Abstract
In the preceding paper (Bevensee, M.O., R.A. Weed, and W.F. Boron. 1997. J. Gen. Physiol. 110: 453–465.), we showed that a Na+-driven influx of HCO3 − causes the increase in intracellular pH (pHi) observed when astrocytes cultured from rat hippocampus are exposed to 5% CO2/17 mM HCO3 −. In the present study, we used the pH-sensitive fluorescent indicator 2′,7′-biscarboxyethyl-5,6-carboxyfluorescein (BCECF) and the perforated patch-clamp technique to determine whether this transporter is a Na+-driven Cl-HCO3 exchanger, an electrogenic Na/HCO3 cotransporter, or an electroneutral Na/HCO3 cotransporter. To determine if the transporter is a Na+-driven Cl-HCO3 exchanger, we depleted the cells of intracellular Cl− by incubating them in a Cl−-free solution for an average of ∼11 min. We verified the depletion with the Cl−-sensitive dye N-(6-methoxyquinolyl)acetoethyl ester (MQAE). In Cl−-depleted cells, the pHi still increases after one or more exposures to CO2/HCO3 −. Furthermore, the pHi decrease elicited by external Na+ removal does not require external Cl−. Therefore, the transporter cannot be a Na+-driven Cl-HCO3 exchanger. To determine if the transporter is an electrogenic Na/ HCO3 cotransporter, we measured pHi and plasma membrane voltage (Vm) while removing external Na+, in the presence/absence of CO2/HCO3 − and in the presence/absence of 400 μM 4,4′-diisothiocyanatostilbene-2,2′-disulphonic acid (DIDS). The CO2/HCO3 − solutions contained 20% CO2 and 68 mM HCO3 −, pH 7.3, to maximize the HCO3 − flux. In pHi experiments, removing external Na+ in the presence of CO2/HCO3 − elicited an equivalent HCO3 − efflux of 281 μM s−1. The HCO3 − influx elicited by returning external Na+ was inhibited 63% by DIDS, so that the predicted DIDS-sensitive Vm change was 3.3 mV. Indeed, we found that removing external Na+ elicited a DIDS-sensitive depolarization that was 2.6 mV larger in the presence than in the absence of CO2/ HCO3 −. Thus, the Na/HCO3 cotransporter is electrogenic. Because a cotransporter with a Na+:HCO3 − stoichiometry of 1:3 or higher would predict a net HCO3 − efflux, rather than the required influx, we conclude that rat hippocampal astrocytes have an electrogenic Na/HCO3 cotransporter with a stoichiometry of 1:2.
Keywords: acid–base transport, glia, intracellular Cl−, N -(6-methoxyquinolyl)acetoethyl ester, patch clamp
INTRODUCTION
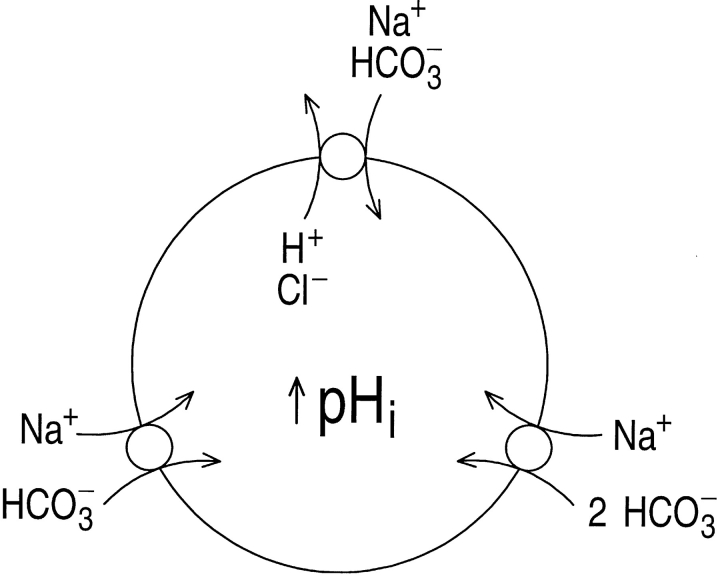
As described in the accompanying paper (Bevensee et al., 1997), exposing rat hippocampal astrocytes to CO2/ HCO3 − causes pHi to decrease initially, due to the influx of CO2, and then generally to increase to a value higher than the initial one prevailing in the nominal absence of CO2/HCO3 −. Because this pHi increase is blocked by the HCO3 −-transport inhibitors 4,4′-diisothiocyanatostilbene-2,2′-disulphonic acid (DIDS)1 and 4-acetamido-4′-isothiocyanatostilbene-2,2′-disulfonic acid (SITS), and requires external Na+, we concluded that the astrocytes have a Na+-driven HCO3 − transporter. The data are consistent with the presence of one or more of three HCO3 − transporters known to exist in other cells (Fig. 1): (a) a Na+-driven Cl-HCO3 exchanger, (b) an electroneutral Na/HCO3 cotransporter with a Na+:HCO3 − stoichiometry of 1:1, and (c) an electrogenic Na/HCO3 cotransporter with a Na+:HCO3 − stoichiometry of 1:2. Theoretically, a fourth possibility is the electrogenic NaHCO3 cotransporter with a 1:3 stoichiometry, as exists in renal proximal tubules (Boron and Boulpaep, 1983; Soleimani et al., 1987). However, given the ion and voltage gradients likely to prevail in an astrocyte, this 1:3 cotransporter would almost certainly mediate net HCO3 − efflux, and not the influx necessary to account for the observed CO2/HCO3 −-induced alkalinization.
Figure 1.
Hippocampal astrocytes could have any of three known Na+-driven HCO3 − transport mechanisms.
Compared with the 1:1 and 1:2 Na/HCO3 cotransporters, the Na+-driven Cl-HCO3 exchanger is unique in requiring internal Cl−. Because the Na+-driven Cl-HCO3 exchanger normally moves Na+ and HCO3 − into a cell and Cl− out, transport in its normal (“forward”) direction should be inhibited by depleting cells of internal Cl−. However, because the K1/2 of Cl− for the Na+-driven Cl-HCO3 exchanger in mammalian cells is not known, even low levels of intracellular Cl− ([Cl−]i) might still support the exchanger in cells preincubated in a Cl−-free solution. Indeed, depleting [Cl−]i has proven difficult in some cells. For example, renal mesangial cells had to be incubated in a Cl−-free solution for 1–2 h to achieve substantial inhibition of their Na+-driven Cl-HCO3 exchanger (Boyarsky et al., 1988b ). In pyramidal neurons from rat hippocampus, the Na+-driven Cl-HCO3 exchanger was active even after the cells were preincubated in a Cl−-free solution for up to 4 h (Schwiening and Boron, 1994). Therefore, one must be cautious when interpreting results from experiments designed to assess the effect of acute extracellular Cl− removal on transporters requiring intracellular Cl−. In the present study, we use the Cl−-sensitive dye N -(6-methoxyquinolyl) acetoethyl ester (MQAE) to study the time course of depletion of [Cl−]i, finding that [Cl−]i falls to very low levels when astrocytes are incubated in a Cl−-free solution for as few as ∼11 min. Under these conditions, the Na+-driven HCO3 − transporter still operates in the forward direction, and moves HCO3 − into cells exposed to CO2/ HCO3 −. Furthermore, in the absence of external Cl−, the transporter still operates in the reverse direction, and moves HCO3 − out of cells exposed to a Na+-free solution.
The other possibilities shown in Fig. 1 are two Na/ HCO3 cotransporters, one that is electroneutral and has a Na+:HCO3 − stoichiometry of 1:1, and one that is electrogenic and has a stoichiometry of 1:2. In the present study, we distinguish between these two by using the perforated patch-clamp technique to record plasma membrane voltage (Vm) in astrocytes, and determine whether the movement of net negative charge accompanies the movement of HCO3 −. We found that removing Na+ elicits a DIDS-sensitive depolarization that is larger in the presence than in the absence of CO2/HCO3 −. Comparing the size of the DIDS-sensitive, HCO3 −-dependent depolarizations with the magnitude of the DIDS-sensitive HCO3 − effluxes measured under similar conditions, we conclude that the Na+-driven HCO3 − transporter in hippocampal astrocytes is an electrogenic Na/HCO3 transporter, presumably with a Na+:HCO3 − stoichiometry of 1:2.
METHODS
Solutions
The solutions used in the present study, with the exception of the ones buffered with 20% CO2/68 mM HCO3 −, are described in the accompanying paper (Bevensee et al., 1997). These 20% CO2 solutions were made by isotonically substituting 68 mM HCO3 salt for HEPES and a Cl salt. The patch-pipette filling solution contained (mM): 105 KCl, 45 NaCl, 1.0 MgCl2, 0.2 CaCl2, 10 EGTA, and 1.0 HEPES. The pH of the patch-pipette solution (pHpip) was adjusted to 7.4 with Tris. pHpip was buffered with only 1 mM HEPES to minimize the effect of pHpip on the pHi of a patched cell. In some early experiments, we used 120 KCl and 30 NaCl in the pipette solution. Pipette solutions contained either nystatin or amphotericin B (80–300 μM). MQAE was obtained from Molecular Probes, Inc. (Eugene, OR). Bumetanide, nystatin, and amphotericin B were obtained from Sigma Chemical Co. (St. Louis, MO). All other reagents were obtained as described in the accompanying paper (Bevensee et al., 1997).
Measurement of [Cl−]i in Astrocytes
Handling of cells.
We measured [Cl−]i in astrocytes using the Cl-sensitive indicator MQAE, developed by Verkman et al. (1989). Astrocytes were grown to confluence, as described in the accompanying paper (Bevensee et al., 1997), and passaged onto 8.5 × 8.5–mm glass coverslips. 2–8.25 h (average 4.7 ± 0.3 h, n = 23) before each experiment, a coverslip with confluent astrocytes was transferred to a HEPES-buffered solution containing 5 mM MQAE.
Optics.
After cells were exposed to dye, the coverslip was placed into a quartz cuvette designed to fit into a SPEX Fluorolog-2 spectrofluorometer (CM1T10E; Spex Industries, Inc., Edison, NJ). The coverslip was mounted at a 30° angle to the excitation light. Solutions flowed through the cuvette from bottom to top through Tygon® or stainless-steel tubing. Both the tubing and the cuvette were maintained at 37°C by a water jacket. The temperature of the cuvette was monitored continually during an experiment with a thermistor placed at the base of the cuvette. During an experiment, we used only one of the excitation monochromators of the dual-beam spectrofluorometer, continuously exciting with light at 320 nm (1.89-nm bandwidth). A photomultiplier tube mounted on an emission monochromator monitored the emitted fluorescence intensity (I320) at 460 nm (4.71-nm bandwidth). During each 3.0-s data collection cycle, the spectrofluorometer integrated the emitted intensity for 1.0 s, and corrected for fluctuations in the arc-lamp intensity (continuously monitored). The background fluorescence of cells containing no dye was measured daily, and subtracted from the total emitted fluorescence. The background fluorescence averaged 10.4 ± 1.5% (n = 23) of total I320 at the beginning of an experiment.
Spectral properties of MQAE.
The fluorescence of the quinolinium dye MQAE is quenched at progressively higher concentrations of halides such as Cl−. Fig. 2 A shows the fluorescence excitation and emission spectra of 5 μM MQAE in HEPES-buffered solutions of different Cl− concentrations. The spectra were obtained at 37°C; all solutions were maintained at a constant ionic strength of ∼305 mosmol by replacing Cl− with cyclamate. The excitation spectra (Fig. 2 A, left) were obtained by measuring the emitted fluorescence at 460 nm while exciting the dye with light from 270 to 420 nm in intervals of 1 nm. For each of the seven excitation spectra, there was a major peak at ∼320 nm and a minor peak at ∼350 nm. The emission spectra (Fig. 2 A, right) were obtained by exciting the dye at 350 nm and measuring the emitted fluorescence from 375 to 600 nm in intervals of 1 nm. For each of the seven emission spectra, the peak was at ∼460 nm. These spectral data are similar to those previously reported (Verkman et al., 1989), except that our 320-nm peaks in the excitation spectra were more prominent.
Figure 2.
