Abstract
AIDS-related non-Hodgkin’s lymphoma (AIDS NHL) comprises a diverse and heterogeneous group of high-grade B cell tumors. Certain classes of AIDS NHL are associated with alterations in oncogenes or tumor-suppressor genes or infections by oncogenic herpesviruses. However, the clinically significant class of AIDS NHL designated immunoblastic lymphoma plasmacytoid (AIDS IBLP) lacks any consistent genetic alterations. We identified the TCL1 oncogene from a set of AIDS IBLP-associated cDNA fragments generated by subtractive hybridization with non-AIDS IBLP. Aberrant TCL1 expression has been implicated in T cell leukemia/lymphoma development, and its expression also has been seen in many established B cell tumor lines. However, TCL1 expression has not been reported in AIDS NHL. We find that TCL1 is expressed in the majority of AIDS IBLP tumors examined. TCL1 protein expression is restricted to tumor cells in AIDS IBLP tissue samples analyzed with immunohistochemical staining. Hyperplastic lymph node and tonsil also exhibit strong TCL1 protein expression in mantle zone B cells and in rare interfollicular zone cells, whereas follicle-center B cells (centroblasts and centrocytes) show weaker expression. These results establish TCL1 as the most prevalent of all of the surveyed oncogenes associated with AIDS IBLP. They also indicate that abundant TCL1 expression in quiescent mantle zone B cells is down-regulated in activated germinal center follicular B cells in parallel to the known expression pattern of BCL-2. High-level expression in nonproliferating B cells suggests that TCL1 may function in protecting naïve preactivated B cells from apoptosis.
AIDS-related non-Hodgkin’s lymphoma (AIDS NHL) occurs in up to 10% of HIV-infected individuals who have moderate to severe immunodeficiency (1–3). These lymphomas are biologically and genetically heterogeneous, are derived from germinal center or postgerminal center B cells, and are classified according to body location and histologic criteria (reviewed in refs. 4–7). Certain AIDS NHL classes are associated with specific oncogenic lesions or viral involvement. For example, AIDS-related Burkitt’s lymphoma usually contain activating c-MYC translocations, whereas AIDS-related primary-effusion lymphoma consistently contain human herpesvirus-8 (8–24). AIDS diffuse large B cell lymphoma accounts for 70% of systemic lymphomas and is the second most common type of cancer after Kaposi’s sarcoma in AIDS patients (25, 26). Systemic AIDS diffuse large B cell lymphoma are further classified into two subclasses (6, 7, 27–29). AIDS large noncleaved-cell lymphoma is postulated to originate from germinal center B cells and often exhibits dysregulated expression of the BCL-6 protooncogene because of chromosomal translocations or promoter mutations. AIDS immunoblastic lymphoma plasmacytoid (AIDS IBLP) is thought to derive from postgerminal center B cells and is not associated with any predominant genetic alteration (30, 31). AIDS IBLP are monoclonal tumors that usually contain Epstein–Barr virus, indicating that they are not simply Epstein–Barr virus-driven polyclonal proliferations. The lack of any consistently associated oncogene involvement in AIDS IBLP strongly suggests that these tumors arise through novel patterns of dysregulated gene expression (7, 15, 32).
We sought to identify differentially expressed genes in AIDS IBLP patient samples versus non-AIDS lymphoma samples by using suppression subtractive hybridizations (SSH) (33, 34). Large cell lymphomas with immunoblastic/plasmacytoid features consistent with postgerminal center derivations (IBLP) were selected for these subtractions from HIV-infected or uninfected patient samples. The TCL1 oncogene was identified among multiple differentially expressed genes isolated from AIDS IBLP in these studies.
TCL1 is developmentally regulated and is normally expressed in fetal thymocytes, in bone marrow pre-B and immature B cells, and weakly in CD19+ peripheral blood lymphocytes (40, 41). Abnormal TCL1 expression caused by chromosomal translocations or inversions near T cell receptor-enhancer elements have been previously demonstrated in T cell leukemia/lymphoma (35–39). Continuous tissue-specific expression of TCL1 also caused transgenic mice to develop a polyclonal T cell expansion with subsequent progression to T cell leukemia (40). Widely variable levels of TCL1 expression have been reported in many established B lymphoblastoid and B cell tumor lines. The combined results of several studies showed that most immortalized B lymphoblastoid cell lines (10/12) as well as B cell tumor lines established from acute lymphoblastic leukemia (15/18), Burkitt’s lymphoma (9/11), and non-Hodgkin’s lymphoma (8/9) expressed TCL1 (40–42). TCL1 expression was not detected in myeloma cell lines (0/9). In contrast to these findings on cell lines, TCL1 expression has not been reported in primary B lymphoid tumors, including AIDS-related B cell malignancies. Comparison of the levels of TCL1 protein expression in AIDS IBLP samples with the normal pattern of expression in hyperplastic lymph node (HYP) suggests that TCL1 is aberrantly regulated and overexpressed in many AIDS IBLP tumors. Up-regulation of TCL1 in AIDS IBLP cannot occur by the same chromosomal alterations involved in T cell leukemia/lymphoma because the T cell receptor loci are transcriptionally silent in normal and malignant B cells.
MATERIALS AND METHODS
Patient Samples, cDNA Manufacture, and Subtractions.
