Abstract
The molecular mechanisms by which Ca2+ and metal ions interact with the binding sites that modulate the tight junctions (TJs) have not been fully described. Metal ions were used as probes of these sites in the frog urinary bladder. Basolateral Ca2+ withdrawal induces the opening of the TJs, a process that is abruptly terminated when Ca2+ is readmitted, and is followed by a complete recovery of the TJ seal. Mg2+ and Ba2+ were incapable of keeping the TJ sealed or of inducing TJ recovery. In addition, Mg2+ causes a reversible concentration-dependent inhibition of the Ca2+-induced TJ recovery. The effects of extracellular Ca2+ manipulation on the TJs apparently is not mediated by changes of cytosolic Ca2+ concentration. The transition elements, Mn2+ and Cd2+, act as Ca2+ agonists. In the absence of Ca2+, they prevent TJ opening and almost immediately halt the process of TJ opening caused by Ca2+ withdrawal. In addition, Mn2+ promotes an almost complete recovery of the TJ seal. Cd2+, in spite of stabilizing the TJs in the closed state and halting TJ opening, does not promote TJ recovery, an effect that apparently results from a superimposed toxic effect that is markedly attenuated by the presence of Ca2+. The interruption of TJ opening caused by Ca2+, Cd2+, or Mn2+, and the stability they confer to the closed TJs, might result from the interaction of these ions with E-cadherin. Addition of La3+ (2 μM) to the basolateral Ca2+-containing solution causes an increase of TJ permeability that fully reverses when La3+ is removed. This effect of La3+, observed in the presence of Ca2+ (1 mM), indicates a high La3+ affinity for the Ca2+-binding sites. This ability of La3+ to open TJs in the presence of Ca2+ is a relevant aspect that must be considered when using La3+ in the evaluation of TJ permeability of epithelial and endothelial membranes, particularly when used during in vivo perfusion or in the absence of fixatives.
Keywords: tight junction, calcium, cadmium, lanthanum, E-cadherin
introduction
Ca2+ is essential for cells to maintain intercellular contacts. When the extracellular Ca2+ is removed, the cell– cell connections generally become loose and multicellular organizations are destroyed. A number of studies emphasize the role of extracellular Ca2+ on the stability of mature tight junctions (TJs)1 in natural epithelia (Sedar and Forte, 1964; Hays et al., 1965; Galli et al., 1976; Meldolesi et al., 1978; Pitelka et al., 1983; Palant et al., 1983) and on the development of new TJs in cell cultures in confluence (Martinez-Palomo et al., 1980; Cereijido et al., 1980, 1981; González-Mariscal et al., 1985). The removal of extracellular Ca2+ causes the opening of previously formed TJs and prevents de novo formation of TJs in confluent cell monolayers. Notwithstanding several studies addressing the role of extracellular Ca2+ in the dynamics of the TJs, major questions are still pending. The relative importance of extracel– lular (Gorodeski et al., 1997; Contreras et al., 1992; González-Mariscal et al., 1990) versus intracellular (Bhat et al., 1993; Jovov et al., 1994; Stuart et al., 1994) Ca2+ concentration on the control of TJs is not yet clearly characterized. The cell adhesion molecule E-cadherin (uvomorulin) (Gumbiner et al., 1988), which is particularly rich at the zonula adhaerens (Boller et al., 1985), plays a key role as the extracellular Ca2+ binding molecule that modulates the formation and maintenance of the epithelial junctional complex (Gumbiner et al., 1988). Ca2+ influences the conformation of E-cadherin and stabilizes it in its adhesive state (Ringwald et al., 1987). In addition, the interaction of Ca2+ with E-cadherin is transduced across the cell membrane by a cascade of reactions involving phospholipase C, G proteins, protein kinase C, and calmodulin (Balda et al., 1991, 1993). The structural and electrostatic mechanisms used by the Ca2+ binding sites of E-cadherin to provide Ca2+ specificity are not yet fully understood, as compared with the knowledge on the EF-hand-like sites (Snyder et al., 1990), in part because insufficient information is available regarding the ion specificity of the Ca2+ binding sites. Only recently has the structure of the epithelial cadherin domain responsible for selective cell adhesion been identified. The Ca2+ binding site of the CAD1 domain of E-cadherin was inferred by nuclear magnetic resonance identification of the amino acid residues whose backbone 13CO, 15N, or 1HN chemical shifts differed between Ca2+-bound and -free forms (Overduin et al., 1995). A negatively charged pocket is formed by three sequences of residues with the side chains of the highly conserved Glu11, Glu69, and Asp100 well positioned to ligate Ca2+ (Overduin et al., 1995). In addition, the homophilic specificity surface is also sensitive to Ca2+ ligation through His79 and Met92, indicating that the Ca2+-induced conformational effect on the homophilic specificity surface may reflect a mechanism by which Ca2+ levels regulate the adhesiveness of cadherins (Overduin et al., 1995).