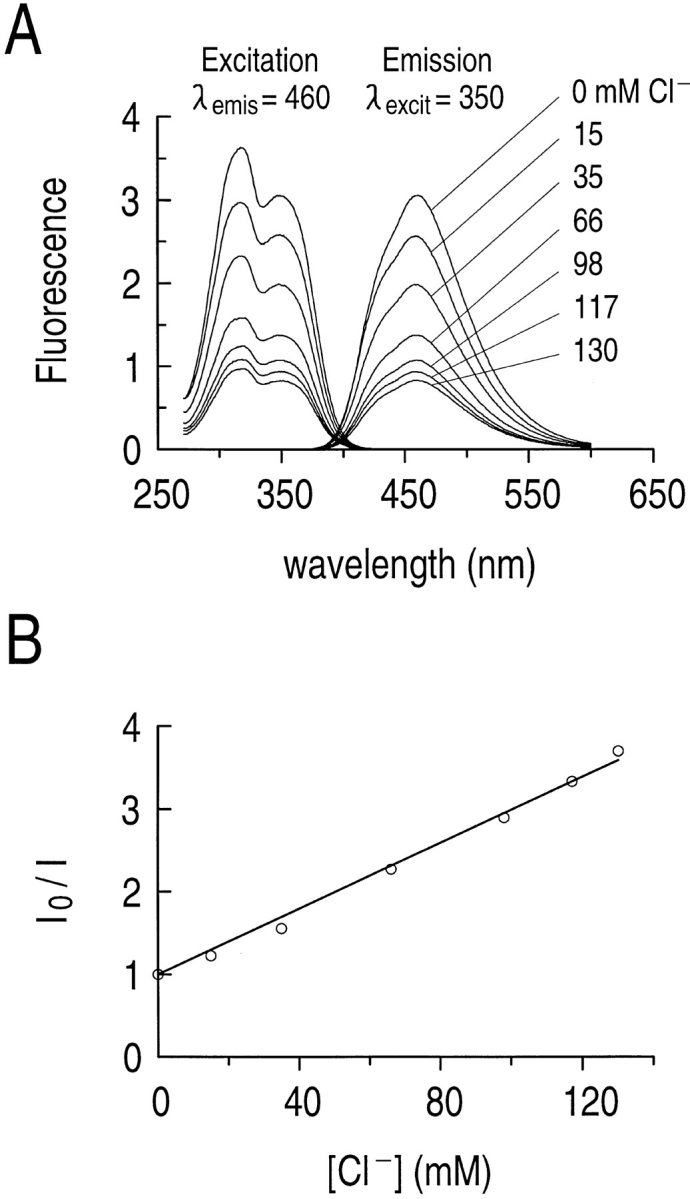
Cl− quenches MQAE fluorescence. (A) The fluorescence excitation and emission spectra of 5 μM MQAE in a cuvette containing solutions of different Cl− concentrations. Both the excitation curves (left) and emission curves (right) were obtained in solutions containing 0, 15, 35, 66, 98, 117, and 130 mM Cl− (top to bottom). (B) The Stern-Volmer relationship for Cl− quenching of MQAE from the emission spectra in A. For the least-square fit to the data points, r 2 = 0.996.
From the emission spectra shown in Fig. 2 A, we plotted the emitted fluorescence as a function of [Cl−] (Fig. 2 B), using the Stern-Volmer equation: I 0/I = 1 + K Cl × ([Cl−]), where I 0/I is the ratio of emitted fluorescence in the absence and presence of Cl−. K Cl is the Stern–Volmer quenching constant for Cl− (in M−1). From the best-fit line to the data (Fig. 2 B), we conclude that K Cl is 21 M−1 for MQAE in a cuvette. Although Verkman et al. (1989) reported a K Cl of 200 M−1 for MQAE, their fluorescence spectra were in solutions containing only 5 mM Na phosphate and variable amounts of NaCl (rather than our standard HEPES buffer at fixed osmolality). Indeed, Koncz and Daugirdas (1994) found that raising osmolality from 5 to 150 mosmol kg−1 caused the K Cl of MQAE to fall by ∼53%. Also, Lau et al. (1994) found that switching from an unbuffered to a HEPES-buffered solution caused the K Cl of intracellular MQAE to fall by ∼57%.
Intracellular calibration of MQAE.
In experiments on astrocytes loaded with MQAE, we calibrated the dye using the high K+/nigericin/tributyltin chloride technique (Chao et al., 1989). At the end of an experiment, astrocytes were exposed to a solution containing (a) 105 mM K+ and 10 μM nigericin to force pHi to approach pHo (Thomas et al., 1979), and (b) 5 μM tributyltin chloride, an ionophore that exchanges Cl− and OH−, and thus forces [Cl−]i to approach [Cl−]o. By exposing astrocytes to a high K+/nigericin/tributyltin chloride solution and changing external Cl−, we determined the Stern–Volmer relationship (see above) for intracellular MQAE in each experiment. The average K Cl of MQAE in hippocampal astrocytes was 8.7 ± 0.8 M−1 (n = 16). Intracellular values in the range of 5.3 to 25 M−1 have been reported for the K Cl of MQAE in other cell types (Lancer et al., 1990; Engblom and Akerman, 1993; Lau et al., 1994; Koncz and Daugirdas, 1994; Martínez-Zaguilán et al., 1994).
In nine experiments on hippocampal astrocytes loaded with MQAE, applying 0.01% saponin caused the fluorescent signal to decrease to 10.7 ± 1.7% of the signal at the start of the experiment. Because 0.01% saponin is thought to permeabilize only the plasma membrane (Lin et al., 1990), we conclude that 89% of intracellular MQAE is located in the cytoplasm.
Measurement of pHi in Single Astrocytes
We measured pHi using the pH-sensitive dye 2′,7′-biscarboxyethyl-5,6-carboxyfluorescein (BCECF) in astrocytes cultured from the hippocampus of the rat, as described in the accompanying paper (Bevensee et al., 1997).
Electrophysiology
Vm was recorded with a patch-clamp amplifier (PC-501A; Warner Instruments Corp., Hamden, CT), using the perforated whole-cell recording technique in current-clamp mode (Horn and Marty, 1988). Recordings were made on the stage of a microscope equipped for epi-fluorescence (IM-35; Carl Zeiss, Inc., Thornwood, NY), as described in the accompanying paper (Bevensee et al., 1997). Signals were filtered at 1 kHz with a 4-pole Bessel filter. Vm was digitized on-line at 50 Hz using an analog-to-digital converter board (Labmaster TL-1; Scientific Solutions Inc., Solon, OH) interfaced with a personal computer (Dell Computers, Austin, TX) based on an Intel-80486 microprocessor. The control of data acquisition and the analysis of data were performed with either a custom-modified version of WinClamp (Indec System, Inc., Capitola, CA) or pClamp (Axon Instruments, Inc., Foster City, CA). LG16 borosilicate glass capillaries (Dagan Corp., Minneapolis, MN) were pulled (PP-83; Narishige Scientific Instruments, Tokyo, Japan) and fire polished (MF-83; Narishige Scientific Instruments, Toyko, Japan) to make patch pipettes with resistances of 2–6 MΩ. In some experiments, the patch pipettes were coated with Sylgard® (Dow Corning Corp., Midland, MI) before being fire polished to minimize noise. Vm recordings were usually made at 37°C, and in no case <34°C.
Statistics
Data are reported as mean ± SEM. Means were compared using the paired and unpaired forms of the Student's t test (one-tail). P < 0.05 is considered significant. The best-fit line to the I 0/I vs. [Cl−] data plotted in Fig. 2 B was determined using a least-squares method. Rates of MQAE loss from cells was determined by fitting a line to the fluorescence vs. time data using a least-squares method. Rates of change in pHi (dpHi/dt) were determined by fitting a line to pHi vs. time data using a least-squares method.
RESULTS
Using MQAE to Measure Intracellular Cl− in Hippocampal Astrocytes
Na/K/Cl cotransport contributes to the high resting [Cl−]i in astrocytes.
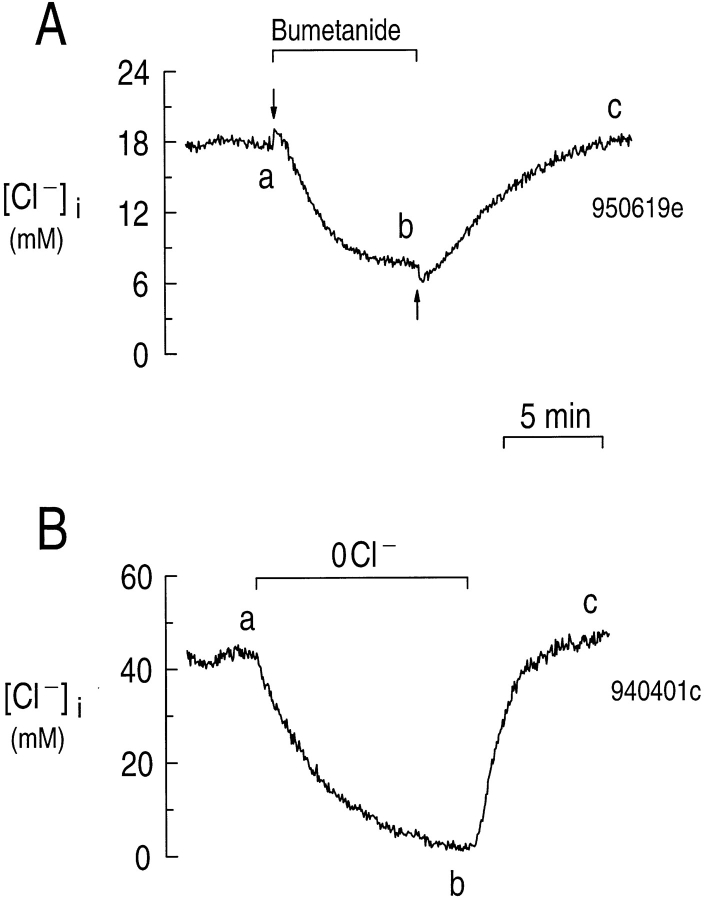
In 16 experiments in which we measured [Cl−]i using the Cl−-sensitive dye MQAE, astrocytes had a resting [Cl−]i of 36 ± 4 mM. This is similar to values in the range 25–30 mM, reported by Walz and Hertz (1983) for mouse astrocytes cultured from the cortex. The average resting [Cl−]i of 36 mM is well above the equilibrium value of 4.0 mM that is predicted from the mean Vm of ∼−88 mV that we observed (see below) for astrocytes in a HEPES-buffered solution containing 130 mM Cl−. Therefore, Cl− must be actively transported into the cells. In mouse astrocytes, a furosemide-sensitive Na/K/Cl cotransporter is responsible for maintaining intracellular Cl− well above the value predicted if Cl− were passively distributed (Walz and Hertz, 1983). In the experiment shown in Fig. 3 A, we determined whether a similar bumetanide-sensitive Na/K/Cl cotransporter contributes to the high resting [Cl−]i in rat hippocampal astrocytes. The [Cl−]i time course shown in Fig. 3 A is the result of a series of steps, outlined in Appendix, for converting MQAE fluorescence into [Cl−]i values. The astrocytes in this experiment had an initial [Cl−]i of ∼18 mM before point a (Fig. 3 A, a). When we exposed the cells to a solution containing 1 μM bumetanide, [Cl−]i decreased to ∼8 mM (Fig. 3 A, ab). Returning the cells to a bumetanide-free solution elicited an increase in [Cl−]i to a value similar to that at the start of the experiment (Fig 3 A, bc). Because 1 μM bumetanide fluoresces when excited at 320 nm, we subtracted the signal due to bumetanide from the total I320. This correction resulted in offsets in the [Cl−]i vs. time trace at the points where bumetanide was applied and removed (Fig. 3 A, arrows). In four experiments, bumetanide caused [Cl−]i to decrease from 23 ± 3 to 15 ± 3 mM (P < 0.005). A bumetanide-insensitive Cl− uptake mechanism could also contribute to the elevated [Cl−]i in the presence of bumetanide. We conclude that a bumetanide-sensitive Na/K/Cl cotransporter contributes to the high resting [Cl−]i in hippocampal astrocytes. Others have found that applying furosemide or bumetanide to cultured hippocampal astrocytes causes a decrease in intracellular Na+ ([Na+]i), suggesting that the Na/K/Cl cotransporter also helps maintain a high resting [Na+]i (Rose and Ransom, 1996).
Figure 3.
Adding bumetanide or removing external Cl− elicits a reversible decrease in [Cl−]i of hippocampal astrocytes. (A) Between a and b, we exposed the cells to 1 μM bumetanide. (B) Between a and b, we replaced the extracellular Cl− with cyclamate.
Removing extracellular Cl− elicits a rapid decrease in [Cl−]i.
In Fig. 3 B, we show the record of [Cl−]i in an experiment in which astrocytes were exposed to a solution in which Cl− was replaced with cyclamate. The cells in this experiment had an initial [Cl−]i of ∼43 mM before point a (Fig. 3 B, a). Removing external Cl− elicited a sharp decrease in [Cl−]i to ∼2 mM (Fig. 3 B, ab). Returning external Cl− caused [Cl−]i to increase to its initial value (Fig. 3 B, bc). In 11 experiments similar to that shown in Fig. 3 B, removing external Cl− caused [Cl−]i to decrease from an average resting value of 40 ± 5 to 2.8 ± 1.5 mM in 10.7 ± 1.9 min. [Cl−]i decreased at an initial rate of 277 ± 71 μM s−1 (n = 11).
Na+-driven HCO3 − Influx: Testing the Intracellular Cl− Dependence
Intracellular Cl− is not required for the forward transport of HCO3 − into astrocytes after an acid load imposed by an NH4 + prepulse.
If the CO2/HCO3 −-induced alkalinization were mediated by a Na+-driven Cl-HCO3 exchanger, then the alkalinization should require the efflux of substantial amounts of Cl−. In the following experiments, we monitored pHi in single astrocytes, using the pH-sensitive dye BCECF. Our first approach was to acid load the astrocytes repeatedly, using the NH4 + prepulse technique (Boron and De Weer, 1976), with 0.9 mM amiloride present to inhibit Na-H exchange. Because the amiloride was added as the Cl− salt, [Cl−]o was ∼ 0.9 mM. After each acid load, we added CO2/HCO3 −to activate the Na+-driven Cl-HCO3 −uptake mechanism. From the magnitude of the pHi recovery and from the known βT, we can compute how much internal Cl− would have to be transported out of the cell to achieve each pHi recovery.
