Abstract
Permeability of the cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel to polyatomic anions of known dimensions was studied in stably transfected Chinese hamster ovary cells by using the patch clamp technique. Biionic reversal potentials measured with external polyatomic anions gave the permeability ratio (PX/PCl) sequence NO3 − > Cl− > HCO3 − > formate > acetate. The same selectivity sequence but somewhat higher permeability ratios were obtained when anions were tested from the cytoplasmic side. Pyruvate, propanoate, methane sulfonate, ethane sulfonate, and gluconate were not measurably permeant (PX/PCl < 0.06) from either side of the membrane. The relationship between permeability ratios from the outside and ionic diameters suggests a minimum functional pore diameter of ∼5.3 Å. Permeability ratios also followed a lyotropic sequence, suggesting that permeability is dependent on ionic hydration energies. Site-directed mutagenesis of two adjacent threonines in TM6 to smaller, less polar alanines led to a significant (24%) increase in single channel conductance and elevated permeability to several large anions, suggesting that these residues do not strongly bind permeating anions, but may contribute to the narrowest part of the pore.
Keywords: pore size, channel selectivity, anion permeation, lyotropic sequence, cystic fibrosis
INTRODUCTION
The cystic fibrosis transmembrane conductance regulator (CFTR)1 is a tightly regulated chloride channel that mediates Cl− transport across epithelia and is mutated in cystic fibrosis (Welsh et al., 1992; Riordan, 1993; Hanrahan et al., 1995). The preceding paper examined halide permeability ratios under biionic conditions and described the unique behavior of I−. In this paper, we examine permeation by polyatomic anions having different dimensions and use the permeability ratios obtained to estimate the functional diameter of the pore.
Mutagenesis studies of CFTR selectivity (Anderson et al., 1991; Tabcharani et al., 1997), conductance (Sheppard et al., 1993; Tabcharani et al., 1993; McDonough et al., 1994), multi-ion pore behavior (Tabcharani et al., 1993), voltage-dependent block (Tabcharani et al., 1993; McDonough et al., 1994; Linsdell and Hanrahan, 1996b ), and susceptibility to hydrophilic sulfhydryl reagents after cysteine substitution mutagenesis (Cheung and Akabas, 1996) suggest that the sixth membrane spanning region of CFTR (TM6) lines the pore. All TM6 mutants that have been characterized at the single channel level have had conductances that are the same, or lower than, that of wild-type CFTR (Sheppard et al., 1993; Tabcharani et al., 1993; McDonough et al., 1994). Some of these low conductance mutations (R334W, R347P, and R347H) occur in cystic fibrosis patients and have been associated with relatively mild disease symptoms. During a preliminary study of channels bearing mutations of polar residues in TM6 that might disrupt hydrogen bonding, we identified a mutant that had significantly higher conductance than wild-type CFTR. This mutant, with two threonine-to-alanine substitutions near the middle of TM6, was also found to have elevated permeability to polyatomic anions, consistent with an increase in the caliber of the narrowest region of the pore. Preliminary reports of this work have appeared (Tabcharani and Hanrahan, 1993; Linsdell et al., 1996).
METHODS
CFTR Mutagenesis and Expression
Chinese hamster ovary cells expressing wild-type CFTR were described previously (Tabcharani et al., 1991; Chang et al., 1993; Tabcharani et al., 1997). The TM6 double mutant (TT338,339AA; see Fig. 4) was constructed by site-directed mutagenesis using the polymerase chain reaction with Vent polymerase (New England Biolabs, Inc., Mississauga, Ontario, Canada), as described previously (Tabcharani et al., 1993). A silent change was introduced in the original CFTR cDNA to create a Stu1 restriction endonuclease site at nucleotide 950. Mutagenesis was carried out using a fragment that extended from this Stu1 site to an Fsp1 site at nucleotide 1169. The manipulated portion of the construct was verified by sequencing with dideoxy chain termination and Sequenase (United States Biochemicals, Cleveland, OH). Cells were transfected and immunoreactive mutant CFTR protein was detected in multiple cell lines after selection in methotrexate as described previously (Tabcharani et al., 1991; Chang et al., 1993).
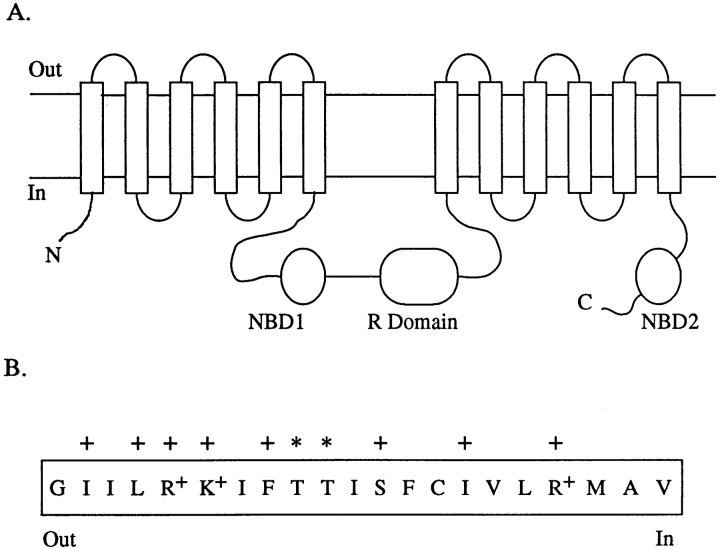
Figure 4.
(A) Proposed topology of CFTR, indicating the 12 transmembrane regions (TM1–12), two nucleotide binding domains (NBD1–2) and the regulatory (R) domain. (B) Amino acid sequence of TM6 according to Riordan et al. (1989). Residues mutated in the present study (T338 and T339) are indicated by asterisks, residues that line the pore according to cysteine scanning mutagenesis (Cheung and Akabas, 1996) are shown by crosses. Not shown are the residues downstream from TM6 (as originally defined), which have also been proposed by Cheung and Akabas (1996) to line the pore (T351, R352, and Q353).
Solutions
The standard recording solutions in the pipette and bath contained (mM): 150 NaCl, 2 MgCl2, 10 Na N -tris[hydroxymethyl]methyl-2-aminoethanesulfonate, pH 7.4. Channel activity was maintained when recording from excised patches by adding 180 nM protein kinase A catalytic subunit and 1 mM MgATP (Sigma Chemical Co., St. Louis, MO) to the bath solution, as described previously (Tabcharani et al., 1997). Permeation was studied under biionic conditions by replacing the chloride salts in the pipette or bath solutions with those of the appropriate anion. Bicarbonate permeation was studied under conditions of high pH 8.3 and 5% CO2 as described previously for the outwardly rectifying anion channel (Tabcharani et al., 1989). Under these conditions, the carbonic acid, bicarbonate, and carbonate concentrations would be ∼1.4, 147, and 1.4 mM, respectively.
