Abstract
The inositol 1,4,5-trisphosphate (InsP3)-gated Ca channel in cerebellum is tightly regulated by Ca (Bezprozvanny, I., J. Watras, and B.E. Ehrlich. 1991. Nature (Lond.). 351:751–754; Finch, E.A., T.J. Turner, and S.M. Goldin. 1991. Science (Wash. DC). 252:443–446; Hannaert-Merah, Z., J.F. Coquil, L. Combettes, M. Claret, J.P. Mauger, and P. Champeil. 1994. J. Biol. Chem. 269:29642–29649; Iino, M. 1990. J. Gen. Physiol. 95:1103–1122; Marshall, I., and C. Taylor. 1994. Biochem. J. 301:591–598). In previous single channel studies, the Ca dependence of channel activity, monitored at 2 μM InsP3, was described by a bell-shaped curve (Bezprozvanny, I., J. Watras, and B.E. Ehrlich. 1991. Nature (Lond.). 351:751–754). We report here that, when we used lower InsP3 concentrations, the peak of the Ca-dependence curve shifted to lower Ca concentrations. Unexpectedly, when we used high InsP3 concentrations, channel activity persisted at Ca concentrations as high as 30 μM. To explore this unexpected response of the channel, we measured InsP3 binding over a broad range of InsP3 concentrations. We found the well-characterized high affinity InsP3 binding sites (with K d < 1 and 50 nM) (Maeda, N., M. Niinobe, and K. Mikoshiba. 1990. EMBO (Eur. Mol. Biol. Organ.) J. 9:61–67; Mignery, G., T.C. Sudhof, K. Takei, and P. De Camilli. 1989. Nature (Lond.). 342:192–195; Ross, C.A., J. Meldolesi, T.A. Milner, T. Satoh, S. Supattapone, and S.H. Snyder. 1989. Nature (Lond.). 339:468–470) and a low affinity InsP3 binding site (K d = 10 μM). Using these InsP3 binding sites, we developed a new model that accounts for the shift in the Ca-dependence curve at low InsP3 levels and the maintained channel activity at high Ca and InsP3 levels. The observed Ca dependence of the InsP3-gated Ca channel allows the cell to abbreviate the rise of intracellular Ca in the presence of low levels of InsP3, but also provides a means of maintaining high intracellular Ca during periods of prolonged stimulation.
Keywords: cerebellum, ligand binding, intracellular calcium channel, channel regulation
introduction
The inositol 1,4,5-trisphosphate (InsP3)1 receptor is an intracellular calcium (Ca) release channel found in virtually all cell types (Berridge, 1993; Bezprozvanny and Ehrlich, 1995; Clapham, 1995; Divecha and Irvine, 1995). Activation of the InsP3-gated channel causes an increase in cytoplasmic Ca by releasing Ca from the endoplasmic reticulum. InsP3-mediated Ca release is important for many cellular processes, including the expression of transcription factors (Negulescu et al., 1994), the formation of the fertilization envelope during egg activation (Nuccitelli et al., 1993), nuclear membrane reformation in mitosis (Sullivan et al., 1995), stimulus-contraction coupling in smooth muscle (Walker et al., 1987), and the development of long term depression (Kasono and Hirano, 1995).
The InsP3-gated channel exists as a complex comprised of four subunits of 260 kD each. To date, three isoforms of the subunits have been cloned (Furuichi et al., 1989; Mignery et al., 1989; Sudhof et al., 1991; Blondel et al., 1993; Maranto, 1994; Morgan et al., 1996). The isoform type and extent of expression is cell-type specific. The cerebellar Purkinje cell expresses almost exclusively type 1 receptor at levels at least 10× greater than other cell types. Hepatocytes express both type 1 and type 2 receptors, pancreatic acinar cells express type 2 and type 3 receptors, and several epithelia express all three receptor types (Bush et al., 1994; Nathanson et al., 1994; Wojcikiewicz, 1995). Further diversity may exist in tissues where different isoforms associate to form heterotetramers (Joseph et al., 1995; Monkawa et al., 1995).
Each subunit of the tetrameric channel complex contains an InsP3 binding site near the NH2 terminus (Mignery and Sudhof, 1990). InsP3 binds to InsP3-gated channels from cerebellum with high affinity (K d ranging from 5 to 50 nM). An additional low affinity site for InsP3 was described using the InsP3 analog InsP3S3, but the location of the site was unclear because crude microsomes were used (Challiss et al., 1991). Ca inhibits InsP3 binding to the InsP3-gated channel (Worley et al., 1987; Danoff et al., 1988). The Ca-dependent inhibition of InsP3 binding can be reversibly removed by purifying the channel by heparin affinity chromatography, suggesting that Ca sensitivity is conferred by an accessory protein (Danoff et al., 1988).
InsP3 is the only known physiological activator of the InsP3-gated channel. Activation of the channel with InsP3 is reversible: channels stop opening if InsP3 is washed out and readdition of InsP3 reactivates the channels (Ehrlich and Watras, 1988). In the presence of InsP3, Ca acts as an allosteric regulator of the InsP3-gated Ca channel (Iino, 1990; Bezprozvanny et al., 1991; Finch et al., 1991; Hannaert-Merah et al., 1994; Marshall and Taylor, 1994). Previous studies using permeabilized smooth muscle (Iino, 1990), Ca release from cerebellar microsomes (Finch et al., 1991), and single channel recordings (Bezprozvanny et al., 1991) showed that the Ca dependence of channel activity is described by a bell-shaped curve. With single channel recordings, maximal channel activity was observed in the presence of 0.25 μM free Ca and there was a steep decline in channel activity on either side of the maximum (Bezprozvanny et al., 1991). Complete inhibition of channel activity occurred when cytoplasmic Ca reached 5 μM. The Ca-dependent activation provides an amplification of the initial signal and the Ca-dependent inhibition of the InsP3-gated channel allows for fast negative feedback of the cytoplasmic Ca concentration.
A number of models have been proposed to describe the regulation of the InsP3-gated channel. Most models assume three regulatory sites on the channel: one site for InsP3, one site for activating Ca, and one site for inhibitory Ca. Under steady state conditions, these models make different predictions of the shape of the Ca-dependence curve as InsP3 concentrations are varied. As the InsP3 concentration is increased, some models predict that the peak of the Ca-dependence curve will (a) shift to lower Ca concentrations (Othmer and Tang, 1993), (b) shift to higher Ca concentrations (De Young and Keizer, 1992), or (c) remain unchanged (Atri et al., 1993; Bezprozvanny and Ehrlich, 1994).