Fresh-frozen patient samples were derived from HIV-infected and uninfected individuals (Table 1). These tissues are part of a catalogued bank of 750 AIDS-associated malignancies and related non-AIDS tissue samples maintained at UCLA. Eighty of the AIDS NHL samples in this bank have been extensively characterized for known oncogenes, tumor-suppressor genes, and viral involvement. Subtraction sample AIDS IBLP 1 was chosen from these characterized tumor samples based on the demonstrated absence of Epstein–Barr virus and herpesvirus-8 and the lack of oncogenic mutations or alterations in p53, RAS, c-MYC, BCL-2, or BCL-6 (19, 43–46). AIDS IBLP, non-AIDS IBLP, and HYP samples were examined histologically, and tissue blocks were trimmed to exclude areas of necrosis or surrounding nonlymphoid tissues. Microtome sections (5–10 μm) were placed directly into 5 ml of RNA STAT-60 for total RNA extraction (Tel-Test, Friendswood, TX). cDNA was synthesized from 0.4 μg of total RNA (Superscript II, GIBCO/BRL) by using a Smart PCR cDNA Synthesis kit and then PCR-amplified 17–19 cycles by using conditions recommended by the manufacturer (CLONTECH) (34).
Table 1.
Features of patient samples
| Tissue type | Site | Lesion(s) |
|---|---|---|
| HYP 1 | LN | HIV(−) |
| HYP 2 | LN | HIV(+) |
| AIDS IBLP 1 | LN | None |
| AIDS IBLP 2 | Muscle | None |
| AIDS IBLP 3 | Parotid | None |
| AIDS IBLP 4 | LN | EBV(+), P53 E6, LOH |
| AIDS IBLP 5 | Bowel | c-MYC, p53 E7, LOH |
| AIDS IBLP 6 | Lung | EBV(+), p53 E7, LOH |
| AIDS IBLP 7 | LN | EBV(+), p53 E6, LOH |
| AIDS IBLP 8 | LN | k-RAS, p53 E5,6,7, LOH |
| AIDS BL | Salivary | Unknown |
| Non-AIDS IBLP 1 | LN | HIV(−) |
| Non-AIDS IBLP 2 | Bowel | HIV(−) |
| Hyperplastic Tonsil | Oropharynx |
Tissue types, biopsy sites, and characteristic genetic lesions and/or viral involvement in patient samples used in this study. AIDS IBLP samples were analyzed for Epstein–Barr virus, human herpesvirus-8, c-MYC, RAS, p53, BCL-2, and BCL-6 genetic alterations (19, 43–46). Lymphomas with p53 mutations in exons 5, 6, and/or 7 terminate translation early or contain frameshift mutations. These five tumors also contain loss-of-heterozygosity (LOH) alterations in the non-mutated alleles.
SSH were performed between AIDS IBLP 1 versus HYP 1 or non-AIDS IBLP 1 by using the PCR-Select cDNA Subtraction kit (CLONTECH) (33). Subtracted gene fragments were cloned into the TA cloning vector (Invitrogen) and transformed into DH5α Escherichia coli. White colonies containing gene inserts were selected by isopropyl-β-d-thiogalactoside/5-bromo-4-chloro-3-indolyl-β-d-galactoside screening and seeded into 96-well microtiter plates for growth with antibiotic selection.
Miniarray Filter Preparation, Hybridization, and Analyses.
Samples of bacterial culture lysates in 96-well plates were stamped with a replicating tool (V&P Scientific, San Diego, CA) into fresh 96-well Thermowell plates (Costar) for PCR amplification of cDNA inserts. PCR was performed for 30 cycles (94°C for 30 s, 68°C for 3 min), and the average size of insert fragments was ≈1 kilobase. These PCR products were identically stamped onto quadruplicate NitroPlus nitrocellulose filters (Micron Separations). The first position on each filter was stamped with an 850-bp PstI fragment of the plant Lemna gibba RuBPCase gene. The filters were denatured and neutralized for 5 min, followed by baking at 80°C for 1 h. Random-primed, radiolabeled probes were made by using a Prime-it II kit (Stratagene) from 50 ng of unsubtracted or subtracted cDNA. Probes were spiked with 0.3 ng of the 850-bp RuBPCase gene fragment before radiolabeling to allow semiquantitative comparisons of hybridization intensities between filters. Hybridizations were performed at 42°C for 18 h, and filters were washed with 0.1× SSC/0.1% SDS for 45 min at 52°C. Hybridization signals were determined visually by autoradiography and quantitatively with a PhosphorImager (Molecular Dynamics) by using the program imagequant.
“Virtual” Northern Analyses of Tissues.
PCR-amplified cDNA (0.5 μg), obtained from equivalent amounts of reverse-transcribed total RNA, was fractionated in a 1% agarose gel and alkaline-transferred for 1 hr to a MagnaCharge nylon membrane by using a TurboBlotter (Schleicher and Schuell). The blots were hybridized with random-primed, radiolabeled TCL1 or BCA-1 cDNA fragment probes. Equal lane loading was determined by ethidium bromide staining (data not shown).
Immunohistochemical Staining of TCL1 and Fascin in Tissues.