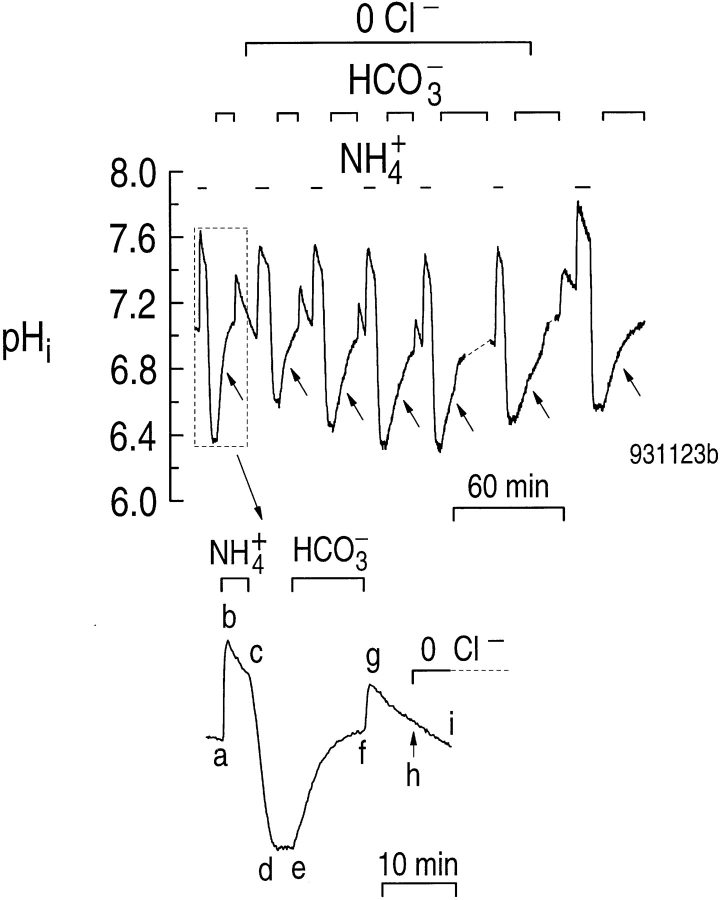
In the experiment of Fig. 4, we acid loaded the astrocytes seven times, the first time in the presence of 130 mM Cl−. As shown in the inset, applying and removing 20 mM NH3/NH4 + caused the usual series of pHi changes (Fig. 4, abcd), as noted in the accompanying study (Bevensee et al., 1997). In the absence of CO2/ HCO3 −, the pHi recovery was blocked by amiloride (Fig. 4, de). However, adding CO2/HCO3 − caused pHi to increase rapidly (Fig. 4, ef ). Removing the CO2/ HCO3 − caused a transient pHi increase (Fig. 4, fg), due to CO2 efflux, followed by a slower decline (Fig. 4, ghi). At point h, we reduced [Cl−]o to ∼0.9 mM and did not return [Cl−]o to 130 mM until the recovery from the sixth NH4 + pulse. Each of the seven CO2/HCO3 −-induced pHi recoveries is indicated by an arrow in Fig. 4. Two technical points are noteworthy. First, after the recovery from the fifth NH4 + pulse, we continued the experiment on a nearby cell on the same cover slip, because of evidence of dye loss from the original astrocyte. Similarly, we switched to a third cell after the sixth NH4 + pulse. We used this third cell, as well as two neighbors, for the calibration of intracellular BCECF. Second, there was a general tendency for the rates of pHi recovery in the presence of CO2/HCO3 − to decrease somewhat over the course of this very long experiment (>4 h). Indeed, the rate of the seventh and final pHi recovery (Cl− present) was ∼1/3 less than that of the first pHi recovery (Cl− present).
Figure 4.
Astrocytes that are acid loaded in HEPES display pHi recoveries when exposed to CO2/HCO3 − in the absence of external Cl−. As shown in the inset, astrocytes were acid loaded by a brief exposure to a solution containing 20 mM NH4Cl (a–d ). At c, the astrocytes were exposed to 0.9 mM amiloride for the duration of the experiment. During the indicated period, the cells were then exposed to 5% CO2/17 mM HCO3 − (ef ). Subsequently, the astrocytes were acid loaded in amiloride and exposed to CO2/ HCO3 − five more times in the absence of Cl− (top). The cells were then returned to the Cl−-containing solution and acid loaded one last time.
The CO2/HCO3 −-induced alkalinization after the second NH4 + pulse in Fig. 4 (i.e., the first in the absence of Cl−) would require the efflux of 4.5 mM Cl−, if it were mediated by Na+-driven Cl-HCO3 exchange. Similarly, the third through sixth CO2/HCO3 −-induced alkalinizations would require effluxes of 4.9, 4.9, 5.5, and 2.5 mM Cl−. However, based on the results from experiments similar to Fig. 3 B, the [Cl−]i just before the addition of CO2/HCO3 − in Fig. 4 was probably only ∼3 mM. Therefore, it appears unlikely that the pHi recoveries in 0 Cl− could be mediated by a Na+-driven Cl-HCO3 exchanger unless substantial amounts of Cl− could recycle back into the cell from the 0.9 mM Cl− in the external solution. However, for the first pHi recovery in the absence of extracellular Cl−, the maximal rate of HCO3 − influx would have required a Cl− efflux of 2.7 mM min−1. Because the Na/K/Cl cotransporter in primary cultures of rat astrocytes has a K m for external Cl− of 40 mM (Tas et al., 1987), it is unlikely that recycling could keep up with the Cl− depletion caused by HCO3 − transport. Thus, [Cl−]i during the CO2/HCO3 −-induced pHi recovery (i.e., Cl− efflux) was probably substantially less than the initial ∼3 mM mentioned above. Even if Cl− recycling were substantial, the five pHi recoveries under low [Cl−]o conditions could have been mediated by a Na+-driven Cl-HCO3 exchanger only if the hypothetical Na+-driven Cl-HCO3 exchanger had an extremely high affinity for intracellular Cl−. In squid axons, the only preparation in which the K m for intracellular Cl− has been determined for the Na+-driven Cl-HCO3 exchanger, K m (Cl−) is about the same as the resting [Cl−]i (Boron and Russell, 1983). Even if the K m (Cl−) for a hypothetical Na+-driven Cl-HCO3 exchanger in astrocytes were substantially less than the resting [Cl−]i of 36 mM, this K m (Cl−) would still be several times higher than the likely [Cl−]i during the CO2/ HCO3 −-induced pHi increase.
Intracellular Cl− does not appear to be necessary for the forward transport of HCO3 − into astrocytes when they are first exposed to CO2/HCO3 −.
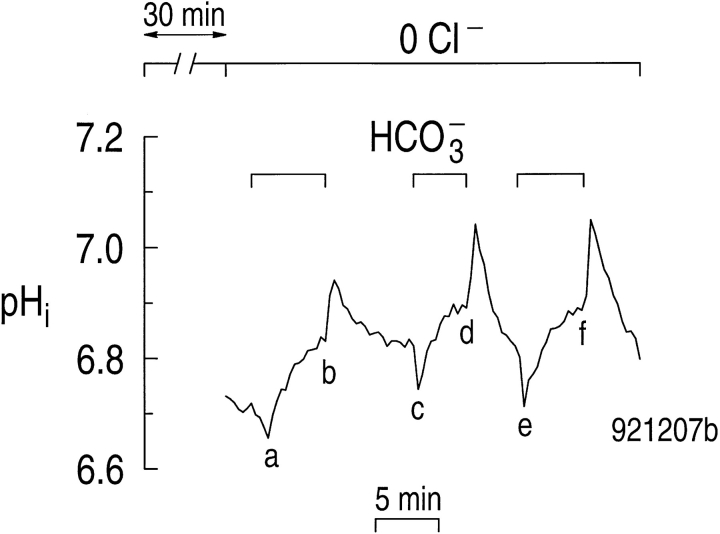
Our second approach for assessing the Cl− dependence of the CO2/HCO3 −-induced alkalinization was to determine if depleting astrocytes of Cl− slows this alkalinization. Before the start of the experiment in Fig. 5, the coverslip containing astrocytes was incubated in a Cl−-free solution for 30 min, enough time to reduce [Cl−]i to an average of ∼3 mM (Fig. 3 B). The Cl−-free solution also contained 1 mM isoguvacine, which stimulates the GABAA-activated Cl− channels known to be present in astrocytes in rat hippocampal slices (MacVicar et al., 1989). Thus, with isoguvacine accelerating the Cl− loss, [Cl−]i should have been extremely low by the outset of the experiment.
Figure 5.
Astrocytes preincubated in a Cl−-free solution still alkalinize when exposed to CO2/HCO3 −. Before the start of the experiment, the astrocyte was incubated for 30 min in a Cl−-free, HEPES-buffered solution containing 1 mM of the GABAA-agonist isoguvacine. During the experiment, 5% CO2/17 mM HCO3 − was applied and removed three times in the continued absence of external Cl−.
In Fig. 5, the astrocyte remained in Cl−-free solutions throughout the experiment. The initial pHi was ∼6.7 in the standard HEPES solution. We then exposed the astrocyte to a CO2/HCO3 −-buffered solution three times. With each exposure, pHi decreased transiently, due to CO2 influx (Fig. 5, a, c, and e), and then increased rapidly to a value higher than in the HEPES solution, due to Na+-driven HCO3 − influx (Fig. 5, b, d, and f ). The three pHi increases were of similar speed, and had a magnitude close to that observed in the accompanying paper (Bevensee et al., 1997) under control conditions (i.e., presence of extracellular Cl−). If the alkalinizations ab, cd, and ef were due to a Na+-driven Cl-HCO3 exchanger, then each alkalinization would further deplete the cell of intracellular Cl−. From the sum of the pHi increases in Fig. 5, ab, cd, and ef, and the average buffering power (βT), we calculate that, at a minimum, 9.5 mM HCO3 − was transported into the cell. If this HCO3 − transport were mediated by a Na+-driven Cl-HCO3 exchanger moving two HCO3 − ions for each Na+ and Cl− ion, then 4.7 mM Cl− would be required.2 As summarized in Table I, the computed Cl− loss was 8.8 and 12.3 in two other experiments. Because it is unlikely that these cells could have had >∼3 mM intracellular Cl− at the outset, it would appear that there was insufficient Cl− to support the CO2/HCO3 −-induced pHi increases, if the alkalinizations were mediated by a Na+-driven Cl-HCO3 exchanger.
Table I.
Intracellular Cl− Dependence of the Alkalinizations When Astrocytes Are Exposed to Multiple CO2/HCO3 − Pulses in the Absence of External Cl−
| Experiment | 0 Cl−-incubation | Exposures to CO2/HCO3 − | ΣΔpHi | Σ[Cl−]i required | ||||
|---|---|---|---|---|---|---|---|---|
| min | mM | |||||||
| 921207b | 30* | 3 | 0.51 | 4.7 | ||||
| 930729c | 12.5 | 8 | 0.91 | 12.3 | ||||
| 940111a | 16 | 3‡ | 0.74 | 8.8 |
0 Cl−-incubation solution contained 1 mM isoguvacine.
Solutions contained 10 μM ethylisopropylamiloride (EIPA).
Na+-driven HCO3 − Efflux: Testing the Extracellular Cl− Dependence
The transporter moves HCO3 − out of astrocytes exposed to a Na+-free, CO2/HCO3 −-buffered solution.
The data in Figs. 4 and 5 suggest that the Na+-driven HCO3 − transporter in astrocytes does not require intracellular Cl− to operate in the forward direction (i.e., HCO3 − influx). We also tested the hypothesis that the transporter does not require extracellular Cl− to operate in the reverse direction (i.e., HCO3 − efflux). This assay has the advantage that one can be reasonably confident that the Cl− has been rapidly and completely removed.