Multiple factors (number, type, and geometry of ligands, electrostatic interactions, cavity size and deformability of the site, dehydration of metal and ligand) are among the variables that must be considered when the metal ion selectivity of protein Ca2+ sites are analyzed (Snyder et al., 1990). The exclusion of Mg2+ from many protein Ca2+ sites, for example, can be explained in part by the fact that Mg2+ prefers a coordination number of six and uses nitrogen as a ligand more frequently (Einspahar and Bugg, 1984; Martin, 1984), while coordination by seven oxygens is observed in protein Ca2+ sites (Snyder et al., 1990; Strynadka and James, 1989).
In a previous study in the frog urinary bladder (Lacaz-Vieira and Kachar, 1996), it was shown that apical Ca2+ may activate the TJ sealing mechanism, an effect that is not impaired by the presence of Ca2+ channel blockers (nifedipine, verapamil, Mn2+, or Cd2+) in the apical solution, indicating that junction resealing in the frog urinary bladder does not depend on Ca2+ entering the cells through the apical membrane. Most likely, this effect results from Ca2+ entering partially disrupted TJs, reaching the zonula adhaerens Ca2+ receptors (E-cadherins). It was also shown that protein kinase C plays a significant role in the control of TJ assembly in the frog urinary bladder since the PKC inhibitor (H7) and the activator (diC8) markedly affect TJ recovery after they are disrupted by apical hypertonicity.
The present study addresses the interactions of the metal ions with the binding sites that affect the function of the TJs in order to characterize their selectivity.
materials and methods
Urinary bladders of the frog Rana catesbeiana were used. Animals were anesthetized by subcutaneous injection of a 2% solution of 3-aminobenzoic acid ethyl ester (methanesulfonate salt) (Sigma Chemical Co., St. Louis, MO) at a dose of 1 ml/100 g body wt. The abdominal cavity was opened, a cannula was passed through the cloaca, and the urinary bladder was inflated with 15–20 ml of air according to the animal size. Plastic rings of 20-mm diameter were glued to the serosal surface of the bladder with ethylcyanoacrylate adhesive (Super Bonder; Loctite, Sáo Paulo, Brazil). The fragment of tissue framed by the plastic ring was excised and immersed in Ringer solution. Subsequently, it was mounted in a modified Ussing's chamber (Castro et al., 1993), exposing an area of 0.5 cm2. Hemichambers with a recessed rim filled with high viscosity silicone grease (High Vacuum Grease; Dow-Corning Corp., Indianapolis, IN) prevented tissue edge damage (Lacaz-Vieira, 1986). Each chamber compartment was perfused with a continuous flow of solution (up to 25 ml/min) driven by gravity from reservoirs through plastic tubings. Unstirred layers on the surfaces of the tissue were minimized by directing the incoming fluid towards the tissue surfaces. Each compartment was drained through a spillway open to the atmosphere so that the pressure inside each compartment was kept fairly constant at the atmospheric level. Rapid solution changes were obtained without interruption of voltage clamping by switching the inlet tubings at their connections with the chamber.
Solutions
Unless otherwise stated, the inner bathing solution was NaCl Ringer's solution. The Ringer's compositions were (mM): NaCl Ringer: 115 NaCl, 2.5 KHCO3, and 1.0 CaCl2. Na2SO4 Ringer: 57.5 Na2SO4, 2.5 KHCO3, 1.0 CaSO4. NaCl HEPES Ringer: 115 NaCl, 2.5 KCl, 2.0 HEPES. All Ringer's solutions had their pH adjusted to 8.2 after aeration. The apical bathing fluids were simple salt solutions, nonbuffered, prepared with glass-distilled water, having pH ∼6.0 and free Ca2+ concentration in the range of 1.5 × 1–7 and 2.0 × 1–7 M (Castro et al., 1993). In the beginning of the experiments, the apical solution was 75 mM KCl.
