Abstract
The role of the voltage sensor positive charges in fast and slow inactivation of the rat brain IIA sodium channel was investigated by mutating the second and fourth conserved positive charges in the S4 segments of all four homologous domains. Both charge-neutralizing mutations (by glutamine substitution) and charge-conserving mutations were constructed in a cDNA encoding the sodium channel α subunit. To determine if fast inactivation altered the effects of the mutations on slow inactivation, the mutations were also constructed in a channel that had fast inactivation removed by the incorporation of the IFMQ3 mutation in the III–IV linker (West, J.W., D.E. Patton, T. Scheuer, Y. Wang, A.L. Goldin, and W.A. Catterall. 1992. Proc. Natl. Acad. Sci. USA. 89:10910– 10914). Most of the mutations shifted the v1/2 of fast inactivation in the negative direction, with the largest effects resulting from mutations in domains I and II. These shifts were in the opposite direction compared with those observed for activation. The effects of the mutations on slow inactivation depended on whether fast inactivation was intact or not. When fast inactivation was eliminated, most of the mutations resulted in positive shifts in the v1/2 of slow inactivation. The largest effects again resulted from mutations in domains I and II. When fast inactivation was intact, the mutations in domains II and III resulted in negative shifts in the v1/2 of slow inactivation. Neutralization of the fourth charge in domain I or II resulted in the appearance of a second component in the voltage dependence of slow inactivation that was only observable when fast inactivation was intact. These results suggest the S4 regions of all four domains of the sodium channel are involved in the voltage dependence of inactivation, but to varying extents. Fast inactivation is not strictly coupled to activation, but it derives some independent voltage sensitivity from the charges in the S4 domains. Finally, there is an interaction between the fast and slow inactivation processes.
Keywords: voltage sensor, slow inactivation, expression, mutagenesis
INTRODUCTION
The voltage-gated sodium channel is essential for the initiation of action potentials in most electrically excitable cells. The channel undergoes at least two distinct gating events, voltage-dependent activation and fast inactivation. Fast inactivation is thought to have only slight voltage dependence, with the apparent voltage dependence resulting from the activation process (Bezanilla and Armstrong, 1977; Nonner, 1980; Aldrich and Stevens, 1987; Zagotta and Aldrich, 1990). Part of the evidence for the lack of voltage dependence is that fast inactivation is not associated with any component of gating current, although it does immobilize about two-thirds of the activation gating charge (Armstrong and Bezanilla, 1977). Fast inactivation is thought to occur by a ball-and-chain–type mechanism (Armstrong and Bezanilla, 1977), in which a portion of the channel on the cytoplasmic side occludes the pore after activation. This model has been verified for the Shaker potassium channel, in which the amino terminus functions as the inactivating particle (Hoshi et al., 1990; Zagotta et al., 1990). In the sodium channel, the cytoplasmic linker connecting domains III and IV is essential for fast inactivation, and it has been proposed that this linker functions as the inactivating particle (Patton et al., 1992; West et al., 1992).
In addition to fast inactivation that occurs over milliseconds, sodium channels can be inactivated by processes that occur over a much longer time scale, from hundreds of milliseconds to minutes (Simoncini and Stühmer, 1987; Ruff et al., 1987, 1988; Ruben et al., 1990). Slow inactivation can be a critical determinant of membrane excitability by decreasing the availability of sodium channels (Ruff et al., 1988). Slow inactivation is voltage dependent (Ruff et al., 1987; Ruben et al., 1990), and it is likely that the voltage dependence is derived, at least in part, from voltage sensors that are not as important for activation. Slow inactivation can occur in the absence of fast inactivation, but the two processes do interact (Featherstone et al., 1996). Disorders of both fast and slow inactivation in either the skeletal muscle or cardiac sodium channel are the cause of a number of human neurological disorders, including hyperkalemic periodic paralysis, paramyotonia congenita, sodium channel myotonia (reviewed by Barchi, 1995; Cannon, 1996) and long QT syndrome (Wang et al., 1995a , 1995 b; Wang et al., 1996).
The roles of some of the S4 charges during activation and inactivation have been investigated previously in both sodium and potassium channels. Stühmer et al. (1989) found that neutralization of S4 positive charges in domains I and II of the rat brain II sodium channel shifted the voltage dependence of inactivation and altered the slope factor, but not necessarily in the same direction as the shifts of activation. Analyses of the effects of S4 mutations in the potassium channel have demonstrated a much stronger concordance between the effects on activation and inactivation (Papazian et al., 1991; Lopez et al., 1991). These results support the original hypothesis of Hodgkin and Huxley (1952) that potassium channels have the same gates for both activation and inactivation, whereas sodium channels have separate activation and inactivation gates. Chen et al. (1996) recently examined the effects of mutations of some of the S4 charges in all four domains of the sodium channel. They concluded that the S4 region of domain IV plays a unique role in the coupling of activation to inactivation, consistent with previous results from O'Leary et al. (1995).
To investigate the roles played by the four S4 segments in the voltage dependence of inactivation, we constructed charge-neutralizing and -conserving substitutions of the second and fourth positive charges in the S4 segment of each domain of the rat brain IIA (RBIIA) sodium channel. To examine the effects on fast and slow inactivation, all of the mutations were constructed both in a wild-type background and in a channel containing the IFMQ3 mutation that eliminates fast inactivation (West et al., 1992). We show that the positively charged residues in all four S4 segments contribute unequally to the voltage dependence of inactivation, and that the effects on slow inactivation differ depending on whether fast inactivation is intact or not.
MATERIALS AND METHODS
Sodium Channel Mutations and Expression in Oocytes
The construction and nomenclature for the wild-type and mutant sodium channels are described in the accompanying paper (Kontis et al., 1997). For the studies described in this paper, the same S4 mutations were constructed in the wild-type sodium channel α subunit background, with fast inactivation intact. The procedures for in vitro transcription and expression in oocytes are described in the accompanying paper (Kontis et al., 1997).
Electrophysiological Recording
Sodium currents were measured by two-microelectrode voltage clamping using an oocyte clamp (OC-725; Warner Instruments, Hamden, CT). Data acquisition and analysis used pCLAMP software (version 6.0.3) and a TL-1 interface (Axon Instruments, Inc., Burlingame, CA). Agarose-tipped borosilicate glass electrodes were filled with 3 M KCl and had impedances between 0.5 and 1.0 MΩ, and currents were measured using a virtual ground circuit. The recording bath solution contained (mM): 96 NaCl, 5 HEPES, pH 7.5, 2 KCl, 1 MgCl2, and 1.8 CaCl2. All experiments were performed at room temperature (20–22°C). Oocytes were clamped at −100 mV and 22°C for at least 5 min to allow temperature equilibration and full recovery from inactivation. Data were acquired at a sampling frequency of 25 kHz with a filter frequency of 3 kHz. Depolarizations were from −90 to +50 mV in 10-mV steps from a holding potential of −100 mV, and lasted 57.5 ms. Capacitive and leak currents were eliminated by subtraction of records obtained in the presence of 400 nM tetrodotoxin for the data used to determine the time constants of fast inactivation. The series resistance varied from 1–2.0 kΩ.
Inactivation data were acquired using two-pulse protocols from a holding potential of −100 mV. For fast inactivation, the conditioning pulses ranged from −90 to +5 mV in 5-mV steps and were 500 ms in duration. These were immediately followed by a test pulse to −10 mV to measure available current. The protocol for slow inactivation included a 60-s conditioning pulse ranging from −90 to +5 mV in 5-mV increments. This was followed by a 20-ms pulse to −150 mV to allow recovery from fast inactivation, followed by a test pulse to −10 mV to measure available current. The test potential was different for those mutants that had shifts in the peak current–voltage relationship. The same slow inactivation protocol was used for the IFMQ3 channels and for the channels with fast inactivation intact. A similar protocol was used to measure the kinetics of slow inactivation, except that the conditioning pulse was to a constant potential of 0 mV and its duration was varied from 1 to 30 s.
