Abstract
ATP-sensitive potassium (KATP) channels link cellular metabolism to electrical activity in nerve, muscle, and endocrine tissues. They are formed as a functional complex of two unrelated subunits—a member of the Kir inward rectifier potassium channel family, and a sulfonylurea receptor (SUR), a member of the ATP-binding cassette transporter family, which includes cystic fibrosis transmembrane conductance regulators and multidrug resistance protein, regulators of chloride channel activity. This recent discovery has brought together proteins from two very distinct superfamilies in a novel functional complex. The pancreatic KATP channel is probably formed specifically of Kir6.2 and SUR1 isoforms. The relationship between SUR1 and Kir6.2 must be determined to understand how SUR1 and Kir6.2 interact to form this unique channel. We have used mutant Kir6.2 subunits and dimeric (SUR1-Kir6.2) constructs to examine the functional stoichiometry of the KATP channel. The data indicate that the KATP channel pore is lined by four Kir6.2 subunits, and that each Kir6.2 subunit requires one SUR1 subunit to generate a functional channel in an octameric or tetradimeric structure.
Keywords: potassium channel, adenosine triphosphate–binding cassette protein, sulfonylurea receptor, inward rectifier
introduction
ATP-sensitive potassium (KATP) channels are unique in requiring two structurally distinct subunits to generate functional channels. One subunit (Kir6.2) is a member of the inward rectifier family of potassium channels (Inagaki et al., 1995a ; Nichols and Lopatin, 1997), while the other (SUR1, a high affinity sulfonylurea receptor; Aguilar-Bryan et al., 1995) is a member of the ATP-binding cassette (ABC)1 superfamily (Higgins, 1995). Expression of either subunit alone normally fails to generate active channels, while coexpression of Kir6.1 or Kir6.2 subunits with SUR1 or SUR2 subunits forms KATP channels with properties identical to those described in pancreatic β cells and cardiac myocytes (Noma, 1983; Ashcroft, 1988; Inagaki et al., 1995a , 1996). The requirement for both subunits raises the possibility that each one contributes to formation of the channel pore in an arrangement other than the homotetrameric array that has been shown for other K+ channels (MacKinnon, 1991; Glowatzki et al., 1995; Yang et al., 1995).
In the present study, we have examined the stoichiometry of channel formation using mutant Kir6.2 subunits and dimeric SUR1-Kir6.2 constructs. Mutation of asparagine to aspartate in the pore of Kir6.2 leads to the generation of strongly inwardly rectifying channels (as previously demonstrated for similar mutations in Kir1.1 channels; Lu and MacKinnon, 1994), as a result of induction of high sensitivity to block by intracellular spermine (Lopatin et al., 1994; Shyng et al., 1997a ). By coexpressing wild-type and mutant Kir6.2 subunits, we generated heteromeric channels with intermediate rectification properties. Examination of current–voltage relationships from these heteromeric channels reveals that the KATP channel pore is likely to be formed as a tetrameric arrangement of Kir6.2 subunits, as shown previously for voltage-gated K+ channels and homomeric inward rectifier channels (MacKinnon, 1991; Glowatzki et al., 1995; Yang et al., 1995). Expression of dimeric SUR1-Kir6.2 constructs results in functional channels with very similar properties to those formed from coexpression of the parental monomers, consistent with a 1:1 SUR1/Kir6.2 stoichiometry being sufficient to form the KATP channel. Additional experiments with coexpression of dimers and monomers indicate that a 1:1 stoichiometry may be preferred, leading to the conclusion that the KATP channel is likely to be formed as an octameric complex of four Kir6.2 subunits and four SUR1 subunits.
materials and methods
Expression of KATP Channels in COSm6 Cells
COSm6 cells were plated at a density of 2.5 × 105 cells per well (30-mm six-well dishes) and cultured in Dulbecco's Modified Eagle Medium plus 10 mM glucose (DMEM-HG), supplemented with fetal calf serum (10%). 24 h later, monomeric hamster SUR1 (in pECE vector; Aguilar-Bryan et al., 1995), mouse Kir6.2 (in pCMV vector, Inagaki et al., 1995a ), or dimeric SUR1-Kir6.2 (in pECE vector) were cotransfected into the COSm6 cells with diethylaminoethyl-dextran (0.5 mg/ml). Cells were incubated for 2 min in HEPES-buffered salt solution containing DMSO (10%), and then for 4 h in DMEM-HG plus 2% FCS and chloroquine (100 μM), and then returned to DMEM-HG plus 10% FCS. For 86Rb+ flux experiments, 86RbCl (1 μCi/ml) was added in fresh DMEM-HG containing FCS (10%) 24 h after transfection. Cells were incubated for 12–24 h before measurement of Rb efflux. For efflux measurements, cells were incubated for 30 min at 25°C in Krebs' Ringer solution, with or without metabolic inhibitors (2.5 μg/ml oligomycin plus 1 mM 2-deoxy-d-glucose) or glibenclamide. At selected time points, the solution was aspirated from the cells and replaced with fresh solution. The 86Rb+ in the aspirated solution was counted.
Patch-Clamp Measurements
1–3 d after transfection, patch-clamp experiments were made at room temperature in an oil-gate chamber (Lederer and Nichols, 1989). Micropipettes were pulled from thin-walled glass (WPI, New Haven, CT) on a horizontal puller (Sutter Instruments, Co., Novato, CA), fire polished, and the tips were coated with a 1:1 mixture of light mineral oil and Parafilm (American National Can Co., Greenwich, CT), to reduce capacitive currents. Electrode resistance was typically 0.5–1 MΩ when filled with K-INT solution (see below). Microelectrodes were ‘sealed' onto cells by applying light suction to the rear of the pipette. Inside-out patches were obtained by lifting the electrode, and then passing the electrode tip through the oil gate. Membrane patches were voltage-clamped with an Axopatch 1B patch-clamp (Axon Instruments, Foster City, CA). PClamp software and a Labmaster TL125 D/A converter (Axon Instruments) were used to generate voltage pulses. Data was normally filtered at 0.5–3 kHz, signals were digitized at 22 kHz (Neurocorder; Neurodata, New York) and stored on video tape. Experiments were replayed onto a chart recorder or digitized into a microcomputer using Axotape software (Axon Instruments). The standard bath (intracellular) solution used throughout these experiments (K-INT) had the following composition (mM): 140 KCl, 10 K-HEPES, 1 K-EGTA, with additions as described. The solution pH was 7.3. The pipette solution was either also K-INT, or Na-INT in which 140 mM KCl was replaced by 140 mM NaCl. Off-line analysis was performed using ClampFit and Microsoft Excel programs.Unless otherwise indicated, data are given as mean ± SEM.