Rabbit antisera were generated against a purified glutathione S-transferase–TCL1 fusion protein (data not shown). Formalin-fixed tissues on glass slides were incubated with blocking buffer and subsequently with TCL1 antisera. Slides were rinsed, and biotinylated goat anti-rabbit Ig (DAKO) was added. Slides were washed, and streptavidin was added, followed by diaminobenzidine HCl incubation and counterstaining in Mayer’s hematoxylin (DAKO). Formalin-fixed tissue sections were also stained with a fascin mAb to detect dendritic reticulum cells (DRC) as described (47, 48).
RESULTS
Identification of TCL1 and BCA-1 in Fresh-Frozen Patient Samples.
Two independent bidirectional SSH were performed to identify AIDS lymphoma-expressed genes. In the first subtraction, AIDS IBLP 1 cDNA was paired with HYP 1 cDNA and in the second, this AIDS IBLP sample was paired with non-AIDS IBLP 1 cDNA (Table 1). The first subtraction reduced genes that were transiently up- or down-regulated because of immune system activation as often occurs in premalignant persistent generalized lymphadenopathy in early HIV infection (4, 49). The second subtraction removed cDNAs expressed in common between two lymphomas of the same histologic type and enriched for up- or down-regulated sequences in severely immunosuppressed individuals. Over 2,000 cloned cDNA fragments from these subtractions were arrayed on nitrocellulose filters and independently hybridized with tester, driver, and subtracted cDNA probes (data not shown). Approximately 120 of the clones that differentially hybridized were sequenced. TCL1, an oncogene implicated in T cell leukemia/lymphoma development, and BCA-1, a recently identified B cell CXC-family chemoattractant, were identified in these characterized isolates and were differentially expressed in opposite directions (40, 50, 51). TCL1 was identified in the AIDS IBLP 1 minus non-AIDS IBLP 1 subtraction, whereas BCA-1 was found in both HYP 1 and non-AIDS IBLP 1 minus AIDS IBLP 1 subtractions (data not shown).
Differential Expression Profiles of TCL1 and BCA-1.
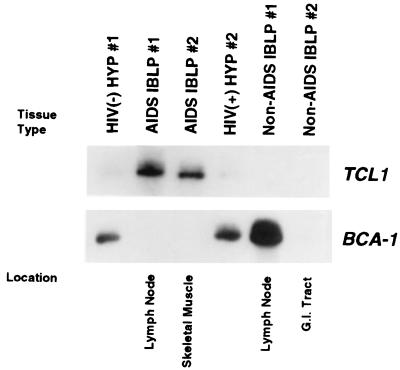
Northern blots to confirm the differential expression of TCL1 and BCA-1 were not possible because of the exceptionally small sizes of the tissue samples used in this study. Therefore, virtual Northern analyses were performed on PCR-amplified cDNA from tissue samples (Fig. 1). TCL1 was abundantly expressed in AIDS IBLP samples 1 and 2 (which both lack known genetic lesions or viral involvement) but was absent in non-AIDS IBLP samples 1 and 2. Low levels of TCL1 were detected in HYP samples from HIV-infected and uninfected individuals. In contrast to TCL1, BCA-1 was highly expressed in HYP samples 1 and 2 as well as in non-AIDS IBLP 1 but was absent in non-lymph node-derived tumors as well as lymph node-derived AIDS IBLP 1. BCA-1 is expressed in secondary human lymphoid tissues (50). Mice also express a BCA-1 homologue, termed BLC, and antisense in situ hybridization has demonstrated a reticular staining pattern for BLC in lymphoid follicles (51). This staining pattern suggested that mouse DRC may secrete BLC and, by analogy, that human DRC also secrete BCA-1, although neither association has been proven.
Figure 1.
Virtual Northern analysis of TCL1 and BCA-1 gene expression in tissue samples. (Upper) Hybridization with a 350-bp random-primed TCL1 cDNA fragment. AIDS IBLP samples from lymph node or muscle expressed abundant TCL1, whereas low levels were expressed in both HYP samples. (Lower) The filter from Upper was stripped and rehybridized with a 200-bp random-primed BCA-1 cDNA fragment. BCA-1 was expressed in both HYP and non-AIDS IBLP 1 derived from a lymph node but was not detected in either AIDS IBLP 1 or 2 or in non-AIDS IBLP 2 derived from the gastrointestinal tract. Ethidium bromide gel staining demonstrated equal lane loading (data not shown).
TCL1 Protein Is Expressed in Distinct Locations in AIDS IBLP, HYP, and Tonsil.