Single Channel Record
Pipettes and recording equipment were as described previously (Tabcharani et al., 1997). The bath agar bridge had the same ionic composition as the pipette solution. Voltages have been corrected for liquid junction potentials measured at the agar bridge using a flowing 3-M KCl electrode as follows (mV): 1 NO3 −, 3 formate, 5 acetate, 5 methane sulfonate, 6 ethane sulfonate, 6 pyruvate, 6 propanoate, 10 gluconate. Permeability ratios were calculated using the equation
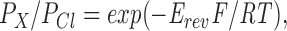 |
1 |
where E rev is the reversal potential and other terms have their usual meanings. The relationship between channel conductance and symmetrical Cl− activity for both wild type and TT338,339AA channels was fitted by a Michaelis-Menten–type hyperbolic function of the form
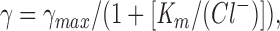 |
2 |
where γ is conductance, γ max the saturating conductance of the channel, K m the apparent affinity of the channel for Cl− ions, and (Cl−) the Cl− activity calculated using the Debye-Hückel theory.
To estimate pore size, the permeation pathway of CFTR was modeled as a cylinder permeated by cylindrical ions (e.g., Dwyer et al., 1980; Bormann et al., 1987; Cohen et al., 1992b ). According to this model, ionic permeability is proportional to the ratio of the diameters of the permeating ion and the pore by an excluded volume effect (Dwyer et al., 1980). The permeability of an ion, relative to Cl−, is then given by
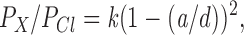 |
3 |
where a is the diameter of the ion, d is the diameter of the pore, and k is a proportionality constant.
Mean values are presented as mean ± SEM. For graphical presentation of mean values, error bars represent ±SEM; where no error bars are shown, this is smaller than the size of the symbol. Experiments were performed at room temperature (22 ± 1°C) unless otherwise indicated.
RESULTS
Biionic Permeability of Extracellular Anions
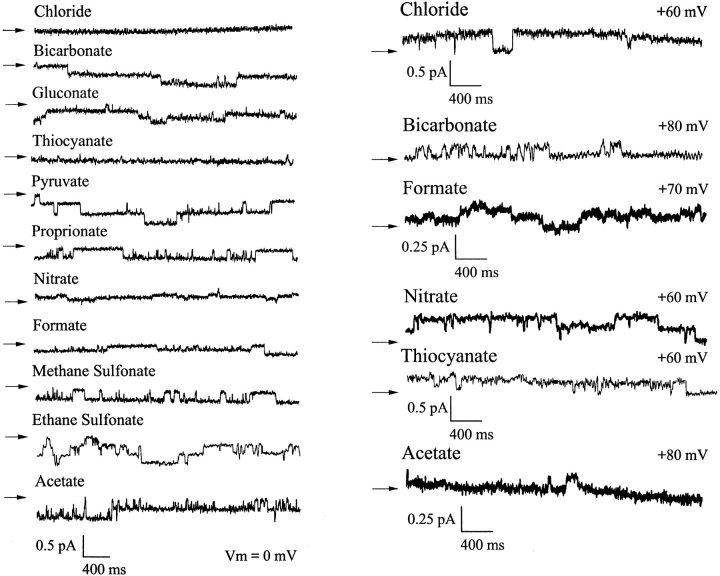
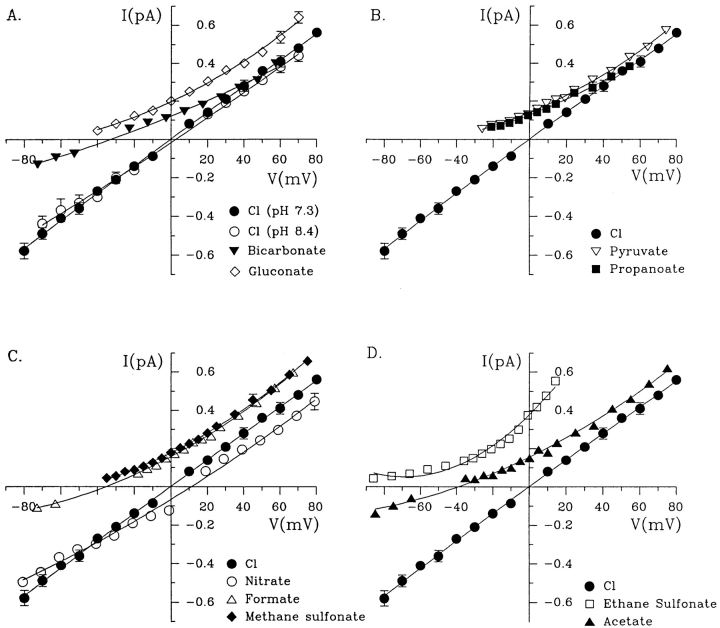
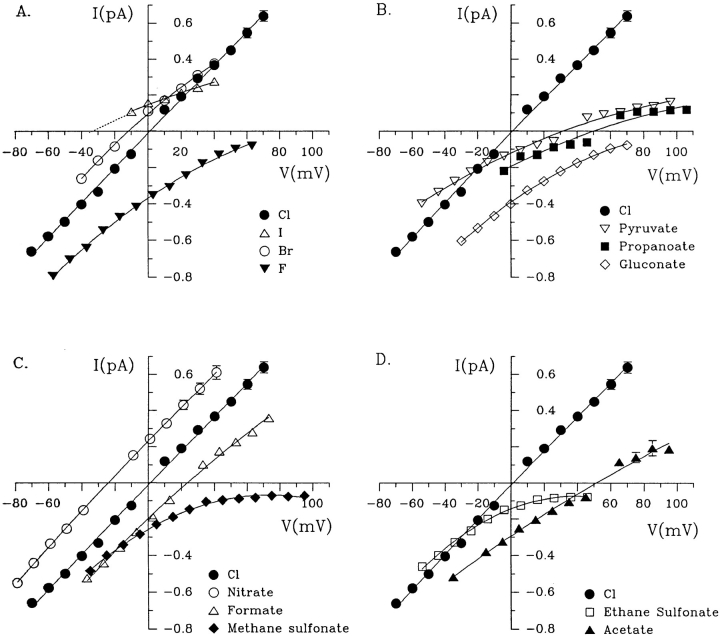
In the first series of experiments, single channel currents were measured using inside-out patches with Cl− solution in the bath and different polyatomic anions in the pipette. Fig. 1 shows recordings under these conditions at 0 mV, and at large positive potentials when measurable currents were carried by test anions. The current–voltage (i/V) relationships are shown in Fig. 2. Four of the nine anions tested in this paper (NO3 −, HCO3 −, formate, and acetate) were clearly permeant and gave reversal potentials within the range ±60 mV (see Table I). When a reversal potential was not observed with a particular anion, the voltage was increased until the seal was lost, which was usually at greater than +100 mV. The control i/V relationship in symmetrical chloride solutions is shown at normal pH 7.4 in Fig. 2, A–D. To allow comparison with results obtained with bicarbonate on one side, Fig. 2 A also shows the i/V relationship with symmetrical 154 mM Cl− at pH 8.3. CFTR-mediated currents were recognizable by their slow gating and activation by PKA plus MgATP, regardless of the anion carrying the current.