Data Analysis
The voltage dependence of inactivation was analyzed by measuring peak currents elicited during the test pulse. The current measurements were normalized to the maximum current observed and plotted against the conditioning pulse potential. The data were fit using the Sigmaplot program (version 4.0; Jandel Scientific, San Rafael, CA), which employs the Marquardt-Levenberg algorithm for nonlinear regression. Normalized current–voltage relationships were fit with a two-state Boltzmann function, I/I max = 1/{1 + exp[(v−v 1/2)/a]}, where I/I max is the normalized current, v 1/2 is the half-maximal voltage of inactivation, and a is the apparent slope factor. The slope factor is inversely related to the steepness of the voltage dependence. In cases where two components of inactivation were observed, the data were fit with the sum of two Boltzmann functions, I/I max = f1/{1 + exp[(v−v 1/2,1)/a 1]} + f2/{1 + exp[(v−v 1/2,2)/a 2]}, where v 1/2 and a have their usual meanings and f1 and f2 are the fractions of the total current represented by each component of inactivation. The average and standard deviation for each voltage-dependent parameter were determined by individually fitting the data, and Student's unpaired t test was used to determine the statistical significance of the differences.
The time constants of fast (τh) and slow (τs) inactivation were determined using the Chebyshev method to fit the current traces with a single or double exponential equation of the form A 1*exp[−(t−K)/τ1] + A 2*exp[−(t−K)/τ2] + C, where A 1 and A 2 represent the amplitudes at the start of the fit region of τ1 and τ2, which are the time constants for inactivation, K is the time shift, and C is the steady state asymptote. The time shift was manually selected by fitting the traces at the time when the currents were just starting to exponentially decrease.
RESULTS
Most of the S4 Substitutions Cause Small Hyperpolarizing Shifts in Fast Inactivation
The voltage dependence of fast sodium channel inactivation is thought to result mainly from the voltage dependence of activation (Aldrich et al., 1983; Aldrich and Stevens, 1987). In the context of a ball-and-chain model for inactivation (Armstrong and Bezanilla, 1977), the coupling of activation and inactivation could result from conformational changes during activation that make the docking site available to bind the inactivation particle. Since the S4 positive charges are believed to comprise at least part of the voltage sensor for activation, it is likely that mutations in the S4 regions would affect fast sodium channel inactivation. We therefore tested the effects on fast inactivation of mutations in which charge neutralizing (glutamine) or charge conserving (arginine or lysine) substitutions were made for the second and fourth residues of each S4 segment. These are the same mutations that were described in the accompanying paper (Kontis et al., 1997), except that they were made in the wild-type sodium channel with inactivation intact. The nomenclature is such that substitution of the second positive charge of the domain I S4 segment with a glutamine is denoted as 1R2Q. Two double mutants were also constructed, one that combines neutralizations of the fourth positive charges in domains I and II (1K4Q and 2K4Q), and the other combining neutralizations of the fourth positive charges in domains III and IV (3R4Q and 4R4Q).
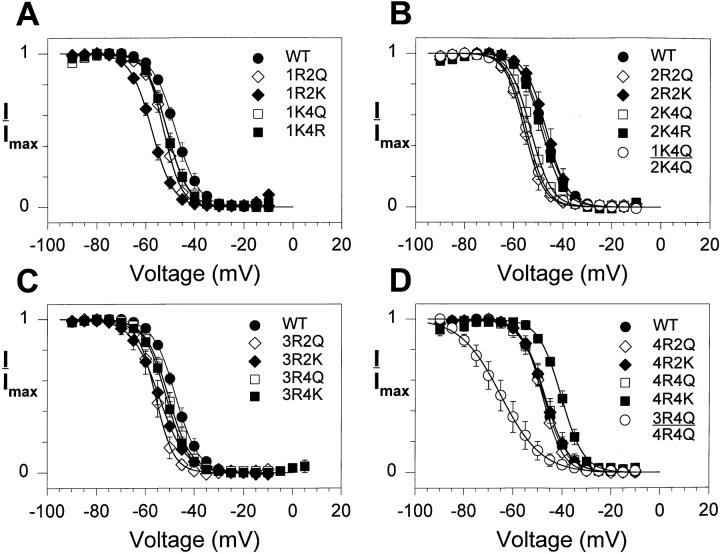
Fig. 1 shows the voltage dependence of steady state inactivation examined with a 500-ms prepulse. The values for v1/2 and slope factor are shown in Table I. Neutralization of either the second or fourth charge in domain I, II, or III resulted in small but statistically significant negative shifts in v1/2. Neutralization of either the second or fourth charge in domain IV had no significant effect on v1/2. Only one mutation (4R4K) shifted the v1/2 in the positive direction. Most of the mutations decreased the slope factor, but not to a large extent. The combination of neutralizations in domains I and II (1K4Q:2K4Q) shifted the v1/2 to a degree consistent with a summation of the slight shifts caused by each substitution individually. In contrast, the 3R4Q:4R4Q double mutation caused a large negative shift in v1/2, which is surprising because the 3R4Q mutation caused only a small negative shift and 4R4Q caused no apparent shift. This double mutant also demonstrated a significant reduction in the steepness of the curve (an increase in the slope factor).
Figure 1.
Effects of S4 mutations on steady state fast inactivation. Xenopus oocytes were injected with RNA encoding the wild-type sodium channel or each of the S4 mutants, along with RNA encoding the β1 subunit. Currents were recorded from a holding potential of −100 mV by 500-ms depolarizations ranging from −90 to +5 mV in 5-mV steps, followed by a test pulse to +10 mV. The normalized peak current during the test pulse is plotted against the prepulse potential. Data are shown for the mutants in domains I (A), II (B), III (C), and IV (D). Data for the double mutants are shown in B and D. The data points represent the means of at least three determinations and the error bars show the standard deviations. The smooth lines are fits to a two-state Boltzmann function, as described in materials and methods. The parameters of the fits are included in Table I.
Table I.
Parameters of the Voltage Dependence of Fast Inactivation
| Channel | v1/2 * | ah * | n * | |||
|---|---|---|---|---|---|---|
| WT | −47.5 ± 0.9 | 4.7 ± 0.4 | 10 | |||
| 1R2Q | −52.6 ± 0.7‡ | 3.7 ± 0.2‡ | 3 | |||
| 1R2K | −57.4 ± 0.9‡ | 4.4 ± 0.2 | 5 | |||
| 1K4Q | −51.2 ± 1.2‡ | 4.3 ± 0.2‡ | 7 | |||
| 1K4R | −51.2 ± 0.7‡ | 4.3 ± 0.4 | 3 | |||
| 2R2Q | −55.6 ± 0.9‡ | 3.9 ± 0.7‡ | 3 | |||
| 2R2K | −47.9 ± 2.7 | 4.3 ± 0.1‡ | 4 | |||
| 2K4Q | −53.2 ± 1.6‡ | 4.1 ± 0.6‡ | 8 | |||
| 2K4R | −48.6 ± 1.4 | 4.4 ± 0.1 | 4 | |||
| 3R2Q | −56.0 ± 1.3‡ | 3.8 ± 0.4‡ | 5 | |||
| 3R2K | −54.8 ± 1.5‡ | 5.5 ± 0.9‡ | 4 | |||
| 3R4Q | −50.7 ± 1.0‡ | 4.7 ± 0.3 | 6 | |||
| 3R4K | −51.7 ± 1.0‡ | 4.6 ± 0.2 | 4 | |||
| 4R2Q | −48.1 ± 0.4 | 4.2 ± 0.1‡ | 3 | |||
| 4R2K | −47.2 ± 1.5 | 4.8 ± 0.2 | 5 | |||
| 4R4Q | −47.3 ± 1.7 | 5.0 ± 0.5 | 7 | |||
| 4R4K | −40.5 ± 0.7‡ | 4.6 ± 0.3 | 3 | |||
| 1K4Q:2K4Q | −54.8 ± 0.9‡ | 4.2 ± 0.4‡ | 8 | |||
| 3R4Q:4R4Q | −64.9 ± 3.4‡ | 8.0 ± 0.5‡ | 7 |
v1/2 and ah are the half-maximal voltage and slope factor for fast inactivation as determined by least-squares fits of the data (n = number of separate determinations) to a two-state Boltzmann function as described in materials and methods.