Electrical Measurements
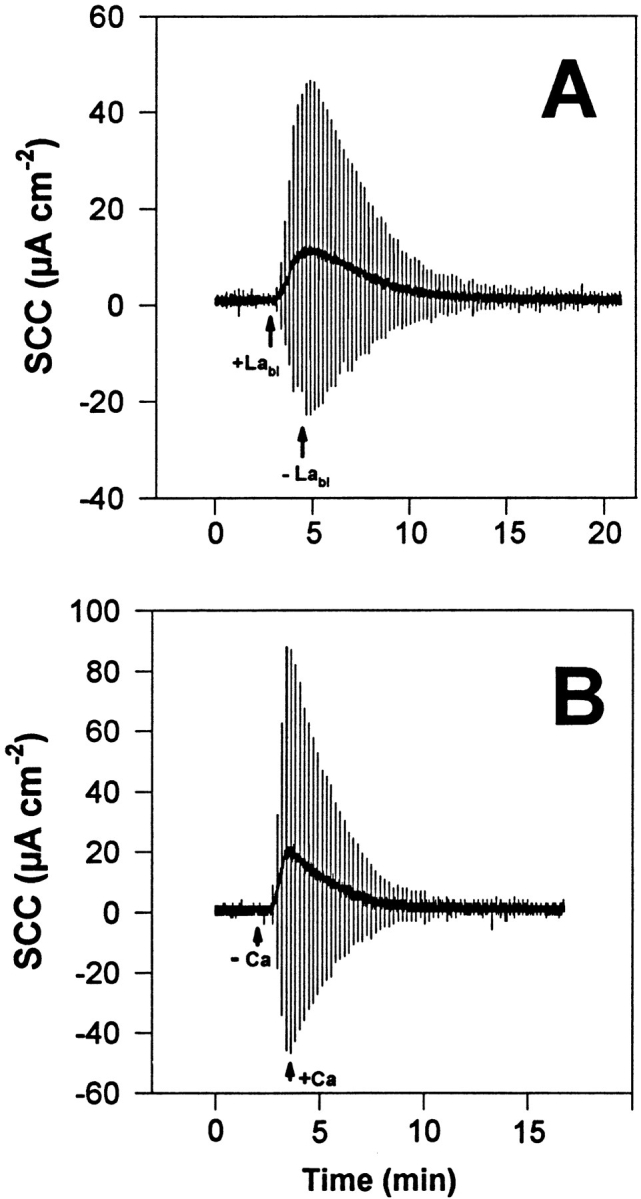
A conventional analog voltage clamp (DVC 1000; WPI, New Haven, CT) was used. Saturated calomel half-cells with 3 M KCl-agar bridges were used to measure the electrical potential difference across the bladder. Current was passed through Ag-AgCl 3 M KCl electrodes and 3 M KCl-agar bridges, adequately placed to deliver a uniform current density across the bladder. The clamping current was continuously recorded by a strip-chart recorder. Clamping current and voltage were also digitized through an analog- to-digital converter (Digidata 1200 and Axotape 2.0; Axon Instruments Inc., Foster City, CA) and stored in a computer for further processing. A digital Gaussian Filter (Colquhoun and Sigworth, 1983) was used to remove high frequency noise of the baseline of all records used in the figures. This digital filter forms output values γi from input values χi by performing the arithmetic mean of three consecutive current values, so that
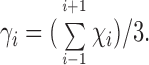 |
Isotopic Flux Measurements
14C-Sucrose (Amersham International, Little Chalfont, UK) was added to the apical or basolateral solution, and the fluid was recirculated with a peristaltic pump at a rate of 5 ml/min. An equilibration period of at least 20 min, during which the solution in the opposite compartment was continuously renewed, was allowed before the sampling in this compartment. During the sampling period, the flow of solution through the compartment was stopped and the solution was stirred by a plastic propeller driven by an electric motor. At the end of the collection interval, the solution was removed for radioactivity assay. Subsequently, the compartment was refilled and a new sampling period started.
TJ Blockade by the Selective Deposition of BaSO4
TJ blockade was induced according to a previously described method (Castro et al., 1993). Tissues were bathed on the basolateral side by Na2SO4 Ringer's solution. To induce the blockade by the selective deposition of BaSO4 in the TJs, the apical bathing fluid was replaced by a solution of BaCl2 (50 mM) and a positive clamping potential (+50 mV) was applied across the tissue to force migration of Ba2+ and SO4 2+ against each other and the formation of BaSO4 precipitate at the TJ level.
Chemicals
All chemicals were obtained from Sigma Chemical Co.
Statistics
The results are presented as mean ± SEM. Comparisons were carried out using Student's paired t test. When more than two groups were compared, significance was determined by two-way analysis of variance followed by appropriate posttest comparison. The P values cited include Bonferroni's correction (Neter and Wasserman, 1974).
results
The experiments were carried out in short-circuited frog urinary bladders bathed on the basolateral side by NaCl-Ringer's solution (or a different Ringer's solution according to the protocol), and on the apical side, in most cases, by a simple solution of KCl (75 mM). The absence of Na+ in the apical solution aimed at abolishing the short-circuit current as well as the role of transcellular Na+ conductance to the overall tissue electrical conductance, so that changes in the transepithelial electrical resistance (TER) reflected changes in the electrical resistance of the tight junctions, as in other tight epithelia (Jovov et al., 1994; Wills and Millinoff, 1990).
Transepithelial Electrical Resistance
TER is shown in Ω cm2, calculated from the deflections of the clamping current induced by shifts of the clamping potential of 300 ms duration, ±1 mV amplitude at 15-s intervals, as TER = ΔVt/ΔIt, where ΔVt and ΔIt are the changes in the electrical potential difference across the tissue and clamping current, respectively. It, clamping current in μA/cm2. Positive (or inward) current corresponds to the transport of positive charges across the tissue, from the apical to the inner bathing solution. Vt, electrical potential difference across the tissue (millivolts). The potential of the apical solution is referred to that of the inner solution.