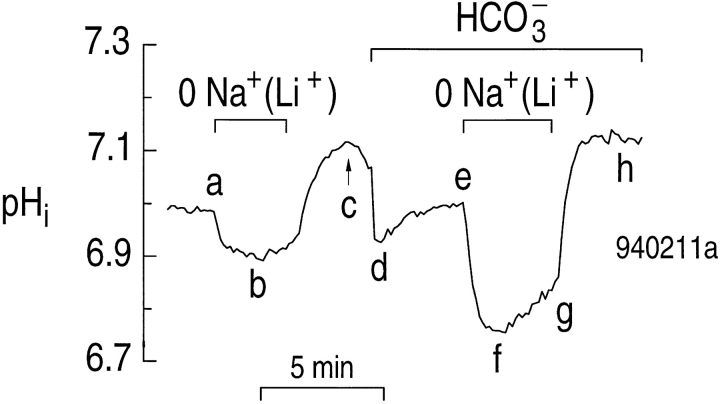
Our first step was to determine if the Na+-driven HCO3 − transporter in astrocytes can be forced to operate in the reverse direction by removing external Na+, thereby causing the net movement of Na+ and HCO3 − out of the cells, and decreasing pHi. Fig. 6 illustrates an experiment on a single astrocyte with a steady state pHi of ∼7.0 in a HEPES solution. Exposing the cell to a solution in which Li+ replaced Na+ as the major cation caused the pHi to decrease by ∼0.1 (Fig. 6, ab), presumably due to reversal of Na-H exchange. The pHi decrease with Li+ as the replacement cation was substantially less than that observed with NMDG+, because Li+, in contrast to NMDG+, can partially substitute for Na+ on the Na-H exchanger (Bevensee et al., 1997). Returning the cell to the Na+-containing solution caused pHi to increase (Fig. 6, bc) and actually overshoot the initial value (Fig. 6, c vs. a). This overshoot could be due to stimulation of Na-H exchange, inasmuch as the exchanger is stimulated by intracellular Li+ in barnacle muscle fibers (Davis et al., 1992). When the astrocyte in Fig. 6 was then switched to a solution buffered with 5% CO2/17 mM HCO3 −, the pHi decreased initially (Fig. 6, cd), and then increased slowly due, in part, to the Na+-driven HCO3 − transporter (Fig. 6, de). Removing external Na+ (replaced with Li+) in the presence of CO2/HCO3 − elicited a decrease in pHi (Fig. 6, ef) that was faster and larger than in HEPES (Fig. 6, ab), presumably because the Na+-driven HCO3 − transporter was operating in the reverse direction and moving HCO3 − out of the cell. As shown in the previous manuscript, Li+ is a poor substitute for Na+ on the Na+-driven HCO3 − transporter in hippocampal astrocytes (Bevensee et al., 1997). After its initial decrease, pHi began to increase slowly (Fig. 6, fg), presumably because external Li+ exchanged with internal H+ via the Na-H exchanger. Returning the astrocyte to the Na+-containing solution caused the pHi to increase (Fig. 6, gh) and overshoot the initial value (Fig. 6, h vs. e). In 14 experiments on astrocytes with an average pHi of 6.98 ± 0.04 in a HEPES solution, removing external Na+ elicited an average initial acid influx (ϕL) of 26.0 ± 3.7 μM s−1. However, in 27 experiments on astrocytes with a similar average pHi of 6.95 ± 0.02 in a CO2/HCO3 −-buffered solution, removing external Na+ elicited a mean ϕL of 102 ± 6 μM s−1. Therefore, ϕL is approximately fourfold greater when Na+ is removed in the presence than in the absence of CO2/HCO3 −, consistent with Na/ HCO3 efflux.
Figure 6.
Removing external Na+ elicits a larger and faster pHi decrease when astrocytes are exposed to CO2/HCO3 − rather than to HEPES. We replaced external Na+ with Li+ when the cell was exposed to a HEPES-buffered solution (ab) and a 5% CO2/17 mM HCO3 −-buffered solution (e–g).
DIDS inhibits the increase in pHi when astrocytes are switched from Li+ to Na+ in the presence of CO2/HCO3 −.
Initially, we attempted to determine if DIDS could block the HCO3 −-dependent pHi decrease observed when astrocytes are exposed to a Na+-free solution. However, we found that exposing cells to DIDS in the presence of CO2/HCO3 − causes a decrease in steady state pHi (not shown), presumably, in part, because DIDS inhibits the Na-dependent HCO3 − transporter that is active in the steady state. Because pHi was lower in the presence of DIDS, it was not possible to compare rates of acidification at similar pHi values in the presence and absence of DIDS. Therefore, we compared rates of alkalinization when astrocytes were reexposed to external Na+ in the presence and absence of DIDS.
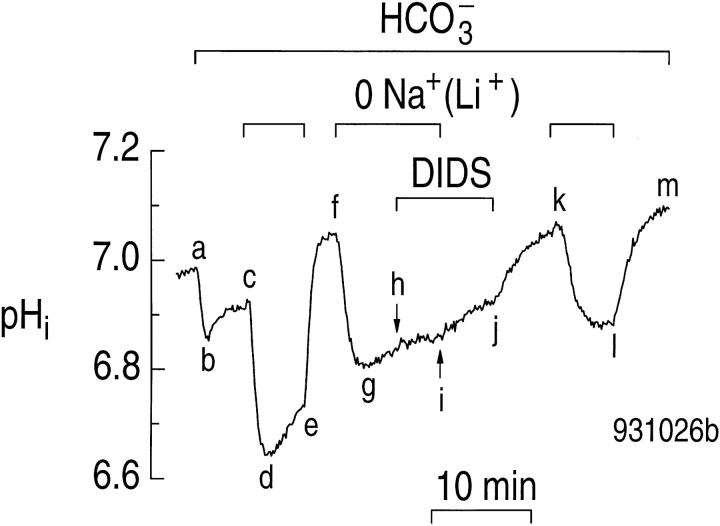
At the start of the experiment in Fig. 7, the astrocyte had a steady state pHi of ∼7.0 in a HEPES-buffered solution (before point a). Exposing the cell to CO2/ HCO3 − caused the usual changes in pHi (Fig. 7, abc). Replacing the Na+ with Li+ in the presence of CO2/ HCO3 − elicited a rapid decrease in pHi (Fig. 7, cd), followed by a slow increase (Fig. 7, de), as seen in Fig. 6. Returning Na+ to the external solution caused pHi to increase (Fig. 7, ef ) to a value higher than that prevailing in the presence of CO2/HCO3 − (Fig. 7, f vs. c). After we replaced Na+ with Li+ a second time, and observed the fall and partial recovery of pHi (Fig. 7, fgh), we added 400 μM DIDS. This addition had little effect on pHi (Fig. 7, hi). When we now returned external Na+ in the presence of DIDS, the acceleration of the pHi recovery (Fig. 7, ij vs. hi) was much smaller than in the absence of DIDS (Fig. 7, ef vs. de). Removing DIDS from the external solution allowed pHi to increase even faster (Fig. 7, jk), indicating that DIDS was partially reversible. Removing external Na+ a third time in the absence of DIDS elicited the expected decrease in pHi (Fig. 7, kl). However, DIDS may not have been entirely reversible, inasmuch as the rate of the pHi decrease (Fig. 7, k) was slower than at Fig. 7, c and f. Similarly, the pHi recovery elicited by returning Na+ the third time (Fig. 7, lm) was slower than for the first time (Fig. 7, ef ).
Figure 7.
DIDS inhibits the pHi increase caused by returning external Na+ to astrocytes bathed in 5% CO2/17 mM HCO3 −. Between a and m, the cell was exposed to 5% CO2/17 mM HCO3 −. We replaced external Na+ with Li+ three times (c–e, f–i, and kl ). 400 μM DIDS was present between points h and j.
Summarizing data from four such experiments, we found that returning Na+ in the presence of DIDS was associated with an acid extrusion rate (ϕE) of 13 ± 9 μM s−1 at a pHi of 6.83 ± 0.03. In paired experiments in the absence of DIDS, ϕE was 127 ± 15 μM s−1 at a slightly lower pHi of 6.75 ± 0.03. Therefore, DIDS inhibits ∼90% of the acid extrusion when astrocytes in CO2/HCO3 − are returned to a solution containing Na+ (P < 0.0003). The effect of DIDS is also CO2/HCO3 − dependent: returning external Na+ to astrocytes bathed in a HEPES solution containing 400 μM DIDS elicited a ϕE of 23 ± 9 μM s−1 at a pHi of 6.82 ± 0.07 (n = 6). In paired experiments at a similar pHi, ϕE in a HEPES solution in the absence of DIDS was nearly the same (25 ± 8 μM s−1, P = 0.26).
Na+-driven HCO3 − transporter can move HCO3 − out of astrocytes even in the absence of external Cl−.
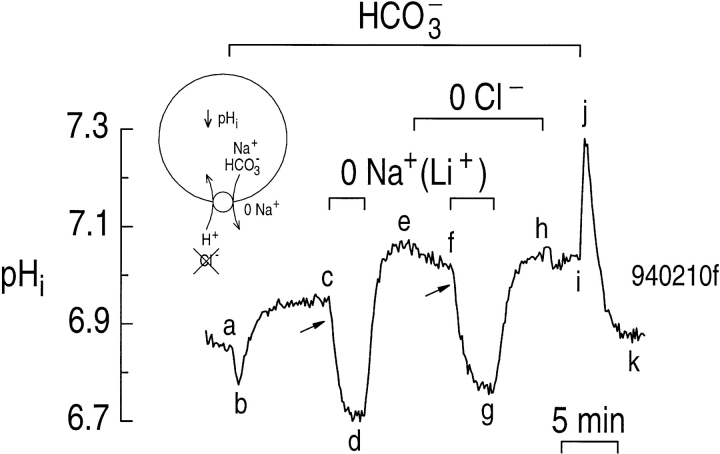
If a Na+-driven Cl-HCO3 exchanger were responsible for the CO2/ HCO3 −-dependent decrease in pHi when astrocytes are exposed to a Na+-free solution, then the pHi decrease should require external Cl− (Fig. 8, inset). In the experiment shown in Fig. 8, the astrocyte at the start of the experiment had a pHi of ∼6.85 in a HEPES solution. Switching to CO2/HCO3 − caused pHi to decrease (Fig. 8, ab), and then to increase promptly (Fig. 8, bc) to a value ∼0.1 higher than in the HEPES solution (Fig. 8, c vs. a). Removing external Na+ elicited a rapid and reversible pHi decrease (Fig. 8, cde), similar to that seen in Figs. 6 and 7. When the astrocyte was exposed to a Cl−-free solution, the pHi decreased slowly (Fig. 8, ef ). If the cells had either a Na+-driven Cl-HCO3 exchanger or a Cl-HCO3 exchanger, one might have expected pHi to increase in the absence of external Cl−, due to exchange of internal Cl− for external HCO3 −. In the continued absence of external Cl−, removing external Na+ elicited a rapid decrease in pHi (Fig. 8, fg) that was of the same rate as observed in the presence of external Cl− (Fig. 8, arrows). Returning the astrocyte to a solution containing Na+, but not Cl− caused pHi to increase to its initial value (Fig. 8, gh). Reintroducing Cl− had little effect on pHi (Fig. 8, hi). Finally, switching back to the HEPES solution elicited a sharp increase in pHi (Fig. 8, ij), followed by a rapid decrease (Fig. 8, jk) to the pHi prevailing at the start of the experiment (Fig. 8, k vs. a). In a total of eight experiments similar to that in Fig. 8, the ϕL caused by Na+ removal in the presence of external Cl− (Fig. 8, cd) was 101 ± 5 μM s−1 at a pHi 6.91 ± 0.03. ϕL in the absence of external Cl− (Fig. 8, fg) was 96 ± 10 μM s−1 (P = 0.32, paired t test) at the same pHi of 6.91 ± 0.03. As noted above, 75% of the acid influx (ϕL) observed during removal of extracellular Na+ in the presence of CO2/HCO3 − is due to reversal of the Na+-driven HCO3 − transporter. Because ϕL was unaffected by removing external Cl−, the Na+-driven HCO3 − transporter could not have been a Na+-driven Cl-HCO3 exchanger.
Figure 8.
The pHi decrease elicited by removing external Na+ in CO2/HCO3 − does not require external Cl−. In the presence of 5% CO2/17 mM HCO3 − (a–i), the cell was exposed twice to a solution in which Li+ replaced external Na+ (cd and fg). During the indicated period, cyclamate replaced external Cl− (e–h). As shown in the inset, a hypothetical cell possessing a Na+-driven Cl-HCO3 exchanger should have exhibited a pHi decrease upon removing external Na+, and this pHi decrease should have required external Cl−. The arrows point to the initial pHi decrease elicited by removing external Cl−.
Na/HCO3 Cotransport in 20% CO2 /68 mM HCO3 −
Because the Na+-driven HCO3 − transporter in hippocampal astrocytes does not require intracellular Cl− to operate in the normal forward direction, nor extracellular Cl− to operate in the reverse direction, we conclude that the transporter cannot be a Na+-driven Cl-HCO3 exchanger. By default (see introduction), the Na+-driven HCO3 − transporter must be a Na/HCO3 cotransporter with a Na+:HCO3 − stoichiometry of either 1:1 (electroneutral) or 1:2 (electrogenic). If the transporter were electrogenic, then the acid influx mediated by the transporter operating in reverse (Fig. 8, cd) should be associated with an inward current that depolarizes the astrocyte. From the initial rate of pHi decrease during Fig. 8, cd, we calculate3 that a 1:2 Na/ HCO3 cotransporter would produce a DIDS-sensitive depolarization of 1.7 mV. Because such a small depolarization could be difficult to detect, we attempted first to enhance the HCO3 − efflux, and thus the predicted depolarization.
DIDS inhibits the Na/HCO3 cotransporter in astrocytes exposed to 20% CO2 /68 mM HCO3 −.
We performed experiments (not shown) identical to that in Fig. 7, except that the CO2/HCO3 − solutions contained 20% CO2 and 68 mM HCO3 −, pH 7.3. The HCO3 − efflux from astrocytes should be greater when the cells, at the same pHi, are incubated in this “high CO2/HCO3 −” solution because the fourfold increase in [HCO3 −]i would increase the efflux (if the cotransporter were not already saturated). In the continued presence of high CO2/HCO3 −, removing external Na+ elicited a rapid decrease in pHi that was reversed upon returning Na+. In paired experiments, returning external Na+ in the presence of DIDS elicited a pHi increase that was substantially slower than in the absence of DIDS. Summarizing the HCO3 −-influx data for astrocytes bathed in 20% CO2/68 mM HCO3 −, DIDS caused ϕE (measured when we returned Na+) to decrease from 239 ± 48 to 87 ± 15 μM s−1 at a pHi of ∼6.7 (n = 6; P < 0.008). Thus, DIDS inhibited acid extrusion less in 20% CO2 (63%) than in 5% CO2 (90%), suggesting that HCO3 − may compete with DIDS.