Values significantly different from WT, with a probability <0.05 resulting from random variation (based on Student's unpaired t test).
S4 Mutations of Domain IV Affect the Kinetics of Fast Inactivation
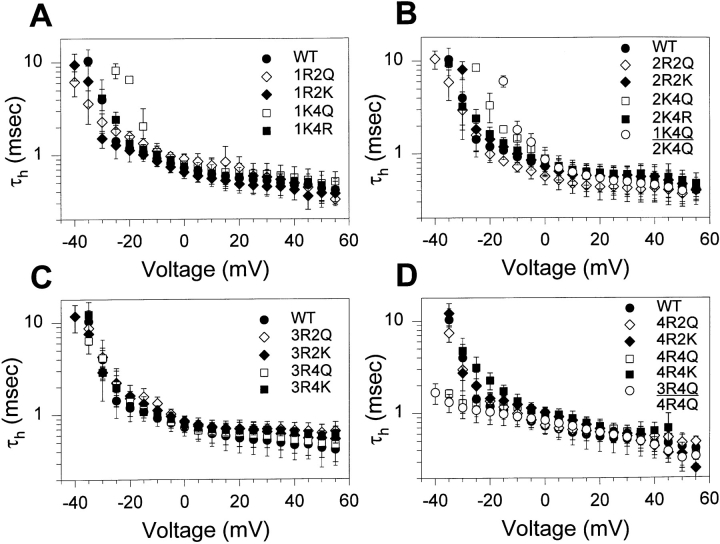
The analysis of steady state inactivation examines the voltage dependence of inactivation from both closed and open states. To determine if any of the mutations specifically affects inactivation from open states, the time constants for fast inactivation (τh) were determined for all of the mutants at potentials ranging from −40 to +55 mV (Fig. 2). Most of the mutations had no significant effects on the kinetics of fast inactivation. The time constants for all of the substitutions in domains I, II, and III were comparable with those observed for the wild-type channel, as were the time constants for all but one of the single substitution mutations in domain IV. However, neutralization of the fourth positive charge in domain IV (4R4Q) resulted in significantly faster inactivation at negative potentials, with a shallower voltage dependence. The double mutant in which the fourth charges in domains III and IV were neutralized was comparable with 4R4Q, demonstrating that this effect depended only on neutralization of the charge in the fourth position. Therefore, neutralization of the fourth positive charge in domain IV decreased the voltage dependence of τh, resulting in faster inactivation than that of the wild-type channel at potentials more negative than −20 mV.
Figure 2.
Effects of S4 mutations on the voltage dependence of fast inactivation time constants. Time constants (τh) of fast inactivation were determined for all of the mutants as described in materials and methods. The time constants are plotted on a log scale versus voltage for the mutants in domains I (A), II (B), III (C), and IV (D). Data for the double mutants are shown in B and D. The data points represent the means of at least three determinations and the error bars show the standard deviations.
Neutralization of Charges in Domains I or II Causes a Significant Shift in the v1/2 of Slow Inactivation in a Channel with Fast Inactivation Removed
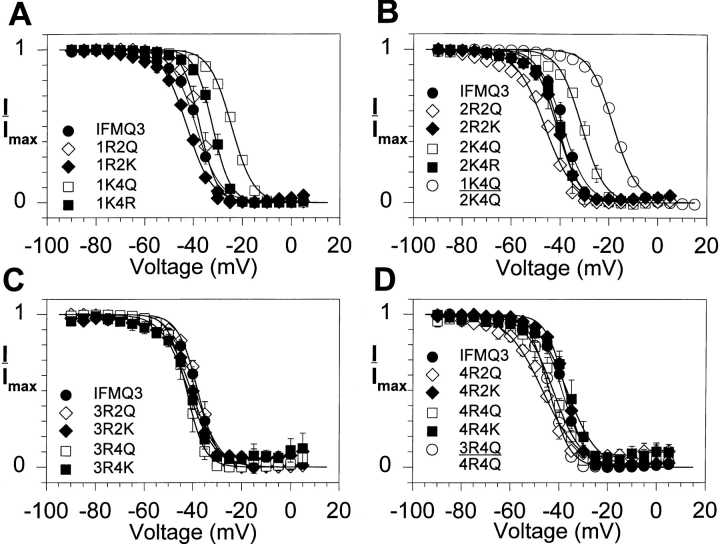
To investigate the role of the S4 positive charges in slow inactivation, the voltage dependence of slow inactivation was measured using the S4 mutants in the IFMQ3 background, with fast inactivation removed. Fig. 3 shows the voltage command protocol and representative traces recorded from an oocyte injected with RNA encoding the wild-type sodium channel with the IFMQ3 mutation. The number of data points between 0 and 60 s is small due to the slow sampling rate used during this portion of the protocol. Peak currents were measured, normalized to the maximum current, and plotted against the conditioning potential. The voltage dependence of slow inactivation curves for the wild-type and mutant channels are shown in Fig. 4, and the values for v1/2 and slope factor are included in Table II.
Figure 3.
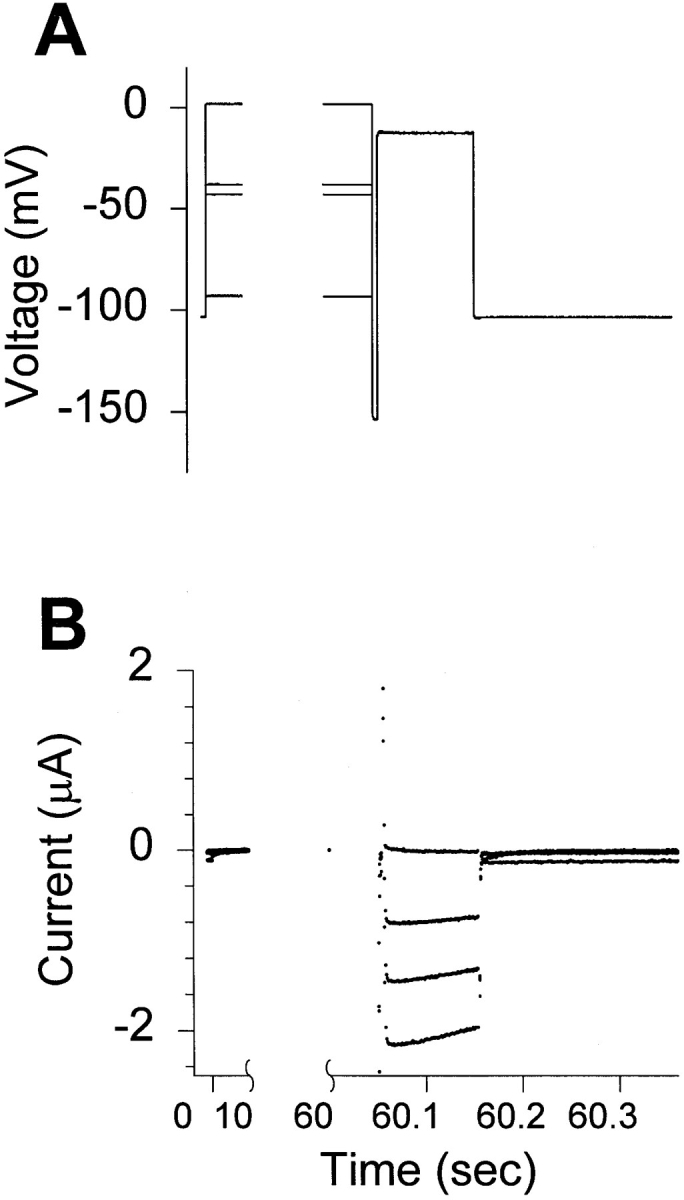
Voltage command protocol and representative current traces showing the voltage dependence of slow inactivation of the IFMQ3 channel. Xenopus oocytes were coinjected with in vitro transcribed RNA encoding the sodium channel α and β1 subunits. After 2 d incubation at 20°C, data were recorded using the two-electrode whole cell voltage clamp as described in materials and methods. Oocytes were held at −100 mV, and slow inactivation was induced by 60-s depolarizations from −90 to +5 mV in 5-mV steps. This was followed by a 20-ms hyperpolarization to −150 mV to allow recovery from fast inactivation (if any, since the channels all contain the IFMQ3 mutation) and a 100-ms test depolarization to −10 mV. (A) Representative traces of the command potential are shown for depolarizations to −90, −40, −35, and +5 mV. (B) The current recorded during the same series of depolarizations is shown. The largest inward current was obtained at a prepulse of −90 mV and essentially no current can be elicited after a +5-mV depolarization.