results
KATP Channels Are Assembled from Four Kir6.2 Subunits
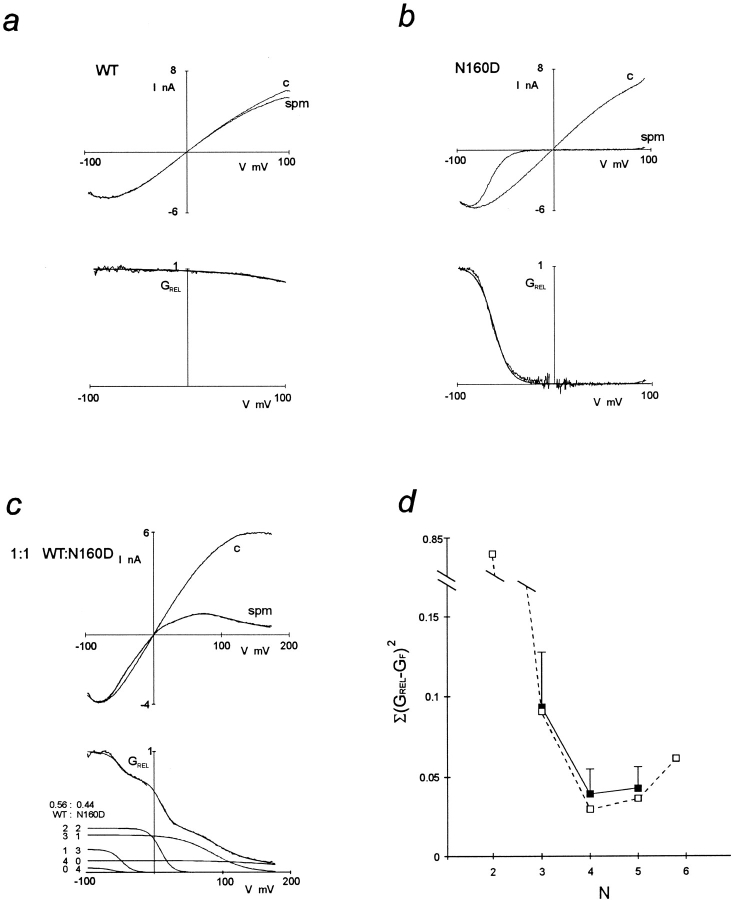
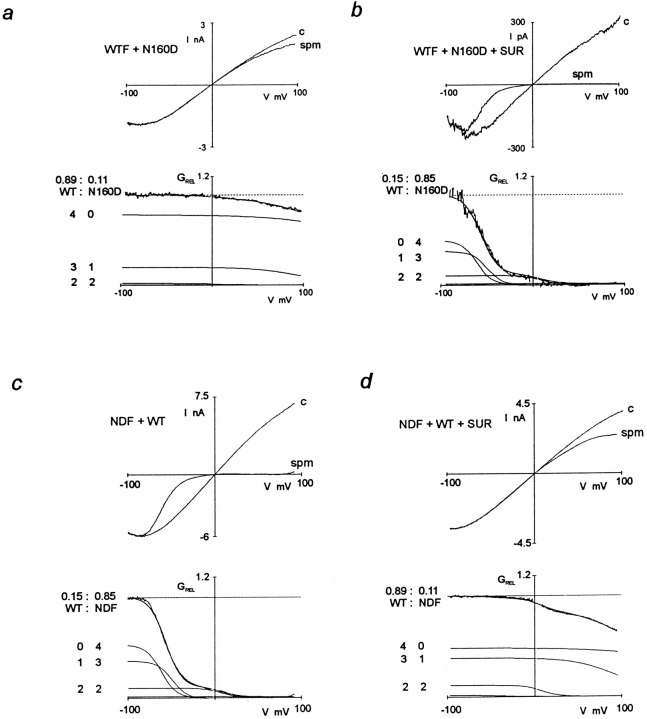
KATP channels in native tissues do not show strong inward rectification (Noma, 1983; Ciani and Ribalet, 1988) and, as demonstrated in Fig. 1 a, neither do KATP channels generated by coexpression of Kir6.2 or SUR1 subunits. Work on cloned inward rectifiers has demonstrated that strong inward rectification is controlled by a pore lining residue in the M2 transmembrane domain (Lu and MacKinnon, 1994; Stanfield et al., 1994), and that the presence of a negative charge at this “rectification controller” position confers strong inward rectification by generation of a high affinity site for the voltage-dependent binding of cytoplasmic polyamines or Mg2+ (Fakler et al., 1994; Ficker et al., 1994; Lopatin et al., 1994). Kir6.2 subunits are homologous to Kir1.1 (ROMK1; Ho et al., 1993) channels in this region of the M2 segment; both contain an asparagine (N160 in Kir6.2) at the rectification controller position. We used a mutant Kir6.2 containing a negatively charged aspartate at this position (Kir6.2[N160D]; Clement et al., 1997; Shyng et al., 1997a ). Expression of this mutant with SUR1 subunits results in generation of KATP channels that rectify strongly in the presence of cytoplasmic spermine (Fig. 1 b), although single channel conductance is unaltered and channels are still sensitive to inhibition by ATP (Ki,ATP = 10.6 μM for wild-type Kir6.2 plus SUR1 channels, and 46.1 μM for Kir6.2[N160D] plus SUR1 channels; Shyng et al., 1997a ). Rb+ flux through both channels is fully inhibited by 1 μM glibenclamide (not shown). The large difference in spermine sensitivity of KATP channels formed from wild-type and Kir6.2[N160D] subunits makes it possible to analyze the hetero-oligomeric assembly of Kir6.2 subunits and directly test the hypothesis that the KATP channel pore involves a tetrameric arrangement of Kir6.2 subunits (MacKinnon, 1991; Glowatzki et al., 1995; Yang et al., 1995).
Figure 1.
KATP channels contain four Kir6.2 subunits. Macroscopic inside-out patch currents in response to voltage ramps (300 ms) between +100 mV (or c, +150 mV) and −100 mV, plotted versus membrane potential, for KATP channels formed from wild-type Kir6.2 (a, WT) or Kir6.2[N160D] (b, N160D) coexpressed with SUR1 subunits (in 1:1 molar ratio), or from mixed wild-type Kir6.2 and Kir6.2[N160D], plus SUR1 (c). (c, top) Currents are shown in the presence and absence of 20 μM spermine (spm), in each case after subtraction of leakage currents in the presence of 5 mM ATP (which reduces channel open probability to <1%). (c, bottom) Relative conductance (GREL)-voltage relationships from the data shown above. The smooth lines in a (and b) are best fits of single Boltzmann functions to the data. For these patches, the unconstrained Boltzmann functions (for i = 5 and 0, respectively) were fitted with V 5 = +220 mV, z 5 = 0.4 (a); V 1 = −54 mV, z 1 = 2.9 (b). In c (bottom), the smooth line that superimposes on the data is the sum of five Boltzmann functions that correspond to different combinations of wild-type Kir6.2 and Kir6.2[N160D] in a tetramer, with fitted probability of wild-type incorporation (P) = 0.56. For this patch, the unconstrained Boltzmann functions (for i = 2–4) components, were fitted with V 2 = −48 mV, z 2 = 2.9; V 3 = +9 mV, z 3 = 2.9; V 4 = +86 mV, z 4 = 1.0. The individual Boltzmann functions are also plotted, with the contributing number of wild-type and Kir6.2[N160D] subunits indicated beside each curve. (d) Residual errors (calculated as the sum of squares of the difference between the fit and the data, every 1 mV, between −80 to +160 mV, excluding −10 to +10 mV), obtained by fitting with n = 2–6 subunits [Σ(GREL − GF)2, where GF is the fitted GREL] plotted against the number of assumed subunits (N). The dashed line and open symbols correspond to the patch illustrated in c. Solid lines and symbols correspond to averaged data for n = 3–5 subunit fits for all patches (n = 9 patches, mean ± SEM). The trimeric model (n = 3) is inadequate to fit the data (see d), and better fits are not obtained by increasing n from 4 to 5. Similar results were obtained when attempting to fit the model to derivative (ΔGREL/ΔV) data (not shown).