In this paper, we measured the Ca dependence of InsP3-gated channel activity as a function of InsP3 concentration. We also compared InsP3 binding and channel activity using the same experimental conditions. Using our measured values, we constructed a model that accounts for the interaction of Ca and InsP3 in regulating the InsP3 receptor. The model is distinct from other models currently applied to the InsP3-gated Ca channel and is consistent with the observed leftward shift of the curve at low InsP3 concentrations. A novel feature of the model is the inclusion of a low affinity InsP3 binding site, which broadens the range of regulation of the channel by InsP3 and explains the observed maintained activity of the channel at high concentrations of Ca and InsP3.
methods
Single Channel Recordings
Canine cerebellar endoplasmic reticulum vesicles were prepared as previously described (Ehrlich and Watras, 1988) and fused with planar lipid bilayers composed of phosphatidylethanolamine and phosphatidylserine (3:1, wt:wt; Avanti Polar Lipids, Alabaster, AL) dissolved in decane (20 mg/ml). Cytoplasmic bilayer solutions contained 500 μM ATP, 500 μM EGTA, 110 mM Tris, and 250 mM HEPES, pH 7.35, and luminal solutions contained 53 mM Ba(OH)2, 250 mM HEPES, pH 7.35. Calibrated CaCl2 was added to the cytoplasmic solution to obtain the desired free Ca concentration (Fabiato, 1988). Because estimation of free Ca was critical, calculations were routinely checked spectrofluorometrically with BTC (Molecular Probes, Inc., Eugene, OR). The number of channels in each experiment was estimated from the maximum number of channels observed simultaneously in the bilayer (Horn, 1991). The InsP3 dependence of the open probability, measured at a fixed Ca concentration, was used to correct for variations in the maximum open probability among individual channels. Transmembrane voltage was maintained at 0 mV and the single channel current amplified (Warner Instruments, Hamden, CT) and stored on VHS tape (Instrutech Corp., Great Neck, NY). Data were filtered at 1 kHz and digitized at 5 kHz for computer analysis using pClamp 6.0 (Axon Instruments, Foster City, CA).
InsP3 Binding Measurements
[3H]InsP3 binding was measured as previously described (Benevolensky et al., 1994) except that binding was measured using solutions similar to the cytoplasmic solution in single channel experiments (500 μM ATP, 500 μM EGTA, 110 mM Tris, 250 mM HEPES, pH 7.35, with the specified free Ca concentration). Radioligand concentrations were varied from 0.4 nM to 30 μM. To achieve this range, stock radioligand (480 nM; New England Nuclear, Boston, MA) was diluted with unlabeled InsP3 (Calbiochem, La Jolla, CA). To assure sufficient reliability in the measurement, the protein concentration was increased as the specific activity decreased. Nonspecific binding was measured in the presence of 2.5 mM unlabeled InsP3 or 1–10 mg/ml heparin. These conditions generated the same value for nonspecific binding. To be included in the analysis, specific binding had to exceed 50% of the nonspecific binding. As Scatchard analysis of InsP3 binding showed the presence of three binding sites, InsP3 binding was modeled as shown:
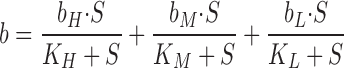 |
1 |
where K H, K M, and K L are the apparent dissociation constants for the 1-nM, 50-nM, and 10-μM sites and b H, b M, and b L refer to the maximum binding at the respective sites.
In most published reports, InsP3 binding experiments were done using conditions that maximize binding, pH 8.0, at 4°C. In contrast, measurements of InsP3-gated channel function have generally been done using conditions closer to physiological conditions, pH 7.3, at 22–37°C. In the experiments described here, measurements were done using similar conditions when possible to compare in vitro channel function with biochemical properties. In addition, InsP3 saturation binding curves were generated at 0 and 22°C to determine the temperature coefficient of binding (Q10). The Q10 was determined to be 2.3 for the 50-nM site and was estimated to be 1.0 for the 10-μM site. Although the Q10 value for the 10-μM site is consistent with our data, it must be called an estimate due to low signal-to-noise ratio in binding measurements at 22°C in combination with the low specific activity obtained when very high InsP3 concentrations are used.
Modeling of Channel Function Using Single Channel Data and Binding
The “2-IP3/2-Ca” model used for the analysis of the open probability data assumes that the InsP3-gated Ca channel complex contains four monomers, and that each monomer of the tetrameric channel complex has two InsP3 binding sites (with apparent dissociation constants K 50nM and K 10μM), one Ca binding site for activation of the channel (CaAC), and one Ca binding site for inhibition of the channel (CaIN). The affinity of the 50-nM site depends upon the occupancy of the CaIN site, as determined from binding experiments in the presence and absence of Ca (see Fig. 3 C and Benevolensky et al., 1994). To fit the model to the data, it was not necessary to include cooperativity of binding among the sites or sequential binding steps.
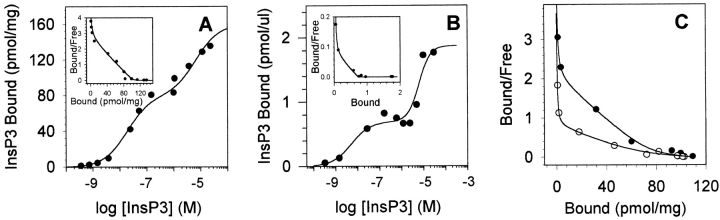
Figure 3.
InsP3 binding reveals three binding sites. (A) Saturation-binding isotherm of InsP3 binding to cerebellar reticular vesicles. InsP3 binding was conducted in the absence of Ca with InsP3 concentrations varying between 0.4 nM and 30 μM. Inset shows the Scatchard analysis. Data were fit assuming the presence of three binding sites for InsP3. Four different vesicle preparations were used; one experiment is displayed. In the absence of Ca, the K d's for the vesicle preparations are 0.47 ± 0.19 nM, 54 ± 5 nM, and 10.2 ± 3.2 μM; x̄ ± SEM, n = 4. (B) Saturation-binding isotherm of InsP3 binding to the type 1 InsP3 receptor purified by heparin-agarose chromatography. Inset shows the Scatchard analysis. Similar results were obtained with a receptor purified from cerebellar microsomes by immunoprecipitation using a type 1-specific antibody (C-19). The purity of the receptor, as assessed from InsP3 binding, was 94% (InsP3 binding was 3,612 pmol/ mg for the 50-nM site, compared with a theoretical maximum binding of 3,846 pmol/mg for a molecular weight of 260 kD). In this panel, the abscissa is expressed as picomoles per microliter of purified InsP3 receptor. K d's for this preparation are 0.5 nM, 29 nM, and 6.8 μM. For comparison, one preparation of InsP3 receptor purified by immunoprecipitation generated K d's of 0.1 nM, 35 nM, and 12 μM. (C) Scatchard analysis of InsP3 binding in the absence of Ca (•) and 10 μM free Ca (○). Increasing the free Ca results in a decrease in the apparent affinity of InsP3 binding from 35 nM in the absence of Ca to 91 nM in the presence of 10 μM Ca without altering binding to the 1-nM or 10-μM sites. The Ca-induced decrease in InsP3 affinity of the 50-nM site was completely reversed upon addition of EGTA.
The presence of four ligands associated with each monomer means there are 16 possible states (24) of each monomer of the tetrameric Ca channel complex. To fit the open probability data over a wide range of InsP3 and Ca concentrations, we had to assume that more than 1 of the 16 states was able to conduct Ca. The simplest model that fit the open probability data and the InsP3 binding data required that 3 of the 16 possible states be capable of conducting Ca. The three possible conducting states are: S1 = InsP3 bound to the K 50nM site, and Ca bound to the CaAC site; S2 = InsP3 bound to the K 50nM and K 10μM sites, and Ca bound to the CaAC site; and S3 = InsP3 bound to the K 50nM and K 10μM sites, and Ca bound to the CaAC and CaIN sites.