Figure 4.
Effects of S4 mutations on steady state slow inactivation when fast inactivation is eliminated. Xenopus oocytes were injected with RNA encoding the IFMQ3 sodium channel or each of the S4 mutants in the IFMQ3 background, along with RNA encoding the β1 subunit. Currents were recorded as described in Fig. 3. The normalized peak current during the test pulse is plotted against the prepulse potential. Data are shown for the mutants in domains I (A), II (B), III (C), and IV (D). Data for the double mutants are shown in B and D. The data points represent the means of at least three determinations and the error bars show the standard deviations. The smooth lines are fits to a two-state Boltzmann function, as described in materials and methods. The parameters of the fits are included in Table II.
Table II.
Parameters of the Voltage Dependence of Slow Inactivation
| Channel | v1/2 * | as * | n * | |||
|---|---|---|---|---|---|---|
| mV | ||||||
| IFMQ3 | −38.9 ± 1.0 | 4.4 ± 0.6‡ | 5 | |||
| 1R2Q | −37.3 ± 1.3 | 3.5 ± 0.1‡ | 3 | |||
| 1R2K | −42.6 ± 0.7‡ | 5.1 ± 0.5 | 3 | |||
| 1K4Q | −24.4 ± 0.5‡ | 4.5 ± 0.1 | 3 | |||
| 1K4R | −32.1 ± 0.9‡ | 3.6 ± 0.2 | 3 | |||
| 2R2Q | −46.3 ± 0.2‡ | 5.9 ± 0.2‡ | 3 | |||
| 2R2K | −41.3 ± 2.6 | 4.5 ± 0.4 | 3 | |||
| 2K4Q | −31.2 ± 1.0‡ | 4.7 ± 0.3 | 4 | |||
| 2K4R | −40.8 ± 0.6‡ | 4.3 ± 0.3 | 5 | |||
| 3R2Q | −38.0 ± 0.8 | 4.1 ± 0.6 | 5 | |||
| 3R2K | −40.8 ± 0.8‡ | 5.5 ± 0.4‡ | 4 | |||
| 3R4Q | −42.5 ± 1.7‡ | 3.6 ± 0.1 | 3 | |||
| 3R4K | −41.9 ± 1.2‡ | 5.6 ± 1.0 | 4 | |||
| 4R2Q | −46.8 ± 1.5‡ | 7.5 ± 0.9‡ | 3 | |||
| 4R2K | −37.4 ± 1.0 | 4.0 ± 0.2 | 3 | |||
| 4R4Q | −42.7 ± 1.6‡ | 6.2 ± 2.1 | 4 | |||
| 4R4K | −36.8 ± 2.3 | 6.1 ± 0.8‡ | 3 | |||
| 1K4Q:2K4Q | −18.2 ± 0.8‡ | 4.9 ± 0.4‡ | 3 | |||
| 3R4Q:4R4Q | −44.4 ± 1.1‡ | 4.2 ± 0.1 | 3 |
v1/2 and as are the half-maximal voltage and slope factor for slow inactivation as determined by least-squares fits of the data (n = number of separate determinations) to a two-state Boltzmann function as described in materials and methods.
Values significantly different from IFMQ3, with a probability <0.05 resulting from random variation (based on Student's unpaired t test).
Neutralization of the fourth positive charge in either domain I (1K4Q) or II (2K4Q) resulted in the largest positive shifts in v1/2. Neither mutation caused a significant change in the slope factor. Combining the two mutations was essentially additive, resulting in a larger positive shift in v1/2 without any significant change in slope. In contrast, neutralization of the second charge in domain II (2R2Q) shifted v1/2 in the negative direction, with an increase in the slope factor. The comparable mutation in domain I had no effect on v1/2, but it decreased the slope factor.
All of the mutations in domain III resulted in slow inactivation curves that were very similar to that of the wild-type channel. Although a number of differences were statistically significant, they were small. The mutations in domain IV also had relatively modest effects. Neutralization of either the second (4R2Q) or fourth (4R4Q) charge in domain IV shifted v1/2 in the negative direction. Combining the mutations that neutralized the fourth charge in domain III or IV (3R4Q:4R4Q) resulted in a small negative shift in v1/2, confirming the lack of dramatic effects with mutations in these two domains.
S4 Mutations Have Different Effects on Slow Inactivation in a Channel with Fast Inactivation Intact
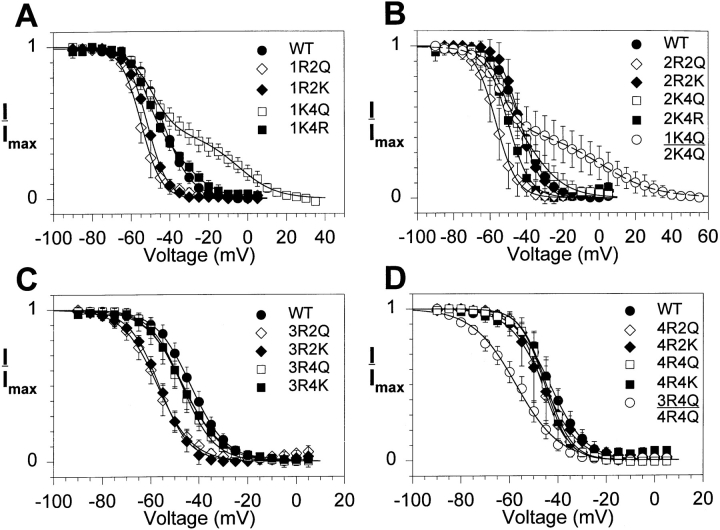
Featherstone et al. (1996) have recently shown that slow inactivation in the rat skeletal muscle sodium channel occurs more quickly when fast inactivation is removed, suggesting that there is an interaction between the two processes. Because of this interaction, the S4 mutations might have different effects on slow inactivation if fast inactivation were intact. To evaluate this possibility, we analyzed slow inactivation of the S4 mutants in a wild-type background with fast inactivation intact. We used the protocol depicted in Fig. 3, which includes a 20-ms hyperpolarization to −150 mV to allow recovery from fast inactivation before measuring available current with the test pulse. The voltage dependence of slow inactivation curves for the wild-type and mutant channels are shown in Fig. 5, and the values for v1/2 and slope factor are included in Table III. In the absence of fast inactivation for the wild-type channel, the v1/2 of slow inactivation was more positive and the slope factor was smaller than when fast inactivation was intact. Therefore, the presence of a functional fast inactivation gate caused a small negative shift in the v1/2 and a decrease in the steepness of the voltage dependence of slow inactivation.