Expression of SUR1 cDNA with a 1:1 mixture of wild-type and Kir6.2[N160D] subunit cDNAs gives rise to channels that differ substantially from the parental homo-oligomeric channels in their sensitivity to block by intracellular spermine (Fig. 1 c). The relative conductance–voltage (GREL-V) curve contains more than two components (Fig. 1 c, bottom), and therefore could not arise from a weighted average of parental GREL-V curves, indicating that multimerization generates channels with novel phenotypes. We attempted to fit GREL-V curves like that in Fig. 1 c, bottom with the sum of multiple Boltzmann functions. Acceptable fits could not be obtained with the sum of four or less Boltzmann functions (see Fig. 1 d), a sum of at least five Boltzmann functions (as used in Fig. 1 c, bottom) was required:
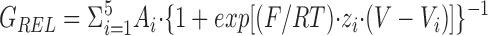 |
1 |
where A i, V i, and z i are the amplitude, voltage of half-maximal inhibition, and effective valency, respectively, of the ith component. With the constraints described below, we did not obtain better fits with the sum of six or more Boltzmann functions, based on least squares residuals (Fig. 1 d), leading us to the conclusion that five different species of tetrameric channels are randomly assembled in these mixing experiments, the channels containing 0, 1, 2, 3, or 4 wild-type Kir6.2 subunits with 4, 3, 2, 1, or 0 Kir6.2[N160D] subunits, respectively. The fitting process was accordingly constrained as follows: (a) we assumed that V 1 and z 1, and V 5 and z 5 are given by the average values for these parameters for SUR1/Kir6.2[N160D] and SUR1/Kir6.2 channels, respectively (Fig. 2, a and b), thus allowing only V i and z i to vary for the i = 2, 3, and 4 components. (b) We assumed that the i = 2, 3, and 4 components represent channels containing 3, 2, and 1 mutant subunits, respectively, and that V 1 < V 2 < V 3 < V 4 < V 5, and z 1 > z 2 > z 3 > z 4 > z 5. These constraints are based on the reasonable assumption that decreasing numbers of mutant subunits should give rise to similar or increasingly shallower and weaker spermine block, and that the ordering of subunits within the tetramer was unimportant. (c) We assumed that the fitted amplitude (A i) corresponds to the probability (P x) of formation of each channel type and that wild-type Kir6.2 and Kir6.2[N160D] subunits are incorporated with equal probability (p) after the binomial distribution:
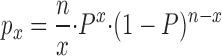 |
2 |
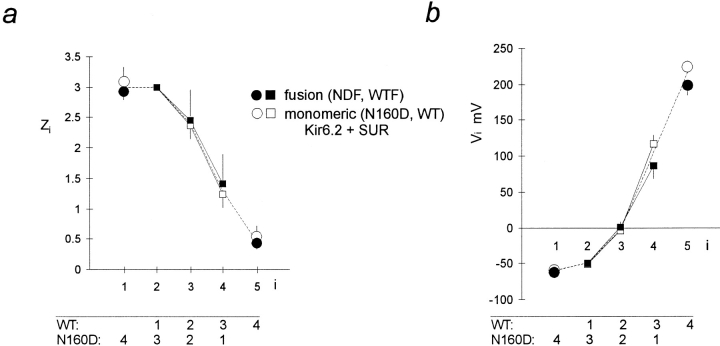
Figure 2.
Fusion channels show identical voltage dependence to parental monomers. (a) Mean ± SEM for z i of single Boltzmann functions fitted to GREL-V relationships obtained from wild-type Kir6.2 or Kir6.2[N160D] subunits coexpressed with SUR1 (○), or from WTF or NDF fusion proteins (•), and for z i of five component Boltzmann functions fitted to GREL-V relationships obtained from mixed wild-type Kir6.2 and Kir6.2[N160D] subunits coexpressed with SUR1 (□), or from mixed expression of WTF and NDF fusion proteins (▪). For these mixed expressions, only z 2, z 3, and z 4 were varied, z 1 and z 5 corresponded to the mean value fitted to homomeric expressions. The dashed line indicates the z i values used to fit the tetrameric model to data in Fig. 6. (b) Mean ± SEM for V i of single Boltzmann functions fitted to GREL-V relationships obtained from wild-type Kir6.2 or Kir6.2[N160D] subunits coexpressed with SUR1 (○, n = 5 in each case), or from WTF or NDF fusion proteins (□, n = 4 in each case), and for V i of five component Boltzmann functions fitted to GREL-V relationships obtained from mixed wild-type Kir6.2 and Kir6.2[N160D] subunits coexpressed with SUR1 (•, n = 4), or from mixed expression of WTF and NDF fusion proteins (▪, n = 4). For these mixed expressions, only V 2, V 3, and V 4 were varied, V 1 and V 5 corresponded to the mean value fitted to homomeric expressions. The dashed line indicates the V i values used to fit the tetrameric model to data in Fig. 6.
where n is the total number of subunits in a functional channel, x is the number, 0 to n, of wild-type subunits in a particular channel, P is the probability of inclusion of a wild-type subunit, and (1 − P) is the probability of inclusion of a mutant subunit. Hence, fits were constrained by allowing P to vary. Only patches with currents >−200 pA at −50 mV (corresponding to ∼50 channels) were included in the analysis, to increase the signal-to-noise ratio to acceptable levels. Leak conductance in the presence of high [ATP] (5 or 10 mM) was typically <10 pS, and patches were rejected from analysis if the leak conductance was >100 pS (i.e., >5% of KATP conductance). The data were well fitted with P values close to 0.5 (0.58 ± 0.04, n = 4 for SUR1 plus 1:1 mixture of Kir6.2[N160D] and wild-type Kir6.2 cDNAs). This agreement between fitted P values and transfected cDNA ratios is reassuring, since the transfection and expres-sion efficiency of the wild-type Kir6.2 and Kir6.2[N160D] cDNAs is not expected to be different.