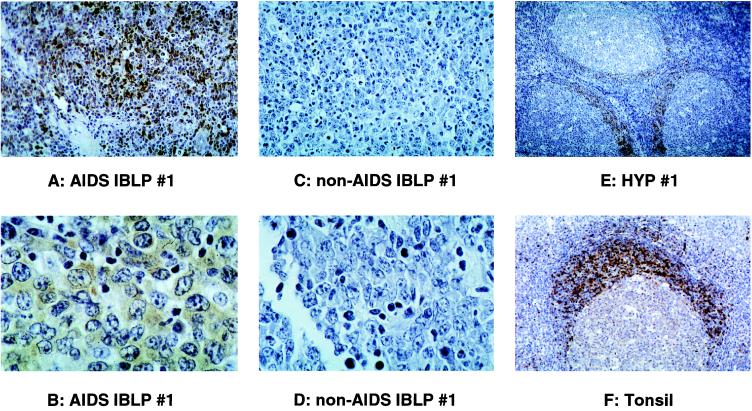
Tissue-localization studies were performed to definitively identify the cell types expressing TCL1 and BCA-1 in AIDS IBLP and non-AIDS IBLP tumor samples. TCL1 rabbit antisera staining of formalin-fixed lymphoma tissues demonstrated intense, widespread expression of TCL1 protein restricted to tumor cells in AIDS IBLP samples (Fig. 2 A and B, data not shown). TCL1 protein staining was mainly cytoplasmic, although some nuclear staining was also seen. TCL1 protein was not detected within heterogeneous nonlymphoma cell elements of these same tissue sections. In contrast, no TCL1 protein expression was detected in non-AIDS IBLP samples (Fig. 2 C and D, data not shown). In agreement with SSH isolation results and virtual Northern analyses (Fig. 1), TCL1 protein was also detected in distinctive locations in HYP from HIV-infected and uninfected individuals. Staining was intense and widespread within mantle zone B cells. Intense TCL1 staining was also detected in rare interfollicular zone cells, none of which morphologically resembled transformed lymphoid cells or immunoblasts (Fig. 2E, data not shown). Lower levels of TCL1 staining was seen in follicle center cells, including both centroblasts and centrocytes. Hyperplastic tonsil showed a pattern of intense TCL1 staining in germinal centers preferentially located in mantle-zone B cells similar to that seen in HYP (Fig. 2F).
Figure 2.
TCL1 antisera staining of formalin-fixed tissues. An intense positive signal was confined to the large immunoblastic/plasmacytic tumor cells in AIDS IBLP 1 (A, ×400X; B, ×1,000 magnification), whereas non-AIDS IBLP 1 (C, ×400; D, ×1,000 magnification) was negative. Non-tumor cell elements were negative for TCL1 protein expression in both samples. TCL1 staining appeared mainly within the cytoplasm. Germinal center B cells also stained with TCL1 antisera (E, ×100; F, ×200 magnification). Both uninfected and HIV-infected (data not shown) HYP contained activated germinal centers that reacted with TCL1 antisera. In these samples, TCL1 staining was most intense in naive, nonproliferating mantle-zone B cells and in rare interfollicular zone cells of unknown phenotype. A reduced staining intensity was seen in follicle-center cells (centroblasts and centrocytes). Overall, the level of staining in the different tissues analyzed correlated with the TCL1 transcript levels detected by virtual Northern analysis.
Although the cells are not distinguishable by routine histologic examination with light microscopy, non-AIDS IBLP 1 tissue contained residual fascin antibody-stained DRC that were not detected in AIDS IBLP 1 (results not shown). AIDS IBLP 1 may lack DRC or, alternatively, may contain DRC that do not express fascin or BCA-1, in agreement with the spectrum of genes isolated by SSH and with virtual Northern results (47, 48). Detection of BCA-1 in non-AIDS IBLP 1, coupled with residual fascin-positive antibody staining in this tumor, compared with a complete lack of both BCA-1 and fascin-stained cells in AIDS IBLP 1, also suggest that DRC express BCA-1. The concordance of BCA-1 expression and fascin antibody staining in these two tumor samples, along with concordant expression in HYP 1 and 2, also further confirms that both subtractions efficiently isolated differentially expressed genes.
TCL1 Expression in AIDS NHL with or Without Genetic/Viral Alterations.
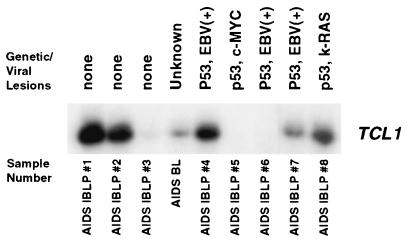
AIDS IBLP samples 1 and 2 expressed abundant TCL1 protein in lymphoma cells without additional genetic alterations or viral involvement. This observation, coupled with detection of high levels of TCL1 protein in nonproliferating mantle-zone B cells and in scattered, rare interfollicular-zone cells of HYP and tonsil, suggests that TCL1 expression occurs early in AIDS lymphomagenesis. We therefore considered whether AIDS NHL tumors with identified oncogenic alterations, (perhaps representing later stage lymphomas) also expressed TCL1. Virtual Northern analyses demonstrated that the large majority (i.e., six of eight) AIDS IBLP patient samples tested expressed TCL1, including three of five lymphoma samples of this class with additional identified genetic aberrations (Fig. 3). These findings indicate that TCL1 is more consistently expressed in AIDS IBLP than any other known oncogene previously analyzed. This prevalent expression strongly supports a possible role for TCL1 in the development of this class of AIDS NHL. Interestingly, an AIDS-related Burkitt’s lymphoma derived from a salivary gland tumor also expressed moderateTCL1 mRNA. This latter observation suggests that additional classes of AIDS NHL should also be examined for TCL1 expression.
Figure 3.
Virtual Northern analysis of TCL1 gene expression in AIDS IBLP and AIDS-related Burkitt’s lymphoma samples. Blots were probed with a 350-bp random-primed TCL1 cDNA fragment. All of the IBLP samples tested were originally classified as B cell immunoblastic lymphomas by Working Formulation criteria, and as AIDS IBLP by the Revised European–American Lymphoma (REAL) AIDS-related classification, except lane 4, which is an AIDS-related Burkitt’s lymphoma. AIDS IBLP and an AIDS-related Burkitt’s lymphoma with and without known genetic or viral lesions express TCL1. Ethidium bromide gel staining demonstrated equal lane loading, and detection of glyceraldehyde-3-phosphate dehydrogenase in each lane showed that the original RNA templates of each tissue sample were of similar quality (data not shown).
