Abstract
The effects on spontaneous ovulation associated with the unilateral or bilateral sectioning of the superior ovarian nerves (SON) were analyzed in guinea pigs at different time intervals of the estrous cycle. Day 1 of the estrous cycle was defined as the day when the animal presents complete loss of the vaginal membrane (open vagina). Subsequent phases of the cycle were determined by counting the days after Day 1. All animals were autopsied on the fifth day of the estrous cycle after surgery. Sectioning the right, left, or both SONs on day 5 (early luteal phase) resulted in a significant increase in the number of fresh corpora lutea. Ovulation increased significantly when the left SON (L-SON) was sectioned during late follicular phase (day 1) and medium luteal phase (day 8). When surgery was performed on days 1 or 8, neither sectioning the right SON (R-SON) nor sectioning the SON bilaterally had an apparent effect on ovulation rates. Similarly, ovulation rates were not affected when unilateral (right or left) or bilateral sectioning of the SON was performed during late luteal phase two (day 12). Unilateral or bilateral sectioning of the SON performed during the early luteal phase (day 5) was associated with a significant decrease in uterine weight. A comparable effect was observed when the L-SON was sectioned during late follicular phase (day 1), or medium luteal phase (day 8). No effects on uterine weight were observed when unilateral or bilateral sectioning of the SON was performed during late luteal phase. Our results suggest that in the guinea pig the SON modulates ovulation, and that the degree of modulation varies along the estrous cycle. The strongest influence of the SONs on ovulation occurs during early luteal phase, and decrease thereafter, being absent by late luteal phase. In addition, sectioning the left or the right SON caused different responses by the ovaries of adult guinea pigs. This paper discusses the mechanisms by which ovulation increased when the SON was surgically cut.
Introduction
Ample documentation on the effects of ovarian innervation in regulating hormone secretion and ovulation [1], the participation of catecholamines in regulating steroidogenesis [2,3] and ovulation [4,5], as well as on the response of follicles to gonadotrophins [6] is available. Sympathetic innervation reaches the ovary, mainly, through the superior ovarian nerve (SON) running along the suspensor ligament, and through some sympathetic fibbers arriving via the ovarian plexus that accompany the ovarian artery.
Evidence that in rats the SON carries noradrenergic and vipergic fibbers has been published [7-9]. In the rat however, the participation of the ovarian innervation is controversial. For instance, no changes in spontaneous or induced ovulation have been observed when both nerves are sectioned or damaged by freezing [10]. However, when unilateral sectioning of the L-SON or R-SON was performed, denervated ovaries released fewer ova than controls did. Furthermore, the sequential administration of gonadotrophins did not restore normal ovulation to the denervated ovary [6,11,12]. These results suggest that the ovarian innervation modulates the responsiveness to gonadotrophins by the ovarian compartments in both, a lateralized and inhibitory ways. For instance, bilateral sectioning of the SON did not affect spontaneous ovulation [11,12], although acute decreases in serum levels of progesterone [13] and estradiol [2] have been described.
The estrous cycles of the rat and the guinea pig are quite different; not only in length, but also in the capacity of the corpora lutea to secrete progesterone, lasting 8 to 10 days in the guinea pig [14], and only several hours in the rat [13]. Another difference is that the ovary of the guinea pig receives a richer sympathetic innervation than ovaries in the rat do [15]. Peripheral sympathetic denervation induced by guanethidine administration to newborn guinea pigs resulted in the advancement of vaginal opening, ovulation, and the response to exogenous gonadotrophins [16,17]. In turn peripheral noradrenergic denervation induced by guanethidine administration to adult guinea pigs resulted in the inhibition of ovulation. No differences in the number of corpora lutea between control and denervated animals injected with guanethidine for three weeks were observed. However, all the corpora lutea from the treated group were old, and a significant decrease in ovarian norepinephrine content was observed. This suggests that sympathetic denervation had an effect on the luteolytic process instead of on ovulation. Based on these results the authors conclude that the ovarian innervation participates in regulating follicular development in an inhibitory way [18]. Consequently, considering the differences between rats and guinea pigs in the regulation of reproductive functions and in ovarian innervation [15], together with the results obtained by sectioning the SON in rats [11,12], we hypothesize that the effects of unilateral and bilateral sectioning of the SON in the guinea pig will produce different results than in the rat.
To analyze such possibility, after performing unilateral or bilateral sectioning of the SON, we analyzed the effects on spontaneous ovulation at the beginning of the estrous cycle after surgery. Sectioning of the SON was performed at different periods of the estrous cycle as a means to establish a time frame for the effects induced by the surgical procedure.