The relative abundance (RA) of the three possible conducting states is determined as follows:
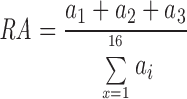 |
2 |
where a i is the calculated fractional abundance of the ith state of the channel based on equilibrium binding constants of the transitions among the 16 states, assuming mass action kinetics. Then, the single channel open probability (P o) is calculated as follows:
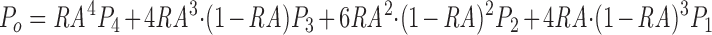 |
3 |
where P i is the probability over time that the channel complex will open assuming that i of the monomers in the channel complex are in one of the conducting states. An iterative curve fitting routine (Sigmaplot; Jandel Scientific, San Rafael, CA) was used to calculate the equilibrium constants of the various transitions that best fit both the single channel data and the InsP3 binding data.
results
InsP3 Shifts the Bell-shaped Calcium-dependence Curve
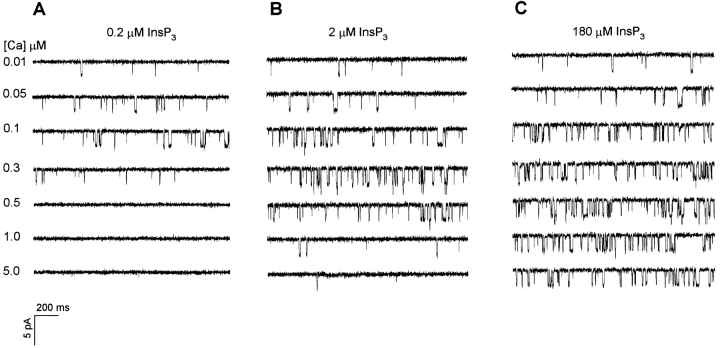
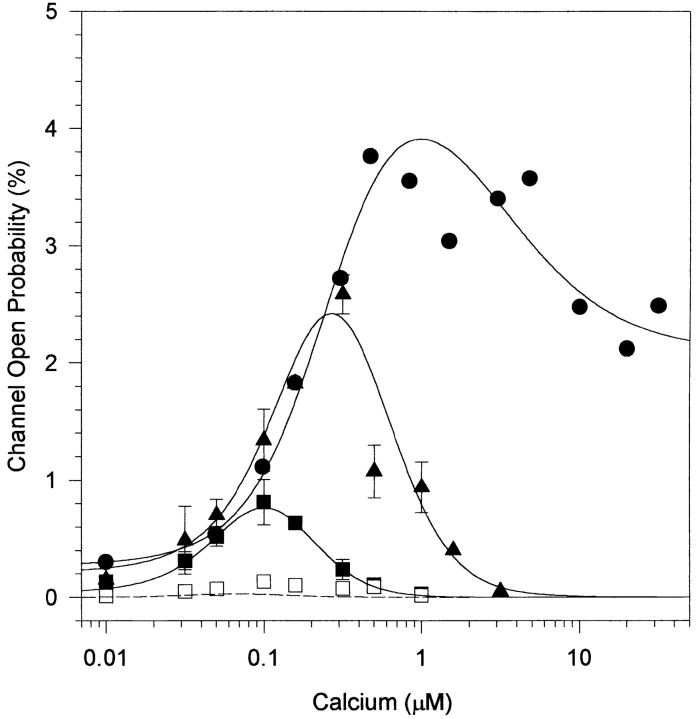
The Ca dependence of the InsP3-gated Ca channel was previously monitored at the single channel level using 2 μM InsP3, a concentration 10× the K d previously determined for InsP3-gated release and channel activity (Watras et al., 1991). Measurements of InsP3-dependent Ca release from vesicles showed that inhibition by Ca varied with InsP3 concentration (Joseph et al., 1989; Combettes et al., 1994; Bootman et al., 1995; Hannaert-Merah et al., 1995). Specifically, as the InsP3 concentration was increased, Ca-dependent inhibition of InsP3-induced Ca release occurred at higher Ca concentrations (Joseph et al., 1989; Combettes et al., 1994; Bootman et al., 1995; Hannaert-Merah et al., 1995). We now report a similar response at the single channel level (Fig. 1). In the presence of either 0.2 or 2 μM InsP3, cytoplasmic Ca activates single channel currents over a similar concentration range (compare Fig. 1, A and B, top 3 traces). As the InsP3 concentration is elevated, activity is maintained at higher cytoplasmic Ca concentrations (Fig. 1, A and B, bottom 4 traces). A comparison of the open probability of the InsP3-gated channel as a function of cytoplasmic Ca concentration shows that the peak channel activity shifts from 0.1 to 0.25 μM Ca when the InsP3 concentration is increased from 0.2 to 2 μM (Fig. 2, ▪ and ▴). However, the activating phase of each curve is quite similar. The predominant effect of raising the InsP3 concentration is an increase in channel activity above 0.1 μM Ca. For example, the channel is essentially closed at 1 μM free Ca in the presence of 0.2 μM InsP3, but there is substantial channel activity in the presence of 2 μM InsP3 .
Figure 1.
Ca dependence of cerebellar InsP3-gated Ca channels at several concentrations of InsP3. Channel activity was observed in the presence of (A) 0.2, (B) 2, and (C) 180 μM InsP3. Free Ca concentrations (μM) are given to the left of each trace. Channel openings are shown as downward deflections. Each panel is taken from one experiment. Activity from multiple channels is seen in many experiments, as in the third and fourth traces of B and C. The single channel conductance is the same at all concentrations of InsP3 tested.
Figure 2.
Open probability of InsP3-gated channels as a function of Ca concentration. Ca-dependence curves were generated at 0.02 (□), 0.2 (▪), 2 (▴), and 180 (•) μM InsP3. Data points represent the mean ± SEM of five experiments for 0.2 and 2 μM InsP3 and one experiment for 0.02 μM InsP3. Data points pooled from three experiments are displayed for 180 μM InsP3 without error bars because the same series of Ca concentrations was not obtained in all experiments. The ordinant shows the open probability for a single channel. Curves through the data were generated by the model described in the text. A dotted line is used to predict channel activity at 0.02 μM InsP3 and solid lines show the fit to the single channel open probability measured at 0.2, 2, and 180 μM InsP3.
If the InsP3 concentration is increased further, an unexpected response of the channel is observed. At 180 μM InsP3, channel activity remains robust at all Ca concentrations tested (Fig. 1 C). Note that the activity measured at 30 μM Ca is essentially the same as that observed at 5 μM Ca (Fig. 2, •). The relatively large open probability of the channel in the presence of 5–30 μM Ca and high InsP3 concentrations was not predicted in published models (De Young and Keizer, 1992; Bezprozvanny, 1994; Tang et al., 1996) of the regulation of the InsP3-gated channel by Ca and InsP3. The persistent activity of the channel at high levels of InsP3 provides a means for the cell to maintain intracellular Ca beyond 1 μM during periods of continued stimulation of the phosphoinositide cascade.
Analysis of [3H]InsP3 Binding Reveals a Low Affinity Site
To investigate the persistent elevation of channel activity in the presence of high concentrations of Ca and InsP3, a series of InsP3 binding experiments were undertaken. When InsP3 concentrations spanning a broad range are used (0.4 nM to 30 μM), the binding data shows three distinct slopes indicating at least three binding sites for InsP3 (Fig. 3 A). Three sites are evident in all four cerebellar preparations tested. The InsP3-gated Ca channel purified by either immunoprecipitation with a type 1-specific InsP3 receptor antibody or by heparin-sepharose column chromatography (Fig. 3 B) also had three distinct binding sites, which indicates that these sites are integral parts of the channel complex. The high affinity (<1 nM) binding site is least abundant, representing <1% of the total sites in all preparations tested. This high affinity site has been observed previously in cerebellum and vascular smooth muscle (Hingorani and Agnew, 1992; Benevolensky et al., 1994). Two binding sites of approximately equal abundance account for the remaining 99% of the InsP3 binding in this tissue; the K d's of the sites when measured at 0°C are 54 nM and 10.2 μM. In this text, these InsP3 binding sites are called the 1-nM, 50-nM, and 10-μM sites. The 50-nM site is the InsP3 binding site that has been shown previously to be concentrated in cerebellar Purkinje cells (Mignery et al., 1989; Ross et al., 1989; Maeda et al., 1990). We report here the existence of a low affinity site that saturates above 10 μM InsP3 (Fig. 3, A and B). The K d of this site is more than 200× higher than those previously shown for the purified InsP3 receptor. The 10-μM site identified in this report may be the site responsible for low affinity InsP3 binding described indirectly in crude cerebellar microsomes (Challiss et al., 1991).
