Abstract
We studied the regulation of intracellular pH (pHi) in single cultured astrocytes passaged once from the hippocampus of the rat, using the dye 2′,7′-biscarboxyethyl-5,6-carboxyfluorescein (BCECF) to monitor pHi. Intrinsic buffering power (βI) was 10.5 mM (pH unit)−1 at pHi 7.0, and decreased linearly with pHi; the best-fit line to the data had a slope of −10.0 mM (pH unit)−2. In the absence of HCO3 −, pHi recovery from an acid load was mediated predominantly by a Na-H exchanger because the recovery was inhibited 88% by amiloride and 79% by ethylisopropylamiloride (EIPA) at pHi 6.05. The ethylisopropylamiloride-sensitive component of acid extrusion fell linearly with pHi. Acid extrusion was inhibited 68% (pHi 6.23) by substituting Li+ for Na+ in the bath solution. Switching from a CO2/HCO3 −-free to a CO2/HCO3 −-containing bath solution caused mean steady state pHi to increase from 6.82 to 6.90, due to a Na+-driven HCO3 − transporter. The HCO3 −-induced pHi increase was unaffected by amiloride, but was inhibited 75% (pHi 6.85) by 400 μM 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS), and 65% (pHi 6.55–6.75) by pretreating astrocytes for up to ∼6.3 h with 400 μM 4-acetamide-4′-isothiocyanatostilbene-2,2′-disulfonic acid (SITS). The CO2/HCO3 −-induced pHi increase was blocked when external Na+ was replaced with N-methyl-d-glucammonium (NMDG+). In the presence of HCO3 −, the Na+-driven HCO3 − transporter contributed to the pHi recovery from an acid load. For example, HCO3 − shifted the plot of acid-extrusion rate vs. pHi by 0.15–0.3 pH units in the alkaline direction. Also, with Na-H exchange inhibited by amiloride, HCO3 − increased acid extrusion 3.8-fold (pHi 6.20). When astrocytes were acid loaded in amiloride, with Li+ as the major cation, HCO3 − failed to elicit a substantial increase in pHi. Thus, Li+ does not appear to substitute well for Na+ on the HCO3 − transporter. We conclude that an amiloride-sensitive Na-H exchanger and a Na+-driven HCO3 − transporter are the predominant acid extruders in astrocytes.
Keywords: H+ concentration, acid–base transport, glia, nervous system, Na-H exchanger
INTRODUCTION
It is well established that the pH of the brain extracellular fluid (pHECF)1 can influence neuronal activity (for reviews, see Chesler and Kaila, 1992; Ransom, 1992), especially because many ion channels are sensitive to changes in extracellular pH (pHo) (for reviews see Moody, 1984; Chesler, 1990). For example, the open probability of the N-methyl-d-aspartate-activated channel decreases at progressively lower pHo values, with an apparent pK in the range 6.7–7.3 (Tang et al., 1990; Traynelis and Cull-Candy, 1990). The relationship between changes in pHECF and neuronal activity is complicated, however, because electrical activity itself can alter the pH of the brain extracellular fluid. The changes in pHECF elicited by neuronal firing are caused by the transport of acid–base equivalents across the plasma membrane of neurons and/or glia cells. This acid–base transport will have two effects. First, it obviously will affect the pHi of the cells doing the transport, as well as the pHECF in the microenvironment. Second, because acid–base transporters on neighboring cells generally are sensitive to such pHECF changes, there will be indirect effects on the pHi of these neighboring cells.
pHECF is thus in the position to mediate a complex interaction among neurons and glial cells. Indeed, the glial cells are thought to play a key role in regulating ECF composition, including pHECF, and the acid–base transporters of both invertebrate and mammalian glial cells are capable of modulating pHECF (see review by Deitmer and Rose, 1996). One such transporter thought to play an important role in regulating pHECF is the electrogenic Na/HCO3 cotransporter. A similar transporter was first described in the cells of the proximal tubule of salamander kidney (Boron and Boulpaep, 1983), where it moves Na+ and HCO3 − across the basolateral membrane from the cell to the blood, with a Na+: HCO3 − stoichiometry of 1:3 (Soleimani et al., 1987). The electrogenic Na/HCO3 cotransporter is independent of Cl−, but is inhibited by disulfonic stilbene derivatives, such as 4-acetamido-4′-isothiocyanatostilbene-2,2′-disulfonic acid (SITS) and 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS). Electrogenic Na/HCO3 cotransporters were identified subsequently in a number of glial cells, including giant neuropile glial cells (Deitmer and Schlue, 1987, 1989) and connective glial cells (Szatkowski and Schlue, 1992) of the leech, astrocytes in the optic nerve of Necturus (Astion and Orkand, 1988), and Müller cells from the salamander retina (Newman, 1991; Newman and Astion, 1991). In the Müller cells, the electrogenic Na/HCO3 cotransporter has a Na+:HCO3 − stoichiometry of 1:3, and moves HCO3 − out of the cells (Newman, 1991; Newman and Astion, 1991). On the other hand, in leech neuropile glial cells, and perhaps others as well, it is thought that the cotransporters have a Na+:HCO3 − stoichiometry of 1:2, and move HCO3 − into the cells (Deitmer and Schlue, 1989; Deitmer, 1992; Munsch and Deitmer, 1994). It is not known whether the electrogenic Na/ HCO3 cotransporters with stoichiometries of 1:3 and 1:2 are different proteins.
There is also evidence that a Na/HCO3 cotransporter might exist in mammalian glial cells. In primary cultures of astrocytes from the cerebral cortex of the mouse, the pHi recovery from an acid load depends on Na+ and HCO3 −, is unaffected by acute Cl− removal, but is inhibited by SITS (Chow et al., 1991). In astrocytes cultured from rat cerebellum, exposure to CO2/ HCO3 − at 25°C causes a transient decrease in pHi, followed by a sustained pHi increase that has properties similar to those of the above mouse astrocytes (Brune et al., 1994). Both the mouse and rat data are consistent with mammalian astrocytes' having a Na/HCO3 cotransporter that moves HCO3 − into the cell and has a stoichiometry of either 1:2 (electrogenic) or 1:1 (electroneutral).
Two groups have used the whole-cell patch-clamp technique, one in rat astrocytes cultured from the cerebellum (Brune et al., 1994) and the other from the hippocampus (O'Connor et al., 1994), to address the question of whether Na/HCO3 cotransporters in mammalian astrocytes are electrogenic. These authors recorded membrane voltage (Vm) and current (Im) in astrocytes, while exposing them to CO2/HCO3 − at room temperature. They found that the exposure to CO2/ HCO3 − elicited hyperpolarizations and outward currents that were at least partially inhibited by removing external Na+ or applying DIDS, but were not inhibited by acutely removing external Cl−. These data are consistent, but as discussed below, do not prove the hypothesis that introducing CO2/HCO3 − initiates the influx of HCO3 − and negative charge via a DIDS-sensitive, electrogenic Na/HCO3 cotransporter.
In this and the accompanying paper on cultured rat hippocampal astrocytes, we examine whether mammalian astrocytes do indeed possess an electrogenic Na/ HCO3 cotransporter. Our approach is significant in three ways. First, we perform the experiments at 37°C, where the activity of the electrogenic Na/HCO3 cotransporter is likely to be greater than at room temperature. Second, we use a more specific assay for the cotransporter than monitoring Vm while adding CO2/HCO3 − under three conditions (control, DIDS, 0 Na+). A potential difficulty with focusing on the DIDS-sensitive electrical changes elicited by exposing the cells to CO2/ HCO3 − is that both DIDS and CO2 are rather nonspecific amino-reactive agents. The isothiocyano groups on DIDS interact with amino groups via the Edman reaction. CO2 interacts with susceptible free amines of proteins, resulting in the formation of carbamino compounds (Morrow et al., 1974), a classic example of which is the formation of carbamino hemoglobin. Moreover, the influx of CO2 into the cell will rapidly lower pHi, especially in the microenvironment on the inner surface of the membrane, and potentially change pHi-sensitive ionic conductances. An additional problem is that switching to a HCO3 −-containing solution could evoke HCO3 − currents per se. In cultured rat astrocytes, GABAA-receptor channels can mediate HCO3 − currents (Kaila et al., 1991).
Therefore, we have chosen to monitor pHi and Vm while executing a maneuver (i.e., Na+ removal) designed to elicit a response (i.e., a rapid depolarization) that is far more specific for the cotransporter than is the addition of CO2/HCO3 −. Although removing Na+ per se is not specific, the resulting rapid depolarization can be attributed to only a few causes: inhibiting a Na+-dependent K+ conductance, inhibiting the Na-K pump, and forcing the electrogenic Na/HCO3 cotransporter to move in the outward direction. Other Na+-coupled transporters would either directly produce rapid Vm changes of the wrong sign, or indirectly produce slow Vm changes by changing cell composition. The Na+- removal assay can be made even more specific by requiring that the rapid depolarization also depend on CO2/HCO3 − and be inhibited by DIDS.
Our third significant approach for identifying an electrogenic Na/HCO3 cotransporter in hippocampal astrocytes is to determine whether the changes in Vm and pHi are quantitatively consistent with electrogenic Na/HCO3 cotransport. Thus, we compare the magnitude of the Vm change (from which we can compute the flux of charge) to the observed rate of pHi change (from which we can compute the flux of HCO3 −).
In the first of our two papers, we use a fluorescent pH-sensitive dye to evaluate the acid–base transporters responsible for regulating pHi in both the presence and absence of CO2/HCO3 −. We found that the astrocytes have both a previously identified amiloride-sensitive Na-H exchanger, as well as a CO2/HCO3 −-dependent transporter that requires external Na+ and can be inhibited by the stilbene derivatives, DIDS and SITS. In the second paper (Bevensee et al., 1997), we provide evidence that the CO2/HCO3 −-dependent transporter does not require Cl−. This rules out a Na+-driven Cl-HCO3 exchanger. In addition, we used the perforated patch-clamp technique to record changes in Vm during the aforementioned Na+-removal protocol, observing Vm changes nearly identical to those predicted from the rates of pHi change. Our data indicate that hippocampal astrocytes have an electrogenic 1:2 Na/HCO3 cotransporter.