Each Kir6.2 Subunit Is Normally Associated with One SUR1 Subunit
We cannot formally discount a role of SUR1 subunits in generating the channel pore, although none of several mutations in the cytoplasmic domains of SUR1 have been shown to affect conductance or rectification of expressed channels (Nichols et al., 1996; Gribble et al., 1997; Shyng et al. 1997 b). Very recently, evidence has been presented that certain Kir6.2 constructs with deletions of 26 or 36 amino acids from the COOH terminus can actually form functional KATP channels in the absence of the sulfonylurea receptor (Tucker et al., 1997). In that study, however, full length Kir6.2 subunits did not form functional channels without SUR1 coexpression, and even truncated constructs generated more current when coexpressed with SUR1, consistent with a normal requirement for association.
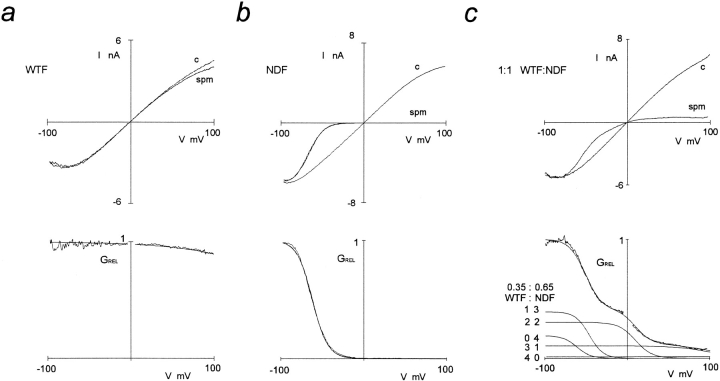
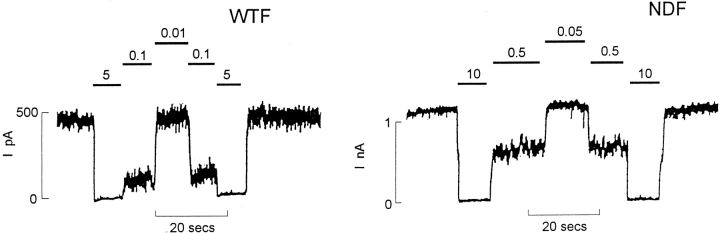
To investigate the stoichiometry of SUR1 and Kir6.2 subunits in functional channels, we used SUR1-Kir6.2 fusion proteins, with a defined 1:1 SUR1/Kir6.2 ratio (Fig. 3), in which the COOH terminus of SUR1 is covalently linked to the NH2 terminus of Kir6.2 using a hexa-glycine (-Gly6-) linker (Clement et al., 1997). Fusion proteins containing wild-type Kir6.2 (wild-type fusion, WTF), or N160D mutated Kir6.2 (N160D fusion, NDF), generate KATP channels that are fully inhibited by 1 μM glibenclamide in Rb+ efflux assays (data not shown), and by ATP in excised patches. There is no obvious difference in the density of channels formed by expression of the fusion proteins versus coexpression of the parent monomers, based on the density of maximally activated KATP conductances in 86Rb+ efflux experiments (see Fig. 5). Fig. 4 shows records of currents through WTF and NDF channels to illustrate ATP sensitivity. Although these channels have wild-type single channel conductance in the absence of internal multivalent cations (75 pS in symmetrical 140 mM K+, not shown), they are approximately five- to eightfold less sensitive to ATP than the parental monomers. The estimated Ki (from n = 3–5 patches) is 45.3 μM for WTF (compare 10.6 μM for wild-type Kir6.2 plus SUR1 channels), and 384.6 μM for NDF (compare 46.1 μM for Kir6.2[N160D] plus SUR1 channels). Because of the reduced ATP sensitivity of fusion protein channels, we routinely used exposure to 10 mM ATP for estimation of leakage current. As shown in Figs. 2 and 3, fusion proteins show identical spermine sensitivity to the channels formed by expression of SUR1 and the respective Kir6.2 monomer type, and when mixtures of WTF and NDF are coexpressed, the spermine sensitivity of heteromultimers is identical to those formed by mixtures of parental monomers (Figs. 2 and 3 c). Expression of a 1:1 mixture of WTF and NDF cDNAs gives rise to the same distribution of spermine block phenotypes as a 1:1 mixture of wild-type Kir6.2 and Kir6.2[N160D] monomer cDNAs with SUR1, in accord with the tetrameric arrangement, the data again being well fitted with P values close to 0.5 for both cases (P = 0.45 ± 0.05, n = 5 for 1:1 WTF/NDF cDNA mixtures, see Fig. 3; compare P = 0.58 ± 0.04, n = 4 for the SUR1 plus nominally 1:1 mixture of Kir6.2[N160D] and wild-type Kir6.2 subunits, above). Averaged data for the fitted z i and V i parameters obtained by expression of mixed wild-type Kir6.2 and Kir6.2[N160D] (plus SUR1) subunits, and by expression of WTF and NDF subunits are shown in Fig. 2, a and b. It is clear that there is no consistent difference in the rectification properties of channels formed by expression of monomeric Kir6.2 subunits, or by expression of dimeric SUR1-Kir6.2 fusion proteins.
Figure 3.
Fusion channels contain four dimeric SUR1-Kir6.2 dimers. (a–c) (top) Macroscopic inside-out patch currents in response to voltage ramps (300 ms) between +100 and −100 mV, plotted versus membrane potential, for KATP channels formed from SUR1-Kir6.2 fusion proteins, containing either wild-type (a, WTF ) or N160D mutant (b, NDF ) Kir6.2 sequences, or from equimolar mixed WTF and NDF constructs (c). (top) Currents are shown in the presence and absence of 20 μM spermine (spm); in each case, currents are shown after subtraction of leakage currents in the presence of 10 mM ATP (which reduces channel open probability to <1%). (bottom) Relative conductance (GREL)-voltage relationships from the data shown above. The continuous lines in b and c are best fits of single Boltzmann functions. For these patches, the unconstrained Boltzmann functions (for i = 5 and 0, respectively) were fitted with V 5 = +200 mV, z 5 = 0.4 (a); V 1 = −58 mV, z 1 = 2.9 (b). In c (bottom), the smooth line that superimposes on the data is the sum of five Boltzmann functions that correspond to different combinations of wild-type Kir6.2 and Kir6.2[N160D] in a tetramer, with fitted probability of wild-type incorporation (P) = 0.35. For this patch, the unconstrained Boltzmann functions (for i = 2–4), components were fitted with V 2 = −46 mV, z 2 = 2.9; V 3 = +10 mV, z 3 = 2.5; V 4 = +99 mV, z 4 = 1.2. The individual Boltzmann functions are also plotted, with the contributing number of wild-type and Kir6.2[N160D] subunits indicated beside each curve.