Summarizing HCO3 −-efflux data in 20% CO2, we found that removing Na+ was associated with a ϕL of 281 ± 31 μM s−1, at a pHi of 6.96 ± 0.03 (n = 14). This efflux is 2.8-fold greater than the ϕL of 101 μM s−1 observed in 5% CO2. Assuming that 63% of the HCO3 − efflux in 20% CO2 is DIDS sensitive, the predicted DIDS-sensitive depolarization would be ∼3.3 mV, approximately twice as large as in 5% CO2. In the next section, we describe experiments in which we attempted to detect this predicted ∼3.3-mV depolarization.
Electrogenic Na/HCO3 Cotransport
Astrocytes exposed to 20% CO2/68 mM HCO3 − had a resting Vm of −92 mV and an input resistance of 236 MΩ in the perforated patch-clamp configuration.
The average resting Vm of astrocytes in a HEPES solution was −87.5 ± 0.8 mV (range: −72.0 to −97.7, n = 52), whereas the average resting Vm of astrocytes in a 20% CO2/68 mM HCO3 −-buffered solution was −92.0 ± 0.7 mV (range: −80.8 to −99.1, n = 40). In 28 experiments in which we switched from HEPES to CO2/HCO3 −, Vm became more negative by an average of 4.3 ± 0.5 mV (P < 0.0001; paired t test). At least qualitatively, this hyperpolarization is consistent with the movement of net negative charge into the cell via electrogenic Na/HCO3 cotransport.
The input resistance of astrocytes was computed at the beginning of some experiments from the current step (ΔI) in response to a 5-ms, 5-mV depolarizing pulse (ΔV) from a holding potential of ∼−80 mV. The average input resistance (ΔV/ΔI) was 236 ± 32 MΩ (n = 18). In other experiments, we computed total resistance at each sample point from the change in Vm in response to current injections. Total resistance did not appear to change when astrocytes were switched from a HEPES- to a CO2/HCO3 −-buffered solution or vice versa.
In the perforated patch-clamp configuration, the pipette contents have little effect on pHi.
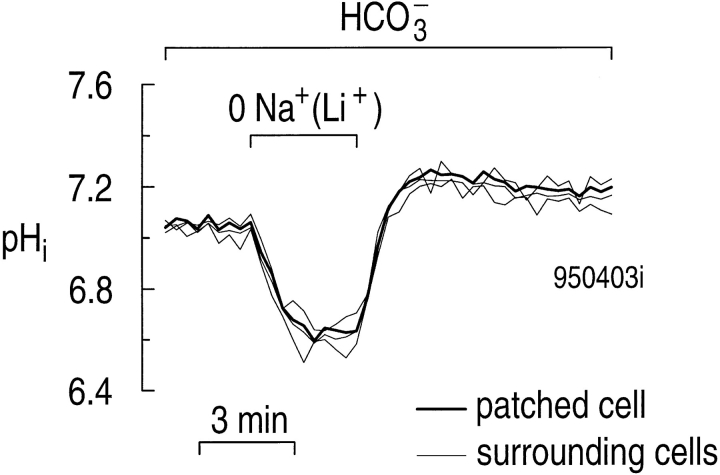
Because the pH of the patch-pipette solution was only weakly buffered (1 mM HEPES), and because a perforated patch limits the diffusion of solutes between cell and pipette, we hoped that patched astrocytes subjected to manipulations of the extracellular solution would display the same pHi changes as unpatched cells. In the experiment shown in Fig. 9, we used a digital-imaging system to monitor pHi in four astrocytes, one of which was attached to the patch pipette (thick trace). In the presence of 5% CO2/17 mM HCO3 −, all cells underwent similar (and expected) pHi changes in response to replacing extracellular Na+ with Li+.
Figure 9.
The patch pipette in the cell-attached configuration has little effect on the pHi changes in astrocytes exposed to a Na+-free (Li+) solution in 5% CO2/17 mM HCO3 −. The patched cell (thick trace), as well as three surrounding cells (thin traces), were exposed to a HCO3 −-containing solution in which we replaced external Na+ with Li+. Similar results were obtained in three other experiments.
Removing external Na+ elicits an HCO3 −-dependent depolarization that is blocked by DIDS.
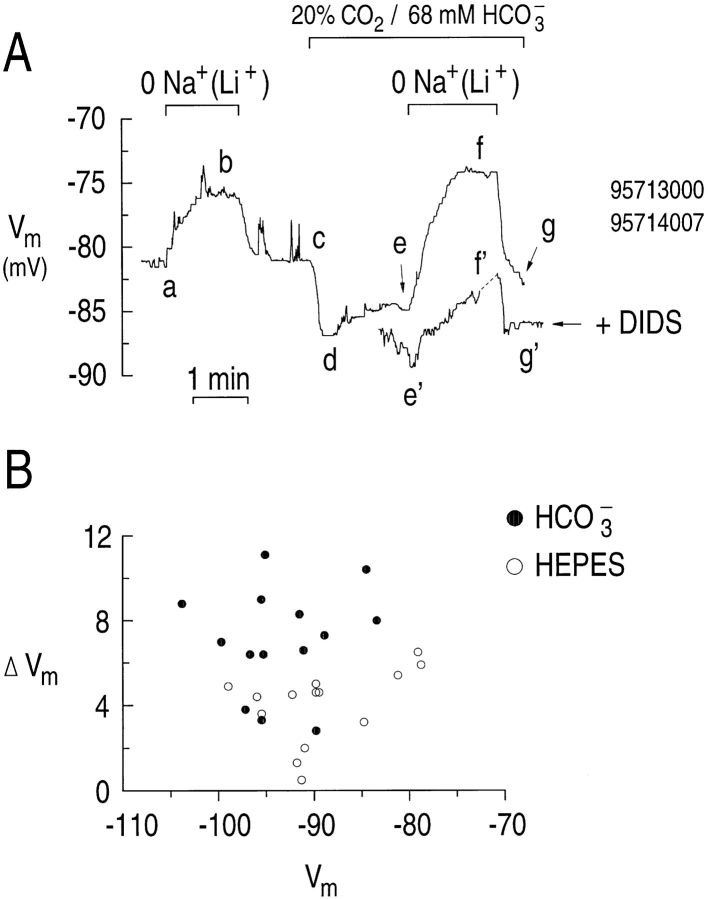
In the experiment shown in Fig. 10 A, the astrocyte had a resting Vm of ∼−82 mV in the nominal absence of CO2/HCO3 − (Fig. 10 A, a). Replacing external Na+ with Li+ elicited a depolarization to ∼−76 mV (Fig. 10 A, ab) that was completely reversed when we returned the Na+ (Fig. 10 A, bc). Some of this depolarization may be due to the inhibition of a Na-dependent K+ conductance (Martin and Dryer, 1989). Subsequently, exposing the cell to 20% CO2/68 mM HCO3 − elicited an initial hyperpolarization to ∼−87 mV (Fig. 10 A, cd), followed by a slower depolarization to ∼−84 mV (Fig. 10 A, de). As noted above, the initial hyperpolarization is qualitatively consistent with the hypothesis that a Na/HCO3 cotransporter moves net negative charge into the cell. When the astrocyte was exposed to a Na+-free solution in the presence of CO2/HCO3 −, the cell depolarized to ∼−73 mV (Fig. 10 A, ef ). The depolarization in the presence of CO2/ HCO3 − was ∼6 mV greater than in the absence of CO2/HCO3 −. Moreover, compared with the depolarization in the nominal absence of CO2/HCO3 −, the one in the presence of CO2/HCO3 − started at a more negative Vm and finished at a more positive Vm, ruling out the possibility that the difference in depolarizations was due to the voltage dependence of the conductances. When Na+ was returned to the external solution, the astrocyte hyperpolarized towards the initial resting Vm in the presence of CO2/HCO3 − (Fig. 10 A, fg). In a separate experiment, the changes in Vm produced by removing and returning external Na+ in the presence of 20% CO2/68 mM HCO3 − were greatly reduced in the presence of DIDS (Fig. 10 A, e ' f ' g ' ).
Figure 10.
Removing external Na+ elicits a depolarization that is inhibited by DIDS or by removing 20% CO2/68 mM HCO3 −. (A) Effect of removing Na+ on the Vm of astrocytes exposed to a “high” CO2/HCO3 − solution. We replaced external Na+ with Li+ when the cell was exposed to a HEPES-buffered solution (ab) and a 20% CO2/68 mM HCO3 −-buffered solution (ef ). In a separate experiment on an astrocyte exposed to 400 μM DIDS, we replaced external Na+ with Li+ in the presence of 20% CO2/68 mM HCO3 − (e ' f ' ). (B) The magnitude of the depolarization plotted as a function of Vm for 14 astrocytes in which external Na+ was removed in either HEPES (○) or 20% CO2/68 mM HCO3 − (•). Some data points represent the average depolarization elicited by removing external Na+ more than once in a single experiment.
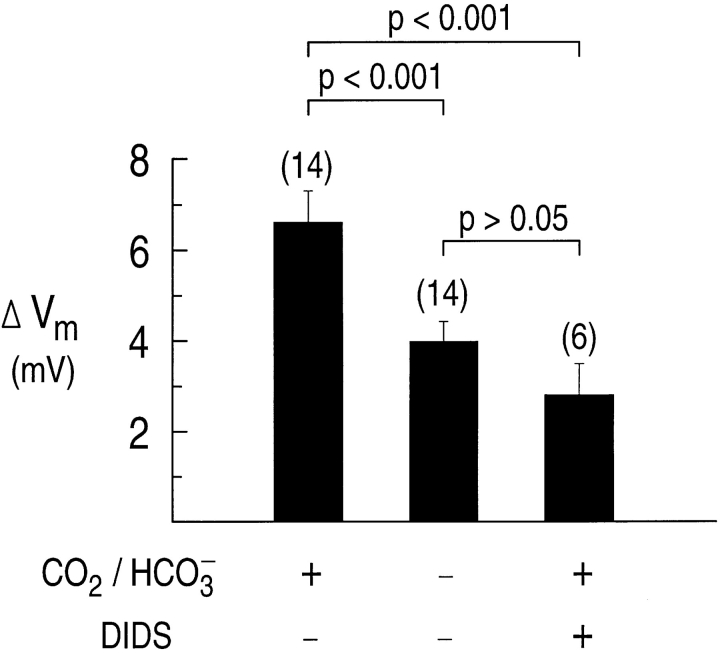
Fig. 10 B summarizes the results from 14 experiments similar to that shown in Fig. 10 A. In each case, the astrocyte was exposed to a Na+-free solution in the presence and absence of 20% CO2/68 mM HCO3 −. The magnitude of the depolarizations in either the presence or absence of CO2/HCO3 − are plotted as a function of the resting Vm before the Na+ removal. In general, the ΔVm in the presence of CO2/HCO3 − (•) is larger than in HEPES (○). In the presence of CO2/ HCO3 − (average resting Vm = −93.4 ± 1.5 mV), removing external Na+ elicited a mean depolarization of 6.6 ± 0.7 mV (Fig. 11). In the absence of CO2/HCO3 − (average resting Vm = −89.0 ± 1.7 mV), removing external Na+ caused a statistically smaller mean depolarization of 4.0 ± 0.5 mV. In the presence of DIDS, removing external Na+ in CO2/HCO3 − elicited a depolarization of only 2.8 ± 0.7 from a resting Vm of −93.3 ± 2.6. Thus, the depolarization in CO2/HCO3 − is larger than in either HEPES or CO2/HCO3 − plus DIDS, and the depolarization in HEPES is similar to that in CO2/ HCO3 − plus DIDS.
Figure 11.
Removing external Na+ from hippocampal astrocytes exposed to a 20% CO2/68 mM HCO3 −-buffered solution elicits a mean depolarization that is larger than that observed for cells exposed to (a) a HEPES-buffered solution, or (b) a 20% CO2/68 mM HCO3 −-buffered solution containing 400 μM DIDS.
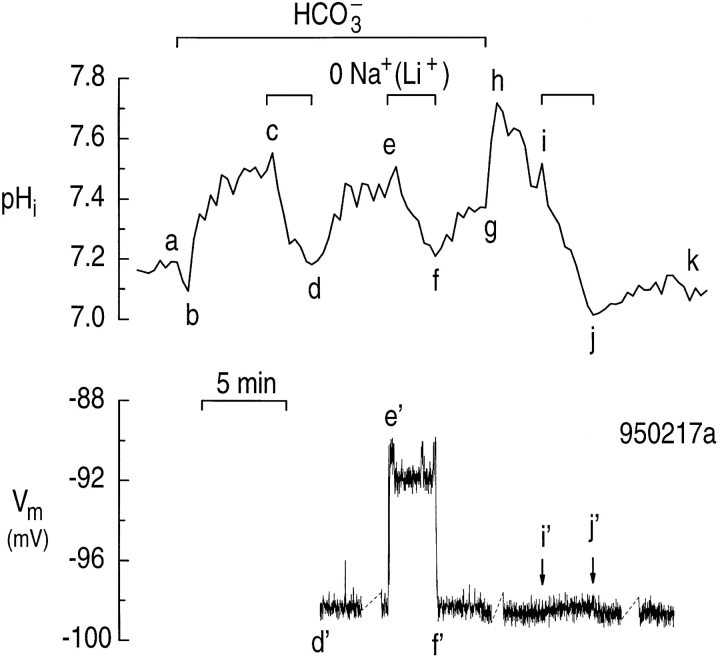
Na/HCO3 cotransport elicited simultaneously recorded changes in pHi and Vm in single astrocytes.