METHODS
Solutions
The standard HEPES-buffered solution contained (mM): 125 NaCl, 3 KCl, 1 CaCl2, 1.2 MgSO4, 2 NaH2PO4, 32 HEPES, and 10.5 glucose, titrated to pH 7.3 at 37°C with NaOH. 5% CO2/17 mM HCO3 −-buffered solutions were made by substituting 17 mM NaHCO3 for HEPES and adding 8.4 mM NaCl to maintain constant ionic strength. In NH3/NH4 +-containing solutions, NaCl was replaced with equimolar NH4Cl. In 0-Na+ solutions, the Na+ substitute was either N-methyl-d-glucammonium (NMDG+) or Li+. In 0-Cl− solutions, the Cl− substitute was cyclamate, and total [Ca2+] was increased threefold to compensate for Ca2+ chelation by the anion substitute. In the 10-μM nigericin solution, KCl was 105 mM and the remainder of the Na+ was replaced with NMDG+. The acetoxymethyl ester of the pH-sensitive dye 2′,7′-biscarboxyethyl-5,6-carboxyfluorescein (BCECF-AM) was obtained from Molecular Probes, Inc. (Eugene, OR). Ethylisopropyl-amiloride (EIPA) was obtained from either E.J. Cragoe, Jr. (Nacogdoches, TX) or Research Biochemicals, Inc. (Natick, MA). DIDS was obtained from either Fluka Chemical Corp. (Ronkon-koma, NY) or Sigma Chemical Co. (St. Louis, MO). All other chemicals were obtained from Sigma Chemical Co.
Cell Isolation and Culturing
Astrocytes were isolated and cultured from the hippocampi of 2–3-d-old rats using a modified version of the procedure described by McCarthy and de Vellis (1980). Rats were decapitated and the brains placed in chilled Dulbecco's PBS supplemented with 33 mM glucose. The hippocampi were removed from each brain by microdissection and placed in fresh, chilled PBS + 33 mM glucose. The hippocampi were triturated with pipettes of decreasing pore diameters until the solution was cloudy in appearance and contained only small hippocampal fragments. A final concentration of ∼0.01% trypsin (GIBCO BRL, Life Technologies Inc., Gaithersburg, MD) was then added to the solution to digest the fragments further. After ∼1 min, a final concentration of 25% fetal calf serum (Gemini Bioproducts, Inc., Calabasas, CA, or GIBCO BRL, Life Technologies Inc.) was added to the solution to inhibit further digestion by the trypsin. The solution was centrifuged (TJ-6; Beckman Instruments, Inc., Fullerton, CA) at 400 g for 5 min, and the cell pellet was resuspended in PBS + 33 mM glucose. This new suspension was again centrifuged and the pellet resuspended in the standard cell culture medium, which consisted of MEM supplemented with 28 mM glucose, 2 mM l-glutamine, 100 U ml−1 penicillin/streptomycin (GIBCO BRL, Life Technologies Inc.), and 10% fetal calf serum (Gemini Bioproducts, Inc. or GIBCO BRL, Life Technologies Inc.). After a final centrifugation, the pellet was resuspended in the culture medium and the cell suspension was plated into cell culture flasks. The cells were grown in a 5% CO2, 37°C incubator, and the culture medium was changed every 3–5 d. To minimize the growth of oligodendrocytes in the cell culture, the flasks were agitated on a variable rotator (R4140; American Hospital Supply Corp., Miami, FL) at ∼100 rpm for ∼2 h before the me33dia was changed on the fourth day after cell plating. 10–12 d after cell plating, the flasks were again agitated at ∼100 rpm for 18–24 h before the confluent monolayer of cells was treated with trypsin-EDTA (GIBCO BRL, Life Technologies Inc.) and passaged onto 22 × 22–mm glass coverslips. Before cell plating, the coverslips were washed with RadiacWash® (Biodex Medical Systems, Shirley, NY), and then 70–100% ethanol several times to rid the glass of grease and optimize cell attachment and growth. Experiments were performed on the cells 1–6 wk after the cells were passaged onto coverslips. The cells grown on the coverslips were immunocytochemically stained routinely with an antibody to the astrocyte-specific glial fibrillary acidic protein (GFAP). Over 95% of the cells stained positive for GFAP.
Measurement of pHi in Single Astrocytes
Coverslips with attached astrocytes were first washed in a standard HEPES-buffered solution before being mounted in a perfusion chamber. The coverslip comprised the floor of the chamber. The chamber was then filled with a HEPES-buffered solution containing 10 μM BCECF-AM. Cells were incubated in this BCECF-AM solution, equilibrated with room air, for 10–20 min at 37°C. The perfusion chamber was then secured to the stage of a microscope equipped for epifluorescence (IM-35; Carl Zeiss, Inc., Thornwood, NY), and perfused for a minimum of 5 min with a HEPES-buffered solution to remove any unhydrolyzed BCECF-AM. Because the optical technique and the data acquisition used to measure pHi have been described extensively elsewhere (Boyarsky et al., 1988), they will be only briefly summarized here. A 63× oil-immersion objective was used to choose a single astrocyte that was typically 20–40 μm in diameter, star shaped, and had a homogeneous distribution of dye inside the cytoplasm. pHi recordings were obtained on cells that were nearly always surrounded by other cells. Dye was excited in an ∼20-μm diameter area in the center of the cell; the light source was a 100-W halogen bulb. Approximately every 8 s, the dye was alternatively excited for 200 ms by light at wavelengths of 490 and 440 nm. The emitted fluorescence at a wavelength of 530 nm was amplified by a photomultiplier tube and recorded as I490 and I440. Because I490 is pH dependent and I440 is relatively pH independent, the ratio of the two (I490/I440) is mainly a function of pH. Normalized I490/I440 values were converted to pHi using a high K+/nigericin technique (Thomas et al., 1979), as modified for a single-point calibration by Boyarsky et al. (1988). Background I490 and I440 levels in the absence of dye were subtracted from the total I490 and I440 values.
In some experiments, pHi was measured using a fluorescence imaging system in which the photomultiplier tube mounted on the microscope was replaced with an intensified CCD camera (ICCD-350F; Video Scope Int., Ltd., Sterling, VA). The system software included custom program macros written by Dr. Michael Apkon (Department of Pediatrics, Yale University, New Haven, CT) for OPTIMAS (Optimas Co., Edmonds, WA).
In nine experiments on hippocampal astrocytes loaded with BCECF, applying 0.01% saponin caused the fluorescent signal to decrease to 4.3 ± 0.6% of the signal at the start of the experiment. Because 0.01% saponin is thought to permeabilize only the plasma membrane (Lin et al., 1990), we conclude that 96% of intracellular BCECF is located in the cytoplasm.
Intracellular Buffering Power
From experiments in which pHi recovered from acute acid loads, we computed acid extrusion as the product of the rate of change in pHi (dpHi/dt) and total intracellular buffering power (βT). Acid-extrusion rate (ϕE) thus has the units of moles per unit volume of cytoplasm per unit time (e.g., μM s−1). In the subsequent manuscript (Bevensee et al., 1997), ϕL refers to acid loading and has similar units. βT is the sum of the buffering power due to CO2/HCO3 − (βHCO3−) and the buffering power due to intrinsic intracellular buffers (βI). We computed the theoretical βHCO3− as 2.3 × [HCO3 −]. As recently confirmed by our laboratory (Zhao et al., 1995), the actual βHCO3− is indistinguishable from the theoretical value. We calculated βI in hippocampal astrocytes from the increases in pHi elicited by increasing the external concentration of the weak base NH3, as described by Roos and Boron (1981).
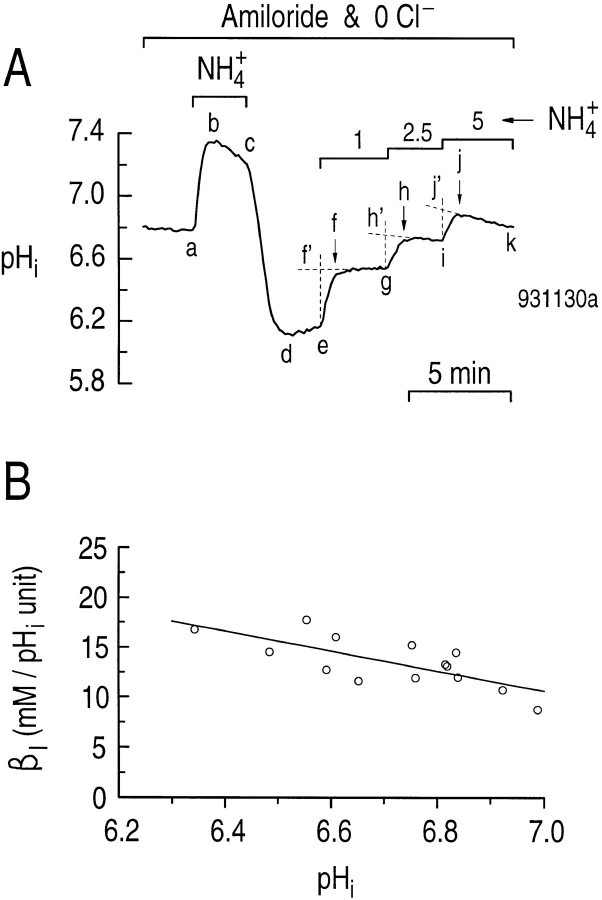
An example of an experiment for determining βI in a single hippocampal astrocyte is shown in Fig. 1 A. We added 0.9 mM amiloride to all solutions to inhibit the Na-H exchanger previously identified in these cells (Pappas and Ransom, 1993), and removed the Cl− to minimize potential Cl-base exchange (Vaughan-Jones, 1982). We acid loaded the astrocyte using the NH4 +-prepulse technique (Boron and De Weer, 1976). Briefly, exposing the cell to a solution containing 20 mM NH3/NH4 + caused pHi to increase rapidly as NH3 entered the cell and consumed H+ to form NH4 + (Fig. 1 A, ab). In the continued presence of the NH3/NH4 + solution, the pHi decreased more slowly due to NH4 + influx and/or stimulation of acid-loading mechanisms (Fig. 1 A, bc). When the NH3/NH4 + solution was removed, the pHi decreased rapidly to a value lower than at the start of the experiment (Fig. 1 A, cd). As discussed below, pHi normally recovers rapidly from such an acid load due to Na-H exchange. In Fig. 1 A, the pHi recovery was greatly slowed by amiloride (Fig. 1 A, de). Exposing the cell sequentially to solutions containing 1, 2.5, and 5 mM NH3/NH4 + caused pHi to increase in a stepwise fashion (Fig. 1 A, ef, gh, and ij, respectively). However, especially at higher pHi values, the rapid increase in pHi in response to elevations of external NH3/NH4 + was often followed by a slower decrease in pHi (e.g., Fig. 1 A, jk). Therefore, we back extrapolated the pHi vs. time record (see Fig. 1 A, f', h', and j') to determine more accurately the pHi changes due to the changes in NH3/ NH4 + (Aickin and Thomas, 1977). In Fig. 1 B, we plotted the results from five experiments similar to that shown in Fig. 1 A. βI is plotted as a function of the average pHi before and after a step change in NH3/NH4 +. βI is 10.5 mM (pH unit)−1 at pHi 7.0. The best-fit line to the data has a slope of −10.0 mM (pH unit)−2. Our βI values are similar to those reported by Pappas and Ransom (1994), although they did not observe a dependence of βI on pHi.
Figure 1.