The general protocol consisted in analyzing the interactions of metal ions with the binding sites that affect the TJ permeability according to a Ca2+-switch assay that consisted of a two-step process: (a) increase of TJ permeability, characterized by a drop in TER, was induced by removing Ca2+ from the basolateral solution. (b) TJ recovery, characterized by return of TER to initial values, was achieved by the reintroduction of Ca2+ into the basolateral solution. Small, short-term perturbations of the TJs were induced to prevent or minimize tardy regulatory responses that might complicate the results. This is exemplified by the fact that the rate of Ca2+-induced TJ recovery depends on the degree of TJ opening, which, in turn, depends on the time the bladders were without Ca2+, in agreement with observations in MDCK (Martinez-Palomo et al., 1980) and A6 (Jovov et al., 1994) cell monolayers. To cope with this problem, the drop of TER in response to Ca2+ withdrawal was normally terminated by the readmission of Ca2+ to the basolateral fluid when TER reached values close to 250 Ω cm2. The experiments were carried out, unless specified, with nominally Ca2+-free apical solution. The presence of Ca2+ in the apical solution is not essential for stability of TJs in A6 cell monolayers (Jovov et al., 1994) or in the frog urinary bladder (Lacaz-Vieira and Kachar, 1996).
Effect of Basolateral Ca2+ on TER
Ca2+ removal from the basolateral solution (NaCl, Na2SO4, or NaCl HEPES Ringer's solution) induces, after a lag time (generally between 30 s and 3 min), a pronounced drop of TER. Once started, the decline of TER shows a rapid progress. Return to Ca2+ promptly stops the decline of TER and triggers a full recovery. In the example of Fig. 1 A, the onset of TER decline has a lag time of 90 s and TER drops to 2% of the initial value in 160 s. Mean values of TER for a group of eight bladders bathed on the apical side by 75 mM KCl and by NaCl Ringer's solution on the basolateral side are: initial condition, 11,729.5 ± 1,532.5 Ω cm2; 120 s after Ca2+ removal from the basolateral medium, 207.3 ± 32.3 Ω cm2; after full recovery of TER in response to the reintroduction of Ca2+ into the basolateral medium, 11,567.1 ± 1,667.0 Ω cm2. The changes in short-circuit current (SCC) that take place in conjunction with the changes in TER result from the movement of ions (mostly Na+ and K+) along the paracellular pathway driven by their concentration differences in the bathing solutions.
Figure 1.
Representative experiments (of a group of eight tissues) carried out in the same piece of bladder showing the effect of Ca2+ withdrawal from the basolateral solution on TER and the reversibility of the process. The urinary bladder was short-circuited and bathed by 75 mM KCl on the apical side and by NaCl Ringer's solution on the basolateral side. (A) The basolateral Ca2+ was removed (−Cabl) and reintroduced (+Cabl). (B) The basolateral Ca2+ was equimolarly replaced by Mg2+ ((Ca × Mg)bl); subsequently, this change was reversed ((Mg × Ca)bl). (C) The basolateral Ca2+ was equimolarly replaced by Ba2+ ((Ca × Ba)bl); subsequently, this change was reversed ((Ba × Ca)bl). The vertical deflections of SCC, caused by pulses of ±1 mV in the clamping potential, are proportional to the overall tissue electrical conductance (G), where G = 1/TER.
A stepwise reduction of basolateral Ca2+ concentration (by addition of EGTA) does not result in a decrease of TER until [Ca2+]bl reaches values in the range of 70–100 μM. A further decrease in concentration resulted in a pronounced decline of TER. The dependence of steady state values of TER on [Ca2+]bl is sigmoidal and conforms with the Hill equation (Rodwell, 1996): TER[Ca] = TER/(1 + [K m/[Ca]]n), with a K m value of 62 ± 28 μM and a Hill coefficient (n) of 8.6 ± 0.9, indicating a steep dependence of TER on [Ca2+]bl. TER[Ca] is the value of TER at any given serosal Ca2+ concentration; TER is the value of TER at 1 mM serosal Ca2+ concentration; K m is the serosal Ca2+ concentration that reduces TER to 50% of the value at normal Ca2+ concentration.
The drop of TER that follows basal Ca2+ withdrawal is caused by a decrease of TJ permeability since it is accompanied by a significant increase of tissue permeability to 14C-sucrose that fully reverses upon reintroduction of Ca2+. The sucrose influx (Jin), which reflects the magnitude of the paracellular permeability, increased from (a) 0.65 ± 0.06 pmol cm−2 min−1 in the control condition (75 mM KCl on the apical side and NaCl Ringer's on the basolateral side) to (b) 3.74 ± 0.09 pmol cm−2 min−1, 5 min after Ca2+ removal from the basolateral solution, and returned to a steady value of (c) 0.68 ± 0.07 pmol cm−2 min−1 10 min after addition of Ca2+ to the basolateral solution. Statistical comparison: a–b, P < 0.01; a–c, P = NS (n = 6).