Fig. 12 illustrates an experiment in which we recorded pHi and Vm simultaneously in a single astrocyte. The cell had an initial pHi of ∼7.2 in a HEPES solution. When the astrocyte was exposed to a solution buffered with 5% CO2/17 mM HCO3 −, the pHi decreased (Fig. 12, ab), and then increased rapidly to a value well above the initial pHi (Fig. 12, bc). Replacing bath Na+ with Li+ elicited a rapid pHi decrease (Fig. 12, cd) that was reversible (Fig. 12, de). The resting Vm was ∼−98 mV when the astrocyte was returned to the Na+-containing solution at Fig. 12, d'. The Vm recording before Fig. 12, d' was unstable and is therefore not shown. When Na+ was removed from the bath a second time, pHi again decreased (Fig. 12, ef ). From the speed of the pHi decrease, we predict a ΔVm of 8.1 mV for an electrogenic Na/HCO3 cotransporter with a 1:2 stoichiometry. The onset of the pHi decrease (Fig. 12, e) coincided with an abrupt depolarization4 of ∼7 mV (Fig. 12, e'). When Na+ was returned to the bath, the pHi increased as expected (Fig. 12, fg). The onset of the pHi increase (Fig. 12, f ) coincided with the return of Vm to the initial resting value (Fig. 12, f '). When the astrocyte was switched to a HEPES-buffered solution, the pHi increased rapidly (Fig. 12, gh), and then decreased (Fig. 12, hi). Switching to a Na+-free solution, now in the nominal absence of CO2/HCO3 −, caused a further decrease in pHi (Fig. 12, ij), but only a small depolarization (Fig. 12, i '). When Na+ was returned to the bath before the pHi stabilized, pHi began to increase (Fig. 12, jk), but the cell hyperpolarized only slightly (Fig. 12, j '). The simultaneous recording of pHi and Vm in hippocampal astrocytes demonstrates that removing/returning external Na+ in the presence of CO2/HCO3 − elicits pHi changes that coincide with Vm changes.
Figure 12.
Removing external Na+ elicits a simultaneous depolarization and pHi decrease in a single hippocampal astrocyte. The astrocyte was exposed to 5% CO2/17 mM HCO3 − between points a and g. During the indicated periods, we replaced external Na+ with Li+ (cd, ef, and ij).
DISCUSSION
Evidence Against Na+-driven Cl-HCO3 Exchange in Hippocampal Astrocytes
The transporter can move HCO3 − into astrocytes when internal Cl− is largely depleted.
In the accompanying paper (Bevensee et al., 1997), we demonstrate that cultured hippocampal astrocytes exposed to CO2/HCO3 − display, on average, an increase in their steady state pHi. The pHi increase is mediated, in part, by a HCO3 − transporter that requires Na+ and is blocked by the stilbene derivatives DIDS and 4-acetamido-4′-isothiocyanatostilbene-2,2′-disulfonic acid (SITS). In the present study, we show that this transporter does not require intracellular Cl− to operate in the normal, forward direction. Our approach was to deplete cells of intracellular Cl−, and then monitor the pHi increase in the cells exposed multiple times to CO2/HCO3 −. Using the Cl−-sensitive indicator MQAE, we found that hippocampal astrocytes could be nearly depleted of intracellular Cl− by incubating the cells in a Cl−-free solution for an average of ∼11 min. This Cl− depletion was probably mediated, at least in part, by a bumetanide-sensitive Na/K/Cl cotransporter. In the absence of external Cl−, cells exposed to CO2/ HCO3 − still displayed an increase in pHi similar to that observed in the presence of Cl−. In fact, astrocytes exposed multiple times to CO2/HCO3 − in the absence of external Cl− displayed multiple increases in pHi. It is unlikely that these pHi increases could have been mediated by a Na+-driven Cl-HCO3 exchanger, inasmuch as such an exchanger would have required substantial amounts of intracellular Cl−.
The transporter can move HCO3 − out of astrocytes in the absence of external Cl−.
We also tested the Cl− dependence of the transporter using a simpler and briefer protocol in which we drove the transporter in the reverse (i.e., HCO3 − outward) direction by removing external Na+ in the presence of CO2/HCO3 −. Under such conditions, we found the rate of pHi decrease to be the same in both the presence and absence of external Cl−. If the transporter were a Na+-driven Cl-HCO3 exchanger, which would require external Cl− to operate in the reverse direction, the pHi decrease should have been substantially reduced in the absence of external Cl−.
Evidence for Electrogenic Na/HCO3 Cotransport in Hippocampal Astrocytes
Switching from HEPES to CO2/HCO3 − causes a hyperpolarization.
Because the Na+-driven HCO3 − transporter is not coupled to Cl−, it presumably is either a 1:1 (electroneutral) or a 1:2 (electrogenic) Na/HCO3 cotransporter. If this astrocyte cotransporter were electrogenic, then it should simultaneously move net negative charge and HCO3 − in the same direction. In the accompanying study (Bevensee et al., 1997), we show that hippocampal astrocytes exposed to 5% CO2/HCO3 − at 37°C first acidify due to CO2 influx, and then display a maximum net acid extrusion (i.e., HCO3 − uptake) rate of 47.7 μM s−1, of which 75% is inhibited by DIDS. Therefore, the DIDS-sensitive HCO3 − influx is 35.8 μM s−1. From the average cell volume and input resistance of astrocytes, we predict5 that such a HCO3 − influx would generate a current of only 2.9 pA and a hyperpolarization of only 0.7 mV. Such small CO2/HCO3 −-induced currents and Vm changes would be difficult to measure.
Our measured net acid extrusion, after the addition of CO2/HCO3 −, could have underestimated the unidirectional DIDS-sensitive HCO3 − influx if the actual HCO3 − influx were partially masked by an acid-loading process (e.g., Cl-HCO3 exchange). Imagine that the DIDS-sensitive HCO3 − influx was in fact 360 μM s−1, 10-fold higher than we suspect, but that this was opposed by a Cl-HCO3 exchange of 324 μM s−1. In this case, the net flux would be only 36 μM s−1, as observed. Whereas masking of a substantial HCO3 − influx is theoretically possible, we think that it is unlikely inasmuch as astrocytes seem to lack significant Cl-HCO3 exchange (see Fig. 8). The potential complexity of the pHi changes associated with exposing a cell to CO2/HCO3 − underscores the difficulty of using the application of CO2/HCO3 − as an assay for electrogenic Na/HCO3 cotransport.
Brune et al. (1994), working on cultured rat cerebellar astrocytes, observed a mean hyperpolarization threefold larger than our prediction, 2.3 mV. O'Connor et al. (1994), working on cultured rat hippocampal astrocytes, observed hyperpolarizations ranging from ∼0 mV (Vm ≅ −75 mV) to ∼25 mV (Vm ≅ −40 mV). Because our calculations show that a depolarization of 0.7 mV would be coupled to a DIDS-sensitive acid-extrusion rate of 35.8 μM s−1, we can conclude that a depolarization of ∼25 mV would be coupled to a DIDS-sensitive acid-extrusion rate of 35.8 × (25/0.7) ≅ 1,280 μM s−1. This figure is substantially greater than any transporter-mediated acid–base flux of which we are aware. For example, it is about eightfold greater than the flux of 160 μM s−1 mediated jointly by Na-H and Na-dependent Cl-HCO3 exchangers in acid-loaded renal mesangial cells (Boyarsky et al., 1988a ), or the flux of 170 μM s−1 mediated by two similar transporters in acid-loaded pyramidal neurons from rat hippocampus (Bevensee et al., 1996), or the flux of 170 μM s−1 induced by basolateral CO2/HCO3 − addition in rabbit S3 proximal tubules (Nakhoul et al., 1993). One could argue that, under the depolarized conditions (∼40 mV) at which the data from O'Connor et al. (1994) were obtained, the electrogenic Na/HCO3 cotransporter influx may have been substantially higher than in our cells (mean Vm ≅ −88 mV). Therefore, we measured the net HCO3 − influx after addition of CO2/HCO3 − in astrocytes depolarized with 2 or 5 mM Ba2+ (Vm ≅ −56 mV). We found no significant difference (not shown) between the net HCO3 − influx under normally polarized and depolarized conditions. The large hyperpolarization observed upon first exposing astrocytes to CO2/HCO3 − may primarily reflect processes other than electrogenic Na/ HCO3 cotransport, such as the influx of HCO3 − through channels (see Bevensee et al., 1997, introduction). This large hyperpolarization may, in fact, inhibit the electrogenic influx of Na/HCO3 after cells are exposed to CO2/HCO3 −.
In addition to the large magnitudes of some of the previously observed CO2/HCO3 −-induced hyperpolarizations, one could argue that the very assay (i.e., an exposure to CO2/HCO3 −) is not sufficiently specific for identifying an electrogenic Na/HCO3 cotransporter. As discussed in the accompanying paper (Bevensee et al., 1997, introduction), CO2 not only lowers pHi rapidly in the unstirred layer on the inner surface of the cell membrane, but also reacts with susceptible amino groups on proteins to form carbamino compounds (Morrow et al., 1974), thereby shifting their net charge in a negative direction. Moreover, adding HCO3 − could induce HCO3 − currents, such as those observed through GABAA-receptor channels in cultured rat astrocytes (Kaila et al., 1991). The sensitivity of CO2/ HCO3 −-induced electrical changes to DIDS, which is not specific for Na-coupled HCO3 − transporters, or their sensitivity to Na+ removal, which changes a wide range of cellular parameters, could be due to the nonspecific effects of these treatments.
Removing external Na+ causes a DIDS-sensitive, CO2/ HCO3 −-dependent depolarization.
Our approach for assaying for the electrogenicity of the cotransporter in hippocampal astrocytes was to expose cells to a Na+-free solution in the presence vs. the absence of CO2/ HCO3 −, and in the presence vs. the absence of DIDS. This was the assay used for the initial identification of the electrogenic Na/HCO3 cotransporter in proximal-tubule cells (Boron and Boulpaep, 1983), and for expression cloning the transporter in Xenopus oocytes (Romero et al., 1997). The advantages of this assay, over the less specific addition of CO2/HCO3 −, are discussed in the accompanying paper (Bevensee et al., 1997). In our initial experiments, we removed Na+ in the continued presence of 5% CO2 and 17 mM HCO3 −, pH 7.3. Under such conditions, however, removing external Na+ elicited an acid influx that predicts only small currents and Vm changes. To enhance the predicted responses to Na+ removal/readdition, we performed experiments in solutions buffered with 20% CO2 and 68 mM HCO3 −, pH 7.3. Under these conditions, DIDS inhibited 63% of the acid extrusion caused by Na+ readdition. Assuming that DIDS inhibits, to the same extent, the acid loading elicited by Na+ removal, then the DIDS-sensitive flux elicited by Na+ removal was 281 μM s−1 × 0.63 = 177 μM s−1. This flux predicts a ΔVm of 3.3 mV, assuming a Na+:HCO3 − stoichiometry of 1:2. Indeed, we found that the depolarization elicited by removing external Na+ was 2.6 mV larger in the presence vs. the absence of CO2/HCO3 −. In the presence of 20% CO2/68 mM HCO3 −, the DIDS-sensitive ΔVm was 4.8 mV (paired experiments). Thus, because the predicted and observed Vm changes were similar quantitatively, our Na+-removal data are consistent with the idea that the Na/HCO3 cotransporter in hippocampal astrocytes is electrogenic.
One would expect that removing Na+ may also alter Vm by influencing the activity of the Na-K pump. Initially, removing external Na+ should drive the pump in its normal forward direction, thereby leading to a hyperpolarization, not a depolarization. In our experiments, we replaced Na+ with Li+, a cation known to promote active efflux of Na+ via the Na-K pump in human red blood cells (McConaghey and Maizels, 1962; Maizels, 1968; Beaugé and Del Campillo, 1976), presumably by substituting for extracellular K+. Therefore, replacing external Na+ with Li+ may actually stimulate the Na-K pump in astrocytes, and elicit a hyperpolarization (J.F. Hoffman, personal communication). In the continual absence of external Na+, however, the cells would eventually become depleted of internal Na+, thereby leading to inhibition of the Na-K pump and a gradual, but continual depolarization. To avoid this inhibition, we added Na+ to the patch pipette solution in experiments in which we measured Vm using the perforated patch-clamp technique.
Electrogenic Na/HCO3 cotransport contributes to depolarization-induced alkalinizations in many preparations.
Because the Na/HCO3 cotransporter we have identified in hippocampal astrocytes is electrogenic, it is reasonable to expect it to be modulated by Vm. When leech glial cells are depolarized by an increase in [K+]o, the electrogenic Na/HCO3 cotransporter drives HCO3 − into the cells and elicits an increase in pHi (Deitmer and Szatkowski, 1990). Such a depolarization-induced alkalinization (DIA) was first described in the proximal tubule of the salamander kidney, where ∼50% of the DIA is believed to be mediated by the electrogenic Na/HCO3 cotransporter, and the remainder is due to stimulation of one of two apparently electroneutral lactate transporters (Siebens and Boron, 1989).