DISCUSSION
Two independent rounds of SSH were performed to isolate genes that are differentially expressed in AIDS IBLP versus non-AIDS IBLP tumors or activated lymph node (33, 34). The differential expression of SSH isolated genes was then confirmed by virtual Northern blots and assigned to specific cell populations by immunohistochemical staining (and antisense in situ hybridizations; results not shown) of tumor and normal tissue samples. These combined steps define a powerful strategy for the isolation of differentially expressed genes from specific cells in heterogeneous surgical or biopsy tissue samples. Among the differentially expressed genes identified with this approach, we focused on TCL1 because of its compelling biology in B cell development and its reported oncogenic activity in T cell leukemia/lymphoma. TCL1 encodes a predominantly cytoplasmic protein of unresolved function that belongs to a novel family of recently identified oncogenes that now includes the MTCP1 and TCL1b genes (42, 52–55). Although its expression was originally reported to be limited to developing lymphocytes (i.e., fetal thymocytes, pre-B cells, and immature surface IgM-expressing B cells in bone marrow) and to endemic (primarily Epstein–Barr virus+) Burkitt’s lymphoma tumor cell lines, TCL1 expression recently has been documented in many immortalized B lymphoblastoid and B cell tumor lines (40–42). TCL1 transcripts have also been reported in CD19+ sorted peripheral-blood lymphocytes and in spleen and lymph nodes, although the cell(s) of origin was not defined in these latter tissues (41). Using immunohistochemical staining, we show that HYP and tonsil express TCL1 protein, with the highest levels of expression in mantle-zone B cells and in rare interfollicular-zone cells (none of which resembled immunoblasts). The mantle zone contains naive, unactivated B cells that may become activated within follicles and differentiate into either mature memory B cells or Ig-secreting plasma cells (ref. 56, reviewed in refs. 57–59). The finding of widespread TCL1 expression in quiescent mantle-zone B cells suggests that TCL1 expression may be involved in B cell survival rather than in proliferation or Ig/T cell receptor gene rearrangements as previously speculated (40). Mantle-zone B cells express abundant BCL-2 protein that serves to protect these resting B cells from apoptosis (58, 60–62). Elevated TCL1 expression within this same quiescent B cell population also may reflect a role in conferring resistance to apoptosis, although this possibility remains to be tested. Finally, the mantle zone is physically juxtaposed to the follicle center, which contains areas of extensive apoptotic activity (along with reduced BCL-2 expression) in which we also find decreased levels of TCL1 protein expression. This concurrence lends further support for an antiapoptotic role for TCL1.
Aberrant TCL1 expression has been previously reported in T cell leukemia/lymphoma with chromosomal rearrangements that juxtapose the TCL1 gene in proximity to the T cell-specific enhancers of either the T cell receptor α/δ or β loci (35–39). Transgenic mice expressing either TCL1 or MTCP1 from promoters that restrict expression to T cells developed early T cell proliferative expansions that progressed to T cell leukemia by 15 months of age (63, 64). Interestingly, BCL-2 transgenic mice also developed B cell lymphomas slowly and in moderate numbers, in a manner paralleling the time course and occurrence of T cell tumors in TCL1 transgenic mice (reviewed in refs. 65 and 66). Humans may also sustain preleukemic TCL1 translocations for many years before overt leukemia/lymphoma formation (67). All of these observations provide strong support for the oncogenic potential of TCL1 and suggest that TCL1 may function to promote cell survival rather than affecting cell proliferation.
AIDS IBLP are derived from postgerminal center cells and not from resting mantle-zone B cells (reviewed in refs. 6 and 7). Elevated TCL1 expression in these cells may result from an abnormal up-regulation of the gene rather than the down-regulation that normally occurs in B cell maturation in germinal centers. Lower protein expression in follicle-center B cells and weak mRNA expression in circulating peripheral-blood lymphocytes is consistent with a physiologic down-regulation of TCL1 from the high levels seen in quiescent mantle-zone B cells. Alternatively, AIDS IBLP may originate from the rare intensely stained interfollicular-zone cells that express high levels of TCL1 protein.
AIDS lymphomas develop under unique and extreme pathological conditions. These include moderate to severe immunodeficiency, chronic antigenic stimulation, profound alterations of cytokine expression profiles, and the loss of normal lymph-node architecture because of progressive immune system destruction (reviewed in refs. 1–3, 6, 7). It is conceivable that abundant TCL1 expression in AIDS lymphoma results from the radically altered cytokine profiles in AIDS or from the absence of critical cell-mediated regulatory influences (e.g., such as those provided by DRC or T cells) that occur in normal lymph nodes. The lack of these cell-mediated regulatory influences may also be responsible for the widely varying levels of TCL1 expression seen in multiple B cell tumor lines. Resolution of the mechanism(s) controlling TCL1 expression in AIDS lymphoma versus normal developing B cells will provide important insights into the contribution of TCL1 expression in AIDS lymphomagenesis.