To circumvent a conceivable objection that sucrose flux measurements, which involve long periods of time, might not provide a clear indication that the initial drop of TER in response to basolateral Ca2+ withdrawal results from an increase of TJ permeability, additional experiments were performed in which open TJs were blocked by the selective deposition of BaSO4 (Castro et al., 1993). The urinary bladders were bathed on the basolateral side by a sulfate-containing solution (Na2SO4 Ringer's, see material and methods) to cause precipitation of BaSO4 in the open TJs when BaCl2 is added to the apical compartment. As soon as TER decreased in response to Ca2+ withdrawal from the basolateral fluid, the addition of Ba2+ to the apical solution leads to a prompt and marked increase of TER that results from the blockade of the permeabilized TJs by precipitation of BaSO4 (Fig. 2). In a control group of bladders bathed by NaCl Ringer's, no effect was observed in response to the addition of Ba2+ to the apical solution, excluding the possibility that the increase of TER caused by apical Ba2+ resulted from the blockade of a transcellular pathway involving K channels (Van Driessche and Zeiske, 1980). The experiments with Ba2+ provide strong evidence that the early drop of TER associated with basolateral Ca2+ withdrawal results from a relaxation of the TJ seal.
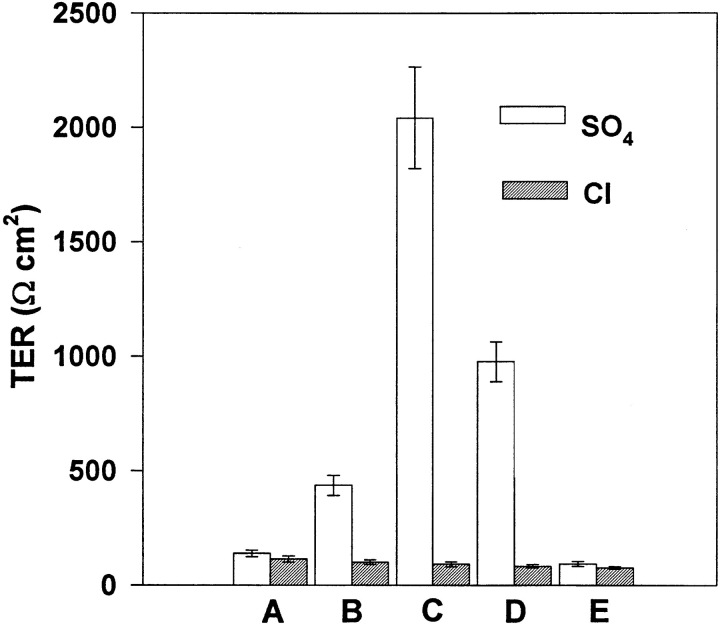
Figure 2.
TJ blockade by the selective deposition of BaSO4. This maneuver permits us to certify that the early drop of TER that takes place in response to basolateral Ca2+ withdrawal results from the opening of TJs. The urinary bladders were initially short-circuited and bathed on the apical side by 75 mM KCl and on the basolateral side by Na2SO4 Ringer's (clear bars) or NaCl Ringer's (hatched bars). (A) TER was evaluated 120 s after the beginning of TER drop in response to basolateral Ca2+ removal. (B) The apical solution was then replaced by 50 mM BaCl2 and TER was evaluated after 20 s. (C) Without removing Ba2+, the clamping potential was set to +50 mV and TER was evaluated 60 s later. (D) Without removing Ba2+, the clamping potential was returned to 0 mV and, after 20 s, TER was evaluated. (E) The apical compartment was rinsed several times with 75 mM KCl and TER was evaluated 10 min later. It can be seen that only tissues bathed on the inner surface by Na2SO4 Ringer's solution showed a marked increase of TER in response to the presence of Ba2+ in the apical compartment, an increase that is enhanced by the imposition of a +50-mV calming potential that favors the interaction of Ba2+ and SO4 2+ at the TJ level and the formation of BaSO4 precipitate. n = 6.
Role of Cytosolic Ca2+ Concentration on TER Responses to Changes in Extracellular Ca2+ Concentration
To ascertain the contribution of cytosolic Ca2+ concentration on TER responses to changes in extracellular Ca2+ concentration, two experimental approaches were used.
BAPTA-AM.
The epithelial cells were loaded with Ca2+ chelator by incubating tissues (n = 5) with the cell-permeant BAPTA-AM ester (10 μM) on both sides for 20 min. No effect was observed on TER. The chelator was then removed together with Ca2+ from the basolateral fluid, leading to a drop of TER that fully recovered upon Ca2+ return to the basolateral fluid. The fact that in the presence of an intracellular Ca2+ chelator, the introduction of Ca2+ into the basolateral fluid triggers TJ recovery is a strong argument in favor of an extracellular effect of Ca2+, most certainly at the level of E-cadherin.