Materials and Methods
One hundred and fifty adult virgin female guinea pigs of the Hartley strain from our own stock were used. Animals were housed under controlled lighting conditions (lights on from 06:00 to 19:00 h) and temperature (20°C), with free access to laboratory chow, alfalfa, and tap water.
Experimental design
Estrous cycles were monitored by daily examination for the presence or absence of the vaginal membrane. Guinea pigs showing three consecutive (16 –18 days) estrous cycles were used, only. All surgeries were performed under ether anesthesia. The day when the animal presented complete loss of the vaginal membrane (open vagina) was considered as day one of the estrous cycle, and the other days of the cycle were subsequently counted. Our strain of guinea pigs ovulates in the second day after vaginal opening, and keeps an open vagina during 4 to 5 days.
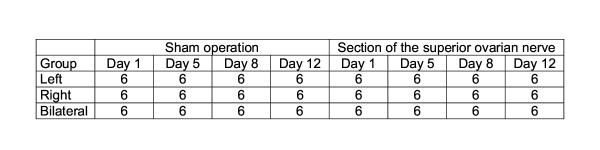
Animals were randomly assigned to groups of six animals that were subject to surgery or sham surgery on day 1 (late follicular phase), day 5 (early luteal phase), day 8 (medium luteal phase), or day 12 (late luteal phase) of the estrous cycle. A group of 6 untreated animals kept under the same food, lighting, and temperature conditions as the treated groups served as the control group. Figure 1 describes the experimental animal allocation setup.
Figure 1.
Distribution of animals in the experimental treatments.
Surgical Procedures
Unilateral or Bilateral Sectioning of the SON
The following surgical procedure was performed on day 1, 5, 8 or 12 of the estrous cycle to animals allotted to each experimental group. Animals were anaesthetized and laparotomized. The right (R- SON group), left (L-SON group) or both (bilateral section group) ovaries were exteriorized. The ovarian pedicle at the suspensor ligament was tied at two different points above the ovary and cut between the two ties with spring scissors. The ovarian pedicle and the ovary were returned to the abdominal cavity and the muscle and skin were sutured.
Sham Surgery
Animals in each of the corresponding phases of the estrous cycle were anaesthetized and laparotomized (the skin and muscle were cut) by the right (R- sham), left (L-sham) or bilateral (bilateral sham) sides of the abdomen. The ovaries were not touched. After these procedures, the muscle and skin were sutured.
Untouched Control Group
Six cyclic guinea pigs were kept under the same living conditions as the treatment groups, but not subject to any surgical procedure. The sequence of the estrous cycle of all animals was monitored before and after surgery. All animals were sacrificed with an overdose of ether anesthesia on the 5th day of the estrous cycle following surgery.
At autopsy, ovaries and uterus were dissected and weighed in a precision balance. The free movement of the ovary in the abdominal cavity was used to verify the complete sectioning of the suspensor ligament. Both ovaries were fixed in Bouin's fluid, embedded in paraffin, serially sectioned at 10 μm, and stained with haematoxylin and eosin. All sections were examined with a light microscope. The presence of fresh corpora lutea was considered evidence of previous ovulation. All fresh corpora lutea were counted, and the number of corpora lutea was used as an ovulation index.
Statistical evaluation
All data are presented as means ± standard error of the mean (SEM). Data on the weight of endocrine organs (ovaries and uterus) were analyzed using analysis of variance (ANOVA), followed by Tukey's test. The duration of the estrous cycle and the number of corpora lutea were analyzed by a Kruskal-Wallis followed by Mann-Whitney U-test. A probability of less than 5% was considered as significant.