NH3/NH4 + solutions can be used to calculate the pHi dependence of intrinsic buffering power (βI) in cultured astrocytes from the rat hippocampus. (A) The effect of step changes in [NH3/NH4 +] on pHi. A single astrocyte was bathed in a Cl−-free, HEPES-buffered solution containing 0.9 mM amiloride. After the cell was acid loaded by a brief exposure to 20 mM NH3/NH4 + (a–d), the cell was subsequently exposed to solutions containing 1, 2.5, and 5 mM NH3/NH4 +. (B) pHi dependence of intrinsic buffering power (βI) in rat hippocampal astrocytes.
Statistics
Data are reported as mean ± SEM. Means were compared using the paired and unpaired forms of the Student's t test (one-tail). P < 0.05 is considered significant. The pHi dependence of βI, as well as the SITS inhibition of the CO2/HCO3 −-induced alkalinization were fitted by a straight line using a least-squares method. Rates of change in pHi (dpHi/dt) were fitted by either a third- order polynomial or a straight line using a least-squares method.
RESULTS
Initial Steady State pHi in the Absence and Presence of CO2/HCO3 −
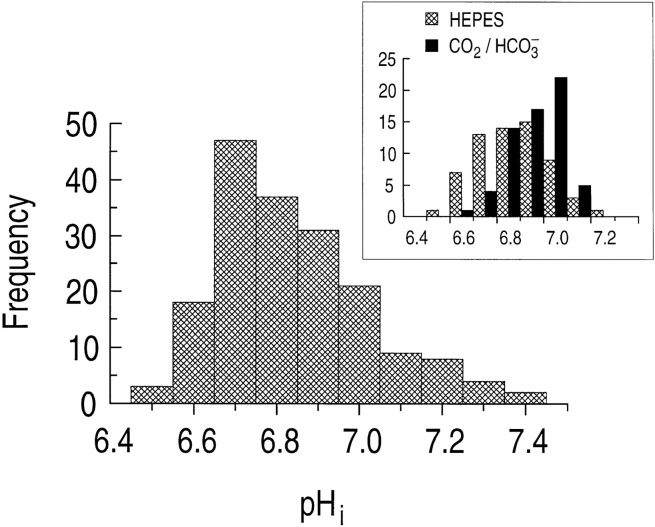
The astrocytes in this study, cultured from the rat hippocampus, had an average initial steady state pHi of 6.83 ± 0.01 (n = 180) when exposed to a nominally CO2/HCO3 −-free, HEPES-buffered solution (pH 7.3). The frequency distribution of these pHi values is slightly skewed to more alkaline values (Fig. 2). For 63 of these 180 astrocytes, we switched the extracellular solution to one buffered with 5% CO2/17 mM HCO3 − (pH 7.3). In the presence of CO2/HCO3 −, these astrocytes had a mean steady state pHi of 6.90 ± 0.01 (n = 63), 0.08 pH units higher, on average, than for the same cells in the absence of CO2/HCO3 − (6.82 ± 0.02). As indicated by the inset to Fig. 2, the frequency distribution of steady state pHi for the 63 astrocytes in the presence of CO2/ HCO3 − (solid bars) is shifted toward more alkaline pHi values, and has a smaller standard deviation than for cells in the absence of CO2/HCO3 − (hatched bars).
Figure 2.
The distribution of steady state pHi is bell-shaped for 180 hippocampal astrocytes bathed in a nominally CO2/HCO3 −-free, HEPES-buffered solution. The bin width is 0.1 pH unit. (inset) The distribution of steady state pHi of 63 astrocytes first exposed to HEPES is alkaline shifted when the cells are subsequently exposed to 5% CO2/17 mM HCO3 −.
pHi Recovery from an Acid Load in the Absence and Presence of CO2/HCO3 −
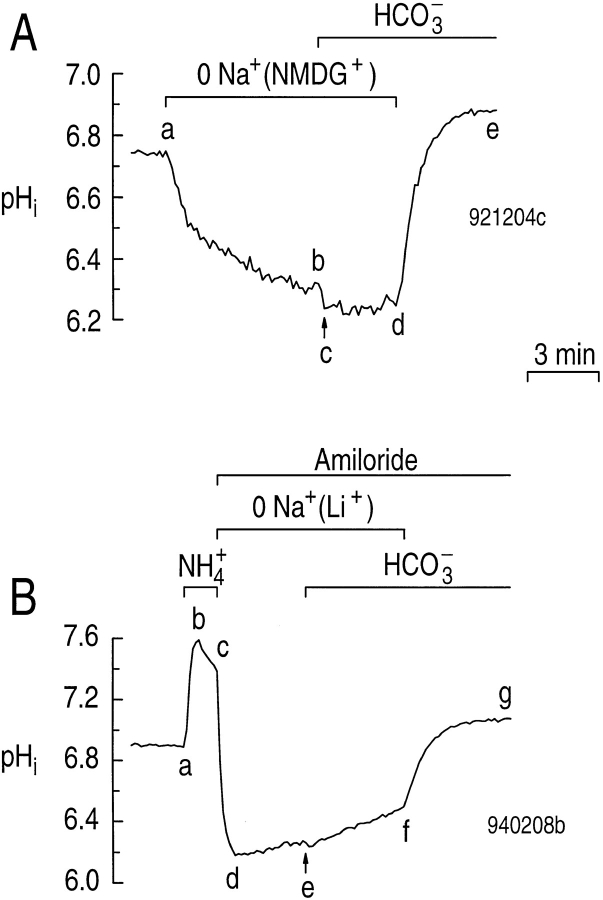
To study further the mechanisms by which astrocytes regulate pHi, we acid loaded cells using the NH4 +-prepulse technique (see methods). As shown in Fig. 3 A, when a single astrocyte in a HEPES-buffered solution was exposed to 20 mM NH3/NH4 +, pHi increased rapidly (Fig. 3 A, ab), and then declined more slowly (Fig. 3 A, bc). When the astrocyte was returned to the standard HEPES-buffered solution, the pHi sharply decreased (Fig. 3 A, cd), and then promptly recovered to a value similar to that at the start of the experiment (Fig. 3 A, de). A large component of the pHi recovery of segment de was likely due to a Na-H exchanger previously identified in rat hippocampal astrocytes (Pappas and Ransom, 1993). Indeed, when the same astrocyte was acid loaded a second time (Fig. 3 A, e–h), the pHi recovery was greatly slowed in the presence of 0.9 mM amiloride (Fig. 3 A, hi). The pHi recovery remaining in the presence of amiloride may reflect incomplete block of the Na-H exchanger by amiloride, or activity of an H+ pump (Pappas and Ransom, 1993). In cultured mouse astrocytes, Wuttke and Walz (1990) also observed an amiloride-insensitive, Na+- and HCO3 −-independent pHi recovery from an acid load, which may be due to acid efflux via lactate-H cotransport. Finally, switching the cell to a solution containing 5% CO2/17 mM HCO3 − led to a rapid pHi increase (Fig. 3 A, ij), even though the Na-H exchanger was inhibited by amiloride.
Figure 3.
CO2/HCO3 − stimulates the pHi recovery from an acid load when the Na-H exchanger is inhibited. (A) Effect of CO2/HCO3 − on the pHi recovery from an acid load in the presence of amiloride. The single astrocyte was acid loaded twice by a brief exposure to a solution containing 20 mM NH4Cl at a constant pHO of 7.3 (a–d and e–h). During the indicated periods, 0.9 mM amiloride was present (hj), and 5% CO2/17 mM HCO3 − was present (ij). (B) The pHi dependence of acid extrusion in a HEPES-buf-fered solution in the absence (circles) and presence (open diamonds) of 0.9 mM amiloride. In principle, the gap between the two groups of diamonds would have been filled had it been practical, in experiments such as that shown in A, to monitor the segment-hi pHi recovery (which generated the diamonds near pHi 6) until pHi had reached the second group of diamonds (near pHi 6.6). (inset) The pHi dependence of the amiloride-sensitive acid extrusion (closed diamonds), obtained by subtracting the acid extrusion rate in the presence from that in the absence of amiloride.
From the segment-de pHi recoveries in experiments similar to that shown in Fig. 3 A, we determined the pHi dependence of total acid extrusion in HEPES (ϕHEPES = dpHi /dt × βT; see methods) in the absence of amiloride and CO2/HCO3 − (“control” conditions). The pHi dependence of ϕHEPES is shown by the open circles in Fig. 3 B. We also determined the pHi dependence of acid extrusion in the presence of amiloride (ϕAmil) from the segment hi pHi increase observed in experiments similar to that shown in Fig. 3 A. These data are the group of three open diamonds near pHi 6 in Fig. 3 B. We also computed ϕAmil from the pHi decrease observed in other experiments in which we unmasked background acid loading by treating naive cells with 0.9 mM amiloride (data not shown). These data are the group of seven diamonds near pHi 6.6 in Fig. 3 B.
At a pHi of 6.05, ϕHEPES averaged 173 ± 12 μM s−1 (n = 19), whereas ϕAmil averaged only 20.1 ± 10.1 μM s−1 (n = 4). Therefore, at this pHi, 88% of ϕHEPES was amiloride sensitive in the nominal absence of CO2/ HCO3 −. The pHi dependence of the amiloride-sensitive acid extrusion rate (ϕAmil-sens), defined as ϕHEPES − ϕAmil, is shown in the inset to Fig. 3 B.
In six experiments in which pHi recovered in the presence of both amiloride and CO2/HCO3 − (e.g., Fig. 3 A, ij), acid extrusion increased 3.8-fold from 32.0 ± 12.9 μM s−1 (e.g., just before i) to 121 ± 29.2 μM s−1 (e.g., just after i) at a pHi of 6.20 ± 0.05 (P = 0.003). The higher βT in the presence of CO2/HCO3 − is factored into the above calculations. The data in Fig. 3 thus suggest that hippocampal astrocytes have as many as three acid-extrusion mechanisms: (a) an amiloride-sensitive Na-H exchanger (Fig. 3 A, de vs. hi); (b) possibly an amiloride-insensitive mechanism (e.g., H+ pump) that functions in the nominal absence of CO2/HCO3 − (Fig. 3 A, hi); and (c) a CO2/HCO3 −-dependent mechanism that can be observed even in the presence of amiloride (Fig. 3 A, ij).