Figure 5.
Effects of S4 mutations on steady state slow inactivation when fast inactivation is intact. Xenopus oocytes were injected with RNA encoding the wild-type sodium channel or each of the S4 mutants in the wild-type background, along with RNA encoding the β1 subunit. Currents were recorded as described in Fig. 3. The normalized peak current during the test pulse is plotted against the prepulse potential. Data are shown for the mutants in domains I (A), II (B), III (C), and IV (D). Data for the double mutants are shown in B and D. The data points represent the means of at least three determinations and the error bars show the standard deviations. The smooth lines are fits to a two-state Boltzmann function, as described in materials and methods. The parameters of the fits are included in Table III.
Table III.
Parameters of the Voltage Dependence of Slow Inactivation with Fast Inactivation Intact
| Channel | v1/2 * | a s * | n * | |||
|---|---|---|---|---|---|---|
| WT | −43.5 ± 2.2 | 7.3 ± 0.7 | 5 | |||
| 1R2Q | −54.8 ± 1.8‡ | 5.3 ± 1.8 | 3 | |||
| 1R2K | −51.8 ± 0.6‡ | 5.2 ± 0.5‡ | 5 | |||
| 1K4Q§ | −49.5 ± 2.1‡ | 7.0 ± 1.6 | 4 | |||
| −4.8 ± 3.7‡ | 9.3 ± 1.5‡ | |||||
| 1K4R§ | −52.7 ± 1.8‡ | 4.3 ± 0.7‡ | 3 | |||
| −33.0 ± 2.5‡ | 8.3 ± 1.7 | |||||
| 2R2Q | −56.6 ± 1.3‡ | 5.3 ± 1.3‡ | 5 | |||
| 2R2K | −43.3 ± 1.7 | 5.7 ± 0.8‡ | 3 | |||
| 2K4Q§ | −52.1 ± 3.8‡ | 5.0 ± 1.5‡ | 7 | |||
| −31.5 ± 4.4‡ | 6.8 ± 3.8 | |||||
| 2K4R | −50.3 ± 1.1‡ | 5.5 ± 0.8‡ | 3 | |||
| 3R2Q | −57.1 ± 4.5‡ | 6.9 ± 1.3 | 3 | |||
| 3R2K | −56.6 ± 1.8‡ | 6.4 ± 0.7 | 4 | |||
| 3R4Q | −47.4 ± 2.5‡ | 7.0 ± 0.9 | 6 | |||
| 3R4K | −45.2 ± 2.4 | 7.3 ± 1.1 | 4 | |||
| 4R2Q | −46.6 ± 0.7 | 5.4 ± 0.3‡ | 3 | |||
| 4R2K | −45.6 ± 7.8 | 6.1 ± 1.3 | 3 | |||
| 4R4Q | −45.1 ± 1.9 | 5.6 ± 0.8‡ | 7 | |||
| 4R4K | −44.9 ± 0.9 | 5.0 ± 0.9‡ | 3 | |||
| 1K4Q:2K4Q§ | −54.8 ± 2.6‡ | 6.2 ± 1.4 | 5 | |||
| −1.2 ± 9.3‡ | 14.9 ± 2.2‡ | |||||
| 3R4Q:4R4Q | −57.5 ± 2.8‡ | 8.9 ± 0.3‡ | 7 |
v1/2 and as are the half-maximal voltage and slope factor for slow inactivation as determined by least-squares fits of the data (n = number of separate determinations) to a two-state Boltzmann function as described in materials and methods.
Values significantly different from WT, with a probability <0.05 resulting from random variation (based on Student's unpaired t test).
The data for these mutants were fit with a two-component Boltzmann function.
Substitution of the S4 positive charges had different effects on slow inactivation when fast inactivation was intact compared with the results when fast inactivation was removed. Most of the mutations shifted the v1/2 for slow inactivation in the negative direction and decreased the steepness of the curve. For example, neutralization of the second charge in domains I, II, or III shifted the v1/2 in the negative direction. However, the charge-conserving substitutions of the second residue in domains I and III resulted in negative shifts of similar magnitude, indicating that the shifts in v1/2 did not result from reduction of the charge in the S4 segment. Neutralization of the second charge in domain IV (4R2Q) had no significant effect on the v1/2 of slow inactivation.
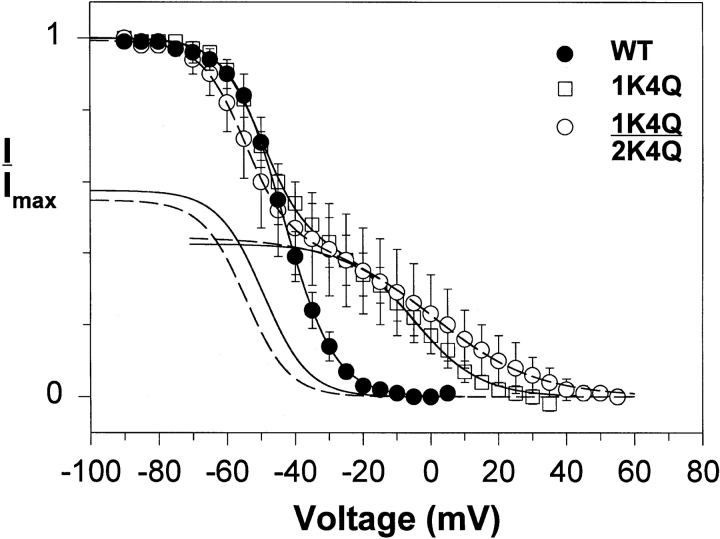
Neutralization of the fourth charge in domain III or IV resulted in small or insignificant negative shifts in v1/2. However, neutralization of the fourth charge in domain I (1K4Q) or II (2K4Q) caused a marked positive shift in v1/2 with a decrease in slope, leading to a curve that could no longer be fit with a single Boltzmann function. Similar results were observed when the fourth charge in domain I was replaced with arginine (1K4R), or when the two charge neutralization mutations were combined (1K4Q:2K4Q). Each of the curves for these mutants could be fit very well with a two-component Boltzmann function, as shown for the 1K4Q and 1K4Q: 2K4Q mutations in Fig. 6. In each case, there is a component of slow inactivation with a v1/2 slightly more negative than that of the wild-type channel, and a second component with a more positive v1/2 and a shallower slope.
Figure 6.
The 1K4Q mutation results in two components of slow inactivation when fast inactivation is intact. Steady-state slow inactivation of the wild-type sodium channel is compared with that of the 1K4Q and 1K4Q:2K4Q mutants. The smooth lines are fits to a single Boltzmann function for the wild-type channel, and the sum of two Boltzmann functions for the mutants. The parameters of the Boltzmann fits are shown in Table III.