As in leech glial cells and salamander proximal-tubule cells, the pHi of rat forebrain astrocytes (Boyarsky et al., 1993) and several other mammalian astrocyte preparations also increases when the cells are depolarized. An exception is C6 glioma cells (Shrode and Putnam, 1994). The DIA generally is larger in the presence than in the absence of CO2/HCO3 −. In cultured astrocytes from the cerebral cortex of the mouse (Brookes and Turner, 1994), astrocytes in gliotic hippocampal slices from the rat (Grichtchenko and Chesler, 1994) and cultured astrocytes from the hippocampus of the rat (Pappas and Ransom, 1994), the DIA was partially or completely inhibited by removing external Na+, and unaffected by acutely removing external Cl−. Stilbene derivatives partially inhibited the DIA in astrocytes from the mouse cortex and the rat hippocampus, but not the DIA in astrocytes from the gliotic hippocampal slices. Thus, the electrogenic Na/HCO3 cotransporter described in the present studies could be responsible for at least a part of the DIAs in these mammalian astrocytes.
Acknowledgments
We thank Dr. W. Knox Chandler for evaluating the manuscript and providing useful suggestions. We also thank Dr. Fred J. Sigworth for useful discussions about our patch-clamp data.
This work was supported by National Institutes of Health Program Project grant PO1HD32573. M.O. Bevensee was supported by a predoctoral training grant (5-T32-GM0752718). M. Apkon was supported by a Physician Postdoctoral Fellowship Award by the Howard Hughes Medical Institute and is an established investigator of the Society for Critical Care Medicine.
APPENDIX
Ratiometric Correction for a Nonratiometric Indicator: Application to the Cl−-sensitive Dye MQAE
Theory and application.
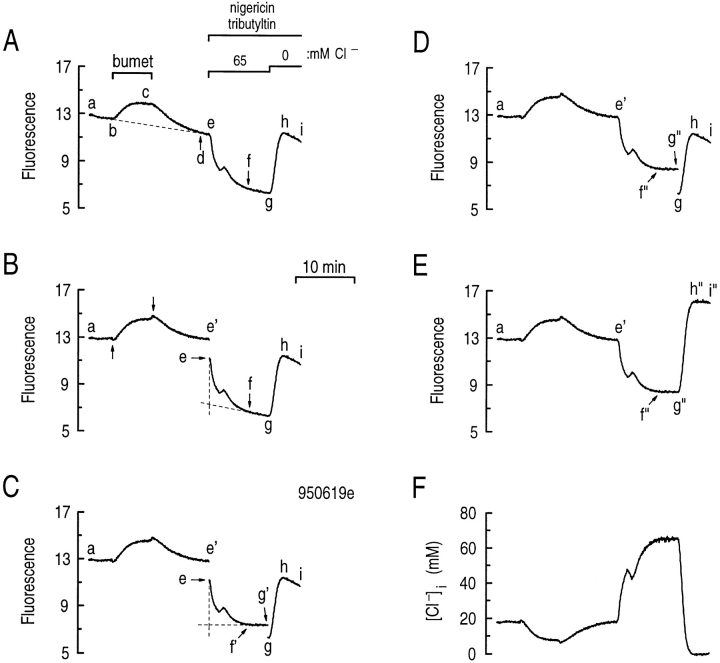
Fig. 13 A is the time course of the raw MQAE fluorescence used to generate the [Cl−]i vs. time trace shown in Fig. 3 A. At the outset of the experiment, I320 decreased slowly (Fig. 13 A, ab) in the astrocytes, which were bathed in the standard HEPES-buffered solution. This slow decrease probably represents both dye loss from cells, as well as loss of cells that become dislodged from the coverslip. When we exposed the cells to 1 μM of bumetanide, an inhibitor of the Na/K/Cl cotransporter, I320 increased (Fig. 13 A, bc), presumably because intracellular Cl− decreased and relieved the quenching of intracellular MQAE. Removing bumetanide caused I320 to decrease (Fig. 13 A, cd) to a level below that before removing external Cl− (Fig. 13 A, d vs. b). Subsequently, I320 continued to decline (Fig. 13 A, de) at about the same rate as in Fig. 13 A, ab. Exposing the cells to a high K+/nigericin/tributyltin calibration solution containing 65 mM Cl− caused I320 to decrease rapidly at first (Fig. 13 A, ef ), and then more slowly (Fig. 13 A, fg). Conversely, exposing the cells to a calibration solution without Cl− caused I320 first to increase rapidly (Fig. 13 A, gh), and then to decline more slowly (Fig. 13 A, hi).
Figure 13.
A corrected MQAE fluorescence loss from hippocampal astrocytes. (A) Raw fluorescence vs. time record for the experiment shown in Fig. 3 A. During the indicated period, the astrocytes were exposed to 1 μM bumetanide (bc). At the end of the experiment, the cells were exposed to a high K+/nigericin/ tributyltin chloride calibration solution containing 65 and 0 mM Cl− (e–g and g–i, respectively). (B) Partially corrected fluorescence vs. time record. Segment ae' was obtained by using the time-dependent, ratiometric correction technique (Eq. A2) to correct for dye loss during segment a–e in A. (C) Partially corrected fluorescence vs. time record. Similarly, ef 'g' was obtained by using Eq. A2 to correct for the dye loss when the astrocytes were exposed to the 65 mM Cl− calibration solution (B, e–g). (D) Partially corrected fluorescence vs. time record. ef 'g' in C was scaled (using Eq. A3) such that ae' was continuous with e'f ”g”. (E) Fully corrected fluorescence vs. time record. Using the ratiometric correction (Eq. A2) and the scaling technique (Eq. A3) as described for segment e–g in B, g–i in D was converted into g”h”i”. (F) [Cl−]i vs. time record. The fully corrected I320 vs. time record in E was converted into the [Cl−]i vs. time record using the Stern-Volmer relationship as described in methods.
In the experiment shown in Fig. 13 A, the cells were probably losing dye at different rates in each of three different time periods. (a) During the main part of the experiment (Fig. 13 A, a–e), dye was lost at a relatively low rate. Notice that a single line (Fig. 13 A) fits the data before and after the application of bumetanide. (b) During the first part of the calibration (Fig. 13 A, e–g), the rate of dye loss was somewhat higher. (c) During the second part of the calibration (Fig. 13 A, g–i), when [Cl−]o was zero, the rate of dye loss was greatest.
We corrected for dye loss separately in each of these three segments using a novel approach that compensates not only for dye loss per se at a particular [Cl−]i, but also for the change in quenching that occurs as [Cl−]i changes. For example, during Fig. 13 A, ab and de, when [Cl−]i is presumably at some fixed initial value, the time-dependent decrease in I320 represents only a linear loss of dye. To correct for this simple dye loss, it would be reasonable to determine the rate of I320 decrease by fitting a line simultaneously to the data in Fig. 13 A, ab and de (broken line). Starting from the original or “raw” I320 values (I t raw) at each time t in Fig. 13 A, one could thus obtain corrected I320 values (It corr) by adding a fraction of the final difference between the intensities at Fig. 13 A, a and e (I a raw – I e raw), assuming that this difference increased linearly with time:
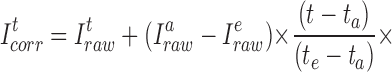 |
A1 |
However, a problem with this simplistic approach is that, at any given time, the computed increment in I320 is independent of [Cl−]i. For example, to compensate for the dye loss during the bumetanide exposure in Fig. 13 A, bc in which [Cl−]i is decreasing, there must be two components to the total increment I320. First, we must increment I320 to compensate for dye loss per se, as in Eq. A1. Second, we must increment I320 by an additional amount because the dye that was lost would have been sensing a lower than normal [Cl−]i, and thus would have been quenched to a lesser extent. Therefore, we did not use Eq. A1. Rather, we introduced an approach in which we simultaneously compensate for both components by multiplying It raw by a time-dependent correction as follows.
Imagine that there had been no dye loss in Fig. 13 A. We will assume that at Fig. 13 A, e, where [Cl−]i has the value x, I320 is 50 arbitrary fluorescence units. We also will assume that when [Cl−]i is zero, I320 will be 100. Thus, at Fig. 13 A, e, the [Cl−]i of x would have produced a Stern-Volmer ratio of I0/I = 100/50 = 2. Now imagine that, in fact, half the dye had been lost between Fig. 13 A, a and e. The Stern-Volmer ratio at e would thus be I0/I = 50/25 = 2. If we were to compensate for dye loss by simply adding 25 to both I0 and I (as in Eq. A1), then I0/I would be 75/50 = 1.5, and we would underestimate [Cl−]i. Thus, to compensate for having lost half the dye by the end of the time interval, we must multiply both I0 and I by 2 at Fig. 13 A, e. At the beginning of the interval, we would have to multiply both I0 and I by 1; between Fig. 13 A, a and e, we would have to multiply by a factor between 1 and 2. The general, time-dependent ratiometric correction is given by the following equation:
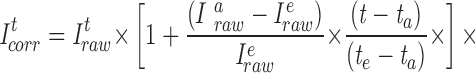 |
A2 |
Applying this formula to Fig. 13 A, a–e yields the corrected curve ae' in Fig. 13 B. Notice that the uncorrected segment e–i in Fig. 13 B is now discontinuous with the corrected segment ae'.
To correct for the dye loss in Fig. 13 A, e–g, we applied the same formula (Eq. A2) that we used to correct for the dye loss in Fig. 13 A, a–e. This yielded the partially corrected curve ef'g' shown in Fig. 13 C. Notice that this curve is discontinuous with both Fig. 13 C, ae' and g–i. To reestablish continuity, one might imagine adding the same correction to all points in Fig. 13 C, ef'g'. Although such an addition would yield the correct [Cl−]i for the transformed point e, the computed [Cl−]i values at all other points would be incorrect. The reason is that Stern-Volmer relationship deals with ratios of intensities. All [Cl−]i values in the transformed segment can be correct only if the transformation maintains its relative ratios. Therefore, we reestablished the continuity of Fig. 13 C, ae' and ef'g' by scaling all the points in segment ef'g' by a factor that made I320 identical at Fig. 13 C, e and e' :
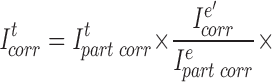 |
A3 |
The result, shown in Fig. 13 D, is that the ratio e'/g” is the same as the ratio e/g' in Fig. 13 C. Thus, the [Cl−]i computed at point g” is the same as that computed at g'.
To compensate for dye loss in Fig. 13 D, g–i, we applied the same two-step correction that we did in Fig. 13 B, e–g. The result is shown as segment g”h”i” in Fig. 13 E. Finally, we used the Stern-Volmer relationship to convert the fully corrected fluorescence data in Fig. 13 E to the [Cl−]i time course shown in Fig. 13 F.
Potential sources of error.
The greatest potential source of error in the time-dependent ratiometric correction described above is in assuming a constant rate of dye loss, especially when a solution change is made during the period of the correction. This assumption of constant dye loss can sometimes be verified directly. For example, in Fig. 13 A, a single best-fit line passes through a and b (before addition of bumetanide) as well as through d and e (after recovery from bumetanide withdrawal). Thus, it is reasonable to conclude that MQAE disappeared at a constant rate throughout the entire segment Fig. 13 A, a–e. On the other hand, we cannot directly verify that the rate of dye loss during Fig. 13 B, ef is the same as during fg, nor that the rate during Fig. 13 B, gh is the same as during hi.
Our ratiometric-correction technique is not well suited to compensate for changes in the dye signal arising from factors other than dye loss or changes in [Cl−]i. For example, our approach would not properly compensate for changes in the dye signal due to cell shrinkage or swelling.
Broader applicability.
The time-dependent ratiometric correction described above for MQAE could be applied to other nonratiometric dyes, such as the Ca2+ indicator Fluo-3. In fact, our ratiometric correction could, in principle, be applied to any indicator whose fluorescence (a) is proportional to dye concentration, and (b) changes with substrate binding. For example, in experiments in which pHi is measured with BCECF, one could apply our ratiometric-correction technique to the I490 (i.e., pH sensitive) signal to arrive at a corrected I490 vs. time record that compensates for dye loss. Thus, without any knowledge of I440, one could use this corrected I490 to arrive at a pHi vs. time record, similar to that obtained using the standard I490/I440 ratiometric approach.
Footnotes
Portions of this work have been previously published in preliminary form (Bevensee, M.O., W.F. Boron, and M. Apkon. 1995. FASEB J. 9: A308.).
Abbreviations used in this paper: BCECF, 2′,7′-biscarboxyethyl-5,6-carboxyfluorescein; DIA, depolarization-induced alkalinization; DIDS, 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid; MQAE, N-(6-methoxyquinolyl)acetoethyl ester; Vm, plasma membrane voltage.