Results
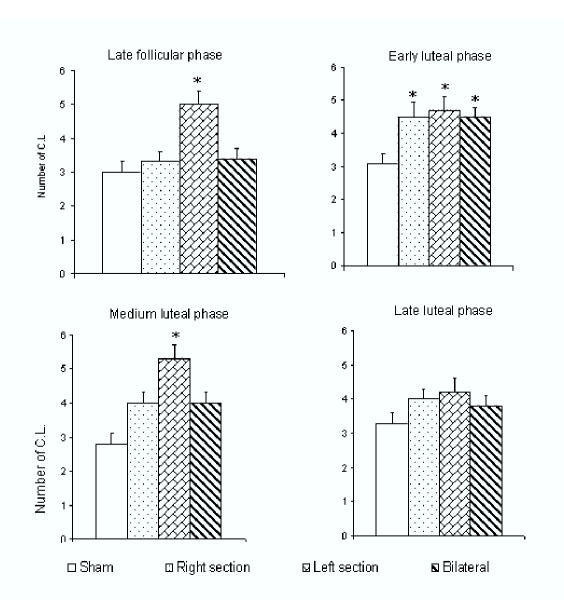
Compared to the control group, sham operation procedures did not cause any apparent change in the parameters measured. When the right SON was sectioned during the late follicular phase, the length of the estrous cycle was prolonged (20.2 ± 1.4 vs. 16.4 ± 0.4 days, p < 0.05). An increase in the number of fresh corpora lutea was observed when sectioning the L-SON was performed during the late follicular phase or during early and medium luteal phase. Similar results were observed when the L-SON or both SONs were sectioned during early luteal phase. No changes in ovulation were observed when surgery was performed during the late luteal phase (Figure 2).
Figure 2.
Number of corpora lutea (Mean ± SEM) in both ovaries of untouched control guinea pigs, L-SON, R-SON, or bilateral SON sectioning performed on day 1 of the estrous cycle (late follicular phase), on day 5 of the estrous cycle (early luteal phase), on day 8 of the estrous cycle (medium luteal phase) and day 12 of the estrous cycle (late luteal phase). *p < 0.05 in comparison with untouched control group (Kruskal-Walis followed by Mann-Whitney U-test)
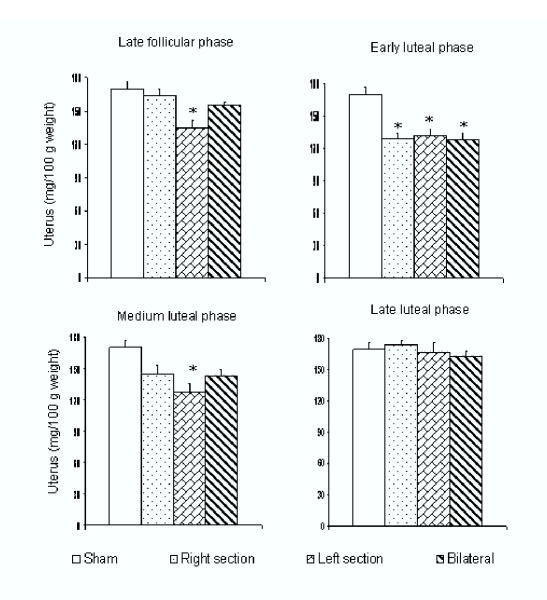
Compared to control animals, uterine weight was lower in animals with the L-SON or both SONs sectioned during early luteal phase; and when the L-SON was sectioned during the late follicular phase, or during early or medium luteal phase. When surgery was performed during the late luteal phase, no changes in uterine weight were observed (Figure 3). The treatments, however, did not yield significant differences in the weight of ovaries (data not shown).
Figure 3.
Mean ± SEM of the uterus weight (mg/100 g body weight) of untouched control guinea-pigs, L-SON, R-SON, or bilateral SON sectioning performed on day 1 of the estrous cycle (late follicular phase), on day 5 of the estrous cycle (early luteal phase), on day 8 of the estrous cycle (medium luteal phase) and day 12 of the estrous cycle (late luteal phase). * p < 0.05 in comparison with untouched control group (ANOVA followed by Tukey's test)
Discussion
The results obtained in the present study suggest that, in the guinea pig, the neural information reaching the ovary through the SON plays an inhibitory role in the regulation of ovulation. Evidence that both, the noradrenergic and the vagal innervation of the ovary modulate the reactivity of the ovary to gonadotropins has been published [6,11,12,20]; and therefore, it may be concluded that the ovary's innervation may participate in regulating ovulation by modulating the reactivity of the follicle to gonadotropins.
According to Bland [21], guinea pigs have a biphasic pattern of follicular growth. The first cohort of follicles reaches its maximum diameter on day 10 or 11 of the estrous cycle, and become atretic thereafter. Since this occurs at the same time as the corpora lutea suffers the luteolitic effect induced by prostaglandin release from the non-pregnant uterus [22], five to six days later, a second cohort of follicles matures and ovulates.
Ovulation rate increases were higher when sectioning the SON procedures were performed close to end of the follicular phase, or during the early and medium luteal phase. These increases in ovulation rates may be the result of a diminution of atresia in the first cohort of follicles and an extension of their viability; explaining the observed increase in ovulation.