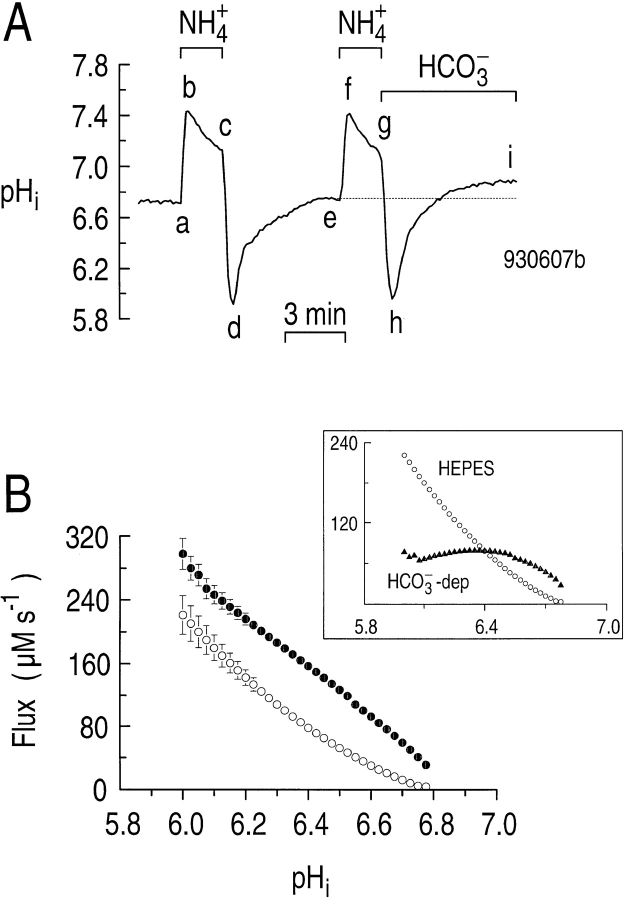
If astrocytes possess both a Na-H exchanger and a HCO3 − transporter, then the acid-extrusion rate should be greater in the presence than in the absence of CO2/ HCO3 −. In the experiment shown in Fig. 4 A, we acid loaded an astrocyte twice in a HEPES-buffered solution, using NH4 + prepulses (Fig. 4 A, a–d and e–h). As we removed the NH3/NH4 + the second time, we simultaneously introduced CO2/HCO3 −. Although the rates of pHi change do not appear substantially different for the two pHi recoveries, recall that βT is substantially higher in the presence of CO2/HCO3 −. Moreover, it is clear from Fig. 4 A that pHi recovered to a higher value in the presence vs. the absence of CO2/HCO3 − (compare points i and e). For six experiments similar to that shown in Fig. 4 A, we determined the pHi dependence of acid extrusion both in the absence (ϕHEPES, open circles) and presence (ϕHCO3, closed circles) of CO2/HCO3 − (Fig. 4 B). Over a wide range of pHi values, ϕE was as much as ∼80 μM s−1 higher in the presence than in the absence of CO2/HCO3 −. Viewed differently, CO2/ HCO3 − shifted the ϕE vs. pHi curve 0.15–0.3 pH units in the alkaline direction.
Figure 4.
During the recovery from an acid load, acid extrusion is greater in the presence than in the absence of CO2/HCO3 −. (A) Effect of CO2/HCO3 − on the pHi recovery from an acid load. An astrocyte was acid loaded twice by applying and withdrawing 20 mM NH3/NH4 + (a–d and e–h). When NH3/NH4 + was removed the second time, the cell was exposed to CO2/HCO3 − simultaneously (g–i). (B) The pHi dependence of acid extrusion in the absence (open circles) and presence (closed circles) of CO2/HCO3 −. The data were taken from experiments similar to those in A. (inset) The open symbols are a replot of the data obtained in HEPES. The closed triangles represent the HCO3 −-dependent component of the flux, obtained by subtracting the open circles from the closed circles in the main panel.
In the inset to Fig. 4 B, we replot the ϕHEPES data from the main portion of Fig. 4 B, and also plot the difference between ϕHCO3 and ϕHEPES, which is the HCO3 −- dependent flux (ϕHCO3−dep). As we saw in Fig. 3 B, ϕHEPES is the algebraic sum of HCO3 −-independent acid extrusion and acid loading mechanisms, and mainly reflects Na-H exchange. ϕHEPES is dominant at very low pHi values. Therefore, astrocytes in the intact brain may predominantly use non-HCO3 − transporters (e.g., Na-H exchange) to extrude acid under pathological conditions when pHi is below ∼6.4. Such low pHi values have been recorded in astrocytes of rat in vivo during conditions of hyperglycemia and ischemia (Kraig and Chesler, 1990). ϕHCO3−dep in Fig. 4 B is the algebraic sum of HCO3 −-dependent acid extrusion and acid loading mechanisms, and thus could underestimate the HCO3 − uptake mechanism. ϕHCO3−dep is dominant in the “normal” pHi range. Astrocytes in the intact brain may predominantly use HCO3 − transporters to extrude acid when pHi is above ∼6.4 (i.e., in a more physiological pH range). Astrocytes in vivo typically have a steady state pHi of ∼7.0 (see review by Chesler, 1990).
Further Characteristics of Na-H Exchange
EIPA also inhibits the Na-H exchanger.
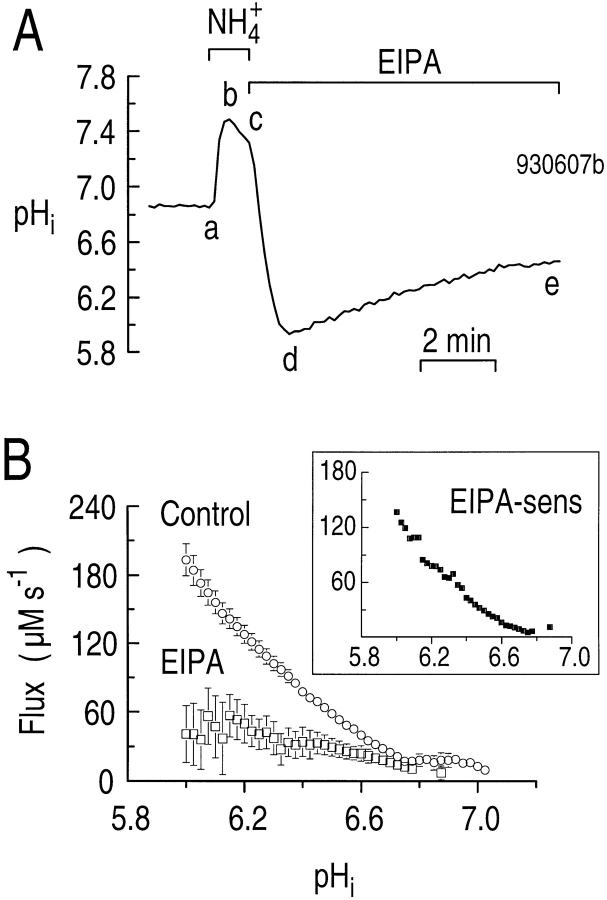
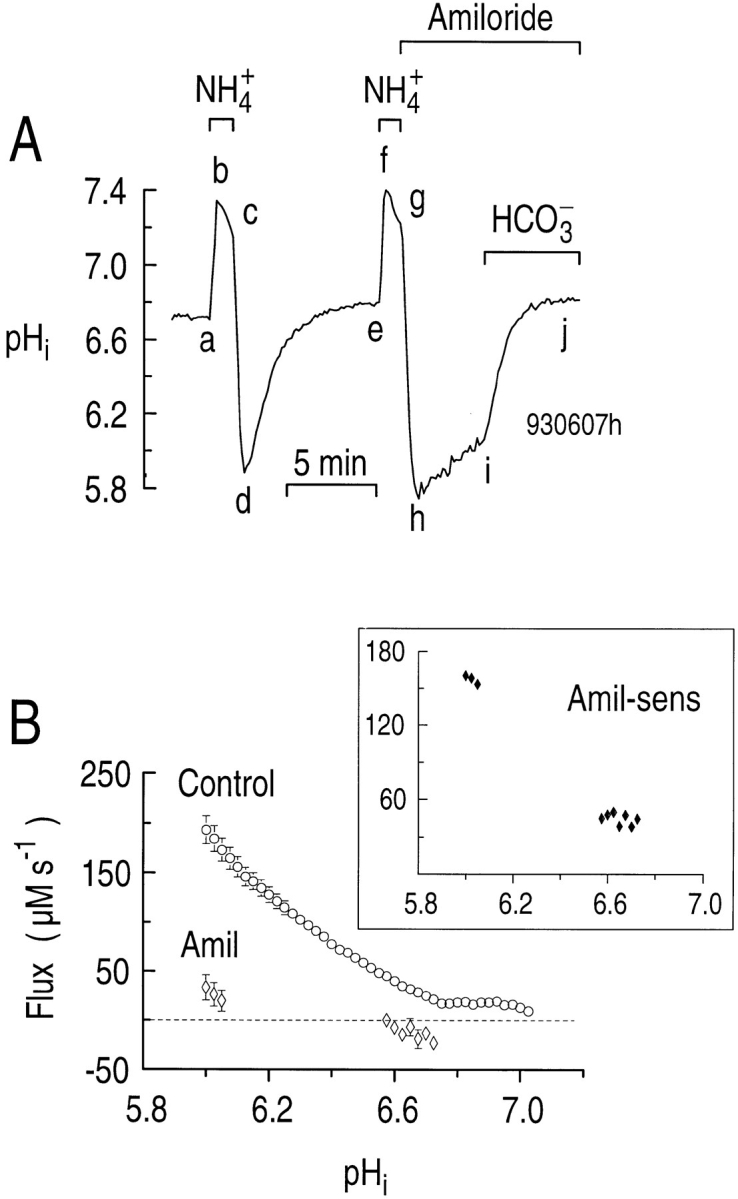
Na-H exchange isoforms can be distinguished by their level of sensitivity to both amiloride and amiloride analogues such as EIPA. To characterize the pharmacology of the Na-H exchanger present in rat hippocampal astrocytes further, we examined how EIPA affects the pHi dependence of acid extrusion in the nominal absence of CO2/HCO3 −. In the experiment represented in Fig. 5 A, we acid loaded an astrocyte by pulsing with 20 mM NH3/NH4 + (Fig. 5 A, a–d), and then monitored the pHi recovery with the cell exposed to 10 μM EIPA (Fig. 5 A, de). Using the segment-de pHi recoveries from 13 similar EIPA experiments and 26 control experiments, we plotted the pHi dependence of acid extrusion in the presence (ϕEIPA, Fig. 5 B, open squares) and absence (ϕHEPES, Fig. 5 B, circles) of EIPA. At a pHi of 6.05, EIPA decreased ϕE from 173 ± 11.7 μM s−1 (n = 19) to 35.9 ± 25.7 μM s−1 (n = 7). Therefore, at a pHi of 6.05, 79% of acid extrusion was inhibited by EIPA. In the four experiments discussed in Fig. 3 B, analyzed at this same pHi, amiloride decreased ϕE to 20.1 ± 10.1 μM s−1 (n = 4), a reduction of 88%. Thus, it seems that amiloride is similar to EIPA in inhibiting Na-H exchange in cultured hippocampal astrocytes. The inset to Fig. 5 B, a plot of the EIPA-sensitive component of ϕE (ϕEIPA-sens), confirms that Na-H exchange activity is highest at low pHi values and falls to nearly zero at a pHi of ∼6.8.
Figure 5.