To investigate the inositol phosphate specificity of the two predominant InsP3 binding sites, competition binding was done at two concentrations of InsP3, 10 nM [3H]InsP3 to examine the 50-nM site and 6 μM [3H]InsP3 to examine the 10-μM site (Fig. 4). For each concentration of InsP3, three competitors were examined: 1,3,4,5-InsP4, 1,4,5-InsP3, and 2,4,5-InsP3. At the 50-nM site, 1,4,5-InsP3 is at least 30× more effective than 1,3,4,5-InsP4 at displacing 10 nM [3H]1,4,5-InsP3 from the receptor (Fig. 4 A). The ability of 2,4,5-InsP3 and 1,3,4,5-InsP4 to displace 30 nM [3H]1,4,5-InsP3 from the 50-nM site was indistinguishable. Thus, 1,4,5-InsP3 has the highest affinity at the 50-nM site and both 2,4,5-InsP3 and 1,3,4,5-InsP4 have 30-fold lower affinity for this site. In contrast, 1,3,4,5-InsP4 appears more effective than 1,4,5-InsP3 in its ability to displace 6 μM [3H]1,4,5-InsP3 from the 10-μM site and 6 μM 2,4,5-InsP3 was unable to displace [3H]1,4,5-InsP3 from the 10-μM site (Fig. 4 B). Therefore, the 10-μM site can be distinguished from the 50-nM site by its relative specificity for inositol phosphates.
Figure 4.
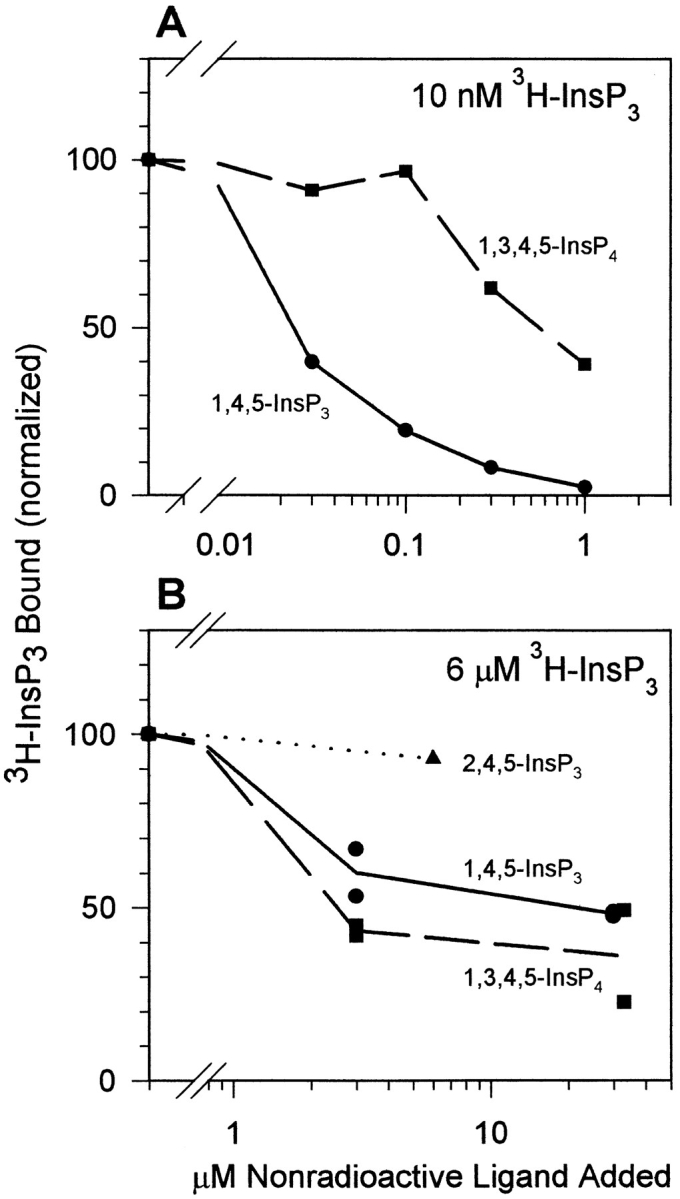
Specificity of inositol phosphate binding to the purified InsP3 receptor. (A) Displacement of 10 nM [3H]1,4,5-InsP3 by unlabeled 1,4,5-InsP3 (•) and 1,3,4,5-InsP4 (▪). This experiment shows that 1,4,5-InsP3 is 30× more effective than 1,3,4,5-InsP4 at the 50-nM site. One of two similar experiments is shown. (B) Displacement of 6 μM [3H]1,4,5-InsP3 by unlabeled 2,4,5-InsP3 (▴), 1,4,5-InsP3 (•), and 1,3,4,5-InsP4 (▪). The two points at each concentration represent two experiments on two different preparations. The points at 30 μM 1,3,4,5-InsP4 were displaced slightly to the right to show that one of the data points falls in the same location as the data points for 30 μM 1,4,5-InsP3. This experiment shows that 2,4,5-InsP3 is ineffective and 1,3,4,5-InsP4 is more effective than 1,4,5-InsP3 at displacing 6 μM [3H]1,4,5-InsP3 from the 10-μM site.
The possibility that other isoforms of the InsP3 receptor were responsible for the 10-μM site was ruled out by comparing the amount of the three InsP3 receptor isoforms in both purified preparations using Western analysis. We obtained strong staining for type 1 and, despite using 10× more protein, virtually no type 2 or 3 InsP3 receptors, confirming published reports that cerebellum contains >90% type 1 InsP3 receptor (Sudhof et al., 1991; Wojcikiewicz, 1995; Morgan et al., 1996). These values need to be compared with the observation that the 50-nM and 10-μM sites are present in approximately equal abundance.
Micromolar concentrations of Ca alter InsP3 binding in a variety of tissues (Pietri et al., 1990; Marshall and Taylor, 1994; Watras et al., 1994). In cerebellum and vascular smooth muscle, Ca decreases InsP3 binding (Worley et al., 1987; Danoff et al., 1988; Benevolensky et al., 1994). Both of these tissues contain predominantly the type 1 InsP3 receptor (Marks et al., 1990; Furuichi et al., 1993; Newton et al., 1994). We find that InsP3 binding to cerebellar membranes under conditions identical to those used for the single channel measurements also shows Ca-dependent inhibition (Fig. 3 C). Only binding to the 50-nM site appears to be Ca sensitive. Elevation of Ca to 10 μM decreases, but does not completely inhibit, InsP3 binding (Fig. 3 C, ○). Similarly, in vascular smooth muscle, Ca concentrations as high as 150 μM failed to completely inhibit InsP3 binding (Benevolensky et al., 1994). In contrast, InsP3 binding to both the 1-nM and 10-μM sites appears insensitive to Ca (Fig. 3 C).