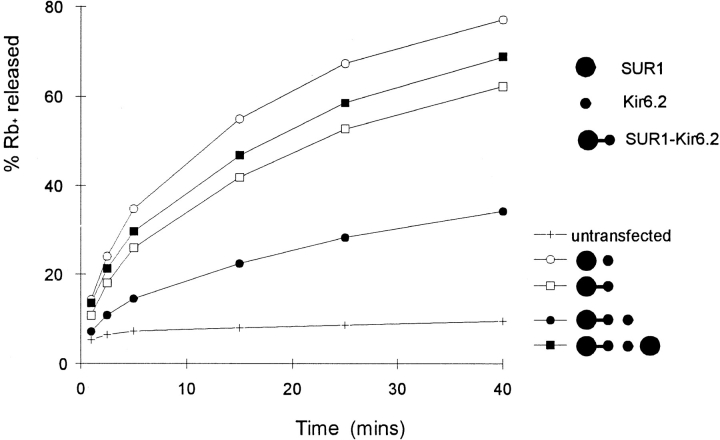
Figure 5.
Monomeric Kir6.2 inhibits fusion protein conductance. 86Rb+ efflux from untransfected COSm6 cells, and cells expressing SUR1-Kir6.2 fusion proteins with or without additional Kir6.2 and SUR1 subunits. Graphs show percent Rb+ released into the medium as a function of time in the presence of metabolic inhibitors (see methods) for a typical experiment (n = 3). The approximate cDNA molar ratio was 1:1:1 for SUR1-Kir6.2 fusion protein/Kir6.2/SUR1.
Figure 4.
Fusion channels show reduced sensitivity to ATP. Representative currents recorded from inside-out membrane patches containing WTF or NDF KATP channels (as indicated) at −50 mV. Patches were exposed to differing [ATP] as indicated (mM). Inward currents are shown as upward deflections
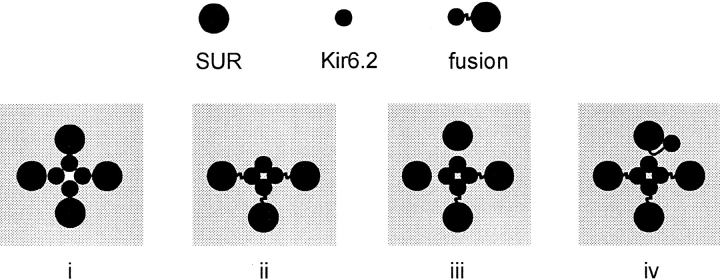
The formation of functional KATP channels by SUR1-Kir6.2 fusion proteins is consistent with a 1:1 SUR1/ Kir6.2 stoichiometry being sufficient for channel formation. To examine whether such a stoichiometry is required, we coexpressed fusion proteins with Kir6.2 monomers to allow generation of channels with stoichiometries where Kir6.2 > SUR1. In 86Rb+ efflux experiments (Fig. 5), transfection of SUR1 and Kir6.2 in approximately equal cDNA ratios produced similar efflux to that resulting from expression of the WTF fusion protein cDNA (at equivalent molar ratio). Coexpression of monomeric Kir6.2 with the fusion protein, at a ratio of Kir6.2/fusion protein cDNA of ∼1:1 (keeping the total amount of Kir6.2 cDNA constant, whether in monomeric or fusion protein construct), suppressed the KATP conductance. This apparent dominant-negative effect may result from incorporation of Kir6.2 monomers and fusion proteins into nonfunctional complexes without a requisite 1:1 (Kir6.2/SUR1) stoichiometry (see Fig. 7). If such an interpretation is correct, then further addition of monomeric SUR1 subunits might rescue the nonfunctional complexes by permitting the requisite stoichiometry to be achieved. Strikingly, this is the case, as shown by 86Rb+ efflux measurements (Fig. 5). Even though coexpression of pore-forming Kir6.2 subunits with fusion proteins reduces the KATP conductance, the additional expression of regulatory SUR1 subunits restores the conductance (cDNA ratio 1:1:1). The fusion/Kir6.2 cDNA ratio was the same in both cases, so, although additional expression of SUR1 subunits might conceivably have reduced the total amount of expressed fusion protein and Kir6.2 subunits (due to overload of the biosynthetic machinery), it could not have altered the expressed fusion protein/ Kir6.2 subunit ratio, and could not have increased the total number of expressed Kir6.2 subunits (i.e., the sum of Kir6.2 monomers and Kir6.2 in fusion proteins). The only reasonable conclusion then is that the increased number of functional channels when SUR1 is coexpressed results from rescue of otherwise nonfunctional Kir6.2 subunits as a result of their association with SUR1 subunits.
Figure 7.
Schematic representation of potential subunit arrangements in the KATP channel complex. The present results indicate that the KATP channel is an octamer consisting of Kir6.2 and SUR1 subunits in a 4:4 stoichiometry with Kir6.2 subunits forming the channel pore (i). The dominant negative effect of Kir6.2 on fusion protein expression suggests that stoichiometries other than 1:1 (between SUR1 and Kir6.2) form (ii) but do not generate functional channels, and that restitution of 1:1 stoichiometry by additional SUR1 subunits (iii) can regenerate functional complexes. However, since monomeric Kir6.2 subunits clearly can incorporate into functional channels with fusion proteins, albeit with low frequency, we suggest that the requisite SUR1 subunit can be provided, inefficiently, by a fusion protein whose Kir6.2 portion “hangs out” of the functional complex (iv).