Unchecked, high-level TCL1 expression is reported to cause early polyclonal, proliferative T cell expansions in transgenic mice. The findings presented here strongly suggest that elevated TCL1 expression also may be involved in an abnormal cellular regulatory program that creates an equivalent, early, or premalignant lymphoproliferative state in B cells. Subsequent transforming events, such as p53 mutations, c-MYC rearrangements, or other genetic alterations could then promote frank B cell tumor formation, in analogy to the effect of introducing c-MYC mutations into BCL-2 transgenic mice (reviewed in refs. 65 and 66).
Acknowledgments
We thank Michael Lee for excellent technical support and members of the Teitell, Denny, and Wall laboratories for insightful discussions. We thank Dr. C. Malone for critical reading of this manuscript. These studies were supported by grants from the UCLA Jonsson Comprehensive Cancer Foundation, Amgen/U.C. BioStar Program, National Institutes of Health (CA74929, CA66533, and GM40185) and the Lymphoma Research Foundation of America (012127). M.T. is a LRFA Fellow. We also specifically acknowledge the generous support and encouragement of Dr. L. Souza (Amgen).
ABBREVIATIONS
- NHL
non-Hodgkin’s lymphoma
- IBLP
immunoblastic lymphoma plasmacytoid
- HYP
hyperplastic lymph node
- SSH
suppression subtractive hybridization
- DRC
dendritic reticulum cell
References
- 1.Hamilton-Dutoit S J, Pallesen G, Franzmann M B, Karkov J, Black F, Skinhoj P, Pedersen C. Am J Pathol. 1991;138:149–163. [PMC free article] [PubMed] [Google Scholar]
- 2.Raphael M, Gentilhomme O, Tulliez M, Byron P A, Diebold J. Arch Pathol Lab Med. 1991;115:15–20. [PubMed] [Google Scholar]
- 3.Raphael M M, Audouin J, Lamine M, Delecluse H J, Vuillaume M, Lenoir G M, Gisselbrecht C, Lennert K, Diebold J. Am J Clin Pathol. 1994;101:773–782. doi: 10.1093/ajcp/101.6.773. [DOI] [PubMed] [Google Scholar]
- 4.Herndier B G, Kaplan L D, McGrath M S. AIDS. 1994;8:1025–1049. doi: 10.1097/00002030-199408000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Diebold J, Raphael M, Prevot S, Audouin J. Cancer Surv. 1997;30:263–293. [PubMed] [Google Scholar]
- 6.Knowles D M. Semin Diagn Pathol. 1997;14:67–82. [PubMed] [Google Scholar]
- 7.Gaidano G, Carbone A, Dalla-Favera R. Am J Pathol. 1998;152:623–630. [PMC free article] [PubMed] [Google Scholar]
- 8.Jaffe E S. Am J Clin Pathol. 1996;105:141–143. doi: 10.1093/ajcp/105.2.141. [DOI] [PubMed] [Google Scholar]
- 9.Carbone A, Gaidano G. Br J Haematol. 1997;97:515–522. doi: 10.1046/j.1365-2141.1997.00064.x. [DOI] [PubMed] [Google Scholar]
- 10.Cesarman E, Knowles D M. Semin Diagn Pathol. 1997;14:54–66. [PubMed] [Google Scholar]
- 11.Chaganti R S, Jhanwar S C, Koziner B, Arlin Z, Mertelsmann R, Clarkson B D. Blood. 1983;61:1265–1268. [PubMed] [Google Scholar]
- 12.Groopman J E, Sullivan J L, Mulder C, Ginsburg D, Orkin S H, O’Hara C J, Falchuk K, Wong-Staal F, Gallo R C. Blood. 1986;67:612–615. [PubMed] [Google Scholar]
- 13.Subar M, Neri A, Inghirami G, Knowles D M, Dalla-Favera R. Blood. 1988;72:667–671. [PubMed] [Google Scholar]
- 14.Ballerini P, Gaidano G, Gong J Z, Tassi V, Saglio G, Knowles D M, Dalla-Favera R. Blood. 1993;81:166–176. [PubMed] [Google Scholar]
- 15.Gaidano G, Parsa N Z, Tassi V, Della-Latta P, Chaganti R S, Knowles D M, Dalla-Favera R. Leukemia. 1993;7:1621–1629. [PubMed] [Google Scholar]
- 16.Cesarman E, Chang Y, Moore P S, Said J W, Knowles D M. N Engl J Med. 1995;332:1186–1191. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- 17.Carbone A, Gloghini A, Vaccher E, Zagonel V, Pastore C, Dalla Palma P, Branz F, Saglio G, Volpe R, Tirelli U, Gaidano G. Br J Haematol. 1996;94:533–543. doi: 10.1046/j.1365-2141.1996.d01-1826.x. [DOI] [PubMed] [Google Scholar]
- 18.Gaidano G, Pastore C, Gloghini A, Cusini M, Nomdedeu J, Volpe G, Capello D, Vaccher E, Bordes R, Tirelli U, et al. AIDS. 1996;10:941–949. doi: 10.1097/00002030-199610090-00003. [DOI] [PubMed] [Google Scholar]