Model of InsP3-gated Channel Function Needs the Novel InsP3 Binding Site
We created a model of InsP3-gated channel function that accounts for the Ca dependence of InsP3 binding (Fig. 3) and the Ca dependence of channel activity (Fig. 2) over a broad range of InsP3 concentrations. This model is named the 2-InsP3/2-Ca model because it incorporates two InsP3 binding sites (50 nM and 10 μM) and two calcium regulatory sites (activating and inhibitory) on each monomer of the tetrameric channel complex. The 1-nM site for InsP3 is not included in the model due to its low abundance. A model incorporating one InsP3 site and two Ca sites previously proposed (De Young and Keizer, 1992) is able to predict channel activity when InsP3 levels are <2 μM, but the one InsP3 binding site model requires parameters that are inconsistent with InsP3 binding data. In addition, this model with one InsP3 binding site cannot predict the persistent activity observed at high concentrations of InsP3 and Ca. In our 2-InsP3/2-Ca model, InsP3 binding to the 10-μM site allows channel activity to be sustained at cytoplasmic Ca concentrations above 5 μM.
Values for the affinity of InsP3 in the absence of Ca were determined from binding experiments (Fig. 3); other parameters were predicted from fits of the model to both the single channel and binding data (Table I). All curves through the experimental points (Figs. 2 and 3) were generated by the 2-InsP3/2-Ca model. Using the parameters generated by the fit of the model to the data, the open probability of the channel can be predicted over a wide range of both InsP3 and Ca. Predictions of channel activity at concentrations of InsP3 and Ca up to 1 mM are shown in Fig. 5.
Table I.
“2-InsP3/2-Ca” Model Parameters
| Affinity for InsP3 binding to the 50-nM site* (−Ca) | 0.3 μM | |
| Affinity for InsP3 binding to the 50-nM site* (+Ca) | 1.5 μM | |
| Affinity for InsP3 binding to the 10-μM site* | 10 μM | |
| Affinity for Ca binding to its activating site | 0.03 μM | |
| Affinity for Ca binding to its inhibitory site | 1.0 μM |
The name of the site corresponds to the apparent affinity obtained at 0°C. The values shown in the table and used for fits to the data of Fig. 2 were, when possible, measured directly at 22°C or calculated from the fits to the data of Fig. 2. The experiments shown in Fig. 2 were performed at 22°C.
Figure 5.
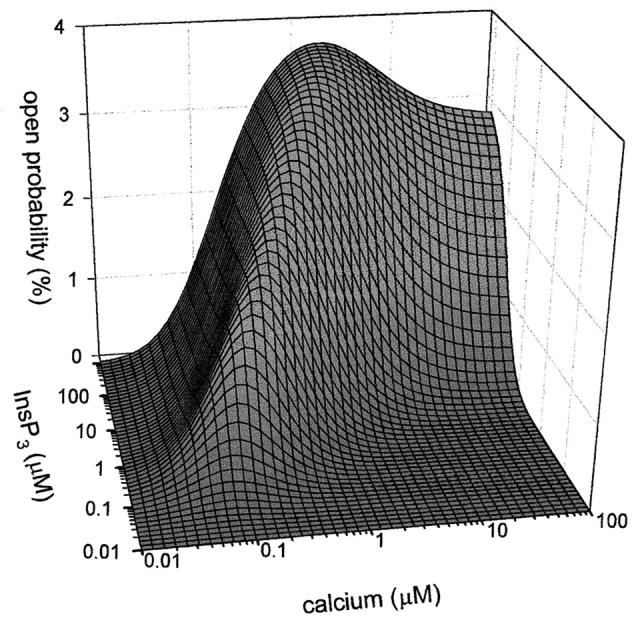
Prediction of the Ca and InsP3 dependence of the open probability using the data in Figs. 2 and 3. The 2-IP3/2-Ca model used for the analysis of the open probability data assumes that the InsP3-gated Ca channel complex contains four monomers, and that each monomer of the tetrameric channel complex has two InsP3 binding sites, one Ca binding site for activation of the channel, and one Ca binding site for inhibition of the channel. Details of the model are included in methods.
An outcome of the 2-IP3/2-Ca model is that at least two of the four monomers of the tetrameric Ca channel complex must be in one of the three possible conducting states for the channel complex to conduct Ca. That is, if only one of the monomers in the tetrameric complex is in one of the conducting states (P 1 in Eq. 3), the predicted value for the probability that the channel will open was 10−11, suggesting that the singly occupied channel rarely opens. In contrast, if two or three monomers are occupied, the predicted values for the probability that the channel will open were 0.06 and 0.04, respectively. The requirement for at least two InsP3 molecules to bind to the receptor is supported by experimental findings. An extension of our earlier experiments describing the InsP3 concentration dependence of the open probability of the channel (Watras et al., 1991) to lower concentration of InsP3 (10 nM, data not shown) generates a curve with a Hill coefficient of 1.8. This result and additional reports (Somlyo et al., 1992; Marchant et al., 1997) support the suggestion that multiple molecules of InsP3 bind to the channel before it opens.
As a further test of the model, single channel behavior was measured at 20 nM InsP3 (Fig. 2, □). At this very low InsP3 concentration, channel activity was difficult to measure because openings were infrequent, but the shape of the Ca dependence and the peak in channel activity were similar to the predicted values. Although other models could be generated, the ability of the 2-InsP3/2-Ca model to fit both the single channel and binding data and to predict InsP3-gated channel function under a variety of conditions lends support for this model of channel function.
discussion
In this paper, we tested the effect of changing the InsP3 concentration on the regulation of the InsP3 receptor. We found that the peak of channel activity shifted to higher Ca concentrations as the InsP3 concentration was increased from 20 nM to 2 μM and that elevating the InsP3 concentration above 2 μM leads to persistent activation of the InsP3-gated channel. To explain this unexpected response, we measured InsP3 binding at InsP3 concentrations from 0.4 nM to 30 μM. We found the well-characterized high affinity InsP3 binding sites (with K d's < 1 and 50 nM) (Mignery et al., 1989; Ross et al., 1989; Maeda et al., 1990) and a low affinity InsP3 binding site (K d = 10 μM). We then developed a new model that accounts for both the channel activity and the InsP3 binding properties over the entire range of InsP3 and Ca concentrations tested.
We measured a large increase in steady state channel activity by elevating the InsP3 concentration (Fig. 1). It is interesting to note that these changes occurred in the absence of other cellular processes that have been implicated in the regulation of InsP3-gated channel activity. For example, it has been proposed that the activating phase of the calcium dependence curve relies upon phosphorylation of the channel by protein kinase C and that the inhibitory phase of the calcium-dependence curve reflects the dephosphorylation of the channel by calcineurin (Cameron et al., 1995). In this series of experiments, the increase in channel activity is unlikely to be attributed to phosphorylation because Mg-ATP was not present (Na-ATP was used in the experimental protocol) and no kinase was added to the system. Similarly, it is unlikely that the decrease in channel activity measured in these experiments is the consequence of calcineurin activity because, in preliminary experiments, we were unable to detect calcineurin in our microsomal preparation. The ability to reverse the effects of elevated Ca in the absence of added kinase or Mg-ATP in our experiments also argues against an absolute requirement for phosphorylation/dephosphorylation in the Ca-dependent regulation of InsP3-gated channel activity.