The pHi recovery from an acid load in the nominal absence of CO2/HCO3 − is sensitive to EIPA. (A) Effect of EIPA on the pHi recovery from an acid load. An astrocyte was acid loaded by applying and withdrawing 20 mM NH3/NH4 + (a–d). EIPA (10 μM) was present during the pHi recovery (de). (B) The pHi dependence of acid extrusion in the absence (circles) and presence (open squares) of 10 μM EIPA. (inset) The pHi dependence of the EIPA-sensitive component of acid extrusion (closed squares). These data were obtained by subtracting the open squares in the main panel from the open circles.
Na-H exchanger can apparently exchange Li+ for H+.
Because Li+ is a substrate for the Na-H exchanger at the brush border of renal proximal tubules (Aronson, 1985), we tested if Li+ could substitute for Na+ on the Na-H exchanger of the astrocyte. In the experiment shown in Fig. 6, we acid loaded a single astrocyte twice by pulsing with 20 mM NH3/NH4 + (Fig. 6 A, a–d and f–i). During the pHi recovery from the first acid load (Fig. 6 A, de), when Li+ rather than Na+ was the major cation, pHi recovered more slowly than normal, and never reached the pHi that prevailed at the start of the experiment (compare Fig. 6 A, a and e). When we returned the Na+, pHi increased further (Fig. 6 A, ef), possibly because the Na-H exchanger has a greater Vmax for Na+ than for Li+ (Aronson, 1985). During the initial portion of the pHi recovery from the second acid load (Fig. 6 A, ij), when Li+ again was the major cation and the solution contained 0.9 mM amiloride, pHi increased very slowly. Removing the amiloride (Fig. 6 A, jk) caused pHi to increase nearly as fast as the recovery from the first acid load (Fig. 6 A, de). Returning the Na+ caused the pHi recovery rate to increase still further, and caused pHi to return to its initial level (compare Fig. 6 A, l and f).
Figure 6.
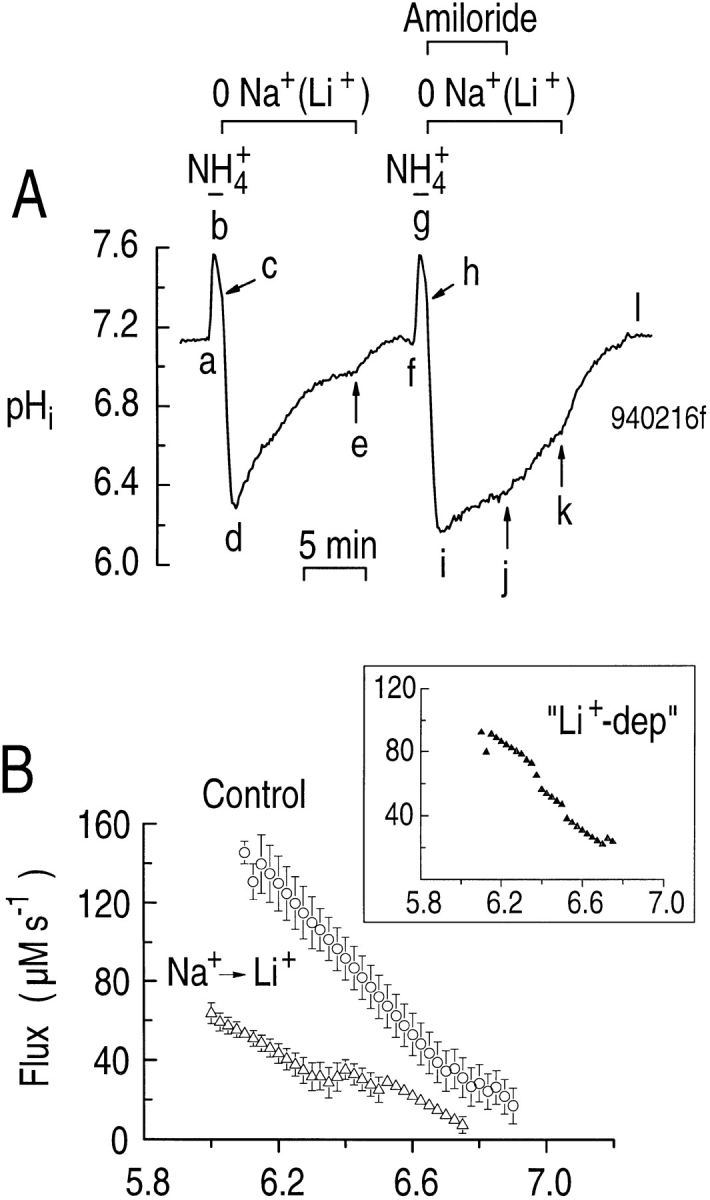
Li+ can exchange with H+ on the Na-H exchanger. (A) Effect of replacing Na+ with Li+ on the pHi recovery from an acid load. An astrocyte was acid loaded twice by applying and withdrawing 20 mM NH3/NH4 + (a–d and f–i). During the indicated times (c–e and h–k), Li+ instead of Na+ was the major extracellular cation. Amiloride (0.9 mM) was added immediately after the NH3/ NH4 + was removed (h–j). (B) The pHi dependence of acid extrusion when Na+ (circles) or Li+ (open triangles) is the major cation. (inset). The pHi dependence of the “Li+-dependent” acid extrusion (closed triangles). These data were obtained by subtracting the open triangles in the main panel from the open circles.
From segment-de data in five experiments, we determined the pHi dependence of acid extrusion during the recovery of pHi from an acid load when Li+ rather than Na+ was the major cation (Fig. 6 B, open triangles). For comparison, we also plot comparable control experiments performed on the same day (day-matched controls) in which Na+ was the major cation (Fig. 6 B, circles). The pHi dependence of the Li+-dependent component of acid extrusion (ϕLi-dep) is shown in the inset to Fig. 6 B. At all pHi values in the range studied, ϕLi-dep in the presence of Li+ was only 1/3 to 1/2 the value seen in the presence of Na+. For example, at a pHi of 6.23, acid extrusion was 125 ± 14 μM s−1 (n = 5) with Na+ as the dominant cation, but only 40.5 ± 5.4 μM s−1 (n = 5) with Li+ as the dominant cation (P < 0.0001). At this same pHi, amiloride (Fig. 6 A, ij) further reduced ϕE in the presence of Li+ to 11.4 ± 1.8 μM s−1 (n = 5), an inhibition of 72% (P = 0.003). Therefore, amiloride-sensitive Li-H exchange appears to contribute to the pHi recovery from an acid load when Li+ rather than Na+ is the major extracellular cation. Our data complement the finding that Li+ can exchange for H+ on the Na-H exchangers of both C6 and NN glial cell lines (Jean et al., 1986), as well as in cultured astrocytes from the cerebral cortex of the rat (Dixon and Wilson, 1995).
CO2/HCO3 −-induced Alkalinization
CO2/HCO3 − elicits an increase in the mean steady state pHi of astrocytes.
Because the pHi to which astrocytes recover after an acid load is higher in the presence than in the absence of CO2/HCO3 − (Fig. 4), one would predict that simply applying CO2/HCO3 − should increase steady state pHi. As shown in the experiment in Fig. 7 A, when a single astrocyte was switched from a HEPES-buffered to a CO2/HCO3 −-buffered solution, the pHi transiently fell, due to the influx of CO2 and production of intracellular H+ and HCO3 − (Fig. 7 A, ab), and then increased to a value higher than the initial one (Fig. 7 A, bc). When the astrocyte was returned to a HEPES-buffered solution, the opposite pHi changes occurred (Fig. 7 A, c–e). The mechanism of the segment- bc pHi increase is the subject of the rest of this paper, as well as the accompanying one (Bevensee et al., 1997). The mechanism of the segment-de decrease in Fig. 7 A is presently unknown. Some have suggested that a similar pHi decrease in other cells could be caused by Cl-HCO3 exchange, with the HCO3 − being provided by intracellular metabolism. However, as discussed in the accompanying paper (Bevensee et al., 1997), hippo-campal astrocytes do not appear to have appreciable Cl-HCO3 exchange activity. Another possibility is that the Fig. 7 A, de pHi decrease is mediated by a K/HCO3 cotransporter, similar to that described in squid axons (Hogan et al., 1995).
Figure 7.
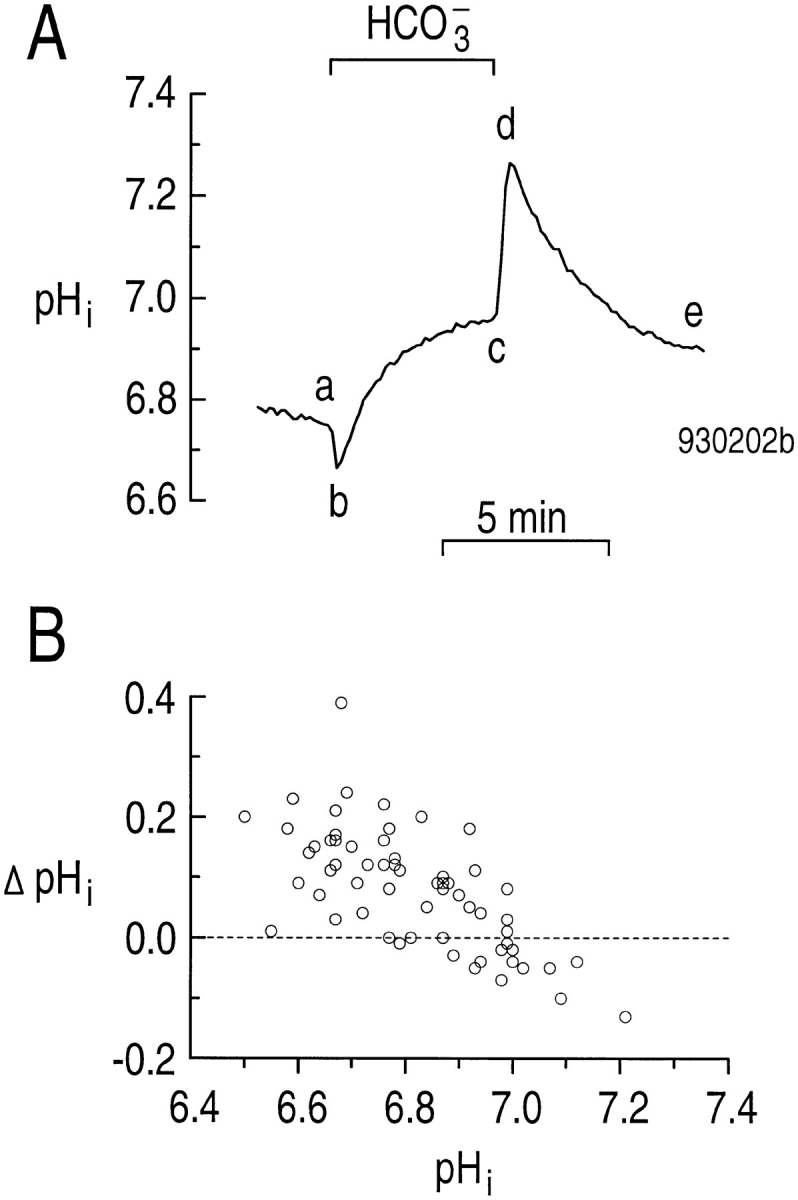
Mean steady state pHi increases when hippocampal astrocytes are switched from HEPES to CO2/HCO3 −. (A) Effect of CO2/HCO3 − on steady state pHi. During the indicated period, we switched to an extracellular solution that was buffered with 5% CO2/17 mM HCO3 − rather than HEPES (a–c). (B) The change in steady state pHi caused by adding CO2/HCO3 −, plotted as a function of steady state pHi in the HEPES-buffered solution.