Sectioning the ovarian pedicle during late luteal phase had no evident effect on the number of fresh corpora lutea, indicating that in the guinea pig, the role of ovarian innervation in modulating follicular growth and atresia varies along the estrous cycle. In the rat, the neural information arriving to the ovary through the SON seems to play a stimulatory role on ovulatory processes; and this role is solely related to the treated ovary [6,11,12,20]. Differences between rats and guinea pigs in the plausible participation of the neural information arriving to the ovary through the SON on ovulation may be related to differences in the length of the estrous cycle (4 to 5 days in the rat and up to 16 to 18 days in the guinea pig).
As previously suggested [6], the results obtained in guinea pigs after unilateral sectioning of the SON seems to support the existence of neural communication between ovaries, since the increase in the number of fresh corpora lutea is observed in both ovaries.
The increase in fresh corpora lutea induced by sectioning either SON may be related to modifications in ovarian norepinephrine concentrations. In the rat, bilateral sectioning of the SON resulted in a partial reduction in ovarian norepinephrine concentration [23]; while sympathetic denervation, induced by guanethidine administration to pre-pubertal and adult guinea-pigs, resulted in a significant decrease in the concentration of this neurotransmitter at the ovarian level [17,18]. Peripheral sympathetic denervation induced by guanethidine injection to prepubertal rats resulted in an increase in the number of ova shed at puberty. The same treatment performed to adult animals results in a decrease in the number of ova shed. These results suggest that the roles played by the sympathetic ovarian innervation in regulating ovulatory processes between pre-pubertal and adult rats are age dependent [24].
In summary, our results suggest that in the adult guinea pig, the SON modulates ovulation in an inhibitory way.
Acknowledgments
Acknowledgements
The authors gratefully acknowledge Bioterio "Claude Bernard", BUAP for giving the guinea pig for this research, and MS A. Domínguez-González for the English revision. This work was supported by a PADEP-UNAM and CONACYT, grant IN1796.
Contributor Information
F Luna, Email: flunam@yahoo.com.mx.
M Cortés, Email: fluna@siu.buap.mx.
M Flores, Email: fluna@siu.buap.mx.
B Hernández, Email: fluna@siu.buap.mx.
A Trujillo, Email: atrujilloh@hotmail.com.
R Domínguez, Email: rdcasala@hotmail.com.
References
- Burden HW. Ovarian innervation. In: Jones RE, editor. The Vertebrate Ovary. Comparative Biology and Evolution. New York: Plenum Press; 1978. pp. 616–638. [Google Scholar]
- Aguado LI, Ojeda SR. Prepuberal ovarian function is finely regulated by direct adrenergic influences. Role of noradrenergic innervation. Endocrinology. 1984;114:1845–1853. doi: 10.1210/endo-114-5-1845. [DOI] [PubMed] [Google Scholar]
- Ahmed CE, Dees WL, Ojeda SR. The immature rat ovary is innervated by vasoactive intestinal peptide [VIP] containing fibers and response to VIP with steroid secretion. Endocrinology. 1986;118:1682–1686. doi: 10.1210/endo-118-4-1682. [DOI] [PubMed] [Google Scholar]
- Domínguez R, Cruz ME, Chávez R. Differences in the ovulatory ability between the right and left ovary are related to ovarian innervation. In: Hirshfield AM, editor. In Growth factors and the ovary. New York: Plenum Press; 1989. pp. 321–325. [Google Scholar]
- Espey LL, Lipner H. Ovulation. In: Knobil E, Neill JD, editor. The Physiology of Reproduction. New York: Raven Press Ltd; 1994. pp. 725–780. [Google Scholar]
- Morales L, Chávez R, Ayala ME, Domínguez R. Effects of the unilateral or bilateral superior ovarian nerve section in prepubertal rats, on the ovulatory response to gonadotrophins administration. J Endocrinol. 1998;158:213–219. doi: 10.1677/joe.0.1580213. [DOI] [PubMed] [Google Scholar]
- Lawrence IE, Burden HW. The origin of the extrinsic adrenergic innervation to the rat ovary. Anat Rec. 1980;196:51–59. doi: 10.1002/ar.1091960106. [DOI] [PubMed] [Google Scholar]