The mechanism underlying the Ca-dependent inhibition of InsP3 binding to the 50-nM InsP3 binding site is unclear, but may involve the presence of an accessory protein associated with the InsP3 receptor (Danoff et al., 1988; Ferris and Snyder, 1992; Benevolensky et al., 1994). Our experiments suggest that the 10-μM site cannot reside on the associated protein that confers Ca sensitivity to the InsP3 receptor. The InsP3 receptor purified with heparin affinity chromatography is calculated to be 94% pure and it lacks Ca sensitivity. The heparin affinity purified channel, however, does retain the 10-μM site in an approximately one-to-one stoichiometry with the 50-nM InsP3 binding site. Other proteins thought to associate with the InsP3 receptor do not appear to bind InsP3 (e.g., FKBP12, calcineurin), strongly implying that the 10-μM site resides on the InsP3 receptor.
Two properties of the 10-μM site are crucial for InsP3-gated channel function. First, binding of InsP3 to the 10-μM site is not Ca dependent (Fig. 3). This allows InsP3 to remain bound to the receptor even in the presence of high Ca. Indeed, even in the presence of 150 μM Ca it was not possible to remove all of the InsP3 (Benevolensky et al., 1994). With this site, the channel can remain open even when cytoplasmic Ca is at micromolar concentrations. Second, the existence of a binding site with affinity orders of magnitude different from previously identified binding sites provides a wider dynamic range over which the channel can function.
Three models have been proposed for the regulation of the InsP3-gated Ca channel (De Young and Keizer, 1992; Atri et al., 1993; Othmer and Tang, 1993; Bezprozvanny and Ehrlich, 1994). Only one of these models (De Young and Keizer, 1992), however, predicts a rightward shift in the Ca dependence as the InsP3 concentration is increased from 0 to 2 μM. This model included only one binding site for InsP3 and it gave reasonable fits of our single channel data over the range of InsP3 concentrations from 0 to 2 μM. However, this model was unable to fit our InsP3 binding data over the same range of InsP3 concentrations with the parameters used to fit the single channel data. Moreover, the one InsP3 binding site model cannot explain the persistence of channel activity at very high concentrations of InsP3 and high levels of Ca.
When InsP3-induced Ca release was measured in Purkinje cells of rat cerebellar slices, the cytoplasmic free Ca was elevated to 26 μM (Khodakhah and Ogden, 1995), well beyond the Ca concentration one would have initially expected for InsP3-mediated Ca release. Indeed, it was thought that this could never happen because the InsP3-gated channel would be closed by 5 μM cytoplasmic Ca. The data in the present paper show that intracellular Ca in the tens of micromolar range could be achieved by an InsP3-dependent pathway. Moreover, the ability of the channel to remain open at high intracellular Ca occurs when InsP3 concentrations are elevated, which is consistent with the need for InsP3 concentrations of 9 μM and higher to induce Ca release in intact Purkinje cells (Khodakhah and Ogden, 1993, 1995). Thus, the values for intracellular Ca predicted by the model presented here (see Fig. 5) are within the range found in intact Purkinje cells.
The interaction between InsP3 and Ca in the regulation of the InsP3-gated channel (Fig. 2) also may explain the pattern of cytoplasmic Ca oscillations evoked by InsP3 in pancreatic acinar cells. These cells generate Ca oscillations in the continued presence of low concentrations of InsP3. The oscillations are a consequence, at least in part, of the Ca-dependent inhibition of Ca release through the InsP3-gated channel. When InsP3 levels are ≥50 μM, sustained elevations in cytoplasmic Ca are observed (Wakui et al., 1989; Petersen et al., 1991). The ability to sustain an elevation in intracellular Ca in the presence of high concentrations of InsP3 is consistent with the response of the InsP3-gated channel seen in the presence of high concentrations of InsP3 (Fig. 2 C).
High concentrations of InsP3 normally exist in a number of cell types under basal (0.1–3 μM InsP3) and agonist-induced (1–20 μM InsP3) conditions (Putney, 1990). These values for intracellular InsP3 concentrations may actually be underestimates when confined portions of the cell, such as dendrites, are considered. That many cell types have resting and stimulated concentrations of InsP3 thought to be super saturating now has a purpose, to provide a prolonged elevation of intracellular Ca and to provide a larger dynamic range for InsP3-mediated Ca signaling.
In summary, we show that the interactions between Ca and InsP3 with the InsP3-gated channel are complex (Fig. 5). The ability of Ca to regulate the activity of the InsP3 receptor within the expected range of intracellular Ca concentrations by interacting directly with the channel complex has been useful for understanding Ca signaling, waves, and oscillations (Allbritton and Meyer, 1993; Berridge, 1993; Bezprozvanny, 1994; Clapham, 1995). The presence of the newly identified InsP3 binding site on the purified InsP3 receptor provides physiological relevance for the seemingly high levels of basal (0.1–3 μM) and agonist-induced InsP3 concentrations (1–20 μM; Putney, 1990). Our results and model show that more complex interactions among the regulatory ligands are needed to explain InsP3-gated channel function. The expanded relationship between InsP3 and Ca demonstrated in the present paper is of great functional importance as a cell is able to overcome Ca-induced channel inhibition during sustained stimulation by producing more InsP3.
Acknowledgments
The authors thank William Dyckman for his assistance in obtaining canine cerebella. We thank Drs. Joel Brown, Knox Chandler, Larry Cohen, and Frank Striggow for critical comments on the manuscript. Dr. Greg Mignery graciously provided antibody specific for the type 2 InsP3 receptor. We thank Dr. Ion Moraru for invaluable discussions regarding the modeling.
This work was supported by National Institutes of Health grants HL-33026 and GM-51480.
Abbreviation used in this paper
- InsP3
inositol 1,4,5-trisphosphate
Footnotes
B.E. Ehrlich's present address is Department of Pharmacology, Yale University, New Haven, CT 06510. E.J. Kaftan's present address is Department of Physiology and Biophysics, University of Washington, Seattle, WA 98195.