As we described in connection with the inset to Fig. 2, we performed 63 experiments similar to that shown in Fig. 7 A, in which we switched astrocytes from a HEPES-buffered to a CO2/HCO3 −-buffered solution. The maximum ϕE at Fig. 7 A, b averaged 47.7 ± 2.4 μM s−1 at a pHi of 6.74 ± 0.02 (n = 63). In Fig. 7 B, we plot the difference between the steady state pHi in CO2/ HCO3 − (Fig. 7 A, c) and the steady state pHi in HEPES (Fig. 7 A, a) as a function of the initial steady state pHi of the 63 astrocytes in HEPES (Fig. 7 A, a). Astrocytes with relatively low initial pHi in HEPES are more likely to undergo a net increase in pHi, whereas those with high initial pHi are more likely to undergo a smaller increase in steady state pHi when exposed to CO2/ HCO3 −. In fact, for cells with an initial pHi in the range of 6.9–7.2, CO2/HCO3 − actually caused the steady state pHi to decrease in about half of the cells. This is why the distribution of steady state pHi in CO2/HCO3 − (Fig. 2, inset) is narrower and alkaline shifted compared with the distribution in HEPES.
Amiloride does not inhibit the CO2/HCO3 −-induced alkalinization.
One possible explanation for the CO2/ HCO3 −-induced alkalinization in Fig. 7 A, bc is that CO2/HCO3 − stimulates Na-H exchange, as has been postulated for the proximal tubule (Chen and Boron, 1995a , 1995b ). In six experiments in which the astrocytes had an average initial pHi of 6.78 ± 0.06 in a HEPES-buffered solution, adding 0.9 mM amiloride caused pHi to decrease to 6.66 ± 0.05 (data not shown), presumably because amiloride inhibited Na-H exchange that actively extruded acid in the steady state (see Fig. 3 B, inset). In contrast, adding 10 μM EIPA had no effect on the steady state pHi (n = 7, data not shown). Introducing CO2/HCO3 − in the continued presence of amiloride elicited a rapid, initial decrease in pHi, followed by a sustained increase (data not shown). In five experiments, the maximum acid extrusion rate was 89.3 ± 11.4 μM s−1 at a pHi of 6.60 ± 0.03 during the CO2/HCO3 −-induced alkalinization in the presence of amiloride. This average ϕE in the presence of amiloride is similar to ϕE in three day-matched control cells in the absence of amiloride (73.2 ± 4.8 μM s−1; P = 0.13) at a similar pHi of 6.67 ± 0.02. Therefore, even when Na-H exchange is substantially inhibited with amiloride, CO2/HCO3 − elicits an alkalinization in hippocampal astrocytes that have relatively low initial pHi.
Stilbene derivatives inhibit the CO2/HCO3 −-induced alkalinization.
Because the most likely explanation for the CO2/HCO3 −-induced alkalinization is stimulation of a HCO3 −-dependent acid extrusion mechanism, we tried inhibiting the alkalinization with stilbene derivatives that block HCO3 − transporters in other preparations. Fig. 8 A shows an experiment on a single hippocampal astrocyte exposed to CO2/HCO3 − in the presence of the stilbene derivative DIDS. Applying 400 μM DIDS in the nominal absence of CO2/HCO3 − elicited a sustained decrease in pHi (Fig. 8 A, ab). The mechanism of this DIDS-stimulated acidification is unknown. In principle, some of the pHi decrease could have been caused by inhibition of a HCO3 −-dependent acid extruder capable of using the small amount of HCO3 − (∼100 μM) present in the nominally CO2/HCO3 −-free solution. However, it seems unlikely that such a mechanism, when inhibited, could produce the rapid segment-ab decrease in pHi (Fig. 8 A). In the presence of DIDS, exposing the cell to CO2/HCO3 − caused a further acidification (Fig. 8 A, bc), followed by a slow recovery of pHi (Fig. 8 A, cd) that is probably mediated by the Na-H exchanger. In the continued presence of CO2/HCO3 −, removing DIDS relieved most of the inhibition, causing pHi to increase (Fig. 8 A, de). Removing the CO2/HCO3 − caused the usual series of pHi changes (Fig. 8 A, e–g). Summarizing mean data, switching astrocytes from HEPES to CO2/HCO3 − in the presence of 400 μM DIDS resulted in a mean ϕE of 12.8 ± 3.2 μM s−1 at a pHi of 6.79 ± 0.06 (n = 7). In day-matched control experiments in the absence of DIDS, ϕE was 51.0 ± 6.5 μM s−1 at a similar pHi of 6.85 ± 0.06 (n = 7). Therefore, 400 μM DIDS inhibited the CO2/ HCO3 −-induced acid extrusion by 75% (P < 0.0004).
Figure 8.
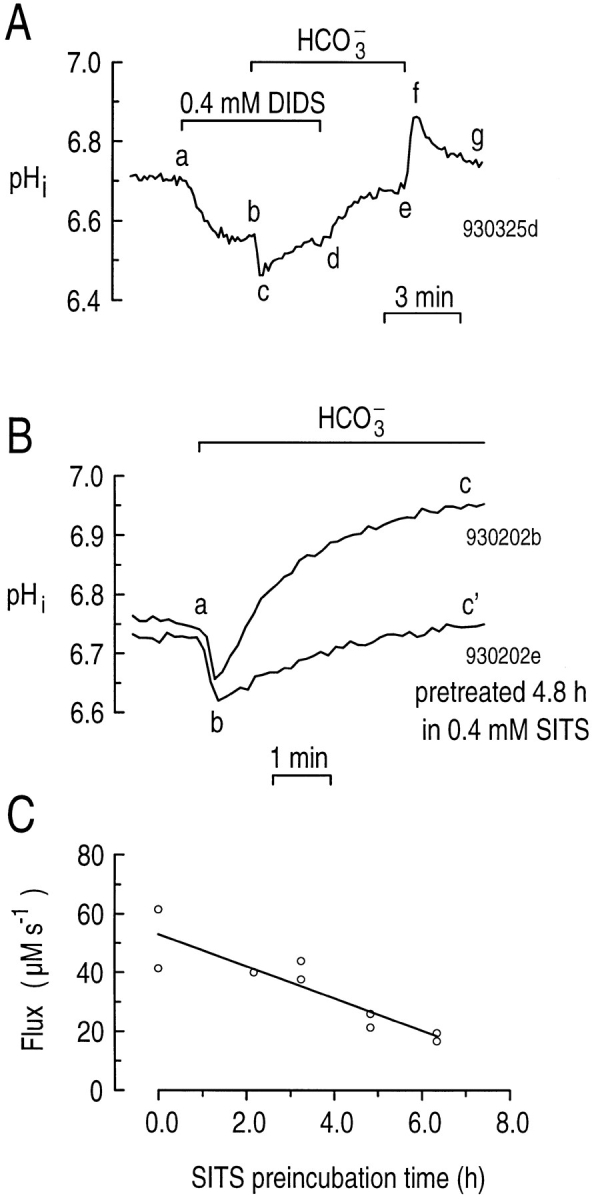
Stilbene derivatives partially inhibit the alkalinization when astrocytes are exposed to CO2/HCO3 −. (A) Inhibition by DIDS. At the indicated time, 400 μM DIDS was present in the extracellular solution (a–d). Between b and e, we switched the extracellular buffer from HEPES to 5% CO2/17 mM HCO3 −. (B) Inhibition by SITS. One of the two astrocytes was preincubated in 400 μM SITS for 4.8 h before the start of the experiment. Both cells were then exposed to CO2/HCO3 − (a–c and a–c'). (C) Maximum acid extrusion (i.e., at point b in B) as a function of the length of time astrocytes were preincubated in 400 μM SITS. All the experiments for this protocol were performed over a 2-d period. The best-fit line to the data has a slope of −5.5 μM s−1 h−1 and a y-intercept of 53.1 μM s−1.
We also determined whether SITS, another commonly used stilbene derivative, can inhibit the CO2/ HCO3 −-induced alkalinization. Because 400 μM SITS has considerable fluorescence when excited at 490 and 440 nm, we exposed astrocytes to SITS in the culture media for up to ∼6.3 h before experiments, and then washed away the drug.2 In mesangial cells, the Na+-driven Cl-HCO3 exchanger is irreversibly inhibited when exposed to SITS for 1–2 h before the start of an experiment (Boyarsky et al., 1988). Fig. 8 B illustrates two day-matched experiments on single hippocampal astrocytes, one of which we pretreated with 400 μM SITS for ∼4.8 h before the experiment. In the control cell, CO2/HCO3 − elicited an initial decrease in pHi (Fig. 8 B, ab), followed by the expected increase (Fig. 8 B, bc). In the SITS-pretreated cell, the initial pHi (before point a) was similar to that in the control cell, but the rate of pHi increase (Fig. 8 B, bc' ) was much slower. From experiments similar to those shown in Fig. 8 B, we plotted the maximum ϕE (pHi 6.55–6.75) for nine astrocytes exposed to CO2/HCO3 − (point b) as a function of the time cells were pretreated with 400 μM SITS (Fig. 8 C). Compared with ϕE in astrocytes not treated with SITS (51.4 μM s−1, n = 2), ϕE in cells treated with SITS for ∼6.3 h (18.1 μM s−1, n = 2) was inhibited 65%.
CO2/HCO3 −-induced alkalinization requires external Na+.