Figure 1.
Single CFTR channel currents recorded with different extracellular anions. Traces are shown at 0 mV (left) and at strongly positive potentials when the extracellular anion carried a measurable current. In each case the arrow on the left represents the current level when all channels were in the closed state.
Figure 2.
Mean single channel i/V relationships determined for wild-type CFTR with different extracellular anions. Note that control i/Vs in symmetrical Cl− at pH 7.3 are shown in each panel for reference. In A, the control i/V relationship obtained with symmetrical Cl− is also shown at pH 8.4 for comparison with the HCO3 − curve, which was obtained immediately after equilibrating the HCO3 − solution at this pH with 5% CO2 (see methods).
Table I.
Comparison of the Permeability of Wild-Type and TT338,339AA Channels to Some Extracellular Anions
| Extracellular anion | Wild-Type CFTR | TT338,339AA | Ion dimensions | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Erev | PX/PCl | n | Erev | PX/PCl | n | |||||||||
| mV | mV | Å | ||||||||||||
| Chloride | 0 | 1.00 | 8 | 0 | 1.00 | 8 | 3.62 × 3.62 × 3.63 | |||||||
| Iodide | −18.1 ± 0.1 | 2.0 | 2 | −37.7 ± 1.0 | 4.42 ± 0.08 | 3 | 4.32 × 4.32 × 4.32 | |||||||
| Bromide | −5.2 ± 1.0 | 1.22 ± 0.05 | 4 | −11.5 ± 0.7 | 1.57 ± 0.7 | 4 | 3.90 × 3.90 × 3.90 | |||||||
| Fluoride | No reversal | <0.06 | 3 | No reversal | <0.06 | 3 | 2.72 × 2.72 × 2.72 | |||||||
| Nitrate | −8.2 ± 2.1 | 1.43 ± 0.10 | 4 | −23.6 ± 0.2 | 2.53 ± 0.02 | 4 | 3.10 × 4.76 × 5.17 | |||||||
| Formate | +43.4 ± 3.7 | 0.18 ± 0.10 | 4 | +22.5 ± 1.0 | 0.42 ± 0.02 | 5 | 3.40 × 4.62 × 4.82 | |||||||
| Bicarbonate | +49.6 ± 1.8 | 0.14 ± 0.02 | 8 | — | — | 0 | 3.40 × 4.95 × 5.96 | |||||||
| Acetate | +61.1 ± 0.2 | 0.09 ± 0.00 | 2 | +50.6 ± 0.2 | 0.14 ± 0.00 | 4 | 3.99 × 5.18 × 5.47 | |||||||
| Propanoate | No reversal | <0.06 | 5 | +53.2 ± 1.0 | 0.12 ± 0.00 | 5 | 4.12 × 5.23 × 7.05 | |||||||
| Pyruvate | No reversal | <0.06 | 5 | +32.7 ± 0.5 | 0.28 ± 0.01 | 5 | 4.09 × 5.73 × 6.82 | |||||||
| Methane sulfonate | No reversal | <0.06 | 6 | No reversal | <0.06 | 5 | 5.08 × 5.43 × 5.54 | |||||||
| Ethane sulfonate | No reversal | <0.06 | 5 | No reversal | <0.06 | 3 | 4.94 × 5.37 × 7.44 | |||||||
| Gluconate | No reversal | <0.06 | 5 | No reversal | <0.06 | 4 | 4.91 × 6.86 × 12.09 | |||||||
| ATP0 | 7.65 × 9.23 × 18.71 | |||||||||||||
| ATP4− | 7.30 × 9.43 × 18.40 | |||||||||||||
Summary of mean reversal potentials (Erev) measured with different extracellular anions in wild-type CFTR and TT338,339AA channels. Relative permeabilities (PX/PCl) were calculated from the reversal potentials using Eq. 1 (see methods). n represents the number of experiments with each ion. Wild-type values for Br−, I−, and F− are from the preceding paper (Tabcharani et al., 1997). Also shown are the minimal unhydrated dimensions of the ions used, which were estimated using Molecular Modeling Pro computer software (WindowChem Software Inc., Fairfield, CA). The estimated minimal dimensions of ATP, both in the fully protonated (ATP0) and fully ionized (ATP4−) forms, are also shown for comparison. Nonhalide ions are shown in the order of their unhydrated diameters, estimated as described in the text.
Although reversal potentials were not observed with external pyruvate, propanoate, methane sulfonate, ethane sulfonate, or gluconate, CFTR might still have some permeability to these anions below the detection threshold of single channel recording. Indeed, the channel must have some permeability to extracellular methanethiosulfonates because they reach cysteine residues when they are engineered near the cytoplasmic end of TM6 (Cheung and Akabas, 1996). Anions that appeared to be impermeant under the conditions used in this study were assigned permeability ratios <0.06. Table I summarizes the reversal potentials and permeability ratios obtained for external anions, and also the estimated minimum unhydrated dimensions of each ion used.
Permeability to Intracellular Anions
Fig. 3 shows i/V relationships obtained when different anions were present on the cytoplasmic side of the membrane. Permeability ratios calculated for cytoplasmic anions were somewhat higher than when the same anions were present extracellularly; for example, the mean Pformate/PCl ratios were 0.18 ± 0.03 and 0.25 ± 0.01 with external and internal formate, respectively (Tables I and II). Acetate displayed a similar asymmetry, with Pacetate/PCl being 0.09 ± 0.00 from the extracellular side and 0.19 ± 0.01 from the intracellular side. Nevertheless, the overall permeability sequence observed was the same regardless of the direction of the anion gradients (NO3 − > Cl− > HCO3 − > formate > acetate). The i/V relationships measured with internal pyruvate, propanoate, methane sulfonate, ethane sulfonate, or gluconate on the cytoplasmic side did not reverse (Fig. 3), indicating negligible permeability to these ions (PX/PCl < 0.06). Reversal potentials and permeability ratios for intracellular anions are summarized in Table II.
Figure 3.
Mean single channel i/V relationships determined for wild-type CFTR with different intracellular anions. See Fig. 1 for details.
Table II.
Permeability of Wild-Type Channels to Intracellular Anions
| Intracellular anion | Erev | PX/PCl | n | |||
|---|---|---|---|---|---|---|
| mV | ||||||
| Chloride | 0 | 1.00 | 8 | |||
| Iodide | +15.5 ± 1.4 | 1.82 ± 0.10 | 6 | |||
| Bromide | +5.8 ± 3.5 | 1.26 ± 0.17 | 4 | |||
| Fluoride | −46.7 ± 11.7 | 0.17 ± 0.07 | 5 | |||
| Nitrate | +11.3 ± 1.4 | 1.61 ± 0.09 | 6 | |||
| Formate | −35.4 ± 0.0 | 0.25 ± 0.00 | 4 | |||
| Bicarbonate | −34.5 ± 1.5 | 0.25 ± 0.01 | 3 | |||
| Acetate | −41.5 ± 0.4 | 0.19 ± 0.01 | 3 | |||
| Propanoate | No reversal | <0.06 | 2 | |||
| Pyruvate | No reversal | <0.06 | 4 | |||
| Methane sulfonate | No reversal | <0.06 | 3 | |||
| Ethane sulfate | No reversal | <0.06 | 5 | |||
| Gluconate | No reversal | <0.06 | 6 |
Summary of mean reversal potentials and permeability ratios measured with different intracellular anions in wild-type channels. See Table I for details.