references
- Allbritton NL, Meyer T. Localized calcium spikes and propagating calcium waves. Cell Calcium. 1993;14:691–697. doi: 10.1016/0143-4160(93)90095-n. [DOI] [PubMed] [Google Scholar]
- Atri A, Amundson J, Clapham D, Sneyd J. A single-pool model for intracellular calcium oscillations and waves in the Xenopus laevisoocyte. Biophys J. 1993;65:1727–1739. doi: 10.1016/S0006-3495(93)81191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benevolensky D, Moraru I, Watras J. Micromolar calcium reduces the affinity of the inositol 1,4,5-trisphosphate receptor in smooth muscle. Biochem J. 1994;299:631–636. doi: 10.1042/bj2990631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ. Inositol trisphosphate and calcium signalling. Nature (Lond) 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I. Theoretical analysis of calcium wave propagation based on inositol (1,4,5)-trisphosphate (InsP3) receptor functional properties. Cell Calcium. 1994;16:151–166. doi: 10.1016/0143-4160(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I, Ehrlich B. Inositol (1,4,5)-trisphosphate gated Ca channels from canine cerebellum: divalent cation conduction properties and regulation by intraluminal Ca. J Gen Physiol. 1994;104:821–856. doi: 10.1085/jgp.104.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny I, Ehrlich BE. The inositol 1,4,5-trisphosphate (InsP3) receptor. J Membr Biol. 1995;145:205–216. doi: 10.1007/BF00232713. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I, Watras J, Ehrlich BE. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature (Lond) 1991;351:751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- Blondel O, Takeda J, Janssen H, Seino S, Bell GI. Sequence and functional characterization of a third inositol trisphosphate receptor subtype, IP3R-3, expressed in pancreatic islets, gastrointestinal tract, and other tissues. J Biol Chem. 1993;268:11356–11363. [PubMed] [Google Scholar]
- Bootman MD, Missiaen L, Parys JB, DeSmedt H, Casteels R. Control of inositol 1,4,5-trisphosphate-induced Ca2+ release by cytosolic Ca2+ . Biochem J. 1995;306:445–451. doi: 10.1042/bj3060445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush KT, Stuart RO, Li SH, Moura LA, Sharp AH, Ross CA, Nigam SK. Epithelial inositol 1,4,5-trisphosphate receptors: multiplicity of localization, solubility, and isoforms. J Biol Chem. 1994;269:23694–23699. [PubMed] [Google Scholar]
- Cameron AM, Steiner JP, Sabatini DM, Kaplin AI, Walensky LD, Snyder SH. Immunophilin FK506 binding protein associated with inositol 1,4,5-trisphosphate receptor modulates calcium flux. Proc Natl Acad Sci USA. 1995;92:1784–1788. doi: 10.1073/pnas.92.5.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challiss RAJ, Smith SM, Potter BVL, Nahorski SR. D- < S-35(U) > inositol 1,4,5-trisphosphorothioate, a novel radioligand for the inositol 1,4,5-trisphosphate receptor. Complex binding to rat cerebellar membranes. FEBS Lett. 1991;281:101–104. doi: 10.1016/0014-5793(91)80368-d. [DOI] [PubMed] [Google Scholar]
- Clapham DE. Calcium signaling. Cell. 1995;80:259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- Combettes L, Hannaert-Merah Z, Coquil JF, Rousseau C, Claret M, Swillens S, Champeil P. Rapid filtration studies of the effect of cytosolic Ca2+ on inositol 1,4,5-trisphosphate-induced 45Ca2+ release from cerebellar microsomes. J Biol Chem. 1994;269:17561–17571. [PubMed] [Google Scholar]
- Danoff SK, Supattapone S, Snyder SH. Characterization of a membrane protein from brain mediating the inhibition of inositol 1,4,5-trisphosphate receptor binding by calcium. Biochem J. 1988;254:701–705. doi: 10.1042/bj2540701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Young GW, Keizer J. A single-pool inositol 1,4,5-trisphosphate-receptor-based model for agonist-stimulated oscillations in Ca concentration. Proc Natl Acad Sci USA. 1992;89:9895–9899. doi: 10.1073/pnas.89.20.9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divecha N, Irvine RF. Phospholipid signaling. Cell. 1995;80:269–278. doi: 10.1016/0092-8674(95)90409-3. [DOI] [PubMed] [Google Scholar]
- Ehrlich BE, Watras J. Inositol 1,4,5-trisphosphate activates a channel from smooth muscle sarcoplasmic reticulum. Nature (Lond) 1988;336:583–586. doi: 10.1038/336583a0. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Ferris CD, Snyder SH. Inositol 1,4,5-trisphosphate-activated calcium channels. Annu Rev Physiol. 1992;54:469–488. doi: 10.1146/annurev.ph.54.030192.002345. [DOI] [PubMed] [Google Scholar]
- Finch EA, Turner TJ, Goldin SM. Calcium as a coagonist of inositol 1,4,5-trisphosphate-induced calcium release. Science (Wash) 1991;252:443–446. doi: 10.1126/science.2017683. [DOI] [PubMed] [Google Scholar]
- Furuichi T, Simon-Chazottes D, Fujino I, Yamada N, Hasegawa M, Miyawaki A, Yoshikawa S, Guenet J, Mikoshiba K. Widespread expression of inositol 1,4,5-trisphosphate receptor type 1 gene (Insp3r1) in the mouse central nervous system. Receptors Channels. 1993;1:11–24. [PubMed] [Google Scholar]
- Furuichi T, Yoshikawa S, Miyawaki A, Wada K, Maeda N, Mikoshiba K. Primary structure and functional expression of the inositol 1,4,5-trisphosphate-binding protein P400 . Nature (Lond) 1989;342:32–38. doi: 10.1038/342032a0. [DOI] [PubMed] [Google Scholar]
- Hannaert-Merah Z, Combettes L, Coquil J-F, Swillens S, Mauger J-P, Claret M, Champeil P. Characterization of the co-agonist effects of strontium and calcium on myo-inositol trisphosphate-dependent ion fluxes in cerebellar microsomes. Cell Calcium. 1995;18:390–399. doi: 10.1016/0143-4160(95)90054-3. [DOI] [PubMed] [Google Scholar]
- Hannaert-Merah Z, Coquil JF, Combettes L, Claret M, Mauger JP, Champeil P. Rapid kinetics of myo-inositol trisphosphate binding and dissociation in cerebellar membranes. J Biol Chem. 1994;269:29642–29649. [PubMed] [Google Scholar]
- Hingorani SR, Agnew WS. Assay and purification of neuronal receptors for inositol 1,4,5-trisphosphate. Methods Enzymol. 1992;207:573–591. doi: 10.1016/0076-6879(92)07041-l. [DOI] [PubMed] [Google Scholar]
- Horn R. Estimating the number of channels in patch recordings. Biophys J. 1991;60:433–439. doi: 10.1016/S0006-3495(91)82069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M. Biphasic Ca2+ dependence of inositol 1,4,5-trisphosphate-induced Ca release in smooth muscle cells of the guinea pig taenia caeci. . J Gen Physiol. 1990;95:1103–1122. doi: 10.1085/jgp.95.6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph SK, Lin C, Pierson S, Thomas AP, Maranto AR. Heteroligomers of type-I and type-III inositol trisphosphate receptors in WB rat liver epithelial cells. J Biol Chem. 1995;270:23310–23316. doi: 10.1074/jbc.270.40.23310. [DOI] [PubMed] [Google Scholar]
- Joseph SK, Rice HL, Williamson JR. The effect of external calcium and pH on inositol trisphosphate-mediated calcium release from cerebellum microsomal fractions. Biochem J. 1989;258:261–265. doi: 10.1042/bj2580261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasono K, Hirano T. Involvement of inositol trisphosphate in cerebellar long-term depression. Neuroreport. 1995;6:569–572. doi: 10.1097/00001756-199502000-00040. [DOI] [PubMed] [Google Scholar]