Insight into the nature of the currents formed by mixed expression of the fusion protein with monomers was obtained from inside-out membrane patches. Channels were formed by coexpression of WTF or NDF fusions with Kir6.2[N160D] or wild-type Kir6.2 monomers, respectively, at equimolar cDNA ratios. As expected from the 86Rb+ efflux experiments, the observed current density in membrane patches was lower than that seen with expression of fusion proteins alone. Although KATP currents were present in 15/15 patches from cells expressing only the fusion proteins, measurable KATP currents were present in only 2/12 patches when Kir6.2[N160D] was coexpressed with WTF (1:1 molar ratio of cDNAs), and in only 3/13 patches when wild-type Kir6.2 was coexpressed with NDF. When the molar ratio of fusion protein/Kir6.2 cDNAs was increased to 8:1, measurable currents were present in 13/21 patches (combined data for both WTF plus Kir6.2[N160D], and for NDF plus wild-type Kir6.2). The phenotype of active channels was predominantly that expected from the fusion protein (Fig. 6, a and b). Using estimates of z i and V i (spermine sensitivity of heteromeric channels containing 0–4 Kir6.2 subunits) obtained above (Fig. 2, a and b, points connected by dashed lines), we fitted the tetrameric model to data from heteromeric channels composed of Kir6.2 and fusion protein, varying only the apparent ratio of wild-type to mutant Kir6.2 subunits (Fig. 6 and Table I). At all fusion/Kir6.2 cDNA molar ratios, fusion proteins contributed predominantly to the pore of functional channels, but the phenotype of functional channels was very different when monomeric SUR1 was included in the fusion protein/ Kir6.2 monomer transfections (molar ratio of cDNAs of 8:1:1 fusion/Kir6.2/SUR1 in Fig. 6, b and d). In these patches, measurable currents were present in 21/21 (100%) patches (combined data for both cases), and the rectification phenotype of the Kir6.2 monomer predominated. Table I shows data obtained from different membrane patches expressing fusion proteins (WTF or NDF) plus monomeric Kir6.2 of the opposite rectification phenotype, with or without additional SUR1 cDNAs. The ratio of fusion protein/Kir6.2 cDNA was varied between 1:1 and 18:1, as indicated. When SUR1 was added to the transfections, the SUR1/Kir6.2 ratio was 1:1. Fits of the tetrameric model to data from these triple transfections showed that the apparent contribution of the fusion protein to formation of active channels was greatly reduced by SUR1 inclusion. At fusion protein/Kir6.2 cDNA ratios of 8:1, the apparent fraction of pore subunits formed by Kir6.2 monomers (f 6.2, corresponding to P, for wild-type Kir6.2 subunits, or 1 − P for Kir6.2[N160D] subunits) increased from 0.15 to 0.84 (Table I) with inclusion of SUR1 subunits, representing an ∼30-fold increase in appearance of Kir6.2 monomers in functional channels, indicating that a 1:1 stoichiometry is strongly preferred, and probably required, to generate functional channel complexes (Fig. 7).
Figure 6.
A 1:1 SUR1/Kir6.2 stoichiometry is preferred. (a and b) Macroscopic inside-out patch currents in response to voltage ramps (300 ms) between +100 and −100 mV, plotted versus membrane potential, for KATP channels formed by coexpression of wild-type fusion proteins (WTF ) with Kir6.2[N160D] monomers (N160D) with (b) or without (a) SUR1 monomers (SUR). The approximate cDNA molar ratio of WTF/Kir6.2 [N160D]/SUR1 in transfection mixtures was 8:1:1. (top) Currents are shown in the presence and absence of 20 μM spermine (spm); in each case, currents are shown after subtraction of leakage currents in the presence of 10 mM ATP. (bottom) Relative conductance (GREL)-voltage relationships from the data shown above. The superimposed continuous line is the sum of five Boltzmann functions with z i and V i parameters constrained as described in Fig. 2, a and b. In the fitting, only the apparent fraction of wild-type (P) and N160D mutant components (1 − P) was varied as indicated. (c and d) Macroscopic inside-out patch currents in response to voltage ramps (300 ms) between +100 and −100 mV, plotted versus membrane potential, for KATP channels formed by coexpression of fusion proteins (NDF ) with wild-type Kir6.2 monomers (WT) with (d) or without (c) SUR1 monomers (SUR). The approximate cDNA molar ratio of NDF/Kir6.2/SUR1 in transfection mixtures was 8:1:1. (top) Currents are shown in the presence and absence of 20 μM spermine (spm); in each case, currents are shown after subtraction of leakage currents in the presence of 5 mM ATP. (bottom) Relative conductance (GREL)-voltage relationships from the data shown above. The superimposed continuous line is the sum of five Boltzmann functions with z i and V i parameters constrained as described in Fig. 3. For fitting, the apparent ratio of wild-type (P) and N160D mutant components (1 − P) was the only parameter varied as indicated.
Table I.
SUR1 Subunit Rescue of Kir6.2 Subunits in Coexpression with Fusion Proteins
| Nominal molar ratio of cDNAs (fusion protein/Kir6.2 monomer) | ||||||
|---|---|---|---|---|---|---|
| 1:1 | 8:1 | 18:1 | ||||
| Apparent fraction of monomeric Kir6.2 in functional channels (f 6.2 = monomer/total) | ||||||
| −SURl subunits | 0.26(w)* | 0.15(m) | 0.06(m) | |||
| (f 6.21) | 0.27(m) | |||||
| 0.16(m) | ||||||
| 0.06(w) | ||||||
| 0.12(w) | ||||||
| mean | 0.26 | 0.15 ± 0.02 | 0.06 | |||
| +SUR subunits‡ | 0.93(m) | 0.84(m) | 0.60(m) | |||
| (f 6.22) | 0.93(w) | 0.83(m) | 0.69(m) | |||
| 0.95(w) | 0.83(m) | 0.51(m) | ||||
| 0.84(m) | 0.51(m) | |||||
| mean | 0.93 ± 0.01 | 0.84 ± 0.01 | 0.58 ± 0.03 | |||
| Fold increase in monomer incorporation with SUR1 = f 6.22/(1−f 6.22)/ [f 6.21/(1-f 6.21)] | ||||||
| 37.8 | 29.8 | 21.6 | ||||
(w) and (m) refer to wild-type fusion (plus Kir6.2[N160D] monomer), and N160D fusion (plus wild-type Kir6.2 monomer), respectively.
SURl/ Kir6.2 at nominal 1:1 molar cDNA ratio.
discussion
KATP channels are unique amongst known potassium channels in requiring an unrelated ABC protein subunit (SUR1) in addition to an inward rectifier K channel (Kir6.2) subunit (Inagaki et al., 1995a ). In other cloned inward rectifiers, strong inward rectification is controlled by a pore-lining residue in the M2 transmembrane segment (Fakler et al., 1994; Ficker et al., 1994; Lopatin et al., 1994; Lu and MacKinnon, 1994; Stanfield et al., 1994). Mutation of the corresponding residue in Kir6.2 from asparagine to aspartate results in generation of KATP channels that rectify strongly in the presence of cytoplasmic spermine (Fig. 1 b; Clement et al., 1997; Shyng et al., 1997), single channel conductance being unaltered and channels remaining sensitive to inhibition by ATP (Shyng et al., 1997). The requirement for SUR1 to form active channels still raises the possibility that the receptor might also contribute to the pore, and perhaps reduce or otherwise alter the number of Kir6.2 subunits involved. The large difference in spermine sensitivity of KATP channels formed from wild-type and Kir6.2[N160D] subunits made it possible for us to directly analyze the hetero-oligomeric assembly of Kir6.2 subunits. As shown in Fig. 1 d, the data could not be fit by assuming a trimer or less, and the fits of pentameric or hexameric models were not better than the tetrameric model. The analysis that we used (Fig. 1) was previously used by Glowatzki et al. (1995), after the original approach of MacKinnon (1991) to demonstrate that homomeric Kir4.1 (BIR10) channels are formed as tetramers. In both cases, the strong rectifying phenotype of the N160D (or equivalent) mutation is dominant, channels containing three wild-type subunits are approximately intermediate between wild-type and homotetrameric mutant channels in spermine sensitivity (Figs. 2 and 3). This suggests that Kir6.2 subunits form tetrameric KATP channel pores in a very similar arrangement to those of homomeric Kir channel pores (Glowatzki et al., 1995; Yang et al., 1995).
