [DOI] [PubMed] [Google Scholar]
- 8.Regnier C H, Tomasetto C, Moog-Lutz C, Chenard M-P, Wendling C, Basset P, Rio M-C. J Biol Chem. 1995;270:25715–25721. doi: 10.1074/jbc.270.43.25715. [DOI] [PubMed] [Google Scholar]
- 9.Nakano H, Oshima H, Chung W, Williams-Abbot L, Ware C F, Yagita H, Okumura K. J Biol Chem. 1996;271:14661–14664. doi: 10.1074/jbc.271.25.14661. [DOI] [PubMed] [Google Scholar]
- 10.Cao Z, Xiong J, Takeuchi M, Kurama T, Goeddel D V. Nature (London) 1996;383:443–446. doi: 10.1038/383443a0. [DOI] [PubMed] [Google Scholar]
- 11.Ishida T, Mizushima S, Azuma S, Kobayashi N, Tojo T, Suzuki K, Aizawa S, Watanabe T, Mosialos G, Kieff E, et al. J Biol Chem. 1996;271:28745–28748. doi: 10.1074/jbc.271.46.28745. [DOI] [PubMed] [Google Scholar]
- 12.Ishida T, Tojo T, Aoki T, Kobayashi N, Ohishi T, Watanabe T, Yamamoto T, Inoue J-I. Proc Natl Acad Sci USA. 1996;93:9437–9442. doi: 10.1073/pnas.93.18.9437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ashkenazi A, Dixit V M. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 14.Rothe M, Sarma V, Dixit V M, Goeddel D V. Science. 1995;269:1424–1427. doi: 10.1126/science.7544915. [DOI] [PubMed] [Google Scholar]
- 15.Malinin N L, Boldin M P, Kovalenko A V, Wallach D. Nature (London) 1997;385:540–544. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- 16.Song H Y, Regnier C H, Kirschning C J, Goeddel D V, Rothe M. Proc Natl Acad Sci USA. 1997;94:9792–9796. doi: 10.1073/pnas.94.18.9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stancovski I, Baltimore D. Cell. 1997;91:299–302. doi: 10.1016/s0092-8674(00)80413-4. [DOI] [PubMed] [Google Scholar]
- 18.Nishitoh H, Saitoh M, Mochida Y, Takeda K, Nakano H, Rothe M, Miyazono K, Ichijo H. Mol Cell. 1998;2:389–395. doi: 10.1016/s1097-2765(00)80283-x. [DOI] [PubMed] [Google Scholar]
- 19.Yeh W-C, Shahinian A, Speiser D, Kraunus J, Billia F, Wakeham A, de la Pompa J L, Ferrick D, Hum B, Iscove N, et al. Immunity. 1997;7:715–725. doi: 10.1016/s1074-7613(00)80391-x. [DOI] [PubMed] [Google Scholar]
- 20.Lee S Y, Reichlin A, Santana A, Sokol K A, Nussenzweig M C, Choi Y. Immunity. 1997;7:703–713. doi: 10.1016/s1074-7613(00)80390-8. [DOI] [PubMed] [Google Scholar]
- 21.Xu Y, Cheng G, Baltimore D. Immunity. 1996;5:407–415. doi: 10.1016/s1074-7613(00)80497-5. [DOI] [PubMed] [Google Scholar]
- 22.Oshima H, Nakano H, Nohara C, Kobata T, Nakajima A, Jenkins N A, Gilbert D J, Copeland N G, Muto T, Yagita H, et al. Int Immunol. 1998;10:517–526. doi: 10.1093/intimm/10.4.517. [DOI] [PubMed] [Google Scholar]
- 23.Wurst W, Joyner A L. Gene Targeting: A Practical Approach. Oxford: IRL; 1993. pp. 33–61. [Google Scholar]
- 24.Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder J C. Proc Natl Acad Sci USA. 1993;90:8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shu H-B, Takeuchi M, Goeddel D. Proc Natl Acad Sci USA. 1996;93:13973–13978. doi: 10.1073/pnas.93.24.13973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lane P, Brocker T, Hubele S, Padovan E, Lanzavecchia A, McConnell F. J Exp Med. 1993;177:1209–1213. doi: 10.1084/jem.177.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akiba H, Nakano H, Nishinaka S, Shindo M, Kobata T, Morimoto C, Ware C F, Malinin N L, Wallach D, Yagita H, et al. J Biol Chem. 1998;273:13353–13358. doi: 10.1074/jbc.273.21.13353. [DOI] [PubMed] [Google Scholar]
- 28.Futterer A, Mink K, Luz A, Kosco-Vilbois M H, Pfeffer K. Immunity. 1998;9:59–70. doi: 10.1016/s1074-7613(00)80588-9. [DOI] [PubMed] [Google Scholar]
- 29.Kawabe T, Naka T, Yoshida K, Tanaka T, Fujiwara H, Suematsu S, Yoshida N, Kishimoto T, Kikutani H. Immunity. 1994;1:167–178. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 30.Xu J, Foy T M, Laman J D, Elliott E A, Dunn J J, Waldschmidt T J, Elsemore J, Noelle R J, Flavell R A. Immunity. 1994;1:423–431. doi: 10.1016/1074-7613(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 31.Koni P A, Sacca R, Lawton P, Browning J L, Ruddle N H, Flavell R A. Immunity. 1997;6:491–500. doi: 10.1016/s1074-7613(00)80292-7. [DOI] [PubMed] [Google Scholar]
- 32.Hostager B S, Hsing Y, Harms D E, Bishop G A. J Immunol. 1996;157:1047–1053. [PubMed] [Google Scholar]
- 33.Kobata T, Jacquot S, Kozlowski S, Agematsu K, Schlossman S F, Morimoto C. Proc Natl Acad Sci USA. 1995;92:11249–11253. doi: 10.1073/pnas.92.24.11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakano H, Shindo M, Yamada K, Yoshida M C, Santee S M, Ware C F, Jenkins N A, Gilbert D J, Yagita H, Copeland N G, et al. Genomics. 1997;42:26–32. doi: 10.1006/geno.1997.4697. [DOI] [PubMed] [Google Scholar]