1K4Q Mutation Primarily Affects One Component of Slow Inactivation
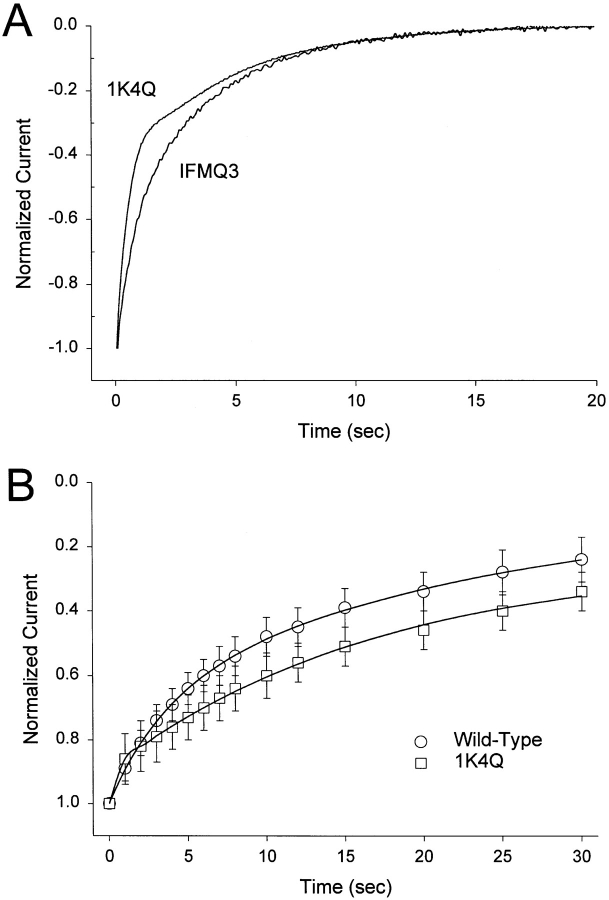
The presence of two components in the voltage dependence of slow inactivation for the 1K4Q and 2K4Q mutants suggests that slow inactivation is not a single process. To evaluate this possibility, the kinetics of slow inactivation were determined for the wild-type, IFMQ3, and 1K4Q mutant channels with and without fast inactivation. For the channels without fast inactivation, the onset of slow inactivation was measured during a 60-s depolarization to 0 mV (Fig. 7 A). Both IFMQ3 and 1K4Q channels inactivated with kinetics that were best fit with two exponential time constants. The slower time constant was not significantly different for the two channels, but the faster time constant for 1K4Q was about half that for IFMQ3 (time constant values are presented in Fig. 7). The presence of two time constants is consistent with the hypothesis that slow inactivation consists of at least two processes.
Figure 7.
The 1K4Q mutation affects one component of slow inactivation. The kinetics of slow inactivation for the 1K4Q mutant channel were compared with those of the wild-type and IFMQ3 channels. (A) Oocytes injected with RNA encoding either the IFMQ3 channel or the 1K4Q mutant channel in the IFMQ3 (noninactivating) background were examined by two-electrode voltage clamping. The oocytes were held at −100 mV and depolarized to 0 mV for 60 s (only the first 20 s of the records are shown). The kinetics of inactivation for both channels were best fit with two exponential time constants. For the IFMQ3 channel, τ1 = 4.9 ± 0.9 s, A1 = 67 ± 9%, τ2 = 1.0 ± 0.2 s, A2 = 33 ± 9% (n = 5). For the 1K4Q channel, τ1 = 5.0 ± 0.5 s, A1 = 51 ± 8%, τ2 = 0.43 ± 0.06 s, A2 = 49 ± 8% (n = 3). (B) Oocytes injected with RNA encoding either the wild-type channel or the 1K4Q mutant channel in the inactivating background were examined by two-electrode voltage clamping. The protocol was similar to that shown in Fig. 3 except that the conditioning pulse was to a constant value of 0 mV and the duration of that pulse varied from 1 to 30 s. The amplitude of the current during the test pulse (normalized to the current obtained without any conditioning pulse) is plotted versus the conditioning pulse duration. The kinetics were best fit with two exponential time constants. For the wild-type channel, τ1 = 32.5 ± 15.3 s, A1 = 71 ± 12%, τ2 = 3.6 ± 0.9 s, A2 = 36 ± 9%, C = −0.07 ± 0.19 (n = 5). For the 1K4Q channel, τ1 = 14.2 ± 2.2 s, A1 = 62 ± 4%, τ2 = 0.2 ± 0.1 s, A2 = 14 ± 7%, C = 0.27 ± 0.05 (n = 4). Symbols represent means and error bars indicate standard deviations.
For the channels with fast inactivation intact, the onset of slow inactivation was determined using the protocol shown in Fig. 3, modified so that the prepulse varied in length from 0 to 30 s at a constant voltage of 0 mV. The presence of fast inactivation significantly delayed the onset of slow inactivation (Fig. 7 B), as previously shown by Featherstone et al. (1996). Both the wild-type and 1K4Q channels with fast inactivation intact demonstrated two exponential time constants for slow inactivation. As was the case for the channels without fast inactivation, the slower time constant was not significantly different between the two channels, but the faster time constant for 1K4Q was much smaller than that for the wild-type channel. In addition, the steady state asymptote for the 1K4Q channel represented 27% of the current, consistent with the finding that the 1K4Q channels did not completely slow inactivate at 0 mV (Fig. 6). These results indicate that slow inactivation consists of at least two components, and that the 1K4Q mutation primarily affects the faster of the two components and causes slow inactivation to be incomplete when fast inactivation is intact.
DISCUSSION
This study examines the effects of mutations in the four putative voltage sensors on the voltage dependence of fast and slow sodium channel inactivation. The effects on fast inactivation have also been studied by Stühmer et al. (1989) and Chen et al. (1996). Stühmer et al. (1989) examined the effects of mutations in domains I and II in the rat brain II channel on steady state inactivation assessed with a 100-ms prepulse. Chen et al. (1996) investigated the effects of mutations of the first and third charges in all four domains of the human heart 1 channel on steady state inactivation assessed with a 500-ms prepulse, which is equivalent to the protocol used in our study. The results of the three studies are summarized in Table IV. Most of the neutralizations in domain I resulted in small negative shifts in v1/2. The only exception is 1R3Q assessed with a 100-ms prepulse. Similarly, all but one of the neutralizations in domain II resulted in negative shifts in v1/2, although these were larger than those observed for the domain I mutations. The one exception is 2R1Q, which demonstrated a significant positive shift. The effects were more pronounced for three of the mutations in domain III, and again all of the shifts in v1/2 were in the negative direction. Three of the neutralizations in domain IV caused very minor shifts, but the other mutation (4R3Q) resulted in a dramatic negative shift in v1/2. The overall trend appears to be that neutralization of S4 positive charges results in negative shifts in v1/2 and small decreases in the slope factor of fast inactivation.
Table IV.
Summary of Charge Neutralization Mutation Effects on Fast Inactivation
| Channel | v1/2 shift* | ah change* | ||
|---|---|---|---|---|
| mV | ||||
| 1R1Q‡ | −0.8 | −0.2 | ||
| 1R1Q§ | −5.0 | −0.8 | ||
| 1R2Q | −5.1‖ | −1.0‖ | ||
| 1R2Q§ | −6.0 | −0.5 | ||
| 1R3Q‡ | −5.8 | −1.1‖ | ||
| 1R3Q§ | +6.0 | +1.4 | ||
| 1K4Q | −3.7‖ | −0.4‖ | ||
| 1K4Q§ | −6.0 | +0.9 | ||
| 2R1Q‡ | +8.2‖ | −0.4 | ||
| 2R2Q | −8.1 | −0.8‖ | ||
| 2R3Q‡ | −9.6‖ | −1.1‖ | ||
| 2K4Q | −5.7‖ | −0.6‖ | ||
| 2K5Q§ | −9.0 | −0.8 | ||
| 3K1Q‡ | −21.0‖ | −0.8‖ | ||
| 3R2Q | −8.5‖ | −0.9‖ | ||
| 3R3Q‡ | −10.6‖ | −0.6 | ||
| 3R4Q | −3.2‖ | 0.0 | ||
| 4R1Q‡ | +4.7‖ | −2.0‖ | ||
| 4R2Q | −0.6 | −0.5‖ | ||
| 4R3Q‡ | −57.5‖ | −2.6‖ | ||
| 4R4Q | +0.2 | +0.3 |
v1/2 and ah are the half-maximal voltage and slope factor for fast inactivation. Data from Stühmer et al. (1989) did not include an assessment of statistical significance.
Data from Chen et al. (1966).
Data from Stühmer et al. (1989). These data were acquired using a 100-ms prepulse, whereas all other data were acquired using a 500-ms prepulse.
Values statistically significant, with a probability <0.05 resulting from random variation.