- Khodakhah K, Ogden D. Fast activation of inositol trisphosphate-evoked Ca2+release in rat cerebellar Purkinje neurones. J Physiol (Camb) 1995;487:343–358. doi: 10.1113/jphysiol.1995.sp020884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodakhah K, Ogden D. Functional heterogeneity of calcium release by inositol trisphosphate in single Purkinje neurones, cultured cerebellar astrocytes, and peripheral tissues. Proc Natl Acad Sci USA. 1993;90:4976–4980. doi: 10.1073/pnas.90.11.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda N, Niinobe M, Mikoshiba K. A cerebellar Purkinje cell marker P400 protein is an inositol 1,4,5-trisphosphate (InsP3) receptor protein. Purification and characterization of InsP3receptor complex. EMBO (Eur Mol Biol Organ) J. 1990;9:61–67. doi: 10.1002/j.1460-2075.1990.tb08080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maranto AR. Primary structure, ligand binding, and localization of the human type 3 inositol 1,4,5-trisphosphate receptor expressed in intestinal epithelium. J Biol Chem. 1994;269:1222–1230. [PubMed] [Google Scholar]
- Marchant JS, Chang Y-T, Chung S-K, Irvine RF, Taylor CW. Rapid kinetic measurements of 45Ca2+ mobilization reveal that Ins(2,4,5)P3 is a partial agonist at hepatic InsP3 receptors. Biochem J. 1997;321:573–576. doi: 10.1042/bj3210573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks AR, Tempst P, Chadwick CC, Riviere L, Fleischer S, Nadal-Ginard B. Smooth muscle and brain inositol 1,4,5-trisphosphate receptors are structurally and functionally similar. J Biol Chem. 1990;265:20719–20722. [PubMed] [Google Scholar]
- Marshall I, Taylor C. Two calcium binding sites mediate the interconversion of liver inositol 1,4,5-trisphosphate receptors between three conformations states. Biochem J. 1994;301:591–598. doi: 10.1042/bj3010591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignery G, Sudhof TC, Takei K, De Camilli P. Putative receptor for inositol 1,4,5-trisphosphate similar to ryanodine receptor. Nature (Lond) 1989;342:192–195. doi: 10.1038/342192a0. [DOI] [PubMed] [Google Scholar]
- Mignery GA, Sudhof TC. The ligand binding site and transduction mechanism in the inositol-1,4,5-triphosphate receptor. EMBO (Eur Mol Biol Organ) J. 1990;9:3893–3898. doi: 10.1002/j.1460-2075.1990.tb07609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monkawa T, Miyawaki A, Sugiyama T, Yoneshima H, Yamamoto-Hino M, Furuichi T, Saruta T, Hasegawa M, Mikoshiba K. Heterotetrameric complex formation of inositol 1,4,5-trisphosphate receptor subunits. J Biol Chem. 1995;270:14700–14704. doi: 10.1074/jbc.270.24.14700. [DOI] [PubMed] [Google Scholar]
- Morgan JM, Gillespie JI, De Smedt H. Identification of three isoforms of the InsP3receptor in human myometrial smooth muscle. Pflügers Arch. 1996;431:697–705. doi: 10.1007/BF02253832. [DOI] [PubMed] [Google Scholar]
- Nathanson MH, Fallon MB, Padfield PJ, Maranto AR. Localization of the type 3 inositol 1,4,5-trisphosphate receptor in the Ca2+wave trigger zone of the pancreatic acinar cells. J Biol Chem. 1994;269:4693–4696. [PubMed] [Google Scholar]
- Negulescu PA, Shastri N, Cahalan MD. Intracellular calcium dependence of gene expression in single T lymphocytes. Proc Natl Acad Sci USA. 1994;91:2873–2877. doi: 10.1073/pnas.91.7.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton C, Mignery G, Sudhof T. Co-expression in vertebrate tissues and cell lines of multiple inositol 1,4,5-trisphosphate (InsP3) receptors with distinct affinities for InsP3 . J Biol Chem. 1994;269:28613–28619. [PubMed] [Google Scholar]
- Nuccitelli R, Yim DL, Smart T. The sperm-induced Ca2+ wave following fertilization of the Xenopusegg requires the production of Ins(1,4,5)P(3) Dev Biol. 1993;158:200–212. doi: 10.1006/dbio.1993.1179. [DOI] [PubMed] [Google Scholar]
- Othmer, H.G., and Y. Tang. 1993. Oscillations and waves in a model calcium dynamics. In Experimental and Theoretical Advances in Biological Pattern Formation. H.G. Othmer, J. Murray, and P. Maini, editors. Plenum Press, London. 295–319.
- Petersen CCH, Toescu EC, Potter BVL, Petersen OH. Inositol trisphosphate produces different patterns of cytoplasmic Ca2+ spiking depending on its concentration. FEBS Lett. 1991;293:179–182. doi: 10.1016/0014-5793(91)81181-7. [DOI] [PubMed] [Google Scholar]
- Pietri F, Hilly M, Mauger J-P. Calcium mediates the interconversion between two states of the liver inositol 1,4,5-trisphosphate receptor. J Biol Chem. 1990;265:17478–17485. [PubMed] [Google Scholar]
- Putney J. The integration of receptor-regulated intracellular calcium release and calcium entry across the plasma membrane. Curr Top Cell Regul. 1990;31:111–127. doi: 10.1016/b978-0-12-152831-7.50004-4. [DOI] [PubMed] [Google Scholar]
- Ross CA, Meldolesi J, Milner TA, Satoh T, Supattapone S, Snyder SH. Inositol 1,4,5-trisphosphate receptor localized to endoplasmic reticulum in cerebellar Purkinje neurons. Nature (Lond) 1989;339:468–470. doi: 10.1038/339468a0. [DOI] [PubMed] [Google Scholar]
- Somlyo AV, Horiuti K, Trentham DR, Kitazawa T, Somlyo AP. Kinetics of Ca2+ release and contraction induced by photolysis of caged d-myo-inositol 1,4,5-trisphosphate in smooth muscle. J Biol Chem. 1992;267:22316–22322. [PubMed] [Google Scholar]
- Sudhof TC, Newton CL, Archer BT, Ushkaryov YA, Mignery GA. Structure of a novel InsP3receptor. EMBO (Eur Mol Biol Organ) J. 1991;10:3199–3206. doi: 10.1002/j.1460-2075.1991.tb04882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan KMC, Lin DD, Agnew W, Wilson KL. Inhibition of nuclear vesicle fusion by antibodies that block activation of inositol 1,4,5-trisphosphate receptors. Proc Natl Acad Sci USA. 1995;92:8611–8615. doi: 10.1073/pnas.92.19.8611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Stephenson JL, Othmer HG. Simplification and analysis of models of calcium dynamics based on IP3-sensitive calcium channel kinetics. Biophys J. 1996;70:246–263. doi: 10.1016/S0006-3495(96)79567-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakui M, Potter BVL, Petersen OH. Pulsatile intracellular calcium release does not depend on fluctuations in inositol trisphosphate concentration. Nature (Lond) 1989;339:317–320. doi: 10.1038/339317a0. [DOI] [PubMed] [Google Scholar]
- Walker JW, Somlyo AV, Goldman YE, Somlyo AP, Trentham DR. Kinetics of smooth and skeletal muscle activation by laser pulse photolysis of caged inositol 1,4,5-trisphosphate. Nature (Lond) 1987;327:249–252. doi: 10.1038/327249a0. [DOI] [PubMed] [Google Scholar]
- Watras J, Bezprozvanny I, Ehrlich BE. Inositol 1,4,5-trisphosphate-gated channels in cerebellum—presence of multiple conductance states. J Neurosci. 1991;11:3239–3245. doi: 10.1523/JNEUROSCI.11-10-03239.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watras J, Moraru I, Costa DJ, Kindman LA. Two inostiol 1,4,5-trisphosphate binding sites in rat basophilic leukemia cells: relationship between receptor occupancy and calcium release. Biochemistry. 1994;33:14359–14367. doi: 10.1021/bi00251a050. [DOI] [PubMed] [Google Scholar]
- Wojcikiewicz RJH. Type I,II,III inositol 1,4,5-trisphosphate receptors are unequally susceptible to down-regulation and are expressed in markedly different proportions in different cell types. J Biol Chem. 1995;270:11678–11683. doi: 10.1074/jbc.270.19.11678. [DOI] [PubMed] [Google Scholar]
- Worley PF, Baraban JM, Supattapone S, Wilson V, Snyder SH. Characterization of inositol trisphosphate receptor binding in brain. J Biol Chem. 1987;262:12132–12136. [PubMed] [Google Scholar]