- Dees WL, Ahmed CE, Ojeda SR. Substance P- and vasoactive intestinal peptide-containing fibers reach the ovary by independent routes. Endocrinology. 1986;119:638–41. doi: 10.1210/endo-119-2-638. [DOI] [PubMed] [Google Scholar]
- Klein CM, Burden HW. Substance P- and vasoactive intestinal polypeptide (VIP)-immunoreactive nerve fibers in relation to ovarian postganglionic perikarya in para- and prevertebral ganglia: evidence from combined retrograde tracing and immunocytochemistry. Cell Tissue Res. 1988;252:403–10. doi: 10.1007/BF00214383. [DOI] [PubMed] [Google Scholar]
- Wylie SN, Roche PJ, Gibson WR. Ovulation after sympathetic denervation of the rat ovary by freezing its nerve supply. J Reprod Fertil. 1985;75:369–373. doi: 10.1530/jrf.0.0750369. [DOI] [PubMed] [Google Scholar]
- Chávez R, Carrizosa L, Domínguez R. Effects of superior ovarian nerve section on spontaneous and induced ovulation in the adult rat. Med Sci Res. 1991;19:41–42. [Google Scholar]
- Chávez R, Domínguez R. Participation of the SON in the regulation of compensatory ovarian hypertrophy: the effects of its section performed on each of the oestrous cycle. J Endocrinol. 1994;140:197–201. doi: 10.1677/joe.0.1400197. [DOI] [PubMed] [Google Scholar]
- Erskine MS, Weaver ChE. The role of ovarian sympathetic innervation in the control of estrous responsiveness in the rat. Horm Behav. 1988;22:1–11. doi: 10.1016/0018-506x(88)90026-8. [DOI] [PubMed] [Google Scholar]
- Blatchley FR, Walker FM, Poyser NL. Progesterone, prostaglandin F2alpha, and oestradiol in the utero-ovarian venous plasma of non-pregnant and early, unilaterally pregnant guinea-pigs. J Endocrinol. 1975;67:225–9. doi: 10.1677/joe.0.0670225. [DOI] [PubMed] [Google Scholar]
- Burden HW. Adrenergic innervation in ovaries of the rat and guinea pig. Am J Anat. 1972;133:455–462. doi: 10.1002/aja.1001330407. [DOI] [PubMed] [Google Scholar]
- Riboni L, Chávez E, Domínguez R. Peripheral sympathetic denervation provoked by guanethidine injection results in the advancement of ovulation induced by sequential gonadotrophin treatment in prepubertal female guinea pig. Med Sci Res. 1997;25:453–454. [Google Scholar]
- Riboni L, Escamilla C, Chavira R, Domínguez R. Effects of peripheral sympathetic denervation induced by guanethidine administration on the mechanisms regulating puberty in the guinea pig. J Endocrinol. 1998;156:91–98. doi: 10.1677/joe.0.1560091. [DOI] [PubMed] [Google Scholar]
- Trujillo A, Riboni L. Effects of functional peripheral sympathetic denervation induced by guanethidine on follicular development and ovulation of the adult female guinea pig. Gen Comp Endocrinol. 2002;127:273–8. doi: 10.1016/S0016-6480(02)00062-X. [DOI] [PubMed] [Google Scholar]
- Morales L, Chávez R, Domínguez R. The participation of superior ovarian nerve in the regulation of ovulation in the prepubertal rat. Differential effects of unilateral and bilateral section of the nerve in the intact and hemiovariectomised animal. Med Sci Res. 1993;21:15–17. [Google Scholar]
- Bland KP. Biphasic follicular growth in the guinea pig oestrous cycle. J Reprod Fertil. 1980;60:73–76. doi: 10.1530/jrf.0.0600073. [DOI] [PubMed] [Google Scholar]
- Horton EW, Poyser NL. Uterine luteolytic hormone: a physiological role for prostaglandin F2alpha. Physiol Rev. 1976;56:595–651. doi: 10.1152/physrev.1976.56.4.595. [DOI] [PubMed] [Google Scholar]
- Greenwald GS, Roy SK. Follicular development and its control. In: Knobil E, Neill JD, editor. The Physiology of Reproduction. 2. New York: Raven Press Ltd; 1994. pp. 629–724. [Google Scholar]
- Chávez R, Morales L, González ME, Domínguez R. Ovarian norepinephrine content in prepubertal rats with SON section: temporal studies. Med Sci Res. 1994;22:789–790. [Google Scholar]
- Flores A, Ayala ME, Domínguez R. Does noradrenergic peripheral innervation have a different role in the regulation ovulation in the pubertal and adult rat? Med Sci Res. 1990;18:817–818. [Google Scholar]