- 19.Nador R G, Cesarman E, Chadburn A, Dawson D B, Ansari M Q, Sald J, Knowles D M. Blood. 1996;88:645–656. [PubMed] [Google Scholar]
- 20.Lyons S F, Liebowitz D N. Semin Oncol. 1998;25:461–475. [PubMed] [Google Scholar]
- 21.Hsi E D, Foreman K E, Duggan J, Alkan S, Kauffman C A, Aronow H D, Nickoloff B J. Am J Surg Pathol. 1998;22:493–499. doi: 10.1097/00000478-199804000-00016. [DOI] [PubMed] [Google Scholar]
- 22.Delecluse H J, Raphael M, Magaud J P, Felman P, Alsamad I A, Bornkamm G W, Lenoir G M. Blood. 1993;82:552–563. [PubMed] [Google Scholar]
- 23.Shiramizu B, Herndier B, Meeker T, Kaplan L, McGrath M. J Clin Oncol. 1992;10:383–389. doi: 10.1200/JCO.1992.10.3.383. [DOI] [PubMed] [Google Scholar]
- 24.Shibata D, Weiss L M, Hernandez A M, Nathwani B N, Bernstein L, Levine A M. Blood. 1993;81:2102–2109. [PubMed] [Google Scholar]
- 25.Beral V, Peterman T, Berkelman R, Jaffe H. Lancet. 1991;337:805–809. doi: 10.1016/0140-6736(91)92513-2. [DOI] [PubMed] [Google Scholar]
- 26.Schulz T F, Boshoff C H, Weiss R A. Lancet. 1996;348:587–591. doi: 10.1016/S0140-6736(95)11033-X. [DOI] [PubMed] [Google Scholar]
- 27.Knowles D M, Chamulak G A, Subar M, Burke J S, Dugan M, Wernz J, Slywotzky C, Pelicci G, Dalla-Favera R, Raphael B. Ann Intern Med. 1988;108:744–753. doi: 10.7326/0003-4819-108-5-744. [DOI] [PubMed] [Google Scholar]
- 28.Gaidano G, Carbone A. Br J Haematol. 1995;90:235–243. doi: 10.1111/j.1365-2141.1995.tb05142.x. [DOI] [PubMed] [Google Scholar]
- 29.Carbone A, Gaidano G, Gloghini A, Larocca L M, Capello D, Canzonieri V, Antinori A, Tirelli U, Falini B, Dalla-Favera R. Blood. 1998;91:747–755. [PubMed] [Google Scholar]
- 30.Gaidano G, Carbone A, Pastore C, Capello D, Migliazza A, Gloghini A, Roncella S, Ferrarini M, Saglio G, Dalla-Favera R. Blood. 1997;89:3755–3762. [PubMed] [Google Scholar]
- 31.Gaidano G, Lo Coco F, Ye B H, Shibata D, Levine A M, Knowles D M, Dalla-Favera R. Blood. 1994;84:397–402. [PubMed] [Google Scholar]
- 32.Ganser A, Carlo-Stella C, Bartram C R, Boehm T, Heil G, Henglein B, Muller H, Raghavachar A, von Briesen H, Griesinger F, et al. Blood. 1988;72:1255–1260. [PubMed] [Google Scholar]
- 33.Diatchenko L, Lau Y F, Campbell A P, Chenchik A, Moqadam F, Huang B, Lukyanov S, Lukyanov K, Gurskaya N, Sverdlov E D, Siebert P D. Proc Natl Acad Sci USA. 1996;93:6025–6030. doi: 10.1073/pnas.93.12.6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peterson L A, Brown M R, Carlisle A J, Kohn E C, Liotta L A, Emmert-Buck M R, Krizman D B. Cancer Res. 1998;58:5326–5328. [PubMed] [Google Scholar]
- 35.Russo G, Isobe M, Pegoraro L, Finan J, Nowell P C, Croce C M. Cell. 1988;53:137–144. doi: 10.1016/0092-8674(88)90495-3. [DOI] [PubMed] [Google Scholar]
- 36.Mengle-Gaw L, Albertson D G, Sherrington P D, Rabbitts T H. Proc Natl Acad Sci USA. 1988;85:9171–9175. doi: 10.1073/pnas.85.23.9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davey M P, Bertness V, Nakahara K, Johnson J P, McBride O W, Waldmann T A, Kirsch I R. Proc Natl Acad Sci USA. 1988;85:9287–9291. doi: 10.1073/pnas.85.23.9287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Virgilio L, Isobe M, Narducci M G, Carotenuto P, Camerini B, Kurosawa N, Abbas ar R, Croce C M, Russo G. Proc Natl Acad Sci USA. 1993;90:9275–9279. doi: 10.1073/pnas.90.20.9275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Narducci M G, Virgilio L, Isobe M, Stoppacciaro A, Elli R, Fiorilli M, Carbonari M, Antonelli A, Chessa L, Croce C M, et al. Blood. 1995;86:2358–2364. [PubMed] [Google Scholar]