There are reports of Kir6.1 and Kir6.2 (COOH-terminal truncated) expressing KATP channels in the absence of SUR1 (Inagaki et al., 1995b ; Tucker et al., 1997), and there are also reports of “promiscuous” coupling of SUR1 to homomeric Kir1.1 channels that form active K currents in the absence of SUR1 (Ammala et al., 1996). While conflicting results have been reported by others (e.g., Inagaki et al., 1995a ), these results suggest a nonobligatory and perhaps nonstoichiometric relationship of SUR1 to Kir6.2. By analyzing channels formed by deliberately generated 1:1 stoichiometry (in SUR1-Kir6.2 fusion proteins), and by altered stoichiometry (mixing fusion proteins with monomeric subunits), we can show (Figs. 2–6, Table I) that a 1:1 stoichiometry is possible and, moreover, may be strongly preferred, indicating a normally octameric assembly. Nevertheless, the results do indicate that a low frequency incorporation of monomeric Kir6.2 subunits in functional channels can still occur when coexpressed with fusion proteins (Fig. 6, a and c). This may be explained by assuming that the necessary SUR1 subunit is provided by a fusion protein, with its Kir6.2 portion “hanging out” of the complex (Fig. 7) as has been suggested to explain formation of Kv and Kir channels by expression of pentameric and trimeric concatamers (Liman et al., 1992; Yang et al., 1995). However, since monomeric SUR1 is 20- to 40-fold more efficient at generating channels with Kir6.2 monomers than is the SUR1-Kir6.2 dimer (Table I), this is clearly an unfavorable arrangement, and not expected to be a significant component of channels formed with equal ratios of Kir6.2 and SUR1 subunits. Using a biochemical approach, Clement et al. (1997) have shown that SUR1 and Kir6.2 are physically associated in COSm6 cells and form a complex with an estimated molecular mass of ∼950,000 D, which is reasonably consistent with four SUR1 subunits (∼170,000 D) and four Kir6.2 subunits (∼45,000 D). Estimates for the size of the complex formed by expression of the fusion protein are similar, consistent with the octameric, or tetradimeric [SUR1-Kir6.2]4 structure that we predict. Using essentially the same biophysical approach used here, Clement et al. (1997) also show that a tetrameric model can fit GREL-V curves from mixed expression of monomeric SUR1 and triple fusion (SUR1-Kir6.2-Kir6.2) constructs (see also below).
The presence of nucleotide binding folds in the sulfonylurea receptor (Aguilar-Bryan et al., 1995) has led to a widespread expectation that ATP inhibition will be shown to occur through binding to these sites. However, while studies with mutations in the nucleotide binding folds of SUR1 have demonstrated that these binding folds are involved in MgADP and diazoxide stimulation of channel activity (Nichols et al., 1996; Gribble et al., 1997; Shyng et al., 1997 b), they provide no evidence for any role in intrinsic ATP inhibition. The recent evidence that the Kir6.2 subunit can control ATP sensitivity, and that a COOH-terminally truncated Kir6.2 (Kir6.2[ΔC26]) forms functional KATP channels in the absence of SUR1 (Shyng et al., 1997a ; Shyng et al., 1997 b; Tucker et al., 1997) raises the possibility that ATP inhibition occurs through binding to Kir6.2 subunits. Examination of the kinetics of ATP sensitivity of KATP channels in cardiac myocytes suggested that four ATP-binding sites were involved in the hallmark inhibition of the channel by ATP (Nichols et al., 1991). These results, together with the present demonstration of a tetrameric arrangement of Kir6.2 subunits, suggests a model in which ATP inhibition results from one ATP molecule binding to each of the four Kir6.2 subunits within the channel complex. The generation of K+ channels by the complexing of Kir channel subunits and an ABC protein is unprecedented, but other ABC proteins, including human p-glycoprotein and cystic fibrosis transmembrane conductance regulators are also known to be associated with separate ion channels (Higgins, 1995), and the bacterial Sap/Trk systems are ABC proteins associated with K+ transport in Salmonella typhimurium and Escherichia coli (Parra-Lopez et al., 1994). It is an intriguing possibility that the stoichiometric association between SUR1 and Kir6.2 to generate the KATP channel may give insight to the architecture of other ion channels generated by complexing ABC proteins with pore-forming subunits. Inagaki et al. (1997) have used a similar strategy with SUR1-Kir6.2 and SUR1-Kir6.2-Kir6.2 fusion constructs to indicate a 1:1 stoichiometry between SUR1 and Kir6.2.
Acknowledgments
We are grateful to Dr. Susumu Seino for the original Kir6.2 clone and to Dr. Joe Bryan for providing us with the original Kir6.2[N160D] mutant and the SUR1-Kir6.2 dimeric constructs.
This work was supported by grants from the National Institutes of Health (HL451231 and HL54171) to C.G. Nichols. Molecular biology support was provided by the Diabetes Research and Training Center at Washington University.
Footnotes
Abbreviations used in this paper: ABC, ATP-binding cassette; DMEM-HG, Dulbecco's Modified Eagle Medium plus 10 mM glucose; GREL-V, relative conductance–voltage; NDF, N160D fusion; WTF, wild-type fusion.