Double Mutation in TM6 Increases Single Channel Conductance
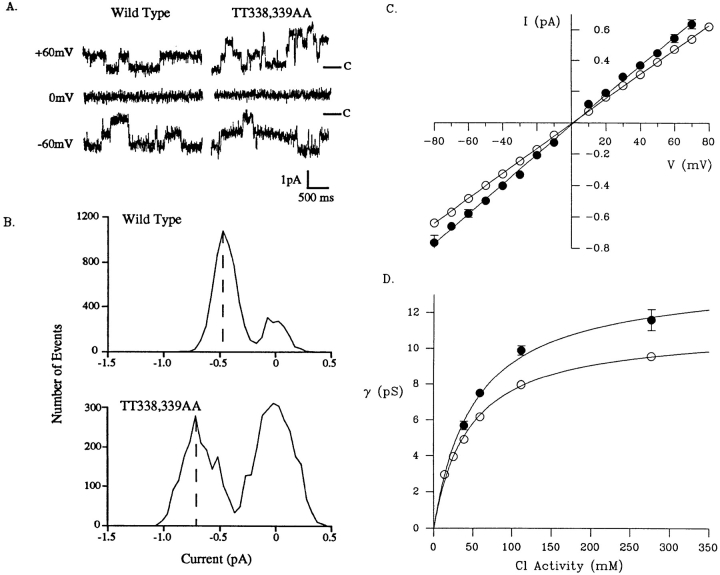
In a second series of experiments, the properties of wild-type channels were compared with those of the mutant TT338,339AA, in which two polar threonine residues near the middle of TM6 were simultaneously replaced by smaller, nonpolar alanines (Fig. 4). Threonines are important potential hydrogen bond–forming residues in the pores of both anion- and cation-selective channels (MacKinnon and Yellen, 1990; Yool and Schwarz, 1991; Cohen et al., 1992a , 1992b ; Sansom, 1992; Villarroel and Sakmann, 1992; Heginbotham et al., 1994; McDonough et al., 1994). Mutation of serine 341, another potential hydrogen-bonding residue in CFTR TM6, to alanine causes pronounced rectification of the macroscopic i/V relationship and reduced sensitivity to block by diphen-ylamine-2-carboxylate (DPC; McDonough et al., 1994). By contrast, mutating threonines 338 and 339 individually to alanines had no effect on the shape of the i/V relationship or DPC block (McDonough et al., 1994). Fig. 5, A–C shows that Cl− currents carried by single TT338,339AA channels were consistently larger than those carried by wild-type channels. Wild-type channels had a linear current–voltage relationship over the range ±80 mV, with a mean slope conductance of 7.97 ± 0.10 pS (n = 6; Fig. 5 C), similar to values reported previously under similar conditions (Tabcharani et al., 1993; Tabcharani et al., 1997). The TT338,339AA mutant also had a linear i/V relationship over the same voltage range, but its conductance was 9.88 ± 0.26 pS (n = 8), significantly higher than that of the wild-type channel (P < 0.05, one-tailed t test; Fig. 5 C).
Figure 5.
Conductance properties of single TT338,339AA channels. (A) Representative single channel current records from wild-type and TT338,339AA channels at the membrane potentials indicated. The closed state is indicated “C” on the right. (B) Examples of single channel amplitude histograms for wild-type and TT338,339AA channels at a membrane potential of −60 mV. In these examples, the mean open state current, indicated by the dashed line, was −0.45 pA for wild type and −0.69 pA for TT338,339AA. The apparent difference in open probability suggested by these histograms is an artifact of the short recording periods used. (C) Mean single channel current–voltage relationships for wild-type (○) and TT338,339AA channels (•). (D) Dependence of channel conductance on symmetrical Cl− activity in wild-type (○) and TT338,339AA channels (•). Each point represents the mean ± SEM (where this is larger than the size of the symbol) of data from three to eight patches. The data points have been fit by Eq. 2 (see methods), giving saturating conductances of 11.1 pS for wild-type CFTR and 14.0 pS for TT338,339AA, and a K m of 45.9 mM for wild type and 52.9 mM for TT338,339AA. Mean conductance was significantly greater in TT338,339AA channels than in wild-type channels at every Cl− concentration studied (P < 0.05, one-tailed t test).
Elevated conductance was also observed when TT338,339AA channels were bathed in symmetrical solutions having different Cl− activities (Fig. 5 D). The relationship between channel conductance and symmetrical Cl− activity for both wild-type and TT338,339AA channels was well fitted by a Michaelis-Menten–type hyperbolic function (Eq. 2; see Fig. 5 D). The fits shown in Fig. 5 D gave γmax = 11.1 pS and Km = 45.9 mM for wild type, and γmax = 14.0 pS and Km = 52.9 mM for TT338,339AA. The fact that this equation fit the data well without correction for local changes in ion concentration suggests that fixed charges on the surface of the channel protein do not greatly influence conductance in wild-type or TT338,339AA channels (for review see Green and Andersen, 1991). The primary functional effect of the TT338,339AA mutation was to increase the saturating conductance of the channel by ∼26%. The Km may also be increased somewhat (∼15%); however, this would probably have little effect at 150 mM Cl−.
Permeability of the TT338,339AA Mutant to Different Anions
To assess whether the increase in conductance and possible decrease in Cl− affinity of TT338,339AA might be associated with a change in pore diameter, permeability of the mutant channel to a number of extracellular anions was tested under biionic conditions as described for wild-type channels (see above; Tabcharani et al., 1997). Mean single channel current–voltage relationships for TT338,339AA obtained with different external anions are shown in Fig. 6. As can be seen from Table I, all permeant anions tested had higher permeability ratios in TT338,339AA than in the wild-type channel. Moreover, two anions that were not measurably permeant in wild-type channels (propanoate and pyruvate) showed significant permeability in TT338,339AA. The small anion F−, which has a high hydration energy and may be unable to interact with “weak field strength” sites in the pore (see below), was not measurably permeant in the TT338,339AA mutant, as reported previously for the wild-type channel (Tabcharani et al., 1997).
Figure 6.
Permeability of TT338,339AA to different extracellular anions. Mean current–voltage relationships were measured under biionic conditions (see methods and Fig. 2). Each point represents the mean ± SEM (where this is larger than the size of the symbol) of data from three to nine patches for Cl−, and from three to seven patches for other anions. Although the shape of the curves for both F− and gluconate suggest current reversal might occur at potentials more positive than those studied, we were unable to record outward F− or gluconate currents at large positive potentials where inward Cl− currents become vanishingly small. Currents recorded with extracellular I− showed a similar hysteresis to that seen in wild-type channels (data not shown; see Tabcharani et al., 1997). The reversal potential for I− was therefore estimated before I− block of the channel occurred (see Tabcharani et al., 1997, for details).