Ionophore A-23187.
Two different protocols were tested. In one (n = 3), the experiments were performed with an apical solution containing 75 mM KCl, 1 mM Ca2+, and 3 μM A23187. The presence of the ionophore in the apical solution caused only a small decrease of TER that soon stabilized. A subsequent removal of basolateral Ca2+ induced a reduction of TER similar to that shown in Fig. 1 A. Recovery was obtained by reintroducing Ca2+ into the basolateral fluid. In another group of experiments (n = 5), the apical solution was 75 mM KCl plus the ionophore (3 μM). TJs were opened by removal of basolateral Ca2+, and then the apical solution was replaced by another containing, in addition to the ionophore, 1 mM Ca2+. Upon addition of Ca2+ to the apical solution, a transient reduction of TER that lasted 1–2 min was observed, followed by a subsequent decline of TER. Return to Ca2+ in the basolateral solution then triggered a complete recovery of TER. These experiments suggest that Ca2+ entering the cells through the pathways created by the ionophore may transiently trigger TER recovery. However, a complete and stable recovery of the TJ seal was only obtained upon addition of Ca2+ to the basolateral solution.
Divalent Metal Ions
These experiments aimed to appraise the degree of interaction of divalent cations with the basolateral Ca2+ sites that affect the sealing of the TJs. Two different aspects were analyzed: (a) the ability of the metal ion to prevent the opening of the TJs when the metal ion replaced the basolateral Ca2+, and (b) the ability of the metal ion to induce the resealing of TJs previously opened by the removal of basolateral Ca2+.
Alkaline earth metals as controls: Mg2+ and Ba2+.
The equimolar substitution of basolateral Ca2+ by Mg2+ causes a drop in TER similar to that induced by the removal of Ca2+ from the basolateral fluid. Return to Ca2+ leads to a full recovery of TER (Fig. 1 B). A similar behavior is observed in response to the substitution of the basolateral Ca2+ by Ba2 (Fig. 1 C). These results indicate that Mg2+ and Ba2+ are ineffective in maintaining the TJ seal in the frog urinary bladder. Experiments (not shown) also indicate that TJs previously opened by Ca2+ withdrawal do not close in response to addition of Mg2+ or Ba2+ (1 mM) to the basolateral medium. Higher concentrations up to 10 mM were tested without effect.
Transition elements: Cd2+ and Mn2+.
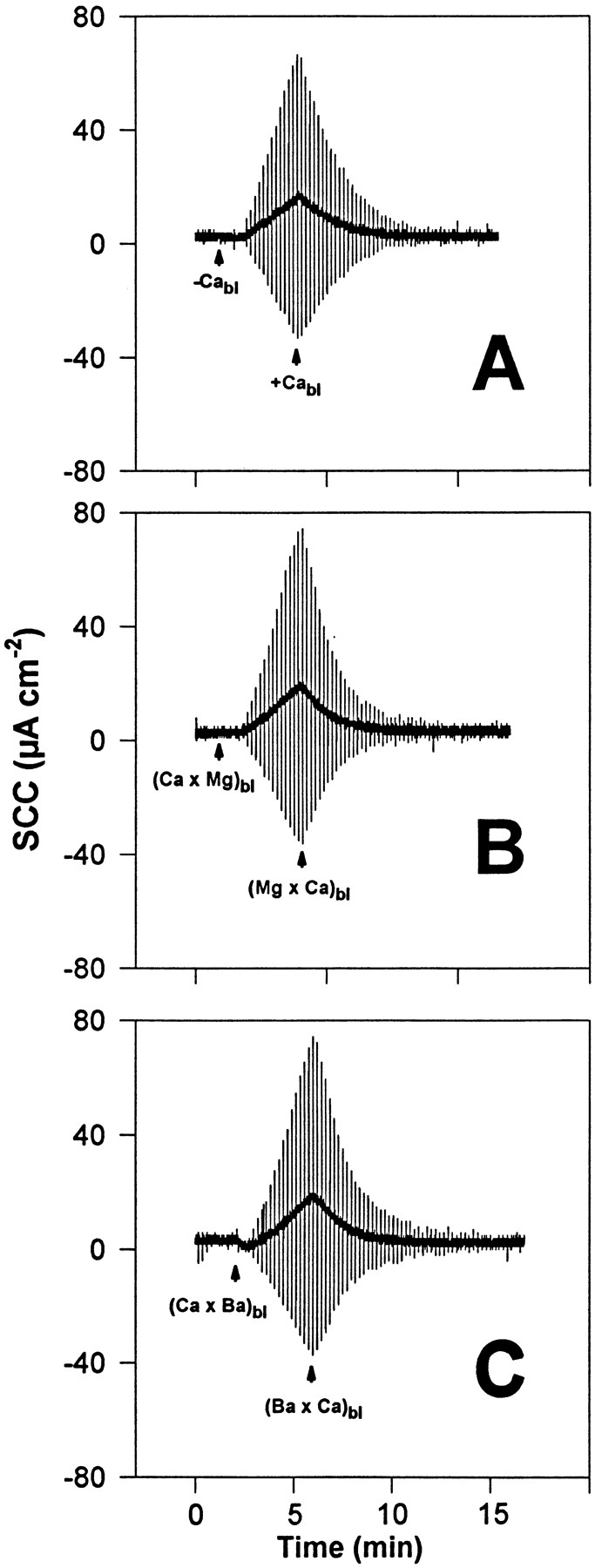