references
- Aguilar-Bryan L, Nichols CG, Wechsler SW, Clement JP, IV, Boyd AE, III, Gonzalez G, Herrera H, Sosa, Nguy K, Bryan J, Nelson DA. Cloning of the β-cell high affinity sulfonylurea receptor: a regulator of insulin secretion. Science. 1995;268:423–426. doi: 10.1126/science.7716547. [DOI] [PubMed] [Google Scholar]
- Ammala C, Moorhouse A, Gribble F, Ashfield R, Proks P, Smith PA, Sakura H, Coles B, Ashcroft SJ, Ashcroft FM. Promiscuous coupling between the sulphonylurea receptor and inwardly rectifying potassium channels. Nature. 1996;379:545–548. doi: 10.1038/379545a0. [DOI] [PubMed] [Google Scholar]
- Ashcroft FM. Adenosine 5′-triphosphate-sensitive potassium channels. Annu Rev Neurosci. 1988;11:97–118. doi: 10.1146/annurev.ne.11.030188.000525. [DOI] [PubMed] [Google Scholar]
- Ciani S, Ribalet B. Ion permeation and rectification in ATP-sensitive channels from insulin-secreting cells (RINm5F): effects of K+, Na+ and Mg2+ . J Membr Biol. 1988;103:171–180. doi: 10.1007/BF01870947. [DOI] [PubMed] [Google Scholar]
- Clement JP, IV, Kunjilwar K, Gonzalez G, Schwanstecher M, Panten U, Aguilar-Bryan L, Bryan J. Association and stoichiometry of KATPchannel subunits. Neuron. 1997;18:827–838. doi: 10.1016/s0896-6273(00)80321-9. [DOI] [PubMed] [Google Scholar]
- Fakler B, Brandle U, Glowatzki E, Konig C, Bond C, Adelman JP, Zenner H-P, Ruppersberg JP. A structural determinant of differential sensitivity of cloned inward rectifier K+channels to intracellular spermine. FEBS Lett. 1994;356:199–203. doi: 10.1016/0014-5793(94)01258-x. [DOI] [PubMed] [Google Scholar]
- Ficker E, Taglialatela M, Wible BA, Henley CM, Brown AM. Spermine and spermidine as gating molecules for inward rectifier K channels. Science. 1994;266:1068–1072. doi: 10.1126/science.7973666. [DOI] [PubMed] [Google Scholar]
- Glowatzki E, Fakler G, Brandle U, Rexhausen U, Zenner HP, Ruppersberg JP, Fakler B. Subunit-dependent assembly of inward-rectifier K+channels. Proc R Soc Lond Ser B Biol Sci. 1995;261:251–261. doi: 10.1098/rspb.1995.0145. [DOI] [PubMed] [Google Scholar]
- Gribble FM, Tucker SJ, Ashcroft FM. The essential role of the Walker A motifs of SUR1 in K-ATP channel activation by Mg-ADP and diazoxide. EMBO (Eur Mol Biol Organ) J. 1997;16:1145–1152. doi: 10.1093/emboj/16.6.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins CF. The ABC of channel regulation. Cell. 1995;82:693–696. doi: 10.1016/0092-8674(95)90465-4. [DOI] [PubMed] [Google Scholar]
- Ho K, Nichols CG, Lederer WJ, Lytton J, Vassilev PM, Kanazirska MV, Herbert SC. Cloning and expression of an inwardly rectifying ATP-regulated potassium channel. Nature. 1993;362:31–38. doi: 10.1038/362031a0. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Gonoi T, Clement JP, IV, Wang CZ, Aguilar-Bryan L, Bryan J, Seino S. A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+channels. Neuron. 1996;16:1011–1017. doi: 10.1016/s0896-6273(00)80124-5. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Gonoi T, Clement JP, IV, Namba N, Inazawa J, Gonzalez G, Aguilar-Bryan L, Seino S, Bryan J. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science. 1995a;270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- Inagaki NT, Gonoi T, Seino S. Subunit stoichiometry of the pancreatic β-cell ATP-sensitive K+channel. FEBS Lett. 1997;409:232–236. doi: 10.1016/s0014-5793(97)00488-2. [DOI] [PubMed] [Google Scholar]
- Inagaki NT, Tsuura Y, Namba N, Masuda K, Gonoi T, Morie M, Seino Y, Mizuta M, Seino S. Cloning and functional characterization of a novel ATP-sensitive potassium channel ubiquitously expressed in rat tissues, including pancreatic islets, pituitary, skeletal muscle and heart. J Biol Chem. 1995b;270:5691–5694. doi: 10.1074/jbc.270.11.5691. [DOI] [PubMed] [Google Scholar]
- Lederer WJ, Nichols CG. Nucleotide modulation of the activity of rat heart KATP channels in isolated membrane patches. J Physiol (Camb) 1989;419:193–211. doi: 10.1113/jphysiol.1989.sp017869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liman ER, Tytgat J, Hess P. Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron. 1992;9:861–871. doi: 10.1016/0896-6273(92)90239-a. [DOI] [PubMed] [Google Scholar]
- Lopatin AN, Makhina EN, Nichols CG. Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature. 1994;372:366–369. doi: 10.1038/372366a0. [DOI] [PubMed] [Google Scholar]
- Lu Z, Mackinnon R. Electrostatic tuning of Mg2+ affinity in an inward rectifier K+channel. Nature. 1994;371:243–246. doi: 10.1038/371243a0. [DOI] [PubMed] [Google Scholar]
- MacKinnon R. Determination of the subunit stoichiometry of a voltage-activated potassium channel. Nature. 1991;350:232–235. doi: 10.1038/350232a0. [DOI] [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- Nichols CG, Lederer WJ, Cannell MB. The ATP- dependence of KATPchannel kinetics in isolated membrane patches from rat ventricle. Biophys J. 1991;60:1164–1177. doi: 10.1016/S0006-3495(91)82152-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols CG, Lopatin AN. Inward rectifier potassium channels. Annu Rev Physiol. 1997;59:171–191. doi: 10.1146/annurev.physiol.59.1.171. [DOI] [PubMed] [Google Scholar]
- Nichols CG, Shyng S-L, Nestorowicz A, Glaser B, Clement JP, IV, Gonzales G, Aguilar-Bryan L, Permutt AM, Bryan J. Adenosine diphosphate as an intracellular regulator of insulin secretion. Science. 1996;272:1785–1787. doi: 10.1126/science.272.5269.1785. [DOI] [PubMed] [Google Scholar]
- Parra-Lopez C, Lin R, Aspedon A, Groisman EA. A Salmonella protein that is required for resistance to antimicrobial peptides and transport of potassium. EMBO (Eur Mol Biol Organ) J. 1994;13:3964–3972. doi: 10.1002/j.1460-2075.1994.tb06712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyng S-L, Clement JP, IV, Bryan J, Nichols CG. Stoichiometry of the KATP channel complex. Biophys J. 1997;72:A251. doi: 10.1085/jgp.110.6.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyng S-L, Ferrigni T, Nichols CG. Control of rectification and gating of cloned KATP channels by the Kir6.2 subunit. J Gen Physiol. 1997a;110:141–153. doi: 10.1085/jgp.110.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyng S-L, Ferrigni T, Nichols CG. Regulation of KATPchannel activity by diazoxide and MgADP: distinct functions of the two nucleotide binding folds of the sulfonylurea receptor. J Gen Phys. 1997b;110:643–654. doi: 10.1085/jgp.110.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield PR, Davies NW, Shelton PA, Sutcliffe MJ, Khan IA, Brammar WJ, Conley EC. A single aspartate residue is involved in both intrinsic gating and blockage by Mg2+of the inward rectifier, IRK1. J Physiol (Camb) 1994;478:1–6. doi: 10.1113/jphysiol.1994.sp020225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker SJ, Gribble FM, Zhao C, Trapp S, Ashcroft FM. Truncation of Kir6.2 produces ATP-sensitive K+channels in the absence of the sulfonylurea receptor. Nature. 1997;387:179–183. doi: 10.1038/387179a0. [DOI] [PubMed] [Google Scholar]
- Yang J, Jan YN, Jan LY. Determination of the subunit stoichiometry of an inwardly rectifying potassium channel. Neuron. 1995;15:1441–1447. doi: 10.1016/0896-6273(95)90021-7. [DOI] [PubMed] [Google Scholar]