Estimates of CFTR Pore Diameter
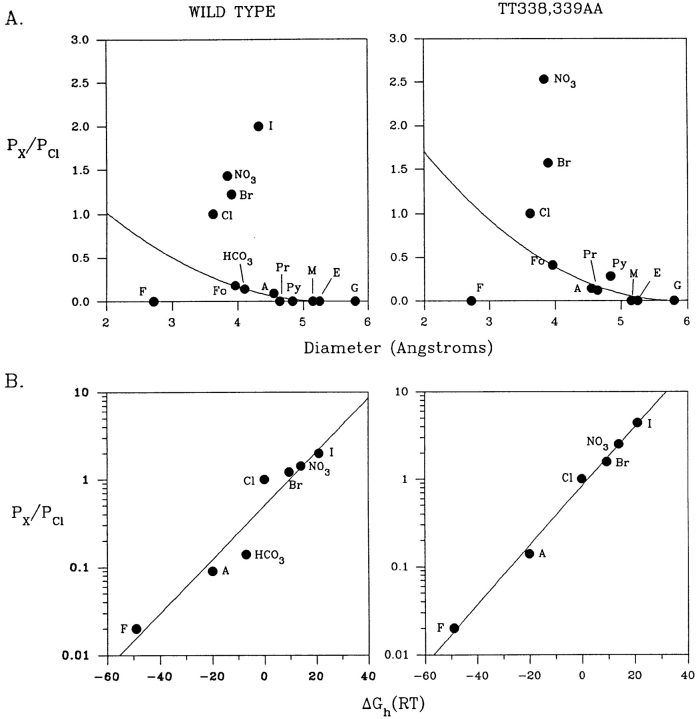
If the ability of large polyatomic ions to permeate depends on their size relative to that of the narrowest region of the pore (Dwyer et al., 1980), then the increased permeability of the TT338,339AA channel to acetate, formate, propanoate, and pyruvate would suggest that this double mutation may widen the narrowest region. The pore of wild-type CFTR accommodates acetate (unhydrated molecular dimensions 3.99 × 5.18 × 5.47 Å; see Table I) but not the slightly larger ions propanoate (4.12 × 5.23 × 7.05 Å) or pyruvate (4.09 × 5.73 × 6.82 Å). Assuming that the narrowest dimensions of the pore are large enough to accommodate the two smaller dimensions of any (unhydrated) permeant anion, this constriction must have minimal dimensions of 3.99 × 5.18 Å and a cross-sectional area of at least 21 Å2. By contrast, the pore of TT338,339AA is permeable to both propanoate and pyruvate but not to methane sulfonate (5.08 × 5.43 × 5.54 Å), suggesting minimal dimensions of 4.12 × 5.73 Å and a cross-sectional area of at least 24 Å2 for the narrowest region. The relative permeability of the different extracellular anions studied as a function of their apparent diameters is shown in Fig. 7 A. The diameter of unhydrated ions is often expressed as the geometric mean of the three minimum dimensions of the ion (e.g., Dwyer et al., 1980; Cohen et al., 1992b ). However, several of the anions studied here are roughly cylindrical in shape and their ability to pass through the CFTR channel may depend on the minimum cross-sectional dimensions of a cylinder that could contain the ion (McDonough et al., 1994; Cheung and Akabas, 1996). The largest dimension of the ion (i.e., the length of the cylinder) would therefore affect its permeability far less than the two smaller dimensions. We therefore took the geometric mean of the two smallest dimensions for each ion (Table I) as our estimate of ionic diameter in Fig. 7 A. Plotting the permeability ratios calculated for extracellular anions (Table I) against these apparent ionic diameters and fitting them with Eq. 3 gave d = 5.34 Å and k = 2.61 for the wild-type channel and d = 5.83 Å and k = 3.94 for TT338,339AA mutant, again consistent with a substantial increase in diameter of the TT338,339AA variant. These diameters would produce cross-sectional areas of ∼22 Å2 for wild type and ∼27 Å2 for TT338,339AA if the pores were cylindrical. Small anions (Cl−, F−, Br−, I−, NO3 −), the permeability of which is dependent more on their hydration energies than their size (see below), were excluded from these fits.
Figure 7.
Dependence of ion permeability on ion dimensions and hydration energy in wild-type and TT338,339AA channels. (A) Permeability (PX/PCl) as a function of mean ion diameter (estimated as described in the text). The data have been fitted by Eq. 3, giving estimated pore diameters of 5.34 Å for wild type and 5.83 Å for TT338,339AA. A, acetate; E, ethane sulfonate; Fo, formate; G, gluconate; M, methane sulfonate; Pr, propanoate; and Py, pyruvate. (B) Permeability as a function of hydration energy, relative to that of Cl− (ΔGh). Values for ΔGh were taken from Halm and Frizzell (1992).
Lyotropic Selectivity
Ionic permeability through channels is thought to involve at least partial dehydration of permeating ions, with ion–solvent interactions being replaced by interactions between the ion and polar groups lining the channel pore. The permeability sequence described for CFTR (I− > NO3 − > Br− > Cl− > HCO3 − > acetate > F−) follows a lyotropic sequence (Dani et al., 1983; Tabcharani et al., 1997), suggesting that hydration energies are mainly responsible for controlling anion permeability (Fig. 7 B). High iodide and thiocyanate permeabilities were reported previously (Tabcharani et al., 1992, 1993). Thus, in CFTR, ion–channel interactions may be relatively weak compared with ion–solvent interactions, indicating a weak field strength selectivity site (Wright and Diamond, 1977). The relationship between permeability and hydration energy is maintained in TT338, 339AA (Fig. 7 B), suggesting that this mutation does not strongly affect the selectivity filter of the channel.
DISCUSSION
This paper describes the most complete permeability sequence of the CFTR Cl− channel measured under biionic conditions, which we find to be I− > NO3 − > Br− > Cl− > HCO3 − > formate > acetate when these ions are present on either side of the membrane. Propanoate, pyruvate, methane sulfonate, ethane sulfonate, and gluconate were not measurably permeant (PX/PCl < 0.06). Our permeability ratios for NO3 − (1.43–1.61), HCO3 − (0.14–0.25), and gluconate (close to zero) are consistent with previous reports for CFTR in different systems (Gray et al., 1990, 1993; Bell and Quinton, 1992; Bajnath et al., 1993; Copello et al., 1993; Overholt et al., 1993; Poulsen et al., 1994; Kottra, 1995; Linsdell and Hanrahan, 1996a ).