Chen et al. (1996) also examined the effects of the S4 positive charge mutations on the kinetics of fast inactivation (τh). They observed that mutations in domains I, II, and III did not significantly affect the kinetics of fast inactivation, similar to our results. With respect to domain IV, Chen et al. (1996) noted that some substitutions of the first and third positive charges decreased the voltage dependence of τh, resulting in slower inactivation at potentials more positive than −40 mV. Our finding that neutralization of the fourth charge in domain IV decreased the voltage dependence of τh is similar to the observations of Chen et al. (1996). However, the net effect of the mutation we studied was to speed up inactivation at potentials more negative than −20 mV, in contrast to the slowing down observed by Chen et al. (1996). We did not observe any significant effects on τh when the second positive charge in domain IV was substituted. These results suggest that the fourth domain plays a unique role in determining inactivation, as concluded by Chen et al. (1996), but indicate that all of the charged residues in the fourth domain are not equivalent.
The effects of the mutations on the voltage dependence of fast inactivation contrast with their effects on activation, as presented in the accompanying manuscript (Kontis et al., 1997) and also examined by Stühmer et al. (1989) and Chen et al. (1996). Most of the S4 mutations in domains I, II, and IV resulted in significant shifts in the v1/2 of activation in the positive direction (Table V). In contrast, the mutations in domains I and II resulted in negative shifts in fast inactivation, and most of the mutations in domain IV had no significant effects on fast inactivation (Table V). There is not much better concordance for the effects of the mutations in domain III. Neutralization of the second (3R2Q) or fourth (3R4Q) charge caused a negative shift in activation and fast inactivation, but 3R2Q resulted in a larger shift in fast inactivation and 3R4Q caused a larger shift in activation. These discrepancies argue against the hypothesis that most of the voltage dependence of fast inactivation in the rat brain sodium channel is derived from activation (Bezanilla and Armstrong, 1977; Nonner, 1980; Aldrich and Stevens, 1987; Zagotta and Aldrich, 1990). Instead, the voltage dependence of fast inactivation may be derived more directly from movement of a subset of the voltage sensors. Consistent with this hypothesis, Chen et al. (1996) observed that the S4 segment of domain IV was uniquely involved in the voltage dependence of inactivation.
Table V.
Comparison of Mutation Effects on Activation and Inactivation
| Mutant | Activation v1/2 shift* | Fast inactivation v1/2 shift‡ | Slow inactivation (no fast) v1/2 shift§ | Slow inactivation (fast intact) v1/2 shift‖ | ||||
|---|---|---|---|---|---|---|---|---|
| mV | mV | mV | mV | |||||
| 1R2Q | −1.0 | −5.1176 | +1.6 | −11.3176 | ||||
| 1R2K | +2.8 | −9.9176 | −3.7176 | −8.3176 | ||||
| 1K4Q | +18.5176 | −3.7176 | +14.7176 | −6.0176 | ||||
| +38.7176 | ||||||||
| 1K4R | +3.8176 | −3.7176 | +6.8176 | −9.2176 | ||||
| +10.5176 | ||||||||
| 2R2Q | +4.4176 | −8.1176 | −7.4176 | −13.1176 | ||||
| 2R2K | +9.1176 | −0.4 | −2.4 | +0.2 | ||||
| 2K4Q | +14.9176 | −5.7176 | +7.7176 | −8.6176 | ||||
| +12.0176 | ||||||||
| 2KRR | +3.8176 | −1.1 | −1.9176 | −6.8176 | ||||
| 3R2Q | −0.3 | −8.5176 | +0.9 | −13.6176 | ||||
| 3R2K | −0.4 | −7.3176 | −1.9176 | −13.1176 | ||||
| 3R4Q | −18.1176 | −3.2176 | −3.6176 | −3.9176 | ||||
| 3R4K | +9.8176 | −4.2176 | −3.0176 | −1.7 | ||||
| 4R2Q | +3.6176 | −0.6 | −7.9176 | −3.1 | ||||
| 4R2K | +4.1176 | +0.3 | +1.5 | −2.1 | ||||
| 4R4Q | +0.4 | +0.2 | −3.8176 | −1.6 | ||||
| 4R4K | +7.4176 | +7.0176 | +2.1 | −1.4 | ||||
| 1K4Q:2K4Q | +41.3176 | −7.3176 | +20.7176 | −11.3176 | ||||
| +42.3176 | ||||||||
| 3R4Q:4R4Q | −10.3176 | −17.4176 | −5.5176 | −14.0176 |
Shifts in v1/2 of activation are from Kontis et al. (1997).
Shifts in v1/2 of fast inactivation were determined from the data in Table I.
Shifts in v1/2 of slow inactivation without fast inactivation were determined from the data in Table III.
Shifts in v1/2 of slow inactivation with fast inactivation intact were determined from the data in Table IV.
Values statistically significant, with a probability < 0.05 resulting from random variation.
The effects of the S4 mutations on slow inactivation depended on whether fast inactivation was intact or not. When fast inactivation was removed by inclusion of the IFMQ3 mutation, most of the mutations resulted in positive shifts in v1/2 (Table V). The effects on slow inactivation of the mutations in domains I and II generally corresponded with the effects of those mutations on activation. For example, neutralization of the fourth residue in either domain I or II shifted both activation and slow inactivation in the positive direction, and combining the two mutations resulted in shifts in both activation and slow inactivation that were essentially additive (Table V). The mutations in domain III had essentially no effect on slow inactivation but did have significant effects on activation, and the mutations in domain IV caused small shifts in slow inactivation but had no significant effects on activation. These results suggest that activation and slow inactivation both derive their voltage dependence from movements of the S4 regions, which is not surprising since both processes most likely represent voltage initiated conformational changes in the channel.
The effects of the mutations on slow inactivation were quite different when fast inactivation was intact. Most of the mutations caused significant shifts of v1/2 in the negative direction, but the magnitude of the effects did not correlate with the reduction in charge. For example, neutralization of the second residue in either domain I or III resulted in negative shifts of slow inactivation when fast inactivation was intact (Table V). The charge-conserving substitutions at the same positions had comparable effects, demonstrating that the shifts were not caused by elimination of the charge. These results indicate that the structure of the residues at these positions in S4 have more important roles in the transition to the slow inactivated state when fast inactivation is present than when it is absent.
The most dramatic effects were observed with mutations of the fourth positive charge in domain I or II (1K4Q, 1K4R, and 2K4Q). Each of these mutations resulted in a second component in the voltage dependence of slow inactivation, but only when fast inactivation was intact. The first component had a v1/2 that was slightly more negative than that of the wild-type channel, and the second component had a pronounced shift in the positive direction and a marked increase in slope factor (Table III). Featherstone et al. (1996) have previously reported that slow inactivation in the skeletal muscle sodium channel differs depending on the presence or absence of fast inactivation. They observed that slow inactivation has a comparable v1/2 in both situations, but that it is only ∼80% complete when fast inactivation is present. Their results are consistent with a model in which fast and slow inactivation are not mutually exclusive, and that fast inactivation interferes with slow inactivation. Our data with the wild-type rat brain IIA channel were somewhat different in that slow inactivation was complete even when fast inactivation was intact, but there was a small negative shift in the v1/2 of slow inactivation.
The second component in the voltage dependence of slow inactivation suggests that slow inactivation does not represent a single process. Consistent with this hypothesis, the kinetics of slow inactivation were best fit with two exponential time constants, regardless of the presence of fast inactivation (Fig. 7). The 1K4Q mutation appeared to affect only the faster component, decreasing the magnitude of the time constant. In addition, this mutation caused incomplete slow inactivation at 0 mV when fast inactivation was intact, as did all of the mutations that caused a second component in the voltage dependence of slow inactivation. These mutations also caused positive voltage shifts in slow inactivation when fast inactivation was eliminated (Table V). One hypothesis to explain these results is that the positive voltage shift resulted in slow inactivation occurring in a voltage region in which fast inactivation was more complete. Therefore, more extensive fast inactivation during the conditioning pulse caused more interference with slow inactivation. The net result would be that some percentage of the channels do not undergo slow inactivation until a more positive membrane potential is reached.