- 40.Virgilio L, Narducci M G, Isobe M, Billips L G, Cooper M D, Croce C M, Russo G. Proc Natl Acad Sci USA. 1994;91:12530–4. doi: 10.1073/pnas.91.26.12530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takizawa J, Suzuki R, Kuroda H, Utsunomiya A, Kagami Y, Joh T, Aizawa Y, Ueda R, Seto M. Jpn J Cancer Res. 1998;89:712–718. doi: 10.1111/j.1349-7006.1998.tb03275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pekarsky Y, Hallas C, Isobe M, Russo G, Croce C M. Proc Natl Acad Sci USA. 1999;96:2949–2951. doi: 10.1073/pnas.96.6.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Said J W, Sassoon A F, Shintaku I P, Corcoran P, Nichols S W. Mod Pathol. 1990;3:659–663. [PubMed] [Google Scholar]
- 44.Nakamura H, Said J W, Miller C W, Koeffler H P. Blood. 1993;82:920–926. [PubMed] [Google Scholar]
- 45.Lones M A, Mishalani S, Shintaku I P, Weiss L M, Nichols W S, Said J W. Hum Pathol. 1995;26:525–530. doi: 10.1016/0046-8177(95)90248-1. [DOI] [PubMed] [Google Scholar]
- 46.Said J W, Rettig M R, Heppner K, Vescio R A, Schiller G, Ma H J, Belson D, Savage A, Shintaku I P, Koeffler H P, et al. Blood. 1997;90:4278–4282. [PubMed] [Google Scholar]
- 47.Said J W, Pinkus J L, Yamashita J, Mishalani S, Matsumura F, Yamashiro S, Pinkus G S. Mod Pathol. 1997;10:421–427. [PubMed] [Google Scholar]
- 48.Said J W, Pinkus J L, Shintaku I P, deVos S, Matsumura F, Yamashiro S, Pinkus G S. Mod Pathol. 1998;11:1–5. [PubMed] [Google Scholar]
- 49.Pelicci P G, Knowles D M d, Arlin Z A, Wieczorek R, Luciw P, Dina D, Basilico C, Dalla-Favera R. J Exp Med. 1986;164:2049–2060. doi: 10.1084/jem.164.6.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Legler D F, Loetscher M, Roos R S, Clark-Lewis I, Baggiolini M, Moser B. J Exp Med. 1998;187:655–660. doi: 10.1084/jem.187.4.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gunn M D, Ngo V N, Ansel K M, Ekland E H, Cyster J G, Williams L T. Nature (London) 1998;391:799–803. doi: 10.1038/35876. [DOI] [PubMed] [Google Scholar]
- 52.Fu T B, Virgilio L, Narducci M G, Facchiano A, Russo G, Croce C M. Cancer Res. 1994;54:6297–6301. [PubMed] [Google Scholar]
- 53.Madani A, Choukroun V, Soulier J, Cacheux V, Claisse J F, Valensi F, Daliphard S, Cazin B, Levy V, Leblond V, et al. Blood. 1996;87:1923–1927. [PubMed] [Google Scholar]
- 54.Stern M H, Soulier J, Rosenzwajg M, Nakahara K, Canki-Klain N, Aurias A, Sigaux F, Kirsch I R. Oncogene. 1993;8:2475–2483. [PubMed] [Google Scholar]
- 55.Hoh F, Yang Y S, Guignard L, Padilla A, Stern M H, Lhoste J M, van Tilbeurgh H. Structure (London) 1998;6:147–155. doi: 10.1016/s0969-2126(98)00017-3. [DOI] [PubMed] [Google Scholar]
- 56.Kuppers R, Zhao M, Hansmann M L, Rajewsky K. EMBO J. 1993;12:4955–4967. doi: 10.1002/j.1460-2075.1993.tb06189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hummel M, Tamaru J, Kalvelage B, Stein H. Blood. 1994;84:403–407. [PubMed] [Google Scholar]
- 58.MacLennan I C. Annu Rev Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 59.Liu Y J, Arpin C. Immunol Rev. 1997;156:111–126. doi: 10.1111/j.1600-065x.1997.tb00963.x. [DOI] [PubMed] [Google Scholar]
- 60.Pezzella F, Tse A G, Cordell J L, Pulford K A, Gatter K C, Mason D Y. Am J Pathol. 1990;137:225–232. [PMC free article] [PubMed] [Google Scholar]
- 61.Chleq-Deschamps C M, LeBrun D P, Huie P, Besnier D P, Warnke R A, Sibley R K, Cleary M L. Blood. 1993;81:293–298. [PubMed] [Google Scholar]
- 62.Liu Y J, Mason D Y, Johnson G D, Abbot S, Gregory C D, Hardie D L, Gordon J, MacLennan I C. Eur J Immunol. 1991;21:1905–1910. doi: 10.1002/eji.1830210819. [DOI] [PubMed] [Google Scholar]
- 63.Virgilio L, Lazzeri C, Bichi R, Nibu K, Narducci M G, Russo G, Rothstein J L, Croce C M. Proc Natl Acad Sci USA. 1998;95:3885–3889. doi: 10.1073/pnas.95.7.3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gritti C, Dastot H, Soulier J, Janin A, Daniel M T, Madani A, Grimber G, Briand P, Sigaux F, Stern M H. Blood. 1998;92:368–373. [PubMed] [Google Scholar]
- 65.Cory S, Vaux D L, Strasser A, Harris A W, Adams J M. Cancer Res. 1999;59:1685s–1692s. [PubMed] [Google Scholar]
- 66.Korsmeyer S J. Cancer Res. 1999;59:1693s–1700s. [PubMed] [Google Scholar]
- 67.Levitt R, Pierre R V, White W L, Siekert R G. Blood. 1978;52:1003–1011. [PubMed] [Google Scholar]