As with halide permeability (Tabcharani et al., 1997), the permeability sequence to polyatomic anions followed a lyotropic or (inverse) Hofmeister sequence (Fig. 7 B). This series is favored when cationic groups or dipoles in proteins attract anions to a region of structured water, such as that found near hydrophobic groups (Von Hippel and Schleich, 1969; Dani et al., 1983; Tabcharani et al., 1997). The same lyotropic sequence has been observed in GABAA and glycine-gated Cl− channels in spinal neurons (Bormann et al., 1987) and hippocampal neurons (Fatima-Shad and Barry, 1993), in a voltage–dependent Cl− channel in hippo-campal neurons (Franciolini and Nonner, 1987), and in the epithelial outwardly rectifying Cl− channel (Reinhardt et al., 1986; Halm and Frizzell, 1992). Although the physical basis of lyotropic anion selectivity has not yet been studied in Cl− channels using mutagenesis, it is likely that a positively charged amino acid and/or cationic dipole within the channel pore is the anion attracting group. As discussed in the companion papers (Tabcharani et al., 1997; Linsdell et al., 1997), one contributor in the CFTR pore may be arginine 347 in TM6, since mutations that remove positive charge at this position drastically reduce selectivity between Cl− and I− (Tabcharani et al., 1997), abolish voltage–dependent inhibition of Cl− currents by the lyotropic anion SCN− (Tabcharani et al., 1993), and reduce channel block by cytoplasmic disulfonic stilbenes (Linsdell and Hanrahan, 1996b ).
Wild-type CFTR channels showed low permeability to formate and acetate ions, and were not measurably permeant to the larger anions propanoate, pyruvate, methane sulfonate, ethane sulfonate, and gluconate. However, these large anions may be able to permeate at rates that are too low to be resolved as single channel currents. Relatively large, hydrophilic sulfhydryl reagents (∼6 Å in diameter) are able to penetrate from the extracellular solution to interact covalently with engineered cysteine residues at the cytoplasmic end of TM6 (Cheung and Akabas, 1996). The irreversible nature of that reaction probably enables permeation by the cysteine reagent to be detected when the flux rates of similar compounds (e.g., ethane sulfonate) are too low to generate measurable current at the single channel level. The anionic channel blockers diphenylamine-2-carboxylate and flufenamic acid are also permeant in CFTR (McCarty et al., 1993).
The relationship between ion diameter and permeability in CFTR (Fig. 7 A) suggests a pore diameter of ∼5.3 Å and a cross-sectional area of ∼21–22 Å2. Other Cl− channel types have been estimated to have pore diameters between 5.2 and 6.4 Å (Bormann et al., 1987; Franciolini and Nonner, 1987; Halm and Frizzell, 1992; Fatima-Shad and Barry, 1993; Arreola et al., 1995). Our estimate of the pore diameter is likely to be a lower limit, since large anions may have permeabilities below our detection threshold (see above). However, our estimates for the pore diameter are less than the diameter of ATP (Table I), which has been reported to diffuse through CFTR channels at high rates (Reisin et al., 1994; Schwiebert et al., 1995), although this has not been observed in all laboratories (Reddy et al., 1996; Li et al., 1996; Grygorczyk et al., 1996). If CFTR can support ATP transport under certain conditions, it seems unlikely that this would involve ATP permeation through the pore.
The increased permeability of large anions in TT338,339AA (Table I) indicates an increase in the dimensions of the narrowest part of the pore in this mutant. We estimate the diameter of the mutated pore to be ∼5.8 Å, with a cross-sectional area of 24–27 Å2. One possible interpretation of these results is that threonine residues 338 and/or 339 might contribute to the narrowest part of the pore, either directly or via an allosteric effect on a constricted region that is physically located elsewhere. Threonine residues have previously been suggested to contribute to the narrowest region of the pore in cation-selective nicotinic acetylcholine receptor channels (Cohen et al., 1992a , 1992b ; Villarroel and Sakmann, 1992). However, substituted cysteine accessibility mutagenesis experiments indicate that the R groups of these two threonine residues are not in contact with the aqueous lumen of the CFTR pore (Cheung and Akabas, 1996).
TT338,339AA had a larger saturating conductance than wild-type CFTR (Fig. 5), suggesting that conductance of the wild-type channel may be limited by the rate of Cl− flux through this narrow region. Conductance could be elevated due to a reduction in nonspecific frictional interactions between the permeating ion and the pore walls (although the smallest estimate of the narrowest part of the pore is still much larger than the diameter of an unhydrated Cl− ion, 3.62 Å). The i/V relationship of TT338,339AA, like wild type, was linear, suggesting anion binding is not strongly altered in this mutant. This agrees with the results of McDonough et al. (1994), who found that mutating each of these threonine residues individually to alanines did not affect the linearity of the macroscopic CFTR Cl− current expressed in Xenopus oocytes. In contrast, mutating serine 341 to alanine produced outward rectification of the i/V relationship, consistent with its proposed role as a binding site for permeating anions (McDonough et al., 1994). Subsequent cysteine mutagenesis also indicated that serine 341 lines the pore (Cheung and Akabas, 1996). The increased conductance of TT338,339AA is unlikely to be a nonspecific effect of mutations in TM6 since many mutations in this region have been studied, but none has previously been found to elevate conductance. Moreover, the fact that the selectivity sequence and channel gating were not affected in the mutant also argues against gross structural alterations, although these cannot be excluded. The altered apparent pore size and conductance of TT338,339AA are consistent with the proposed key role of TM6 in forming the CFTR pore (Anderson et al., 1991; Sheppard et al., 1993; Tabcharani et al., 1993, 1997; McDonough et al., 1994; Cheung and Akabas, 1996; Linsdell and Hanrahan, 1996b ).
A CFTR variant with increased conductance might be useful in maximizing Cl− transport in gene or protein replacement therapy for cystic fibrosis, particularly where the efficiency of gene or protein delivery was low. The 24% increase in channel conductance seen in TT338,339AA might not be therapeutically significant and would also have to be weighed against the possibly deleterious increased permeability to large organic anions. Nevertheless, since it is the first CFTR mutation to increase channel conductance, it suggests that other mutations in this region may allow the development of therapeutically advantageous forms of CFTR.
The lyotropic sequence of permeability ratios is the same in both wild-type and TT338,339AA channels (I− > NO3 − > Br− > Cl− > acetate > F−; Fig. 7 B). This suggests that the narrow region disrupted in the TT338,339AA mutant is not a major determinant of selectivity in CFTR, unlike voltage-gated Na+ (Lipkind and Fozzard, 1994) and K+ channels (Lipkind et al., 1995), where a selectivity filter has been proposed in the narrowest part of the pore. Nevertheless, permeability ratios for I−, NO3 −, and Br− are all increased relative to the smaller Cl− ion. Thus, in wild-type channels, the narrow region may interact preferentially with Cl− compared with these other ions.
Acknowledgments
We thank Jenny Eng and Shu-Xian Zheng for technical assistance.
This work was supported by the Canadian Cystic Fibrosis Foundation (CCFF), the Medical Research Council (MRC; Canada), and the National Institute of Diabetes and Digestive and Kidney Diseases. P. Linsdell is a CCFF postdoctoral fellow. J.W. Hanrahan is an MRC Scientist.