The equimolar substitution of basolateral Ca2+ by Cd2+ (Fig. 3 A) or Mn2+ (Fig. 4) does not lead, as observed for the alkaline earth metals, to a reduction of TER, indicating that these transition elements show a Ca2+ agonistic effect in short term experiments, characterized by their ability to keep the TJs closed in the absence of basolateral Ca2+. A subsequent withdrawal of Cd2+ (Fig. 3 A) or of Mn2+ (Fig. 4) triggers junction opening, indicated by a decline of TER that follows a time course comparable with that observed in response to basolateral Ca2+ removal. TJ opening that follows Cd2+ removal is promptly halted upon reintroduction of Ca2+ (Fig. 3 A) or even Cd2+ into the basolateral solution. Recovery is, however, incomplete even in response to Ca2+, suggesting a residual, apparently toxic effect of Cd2+. This toxic effect of Cd2+ is greatly reduced or even eliminated if Ca2+ is also present, as shown in Fig. 3 B. Tissues exposed for several minutes to basolateral Cd2+ (1 mM) in the presence of a normal basolateral Ca2+ concentration behave, after Cd2+ removal, as control tissues not exposed to Cd2+.
Figure 3.
Representative experiments (of a group of seven tissues) of the action of Cd2+ added to the basolateral solution on the dynamics of TJ opening and closing. The urinary bladders were short-circuited and bathed on the apical side by 75 mM KCl and on the basolateral side by NaCl Ringer's solution. (A) Basolateral Ca2+ was replaced by 1 mM Cd2+ ((Ca × Cd)bl) with no resulting increase of TER. Afterwards, Cd2+ was removed from the basolateral solution (−Cdbl) leading, after a few seconds, to an increase of TER that can be terminated by reintroduction of Ca2+ (1 mM) into the basolateral fluid (+Cabl). No recovery of TER was observed in response to reintroduction of Ca2+, in contrast to control tissues. (B) Cd2+ was added to the basolateral solution (in the presence of 1 mM Ca2+) at concentrations of 0.2 mM (+Cdbl 0.2) and 1.0 mM (+Cdbl 1.0). Subsequently, Cd2+ was removed (−Cdbl) without any noticeable effect. Afterwards, basolateral Ca2+ was also removed (−Cabl), leading to a conspicuous decrease of TER that fully reverts upon readmission of Ca2+ into the basolateral fluid (+Cabl). The vertical deflections of SCC, caused by pulses of ±1 mV in the clamping potential, are proportional to the overall tissue electrical conductance (G), where G = 1/TER.
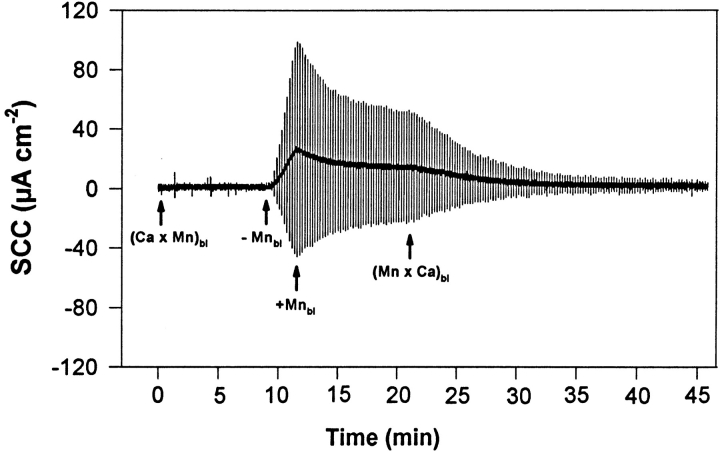
Figure 4.
Representative experiments (of a group of eight tissues) of the action of Mn2+ added to the basolateral solution on the dynamics of TJ opening and closing. The urinary bladders were short-circuited and bathed on the apical side by 75 mM KCl and on the basolateral side by NaCl Ringer's solution. Basolateral Ca2+ was replaced by 1 mM Mn2+ ((Ca × Mn)bl) with no resulting increase of TER. Afterwards, Mn2+ was removed from the basolateral solution (−Mnbl) leading, after a few seconds, to an increase of TER. Reintroduction of Mn2+ (1 mM) into the basolateral fluid triggers a slow recovery of TER that speeds up considerably when this ion is replaced by Ca2+ ((Mn × Ca)bl). The vertical deflections of SCC, caused by pulses of ±1 mV in the clamping potential, are proportional to the overall tissue electrical conductance (G), where G = 1/TER.