In summary, the data are consistent with a model in which the S4 regions of all four domains of the sodium channel are involved in both activation and inactivation gating. Each S4 segment has a different role in both processes, however, so that mutations in any one domain differentially affect each process. Fast inactivation is not completely coupled to activation, but may derive its voltage dependence from movements of the S4 segments that are not rate limiting for activation. The true test of the importance of individual charges as voltage sensing residues for both activation and inactivation will be to examine the effects of S4 mutations on the gating currents of the channel.
Acknowledgments
We thank Dr. Raymond Smith, Dr. Michael Pugsley, Ted Shih, Marianne Smith, and Dan Allen for helpful discussions during the course of this work, Dan Allen and Ian Jester for help with the experiments, and Mimi Reyes for excellent technical assistance.
This work was supported by grants from the National Institutes of Health (NS-26729) and the National Science Foundation (IBN9221984). A.L. Goldin is an Established Investigator of the American Heart Association.
Footnotes
Dr. Kontis' present address is Hycor Biomedical Inc., Garden Grove, CA 92841.
REFERENCES
- Aldrich RW, Corey DP, Stevens CF. A reinterpretation of mammalian sodium channel gating based on single channel recording. Nature (Lond) 1983;306:436–441. doi: 10.1038/306436a0. [DOI] [PubMed] [Google Scholar]
- Aldrich RW, Stevens CF. Voltage-dependent gating of single sodium channels from mammalian neuroblastoma cells. J Neurosci. 1987;7:418–431. doi: 10.1523/JNEUROSCI.07-02-00418.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong CM, Bezanilla F. Inactivation of the sodium channel. II. Gating current experiments. J Gen Physiol. 1977;70:567–590. doi: 10.1085/jgp.70.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barchi RL. Molecular pathology of the skeletal muscle sodium channel. Annu Rev Physiol. 1995;57:355–385. doi: 10.1146/annurev.ph.57.030195.002035. [DOI] [PubMed] [Google Scholar]
- Bezanilla F, Armstrong CM. Inactivation of the sodium channel–sodium current experiments. J Gen Physiol. 1977;70:549–566. doi: 10.1085/jgp.70.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon SC. Ion-channel defects and aberrant excitability in myotonia and periodic paralysis. Trends Neurosci. 1996;19:3–10. doi: 10.1016/0166-2236(96)81859-5. [DOI] [PubMed] [Google Scholar]
- Chen L-Q, Santarelli V, Horn R, Kallen RG. A unique role for the S4 segment of domain 4 in the inactivation of sodium channels. J Gen Physiol. 1996;108:549–556. doi: 10.1085/jgp.108.6.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Featherstone DE, Richmond JE, Ruben PC. Interaction between fast and slow inactivation in Skm1 sodium channels. Biophys J. 1996;71:3098–3109. doi: 10.1016/S0006-3495(96)79504-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol (Cambr) 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T, Zagotta WN, Aldrich RW. Biophysical and molecular mechanisms of Shakerpotassium channel inactivation. Science (Wash DC) 1990;250:533–538. doi: 10.1126/science.2122519. [DOI] [PubMed] [Google Scholar]
- Kontis KJ, Rounaghi A, Goldin AL. Sodium channel activation gating is affected by substitutions of voltage sensor positive charges in all four domains. J Gen Physiol. 1997;110:391–401. doi: 10.1085/jgp.110.4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez GA, Jan YN, Jan LY. Hydrophobic substitution mutations in the S4 sequence alter voltage-dependent gating in Shaker K+channels. Neuron. 1991;7:327–336. doi: 10.1016/0896-6273(91)90271-z. [DOI] [PubMed] [Google Scholar]
- Nonner W. Relations between the inactivation of sodium channels and the immobilization of gating charge in frog myelinated nerve. J Physiol (Cambr) 1980;299:573–603. doi: 10.1113/jphysiol.1980.sp013143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary ME, Chen L-Q, Kallen RG, Horn R. A molecular link between activation and inactivation of sodium channels. J Gen Physiol. 1995;106:641–658. doi: 10.1085/jgp.106.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papazian DM, Timpe LC, Jan YN, Jan LY. Alteration of voltage-dependence of Shakerpotassium channel by mutations in the S4 sequence. Nature (Lond) 1991;349:305–310. doi: 10.1038/349305a0. [DOI] [PubMed] [Google Scholar]
- Patton DE, West JW, Catterall WA, Goldin AL. Amino acid residues required for fast sodium channel inactivation. Charge neutralizations and deletions in the III-IV linker. Proc Natl Acad Sci USA. 1992;89:10905–10909. doi: 10.1073/pnas.89.22.10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruben PC, Starkus JG, Rayner MD. Holding potential affects the apparent voltage-sensitivity of sodium channel activation in crayfish giant axons. Biophys J. 1990;58:1169–1181. doi: 10.1016/S0006-3495(90)82458-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff RL, Simoncini L, Stühmer W. Comparison between slow sodium channel inactivation in rat slow- and fast-twitch muscle. J Physiol. 1987;383:339–348. doi: 10.1113/jphysiol.1987.sp016412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff RL, Simoncini L, Stühmer W. Slow sodium channel inactivation in mammalian muscle: a possible role in regulating excitability. Muscle Nerve. 1988;11:502–510. doi: 10.1002/mus.880110514. [DOI] [PubMed] [Google Scholar]
- Simoncini L, Stühmer W. Slow sodium channel inactivation in rat fast-twitch muscle. J Physiol. 1987;383:327–337. doi: 10.1113/jphysiol.1987.sp016411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stühmer W, Conti F, Suzuki H, Wang X, Noda M, Yahagi N, Kubo H, Numa S. Structural parts involved in activation and inactivation of the sodium channel. Nature (Lond) 1989;339:597–603. doi: 10.1038/339597a0. [DOI] [PubMed] [Google Scholar]
- Wang DW, Yazawa K, George AL, Jr, Bennett PB. Characterization of human cardiac Na+channel mutations in the congenital long QT syndrome. Proc Natl Acad Sci USA. 1996;93:13200–13205. doi: 10.1073/pnas.93.23.13200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Shen J, Li Z, Timothy K, Vincent GM, Priori SG, Schwartz PJ, Keating MT. Cardiac sodium channel mutations in patients with long QT syndrome, an inherited cardiac arrhythmia. Hum Mol Genet. 1995a;4:1603–1607. doi: 10.1093/hmg/4.9.1603. [DOI] [PubMed] [Google Scholar]
- Wang Q, Shen J, Splawski I, Atkinson D, Li Z, Robinson JL, Moss AJ, Towbin JA, Keating MT. b. SCN5Amutations associated with an inherited cardiac arrhythmia, long QT syndrome. Cell. 1995;80:805–811. doi: 10.1016/0092-8674(95)90359-3. [DOI] [PubMed] [Google Scholar]
- West JW, Patton DE, Scheuer T, Wang Y, Goldin AL, Catterall WA. A cluster of hydrophobic amino acid residues required for fast Na+channel inactivation. Proc Natl Acad Sci USA. 1992;89:10910–10914. doi: 10.1073/pnas.89.22.10910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagotta WN, Hoshi T, Aldrich RW. Restoration of inactivation in mutants of Shakerpotassium channels by a peptide derived from ShB. Science (Wash DC) 1990;250:568–571. doi: 10.1126/science.2122520. [DOI] [PubMed] [Google Scholar]
- Zagotta WN, Aldrich RW. Voltage-dependent gating of Shaker A-type potassium channels in Drosophilamuscle. J Gen Physiol. 1990;98:29–60. doi: 10.1085/jgp.95.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]