Abbreviation used in this paper
- CFTR
cystic fibrosis transmembrane conductance regulator
REFERENCES
- Anderson MP, Gregory RJ, Thompson S, Souza DW, Paul S, Mulligan RC, Smith AE, Welsh MJ. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science (Wash DC) 1991;253:202–205. doi: 10.1126/science.1712984. [DOI] [PubMed] [Google Scholar]
- Arreola J, Melvin JE, Begenisich T. Volume-activated chloride channels in rat parotid acinar cells. J Physiol (Cambr) 1995;484:677–687. doi: 10.1113/jphysiol.1995.sp020695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajnath RB, Groot JA, De Jonge HR, Kansen M, Bijman J. Synergistic activation of non-rectifying small-conductance chloride channels by forskolin and phorbol esters in cell- attached patches of the human colon carcinoma cell line HT-29cl.19A. Pflügers Arch. 1993;425:100–108. doi: 10.1007/BF00374509. [DOI] [PubMed] [Google Scholar]
- Bell CL, Quinton PM. T84 cells: anion selectivity demonstrates expression of Cl−conductance affected in cystic fibrosis. Am J Physiol. 1992;262:C555–C562. doi: 10.1152/ajpcell.1992.262.3.C555. [DOI] [PubMed] [Google Scholar]
- Bormann J, Hamill OP, Sakmann B. Mechanism of anion permeation through channels gated by glycine and γ-aminobutyric acid in mouse cultured spinal neurones. J Physiol (Cambr) 1987;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang X-B, Tabcharani JA, Hou Y-X, Jensen TJ, Kartner N, Alon A, Hanrahan JW, Riordan JR. Protein kinase A (PKA) still activates CFTR chloride channel after mutagenesis of all 10 PKA consensus phosphorylation sites. J Biol Chem. 1993;268:11304–11311. [PubMed] [Google Scholar]
- Cheung M, Akabas MH. Identification of cystic fibrosis transmembrane conductance regulator channel-lining residues in and flanking the M6 membrane-spanning segment. Biophys J. 1996;70:2688–2695. doi: 10.1016/S0006-3495(96)79838-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen BN, Labarca C, Czyzyk L, Davidson N, Lester HA. Tris+/Na+permeability ratios of nicotinic acetylcholine receptors are reduced by mutations near the intracellular end of the M2 region. J Gen Physiol. 1992a;99:545–572. doi: 10.1085/jgp.99.4.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen BN, Labarca C, Davidson N, Lester HA. Mutations in M2 alter the selectivity of the mouse nicotinic acetylcholine receptor for organic and alkali metal cations. J Gen Physiol. 1992b;100:373–400. doi: 10.1085/jgp.100.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copello J, Heming TA, Segal Y, Reuss L. cAMP-activated apical membrane chloride channels in Necturus gallbladder epithelium. Conductance, selectivity, and block. J Gen Physiol. 1993;102:177–199. doi: 10.1085/jgp.102.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JA, Sanchez JA, Hille B. Lyotropic anions. Na channel gating and Ca electrode response. J Gen Physiol. 1983;81:255–281. doi: 10.1085/jgp.81.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer TM, Adams DJ, Hille B. The permeability of the endplate channel to organic cations in frog muscle. J Gen Physiol. 1980;75:469–492. doi: 10.1085/jgp.75.5.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatima-Shad K, Barry PH. Anion permeation in GABA- and glycine-gated channels of mammalian hippocampal neurons. Proc R Soc Lond Ser B. 1993;253:69–75. doi: 10.1098/rspb.1993.0083. [DOI] [PubMed] [Google Scholar]
- Franciolini F, Nonner W. Anion and cation permeability of a chloride channel in rat hippocampal neurons. J Gen Physiol. 1987;90:453–478. doi: 10.1085/jgp.90.4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray MA, Plant S, Argent BE. cAMP-regulated whole cell chloride currents in pancreatic duct cells. Am J Physiol. 1993;264:C591–C602. doi: 10.1152/ajpcell.1993.264.3.C591. [DOI] [PubMed] [Google Scholar]
- Gray MA, Pollard CE, Harris A, Coleman L, Greenwell JR, Argent BE. Anion selectivity and block of the small-conductance chloride channel on pancreatic duct cells. Am J Physiol. 1990;259:C752–C761. doi: 10.1152/ajpcell.1990.259.5.C752. [DOI] [PubMed] [Google Scholar]
- Green WN, Andersen OS. Surface charges and ion channel function. Annu Rev Physiol. 1991;53:341–359. doi: 10.1146/annurev.ph.53.030191.002013. [DOI] [PubMed] [Google Scholar]
- Grygorczyk R, Tabcharani JA, Hanrahan JW. CFTR channels expressed in CHO cells do not have detectable ATP conductance. J Membr Biol. 1996;151:139–148. doi: 10.1007/s002329900065. [DOI] [PubMed] [Google Scholar]
- Halm DR, Frizzell RA. Anion permeation in an apical membrane chloride channel of a secretory epithelial cell. J Gen Physiol. 1992;99:339–366. doi: 10.1085/jgp.99.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanrahan, J.W., J.A. Tabcharani, F. Becq, C.J. Mathews, O. Augustinas, T.J. Jensen, X.-B. Chang, and J.R. Riordan. 1995. Function and dysfunction of the CFTR chloride channel. In Ion Channels and Genetic Diseases. D.C. Dawson and R.A. Frizzell, editors. Rockefeller University Press, New York. 125–137. [PubMed]
- Heginbotham L, Lu Z, Abramson T, MacKinnon R. Mutations in the K+channel signature sequence. Biophys J. 1994;66:1061–1067. doi: 10.1016/S0006-3495(94)80887-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottra G. Calcium is not involved in the cAMP-mediated stimulation of Cl−conductance in the apical membrane of Necturus gallbladder epithelium. Pflügers Arch. 1995;429:647–658. doi: 10.1007/BF00373985. [DOI] [PubMed] [Google Scholar]
- Li CH, Ramjeesingh M, Bear CE. Purified cystic fibrosis transmembrane conductance regulator (CFTR) does not function as an ATP channel. J Biol Chem. 1996;271:11623–11626. doi: 10.1074/jbc.271.20.11623. [DOI] [PubMed] [Google Scholar]
- Linsdell P, Hanrahan JW. Flickery block of single CFTR chloride channels by intracellular anions and osmolytes. Am J Physiol. 1996a;271:C628–C634. doi: 10.1152/ajpcell.1996.271.2.C628. [DOI] [PubMed] [Google Scholar]
- Linsdell P, Hanrahan JW. Disulphonic stilbene block of cystic fibrosis transmembrane conductance regulator Cl−channels expressed in a mammalian cell line, and its regulation by a critical pore residue. J Physiol (Cambr) 1996b;496:687–693. doi: 10.1113/jphysiol.1996.sp021719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsdell P, Rommens JM, Hou Y-X, Chang X-B, Tsui L-C, Riordan JR, Hanrahan JW. Mutation of the narrow region of the CFTR channel pore. Biophys J. 1996;70:A72. . (Abstr.) [Google Scholar]