Because several HCO3 −-dependent acid extruders also require Na+, we performed experiments similar to those shown in Fig. 9 A to determine if Na+ is required for the CO2/HCO3 −-induced alkalinization in single hippocampal astrocytes. When we replaced external Na+ with NMDG+ in a HEPES-buffered solution, pHi rapidly decreased (Fig. 9 A, ab), presumably due at least in part to inhibition and/or reversal of Na-H exchange. Reversal of Na-Ca exchange, by elevating intracellular Ca2+ and displacing H+ from Ca2+ buffers, might also cause pHi to decrease (Meech and Thomas, 1977). Switching to a CO2/HCO3 − buffer in the continued absence of external Na+ caused pHi to decrease further by ∼0.06 (Fig. 9 A, bc). This pHi decrease is small compared with Fig. 7, ab because the low pHi in 0 Na+ is closer to the pK of carbonic acid. However, there was no CO2/HCO3 −-induced alkalinization (Fig. 9 A, cd). Switching the cell to a solution containing both Na+ and CO2/HCO3 − elicited a rapid increase in pHi to a value above that in the HEPES-buffered solution (compare Fig. 9 A, e and a). In five of six such experiments, removing extracellular Na+ (Fig. 9 A, ab) caused pHi to decrease from an average of 6.84 ± 0.08 to 6.50 ± 0.14. In one of the six experiments, the pHi did not change appreciably when the cell was exposed to the Na+-free solution. Exposing the six cells to CO2/ HCO3 − in the continued absence of external Na+ (Fig. 9 A, bc) caused a further decrease in the mean pHi to 6.45 ± 0.11. The mean ϕE computed for Fig. 9 A, cd was only 4.0 ± 4.4 μM s−1, compared with the maximum ϕE of 47.7 ± 2.4 μM s−1 at a pHi of 6.74 ± 0.02 (n = 63) in experiments in which we added the CO2/HCO3 − in the presence of Na+ (e.g., Fig. 7 A, bc). Because removing external Na+ elicited a large decrease in pHi, it was not possible to compare ϕE values in the presence and absence of Na+ at the same pHi. However, because other HCO3 −-dependent acid extruders are stimulated by low pHi, we would have expected ϕE to be even larger at lower pHi values prevailing in the presence of external Na+. Therefore, the CO2/HCO3 −-induced alkalinization requires external Na+.
Figure 9.
The HCO3 − transporter is blocked when Na+ is replaced with NMDG+ or Li+. (A) The effect of replacing Na+ with NMDG+ on the CO2/HCO3 −-induced alkalinization. Between a and d, we replaced the extracellular Na+ with NMDG+. During b–e, we switched the extracellular solution to one buffered with 5% CO2/17 mM HCO3 −. (B) The effect of replacing Na+ with Li+ on the CO2/HCO3 −-induced pHi recovery from an acid load in the presence of amiloride. We exposed the astrocyte to 20 mM NH3/ NH4 + during a–c. Amiloride (0.9 mM) was present between c and g. Between c and f, Li+ replaced extracellular Na+. Finally, between e and g, the extracellular buffer was 5% CO2/17 mM HCO3 −.
As discussed above, Li+ can partially substitute for Na+ on the Na-H exchanger in hippocampal astrocytes. To see if Li+ could substitute for Na+ on the transporter responsible for the CO2/HCO3 −-induced alkalinization, we performed the experiment shown in Fig. 9 B. Our approach was to use an NH4 + prepulse to acid load an astrocyte in a CO2/HCO3 −-free solution (Fig. 9 B, a–d), driving pHi to a value similar to that prevailing in the absence of Na+ (Fig. 9 A, b). With amiloride present and Li+ as the dominant extracellular cation, pHi recovered very slowly in the absence of CO2/ HCO3 − (Fig. 9 A, de), presumably because the Na-H exchanger was almost completely blocked. Switching the cell to a solution buffered with CO2/HCO3 − increased the pHi recovery rate only slightly (Fig. 9 A, ef). However, when we replaced the Li+ with Na+, pHi rapidly increased (Fig. 9 A, fg) to a value higher than the pHi at the start of the experiment (compare Fig. 9 A, g and a). In five experiments similar to that shown in Fig. 9 B, adding CO2/HCO3 − in the continued presence of amil-oride and Li+ increased ϕE only slightly, from 11.8 ± 1.6 μM s−1 (Fig. 9 B, de) to 20.0 ± 1.8 μM s−1 (Fig. 9 B, ef) at a pHi of 6.23 (P = 0.02). On the other hand, returning Na+ caused the mean ϕE to increase approximately sevenfold, to 145 ± 18 μM s−1 (Fig. 9 B, fg ; P < 0.001). Therefore, Li+ is a poor Na+ substitute on the transporter responsible for the CO2/HCO3 −-induced alkalinization in hippocampal astrocytes.
DISCUSSION
Properties of the Na-H Exchanger in Hippocampal Astrocytes
Na-H exchanger mediates the pHi recovery from an acid load and contributes to the steady state pHi.
In this manuscript, we have investigated the major acid extruders responsible for regulating pHi in astrocytes cultured from the hippocampus of the rat. Although we have focused predominantly on a HCO3 −-dependent acid extruder, we have also further characterized the Na-H exchanger that mediates almost the entire pHi recovery from an acid load in the nominal absence of CO2/HCO3 − (Pappas and Ransom, 1993). We found that, at pHi 6.05, amiloride inhibits 88% of acid extrusion during the pHi recovery from an acid load. Moreover, our observation that applying amiloride leads to a decrease in steady state pHi implies that the Na-H exchanger contributes to maintaining the steady state pHi of the astrocytes, at least in the nominal absence of CO2/HCO3 −. In three experiments (not shown) on cells exposed to CO2/ HCO3 −, we found that removing amiloride caused pHi to increase by 0.03 ± 0.01, consistent with a modest role for the Na-H exchanger in maintaining steady state pHi even in the presence of CO2/HCO3 −. Recently, Pizzonia et al. (1996) demonstrated that rat hippocampal astrocytes express the Na-H exchanger, NHE-1.
Although 10 μM EIPA inhibits the pHi recovery from an acid load, it does not lower steady state pHi.
Because Na-H exchangers typically are sensitive to amiloride analogues such as EIPA, we studied the effect of EIPA both on the pHi recovery from an acid load and on the steady state pHi in hippocampal astrocytes. At very low pHi values (i.e., 6.05), 10 μM EIPA inhibited the pHi recovery from an acid load about as well as amiloride (79 vs. 88%). On the other hand, at progressively higher pHi values, the amiloride-sensitive flux (Fig. 3 B, inset) fell towards zero more gradually than the EIPA-sensitive flux (Fig. 5 B, inset). Indeed, applying amiloride to a naive cell consistently elicited a pHi decrease, presumably because it inhibited Na-H exchange and unmasked background acid loading, whereas applying EIPA did not. One explanation for these findings is that the astrocytes have two Na-H exchangers, one of which is less EIPA sensitive, particularly at high pHi. Another explanation is that a single Na-H exchanger has a differential sensitivity to amiloride and EIPA that becomes especially apparent at high pHi. A third possibility is that, at high pHi, 10 μM EIPA simultaneously blocks Na-H exchange and alkalinizes the cell by an independent mechanism. Indeed, high levels of EIPA (i.e., 50 μM) elicit paradoxical alkalinizations in NIH-3T3 fibroblasts (Kaplan and Boron, 1994), rat osteoclasts (Ravesloot et al., 1995), and rat hippocampal CA1 neurons (Bevensee et al., 1996), as well as cultured astrocytes from the forebrain (Boyarsky et al., 1993; Bevensee, M.O., G. Frey, and W.F. Boron, unpublished data) and hippocampus (Pizzonia et al., 1996).
Li+ can substitute for Na+ and exchange with H+ on the Na-H exchanger.
The astrocyte Na-H exchanger appears to exchange Na+ for H+ approximately fourfold faster than it exchanges Li+ for H+. In apical membranes of renal proximal tubules, the Vmax for Li-H exchange is less than for Na-H exchange, although the affinity for Li-H exchange is higher (see review by Aronson, 1985). In NN and C6 glioma cells, the K1/2 for Na+ activation of the EIPA-sensitive 22Na+ uptake was 17 and 50 mM, respectively. However, the K1/2 for Li+ inhibition of the EIPA-sensitive 22Na+ uptake was only 5 and 9 mM, respectively (Jean et al., 1986).
Evidence for a Na+-driven, HCO3 −-dependent Acid Extruder in Hippocampal Astrocytes
Five observations suggest that hippocampal astrocytes possess a Na+-driven, HCO3 −-dependent acid extruder. (a) Acid extrusion during the recovery from an acid load is greater in the presence than in the absence of a CO2/HCO3 −-buffered solution (Fig. 4). (b) When the pHi recovery from an acid load is inhibited by amiloride in the nominal absence of CO2/HCO3 −, adding CO2/HCO3 − stimulates pHi recovery (Fig. 3 A). (c) When astrocytes are switched from a solution buffered with HEPES to one buffered with CO2/HCO3 −, the average steady state pHi increases (Fig. 7). (d) The CO2/ HCO3 −-induced alkalinization is inhibited by the HCO3 −-transport inhibitors DIDS (Fig. 8 A) and SITS (Fig. 8, B and C), but not by the Na-H exchange inhibitor amiloride. (e) The CO2/HCO3 −-induced alkalinization requires external Na+ (Fig. 9). These data are consistent with the Na+-driven HCO3 − transporter being either a Na+-driven Cl-HCO3 exchanger, or a Na/ HCO3 cotransporter. We address these possibilities in the subsequent manuscript (Bevensee et al., 1997).
Acknowledgments
We thank Dr. W. Knox Chandler for evaluating the manuscript and providing useful suggestions.
This work was supported by National Institutes of Health Program Project Grant PO1HD32573. M.O. Bevensee was supported by a predoctoral training grant (5-T32-GM0752718).
Footnotes
Portions of this work have been published in preliminary form (Bevensee, M.O., R.A. Weed, and W.F. Boron. 1993. FASEB J. 7:A186.).
Abbreviations used in this paper: BCECF-AM, acetoxymethyl ester of the pH-sensitive dye 2′,7′-biscarboxyethyl-5,6-carboxyfluorescein; DIDS, 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid; EIPA, ethylisopropylamiloride; NMDG+, N-methyl-d-glucammonium; pHECF, pH of the brain extracellular fluid; SITS, 4-acetamido-4′-isothiocyanatostilbene-2,2′-disulfonic acid.
We noted that SITS-treated astrocytes assumed a more spherical shape, rather than the flat polygonal shape that characterized the other cells in this study.