The sum of pHi increases in Fig. 5, ab, cd, and ef was 0.51. βT is the sum of βI (12.7 mM [pH unit]−1) and βHCO3 − (12.1 mM [pH unit]−1), or 24.8 mM (pH unit)−1 at an average pHi of 6.79. 0.51 pH units × 24.8 mM (pH unit)−1 × 0.75 (DIDS-sensitive fraction, as described in the accompanying paper; Bevensee et al., 1997) = 9.5 mM HCO3 − moved into the cell by the Na-driven HCO3 − transporter. For a Na-driven Cl-HCO3 exchanger, two HCO3 − ions would move into the cell in exchange for one Cl− ion out of the cell. Therefore, the movement of 9.5 mM HCO3 − into the cell by this exchanger would result in the movement of 4.7 mM Cl− out of the cell.
Removing external Na+ in CO2/HCO3 − elicited a HCO3 − efflux of 101 μM s−1, of which 90% or 91 μM s−1 was DIDS sensitive. This DIDS-sensitive HCO3 − efflux corresponds to the net movement of 4.55 C (liter cell vol)−1 s−1 for a Na/HCO3 cotransporter that moves one charge equivalent per two HCO3 − ions. We calculated the average cell volume of an astrocyte to be 1.6 pl, assuming the geometry of an astrocyte to be a right-angled cone (V = [area × h]/3) with a height (h) of 5 μm (O'Connor et al., 1993). We imaged BCECF-loaded astrocytes to obtain an average cell area of 973 ± 70 μm2 (n = 17). Therefore, the current flow through the Na/HCO3 cotransporter in a typical astrocyte would be 7.3 pA. Given an average input resistance for hippocampal astrocytes of 236 MΩ (see below), this 7.3-pA current would depolarize astrocytes by 1.7 mV.
We sometimes observed such abrupt depolarizations in experiments in which we monitored only Vm, as in Fig. 10 A. Conversely, we sometimes observed slower depolarizations in experiments in which we monitored Vm and pHi simultaneously. One would expect such depolarizations due to electrogenic Na/HCO3 cotransport to be instantaneous under conditions in which the bath solution could be instantaneously changed. However, to maintain seals in our patch-clamp experiments performed at 37°C, we were often forced to reduce our solution flow rate. During such experiments, we often observed the slower depolarizations in response to Na+ removal (e.g., Fig. 10 A).
35.8 μM s−1 corresponds to the net movement of 1.79 C (liter cell vol)−1 s−1 for a Na/HCO3 cotransporter that moves one charge equivalent per two HCO3 − ions. The current flow through the Na/HCO3 cotransporter in a typical astrocyte with a volume of 1.6 pl would be 2.9 pA. This current flow would depolarize hippocampal astrocytes with an input resistance of 236 MΩ by only 0.7 mV.
REFERENCES
- Beaugé LA, Del Campillo E. The ATP dependence of a ouabain-sensitive sodium efflux activated by external sodium, potassium and lithium in human red cells. Biochim Biophys Acta. 1976;433:547–554. doi: 10.1016/0005-2736(76)90280-7. [DOI] [PubMed] [Google Scholar]
- Bevensee MO, Weed RW, Boron WF. Intracellular pH regulation in cultured astrocytes from rat hippocampus. I. Role of HCO3 − . J Gen Physiol. 1997;110:453–465. doi: 10.1085/jgp.110.4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevensee MO, Cummins TR, Haddad GG, Boron WF, Boyarsky G. pH regulation in single CA1 neurons acutely isolated from the hippocampi of immature and mature rats. J Physiol. 1996;494:315–328. doi: 10.1113/jphysiol.1996.sp021494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron WF, Boulpaep EL. Intracellular pH regulation in the renal proximal tubule of the salamander: basolateral HCO3 −transport. J Gen Physiol. 1983;81:53–94. doi: 10.1085/jgp.81.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron WF, De Weer P. Intracellular pH transients in squid giant axons caused by CO2, NH3and metabolic inhibitors. J Gen Physiol. 1976;67:91–112. doi: 10.1085/jgp.67.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron WF, Russell JM. Stoichiometry and ion dependencies of the intracellular-pH-regulating mechanism in squid giant axons. J Gen Physiol. 1983;81:373–399. doi: 10.1085/jgp.81.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyarsky G, Ganz MB, Sterzel B, Boron WF. pH regulation in single glomerular mesangial cells. I. Acid extrusion in absence and presence of HCO3 . Am J Physiol. 1988a;255:C844–C856. doi: 10.1152/ajpcell.1988.255.6.C844. [DOI] [PubMed] [Google Scholar]
- Boyarsky G, Ganz MB, Sterzel B, Boron WF. pH regulation in single glomerular mesangial cells. II. Na-dependent and -independent Cl-HCO3exchangers. Am J Physiol. 1988b;255:C857–C869. doi: 10.1152/ajpcell.1988.255.6.C857. [DOI] [PubMed] [Google Scholar]
- Boyarsky G, Ransom B, Schlue W-R, Davis MBE, Boron WF. Intracellular pH regulation in single cultured astrocytes from rat forebrain. Glia. 1993;8:241–248. doi: 10.1002/glia.440080404. [DOI] [PubMed] [Google Scholar]
- Brookes N, Turner RJ. K+-induced alkalinization in mouse cerebral astrocytes mediated by reversal of electrogenic Na+-HCO3 −cotransport. Am J Physiol. 1994;36:C1633–C1640. doi: 10.1152/ajpcell.1994.267.6.C1633. [DOI] [PubMed] [Google Scholar]
- Brune T, Fetzer S, Backus KH, Deitmer JW. Evidence for electrogenic Na-HCO3cotransport in cultured rat cerebellar astrocytes. Pflugers Archiv. 1994;429:64–71. doi: 10.1007/BF02584031. [DOI] [PubMed] [Google Scholar]
- Chao AC, Dix JA, Sellers MC, Verkman AS. Fluorescent measurement of chloride transport in monolayer cultured cells. Biochem J. 1989;56:1071–1081. doi: 10.1016/S0006-3495(89)82755-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BA, Hogan EM, Boron WF. Activation of Na-H exchange by intracellular lithium in barnacle muscle fibers. Am J Physiol. 1992;263:C246–C256. doi: 10.1152/ajpcell.1992.263.1.C246. [DOI] [PubMed] [Google Scholar]
- Deitmer JW, Szatkowski M. Membrane potential dependence of intracellular pH regulation by identified glial cells in the leech central nervous system. J Physiol. 1990;421:617–631. doi: 10.1113/jphysiol.1990.sp017965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engblom AC, Akerman KEO. Determination of the intracellular free chloride concentration in rat brain synaptoneurosomes using a chloride-sensitive fluorescent indicator. Biochim Biophys Acta. 1993;1153:262–266. doi: 10.1016/0005-2736(93)90414-u. [DOI] [PubMed] [Google Scholar]
- Grichtchenko II, Chesler M. Depolarization-induced alkalinization of astrocytes in gliotic hippocampal slices. Neuroscience. 1994;62:1071–1078. doi: 10.1016/0306-4522(94)90344-1. [DOI] [PubMed] [Google Scholar]
- Horn R, Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988;92:145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaila K, Panula P, Karhunen T, Heinonen E. Fall in intracellular pH mediated by GABAAreceptors in cultured rat astrocytes. Neurosci Lett. 1991;126:9–12. doi: 10.1016/0304-3940(91)90358-z. [DOI] [PubMed] [Google Scholar]
- Koncz C, Daugirdas JT. Use of MQAE for measurement of intracellular [Cl−] Am J Physiol. 1994;267:H2114–H2123. doi: 10.1152/ajpheart.1994.267.6.H2114. [DOI] [PubMed] [Google Scholar]
- Lancer WI, Weyer P, Verkman AS, Ausiello D, Brown D. FITC-dextran as a probe for endosome function and localization in kidney. Am J Physiol. 1990;258:C309–C317. doi: 10.1152/ajpcell.1990.258.2.C309. [DOI] [PubMed] [Google Scholar]
- Lau KR, Evans RL, Case RM. Intracellular Cl−concentration in striated intralobular ducts from rabbit mandibular salivary glands. Pflugers Archiv. 1994;427:24–32. doi: 10.1007/BF00585938. [DOI] [PubMed] [Google Scholar]
- Lin A, Krockmalnic G, Penman S. Imaging cytoskeleton-mitochondrial membrane attachments by embedment-free electron microscopy of saponin-extracted cells. Proc Natl Acad Sci USA. 1990;87:8565–8569. doi: 10.1073/pnas.87.21.8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacVicar BA, Tse FWY, Crichton SA, Kettenmann H. GABA-activated Cl−channels in astrocytes of hippocampal slices. J Neurosci. 1989;9:3577–3583. doi: 10.1523/JNEUROSCI.09-10-03577.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizels M. Effect of sodium content on sodium efflux from human red cells suspended in sodium-free media containing potassium, rubidium, caesium or lithium chloride. J Physiol. 1968;195:657–679. doi: 10.1113/jphysiol.1968.sp008481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AR, Dryer SE. Potassium channels activated by sodium. Quart J Exp Physiol. 1989;74:1033–1041. doi: 10.1113/expphysiol.1989.sp003331. [DOI] [PubMed] [Google Scholar]
- Martínez-Zaguilán R, Gillies RJ, Sánchez-Armass S. Regulation of pH in rat brain synaptosomes. II. Role of Cl− . J Neurophysiol. 1994;71:2249–2257. doi: 10.1152/jn.1994.71.6.2249. [DOI] [PubMed] [Google Scholar]
- McConaghey PD, Maizels M. Cation exchanges of lactose-treated human red cells. J Physiol. 1962;162:485–509. doi: 10.1113/jphysiol.1962.sp006946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow, J.S., R.S. Gurd, and F.R.N. Gurd. 1974. The chemical basis and possible role of carbamino homeostatic mechanisms. In Peptides, Polypeptides, and Proteins. E.R. Blout, F.A. Bovey, M. Goodman, and N. Lotan, editors. John Wiley and Sons Inc., New York. 594–604.
- Nakhoul NL, Chen LK, Boron WF. Effect of basolateral CO2/HCO3 −on intracellular pH regulation in the rabbit S3 proximal tubule. J Gen Physiol. 1993;102:1171–1205. doi: 10.1085/jgp.102.6.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor E, Sontheimer H, Ransom BR. Rat hippocampal astrocytes exhibit electrogenic sodium-bicarbonate cotransport. J Neurophysiol. 1994;72:2580–2589. doi: 10.1152/jn.1994.72.6.2580. [DOI] [PubMed] [Google Scholar]
- O'Connor ER, Kimelberg HK, Keese CR, Giaever I. Electrical resistance method for measuring volume changes in monolayer cultures applied to primary astrocyte cultures. Am J Physiol. 1993;264:C471–C478. doi: 10.1152/ajpcell.1993.264.2.C471. [DOI] [PubMed] [Google Scholar]
- Pappas CA, Ransom BR. Depolarization-induced alkalinization (DIA) in rat hippocampal astrocytes. J Neurophysiol. 1994;72:2816–2826. doi: 10.1152/jn.1994.72.6.2816. [DOI] [PubMed] [Google Scholar]
- Romero MF, Hediger MA, Boulpaep EL, Boron WF. Expression cloning and characterization of a renal electrogenic Na+/HCO3 −cotransporter. Nature (Lond) 1997;387:409–413. doi: 10.1038/387409a0. [DOI] [PubMed] [Google Scholar]
- Rose CR, Ransom BR. Intracellular sodium homeostasis in rat hippocampal astrocytes. J Physiol. 1996;491:291–305. doi: 10.1113/jphysiol.1996.sp021216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwiening CJ, Boron WF. Regulation of intracellular pH in pyramidal neurones from the rat hippocampus by Na+- dependent Cl−-HCO3 −exchange. J Physiol. 1994;475:59–67. doi: 10.1113/jphysiol.1994.sp020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrode LD, Putnam RW. Intracellular pH regulation in primary rat astrocytes and C6 glioma cells. Glia. 1994;12:196–210. doi: 10.1002/glia.440120305. [DOI] [PubMed] [Google Scholar]
- Siebens AW, Boron WF. Depolarization-induced alkalinization in proximal tubules. I. Characteristics and dependence on Na+ . Am J Physiol. 1989;256:F342–F353. doi: 10.1152/ajprenal.1989.256.2.F342. [DOI] [PubMed] [Google Scholar]
- Soleimani M, Grassl SM, Aronson PS. Stoichiometry of Na+-HCO3 −cotransport in basolateral membrane vesicles isolated from rabbit renal cortex. J Clin Invest. 1987;79:1276–1280. doi: 10.1172/JCI112948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tas PWL, Massa PT, Kress HG, Koschel K. Characterization of an Na+/K+/Cl−co-transport in primary cultures of rat astrocytes. Biochim Biophys Acta. 1987;903:411–416. doi: 10.1016/0005-2736(87)90047-2. [DOI] [PubMed] [Google Scholar]
- Thomas JA, Buchsbaum RN, Zimniak A, Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979;81:2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Verkman AS, Sellers MC, Chao AC, Leung T, Ketcham R. Synthesis and characterization of improved chloride-sensitive fluorescent indicators for biological applications. Anal Biochem. 1989;178:355–361. doi: 10.1016/0003-2697(89)90652-0. [DOI] [PubMed] [Google Scholar]
- Walz W, Hertz L. Comparison between fluxes of potassium and of chloride in astrocytes in primary culture. Brain Res. 1983;227:321–328. doi: 10.1016/0006-8993(83)90940-x. [DOI] [PubMed] [Google Scholar]