- Linsdell P, Tabcharani JA, Hanrahan JW. A multi-ion mechanism for ion permeation and block in the CFTR chloride channel. J Gen Physiol. 1997;110:365–377. doi: 10.1085/jgp.110.4.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkind GM, Fozzard HA. A structural model of the tetrodotoxin and saxitoxin binding site of the Na+channel. Biophys J. 1994;66:1–13. doi: 10.1016/S0006-3495(94)80746-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkind GM, Hanck DA, Fozzard HA. A structural motif for the voltage-gated potassium channel pore. Proc Natl Acad Sci USA. 1995;92:9215–9219. doi: 10.1073/pnas.92.20.9215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon R, Yellen G. Mutations affecting TEA blockade and ion permeation in voltage-activated K+channels. Science (Wash DC) 1990;250:276–279. doi: 10.1126/science.2218530. [DOI] [PubMed] [Google Scholar]
- McCarty NA, McDonough S, Cohen BN, Riordan JR, Davidson N, Lester HA. Voltage-dependent block of the cystic fibrosis transmembrane conductance regulator Cl−channel by two closely related arylaminobenzoates. J Gen Physiol. 1993;102:1–23. doi: 10.1085/jgp.102.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough S, Davidson N, Lester HA, McCarty NA. Novel pore-lining residues in CFTR that govern permeation and open-channel block. Neuron. 1994;13:623–634. doi: 10.1016/0896-6273(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Overholt JL, Hobert ME, Harvey RD. On the mechanism of rectification of the isoproterenol-activated chloride current in guinea-pig ventricular myocytes. J Gen Physiol. 1993;102:871–895. doi: 10.1085/jgp.102.5.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen JH, Fischer H, Illek B, Machen TE. Bicarbonate conductance and pH regulatory capability of cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci USA. 1994;91:5340–5344. doi: 10.1073/pnas.91.12.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy MM, Quinton PM, Haws C, Wine JJ, Grygorczyk R, Tabcharani JA, Hanrahan JW, Gunderson KL, Kopito RR. Failure of the cystic fibrosis transmembrane conductance regulator to conduct ATP. Science (Wash DC) 1996;271:1876–1879. doi: 10.1126/science.271.5257.1876. [DOI] [PubMed] [Google Scholar]
- Reinhardt R, Bridges RJ, Rummel W, Lindemann B. Properties of an anion-selective channel from rat colonic enterocytes plasma membranes reconstituted into planar phospholipid bilayers. J Membr Biol. 1987;95:47–54. doi: 10.1007/BF01869629. [DOI] [PubMed] [Google Scholar]
- Reisin IL, Prat AG, Abraham EH, Amara JF, Gregory RJ, Ausiello DA, Cantiello HF. The cystic fibrosis transmembrane conductance regulator is a dual ATP and chloride channel. J Biol Chem. 1994;269:20584–20591. [PubMed] [Google Scholar]
- Riordan JR. The cystic fibrosis transmembrane conductance regulator. Annu Rev Physiol. 1993;55:609–630. doi: 10.1146/annurev.ph.55.030193.003141. [DOI] [PubMed] [Google Scholar]
- Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plasvik N, Chou J-L, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science (Wash DC) 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Sansom MSP. The roles of serine and threonine sidechains in ion channels: a modelling study. Eur Biophys J. 1992;21:281–298. doi: 10.1007/BF00185123. [DOI] [PubMed] [Google Scholar]
- Schwiebert EM, Egan ME, Hwang T-H, Fulmer SB, Allen SS, Cutting GR, Guggino WB. CFTR regulates outwardly rectifying chloride channels through an autocrine mechanism involving ATP. Cell. 1995;81:1063–1073. doi: 10.1016/s0092-8674(05)80011-x. [DOI] [PubMed] [Google Scholar]
- Sheppard DN, Rich DP, Ostedgaard LS, Gregory RJ, Smith AE, Welsh MJ. Mutations in CFTR associated with mild-disease-form Cl−channels with altered pore properties. Nature (Lond) 1993;362:160–164. doi: 10.1038/362160a0. [DOI] [PubMed] [Google Scholar]
- Tabcharani JA, Chang X-B, Riordan JR, Hanrahan JW. Phosphorylation-regulated Cl−channel in CHO cells stably expressing the cystic fibrosis gene. Nature (Lond) 1991;352:628–631. doi: 10.1038/352628a0. [DOI] [PubMed] [Google Scholar]
- Tabcharani JA, Chang X-B, Riordan JR, Hanrahan JW. The cystic fibrosis transmembrane conductance regulator chloride channel. Iodide block and permeation. Biophys J. 1992;62:1–4. doi: 10.1016/S0006-3495(92)81759-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabcharani JA, Hanrahan JW. Permeation in the cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel. Biophys J. 1993;64:A17. doi: 10.1016/S0006-3495(92)81759-9. . (Abstr.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabcharani JA, Jensen TJ, Riordan JR, Hanrahan JW. Bicarbonate permeability of the outwardly rectifying anion channel. J Membr Biol. 1989;112:109–122. doi: 10.1007/BF01871272. [DOI] [PubMed] [Google Scholar]
- Tabcharani JA, Linsdell P, Hanrahan JW. Halide permeation in wild-type and mutant CFTR chloride channels. J Gen Physiol. 1997;110:341–354. doi: 10.1085/jgp.110.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabcharani JA, Rommens JM, Hou Y-X, Chang X-B, Tsui L-C, Riordan JR, Hanrahan JW. Multi-ion pore behaviour in the CFTR chloride channel. Nature (Lond) 1993;366:79–82. doi: 10.1038/366079a0. [DOI] [PubMed] [Google Scholar]
- Villarroel A, Sakmann B. Threonine in the selectivity filter of the acetylcholine receptor channel. Biophys J. 1992;62:196–208. doi: 10.1016/S0006-3495(92)81805-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Hippel, P.H., and T. Schleich. 1969. The effects of neutral salts on the structure and conformational stability of macromolecules in solution. In Biological Macromolecules. Vol. 2, Structure and Stability of Biological Macromolecules. S.N. Timasheff and G. Fasman, editors. Marcel Dekker, Inc., Monticello, New York. 417–574.
- Welsh MJ, Anderson MP, Rich DP, Berger HA, Denning GM, Ostedgaard LS, Sheppard DN, Cheng SH, Gregory RJ, Smith AE. Cystic fibrosis transmembrane conductance regulator: a chloride channel with novel regulation. Neuron. 1992;8:821–829. doi: 10.1016/0896-6273(92)90196-k. [DOI] [PubMed] [Google Scholar]
- Wright EM, Diamond JM. Anion selectivity in biological systems. Physiol Rev. 1977;57:109–156. doi: 10.1152/physrev.1977.57.1.109. [DOI] [PubMed] [Google Scholar]
- Yool AJ, Schwarz TL. Alteration of ionic selectivity of a K+channel by mutation of the H5 region. Nature (Lond) 1991;349:700–704. doi: 10.1038/349700a0. [DOI] [PubMed] [Google Scholar]