REFERENCES
- Aickin CC, Thomas RC. Microelectrode measurement of the intracellular pH and buffering power of mouse soleus muscle fibres. J Physiol. 1977;267:791–810. doi: 10.1113/jphysiol.1977.sp011838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson PS. Kinetic properties of the plasma membrane Na+-H+exchanger. Annu Rev Physiol. 1985;47:545–560. doi: 10.1146/annurev.ph.47.030185.002553. [DOI] [PubMed] [Google Scholar]
- Astion ML, Orkand RK. Electrogenic Na+/HCO3 −cotransport in neuroglia. Glia. 1988;1:355–357. doi: 10.1002/glia.440010508. [DOI] [PubMed] [Google Scholar]
- Bevensee MO, Apkon M, Boron WF. Intracellular pH regulation in cultured astrocytes from rat hippocampus. II. Electrogenic Na/HCO3cotransport. J Gen Physiol. 1997;110:000–000. doi: 10.1085/jgp.110.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevensee MO, Cummins TR, Haddad GG, Boron WF, Boyarsky G. pH regulation in single CA1 neurons acutely isolated from the hippocampi of immature and mature rats. J Physiol. 1996;494:315–328. doi: 10.1113/jphysiol.1996.sp021494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron WF, Boulpaep EL. Intracellular pH regulation in the renal proximal tubule of the salamander: basolateral HCO3 −transport. J Gen Physiol. 1983;81:53–94. doi: 10.1085/jgp.81.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron WF, De Weer P. Intracellular pH transients in squid giant axons caused by CO2, NH3, and metabolic inhibitors. J Gen Physiol. 1976;67:91–112. doi: 10.1085/jgp.67.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyarsky G, Ganz MB, Sterzel B, Boron WF. pH regulation in single glomerular mesangial cells. I. Acid extrusion in absence and presence of HCO3 − . Am J Physiol. 1988;255:C844–C856. doi: 10.1152/ajpcell.1988.255.6.C844. [DOI] [PubMed] [Google Scholar]
- Boyarsky G, Ransom B, Schlue W-R, Davis MBE, Boron WF. Intracellular pH regulation in single cultured astrocytes from rat forebrain. Glia. 1993;8:241–248. doi: 10.1002/glia.440080404. [DOI] [PubMed] [Google Scholar]
- Brune T, Fetzer S, Backus KH, Deitmer JW. Evidence for electrogenic Na/HCO3cotransport in cultured rat cerebellar astrocytes. Pflügers Archiv. 1994;429:64–71. doi: 10.1007/BF02584031. [DOI] [PubMed] [Google Scholar]
- Chen LK, Boron WF. Acid extrusion in S3 segment of rabbit proximal tubule I. Effect of bilateral CO2/HCO3 − . Am J Physiol. 1995a;268:F179–F192. doi: 10.1152/ajprenal.1995.268.2.F179. [DOI] [PubMed] [Google Scholar]
- Chen LK, Boron WF. Acid extrusion in S3 segment of rabbit proximal tubule. II. Effect of basolateral CO2/HCO3 − . Am J Physiol. 1995b;268:F193–F203. doi: 10.1152/ajprenal.1995.268.2.F193. [DOI] [PubMed] [Google Scholar]
- Chesler M. The regulation and modulation of pH in the nervous system. Prog Neurobiol (Oxf) 1990;34:401–427. doi: 10.1016/0301-0082(90)90034-e. [DOI] [PubMed] [Google Scholar]
- Chesler M, Kaila K. Modulation of pH by neuronal activity. TINS (Trends Neurosci) 1992;15:396–402. doi: 10.1016/0166-2236(92)90191-a. [DOI] [PubMed] [Google Scholar]
- Chow SY, Yen-Chow YC, White HS, Woodbury DM. pH regulation after acid load in primary cultures of mouse astrocytes. Dev Brain Res. 1991;60:69–78. doi: 10.1016/0165-3806(91)90156-d. [DOI] [PubMed] [Google Scholar]
- Deitmer JW. Bicarbonate-dependent changes of intracellular sodium and pH in identified leech glial cells. Pflügers Arch. 1992;420:584–589. doi: 10.1007/BF00374637. [DOI] [PubMed] [Google Scholar]
- Deitmer JW, Rose CR. pH regulation and proton signalling by glial cells. Prog Neurobiol (Oxf) 1996;48:73–103. doi: 10.1016/0301-0082(95)00039-9. [DOI] [PubMed] [Google Scholar]
- Deitmer JW, Schlue W-R. The regulation of intracellular pH by identified glial cells and neurones in the central nervous system of the leech. J Physiol (Camb) 1987;388:261–283. doi: 10.1113/jphysiol.1987.sp016614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitmer JW, Schlue W-R. An inwardly directed electrogenic sodium-bicarbonate cotransport in leech glial cells. J Physiol (Camb) 1989;411:179–194. doi: 10.1113/jphysiol.1989.sp017567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon, S.J., and J.X. Wilson. 1995. Fluorescence measurement of cytosolic pH in cultured rodent astrocytes. In Methods in Neurosciences. Vol. 27. J. Kraicer and S.J. Dixon, editors. Academic Press, Inc., San Diego, CA. 196–213.
- Hogan EM, Cohen MA, Boron WF. K+ and HCO3 −-dependent acid–base transport in squid giant axons: base efflux. J Gen Physiol. 1995;106:821–844. doi: 10.1085/jgp.106.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean T, Frelin C, Vigne P, Lazdunski M. The Na/H exchange system in glial cell lines. Eur J Biochem. 1986;160:211–219. doi: 10.1111/j.1432-1033.1986.tb09959.x. [DOI] [PubMed] [Google Scholar]
- Kaila K, Panula P, Karhunen T, Heinonen E. Fall in intracellular pH mediated by GABAAreceptors in cultured rat astrocytes. Neurosci Lett. 1991;126:9–12. doi: 10.1016/0304-3940(91)90358-z. [DOI] [PubMed] [Google Scholar]
- Kaplan D, Boron WF. Long-term expression of c-ras stimulates Na-H and Na+-dependent Cl−-HCO3 −exchange in NIH-3T3 fibroblasts. J Biol Chem. 1994;269:4116–4124. [PubMed] [Google Scholar]
- Kraig RP, Chesler M. Astrocytic acidosis in hyperglycemic and complete ischemia. J Cereb Blood Flow Metab. 1990;10:104–114. doi: 10.1038/jcbfm.1990.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A, Krockmalnic G, Penman S. Imaging cytoskeleton-mitochondrial membrane attachments by embedment-free electron microscopy of saponin-extracted cells. Proc Natl Acad Sci USA. 1990;87:8565–8569. doi: 10.1073/pnas.87.21.8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissues. J Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech RW, Thomas RC. The effect of calcium injection on the intracellular sodium and pH of snail neurones. J Physiol (Oxf) 1977;265:867–879. doi: 10.1113/jphysiol.1977.sp011749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody W., Jr Effects of intracellular H+on the electrical properties of excitable cells. Annu Rev Neurosci. 1984;7:257–278. doi: 10.1146/annurev.ne.07.030184.001353. [DOI] [PubMed] [Google Scholar]
- Morrow, J.S., R.S. Gurd, and F.R.N. Gurd. 1974. The chemical basis and possible role of carbamino homeostatic mechanisms. In Peptides, Polypeptides, and Proteins. E.R. Blout, F.A. Bovey, M. Goodman, and N. Lotan, editors. John Wiley & Sons Inc., New York. 594–604.
- Munsch T, Deitmer JW. Sodium-bicarbonate cotransport current in identified leech glial cells. J Physiol (Oxf) 1994;474:43–53. doi: 10.1113/jphysiol.1994.sp020001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA. Sodium-bicarbonate cotransport in retinal Müller (glial) cells of the salamander. J Neurosci. 1991;11:3972–3983. doi: 10.1523/JNEUROSCI.11-12-03972.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA, Astion ML. Localization and stoichiometry of electrogenic sodium bicarbonate cotransport in retinal glial cells. Glia. 1991;4:424–428. doi: 10.1002/glia.440040411. [DOI] [PubMed] [Google Scholar]
- O'Connor ER, Sontheimer H, Ransom BR. Rat hippocampal astrocytes exhibit electrogenic sodium-bicarbonate co-transport. J Neurophysiol. 1994;72:2580–2589. doi: 10.1152/jn.1994.72.6.2580. [DOI] [PubMed] [Google Scholar]
- Pappas CA, Ransom BR. A depolarization-stimulated, bafilomycin-inhibitable H+pump in hippocampal astrocytes. Glia. 1993;9:280–291. doi: 10.1002/glia.440090406. [DOI] [PubMed] [Google Scholar]
- Pappas CA, Ransom BR. Depolarization-induced alkalinization (DIA) in rat hippocampal astrocytes. J Neurophysiol. 1994;72:2816–2826. doi: 10.1152/jn.1994.72.6.2816. [DOI] [PubMed] [Google Scholar]
- Pizzonia JH, Ransom BR, Pappas CR. Characterization of Na+/H+exchange activity in cultured rat hippocampal astrocytes. J Neurosci Res. 1996;44:191–198. doi: 10.1002/(SICI)1097-4547(19960415)44:2<191::AID-JNR12>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Ransom, B.R. 1992. Glial modulation of neural excitability mediated by extracellular pH: a hypothesis. In Progress Brain Research. Vol. 94. A. Yu, L. Hertz, M. Norenberg, E. Sykova, and S. Waxman, editors. Elsevier Science, Amsterdam, Netherlands. 37– 46. [DOI] [PubMed]
- Ravesloot JH, Eisen T, Baron R, Boron WF. Role of Na-H exchangers and vacuolar H+pumps in intracellular pH regulation in neonatal rat osteoclasts. J Gen Physiol. 1995;105:177–207. doi: 10.1085/jgp.105.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos A, Boron WF. Intracellular pH. Physiol Rev. 1981;61:296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Soleimani M, Grassl SM, Aronson PS. Stoichiometry of Na+-HCO3 −cotransport in basolateral membrane vesicles isolated from rabbit renal cortex. J Clin Invest. 1987;79:1276–1280. doi: 10.1172/JCI112948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatkowski M, Schlue W-R. Mechanisms of pH recovery from intracellular acid loads in the leech connective glial cell. Glia. 1992;5:193–200. doi: 10.1002/glia.440050305. [DOI] [PubMed] [Google Scholar]
- Tang C-M, Dichter M, Morad M. Modulation of the N-methyl-d-aspartate channel by extracellular H+ . Proc Natl Acad Sci USA. 1990;87:6445–6449. doi: 10.1073/pnas.87.16.6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JA, Buchsbaum RN, Zimniak A, Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979;81:2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Cull-Candy SG. Proton inhibition of N-methyl-d-aspartate receptors in cerebellar neurons. Nature (Lond) 1990;345:347–350. doi: 10.1038/345347a0. [DOI] [PubMed] [Google Scholar]
- Vaughan-Jones, R.D. 1982. Chloride-bicarbonate exchange in the sheep cardiac Purkinje fibre. In Intracellular pH: Its Measurement, Regulation and Utilization in Cellular Function. Vol. 15. R. Nuccitelli and D.W. Deamer, editors. Alan R. Liss, Inc., New York. 239–252.
- Wuttke WA, Walz W. Sodium- and bicarbonate-independent regulation of intracellular pH in cultured mouse astrocytes. Neurosci Lett. 1990;117:105–110. doi: 10.1016/0304-3940(90)90127-u. [DOI] [PubMed] [Google Scholar]
- Zhao J, Hogan EM, Bevensee MO, Boron WF. Out-of-equilibrium CO2/HCO3 −- solutions and their use in characterizing a new K/HCO3cotransporter. Nature (Lond) 1995;374:636–639. doi: 10.1038/374636a0. [DOI] [PubMed] [Google Scholar]