The effect of basolateral Mn2+ (Fig. 4) is different from that of Cd2+ since, in addition to promptly halting the decline of TER, Mn2+ leads to a slower but well characterized recovery of TER, a process that is accelerated and reaches completion if Mn2+ is replaced by Ca2+.
Apical Ca2+ May Reach the Binding Sites that Affect the TJs
The results presented so far show reversible changes of TER due to manipulation of basolateral Ca2+ in the absence of apical Ca2+. Similar results can be obtained in the presence of 1 mM Ca2+ in the apical solution. Higher concentrations of apical Ca2+, however, may curb the increase of TJ permeability that results from basolateral Ca2+ withdrawal. Thus, the presence of 10 mM Ca2+ in the apical solution markedly depresses (Fig. 5 A) or even abolishes (Fig. 5 B) TER decrease in response to basolateral Ca2+ withdrawal. A subsequent removal of apical Ca2+ speeds up (Fig. 5 A) or triggers (Fig. 5 B) a TER decrease that had been blocked by the high apical Ca2+ concentration. TJ permeability increase induced by withdrawal of basolateral Ca2+ stops promptly and reverts almost completely in response to addition of 10 mM Ca2+ to the apical solution (Fig. 5 B). Ca2+ channel blockers (Nifedipine, 1 and 3 μM; Verapamil, 0.3 mM) added to the apical solution have no influence on the effect of a high apical Ca2+ concentration, confirming previous findings (Lacaz-Vieira and Kachar, 1996) that the effect of apical Ca2+ is not mediated by Ca2+ entering the cells through apical Ca2+ channels. In conclusion, these results support the notion that apical Ca2+, crossing the open TJs, may reach the binding sites affecting TJ permeability. When a sufficient Ca2+ concentration is present in the apical solution, diffusion through normally closed TJs may be sufficient to raise the Ca2+ concentration at the binding sites as to overcome the withdrawal of Ca2+ from the basolateral solution.
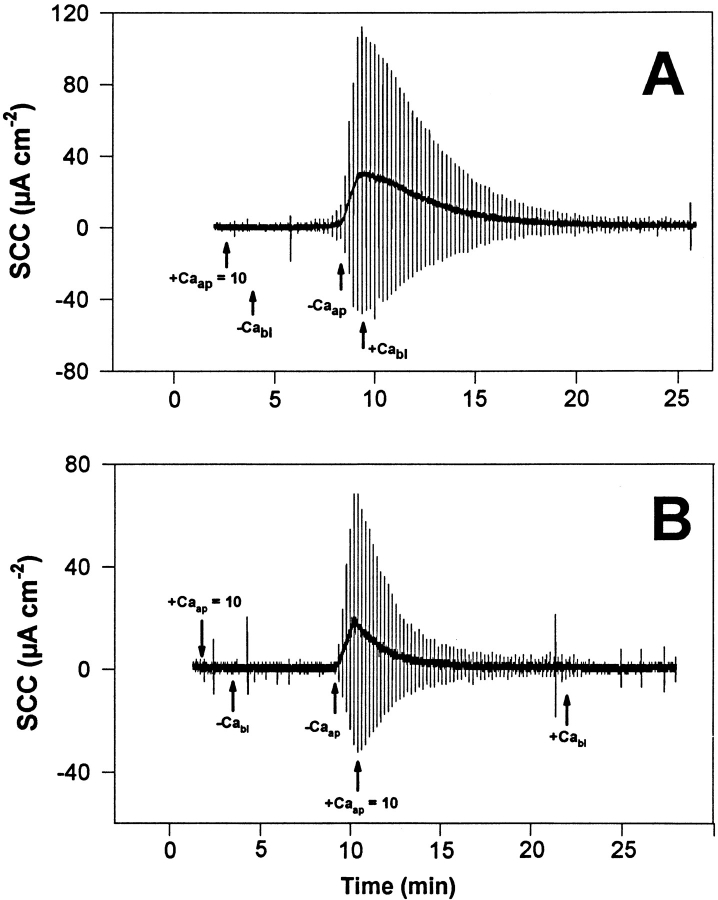
Figure 5.
Representative experiment (of a group of 10 tissues) of the action of apical Ca2+ on the TJ response to withdrawal of basolateral Ca2+. The urinary bladders were short-circuited and bathed on the apical side by 75 mM KCl and on the basolateral side by NaCl Ringer's solution. (A) The presence of 10 mM Ca2+ in the apical bathing fluid (+Caap = 10) markedly reduces the rate of TER decrease in response to Ca2+ removal from the basolateral solution (−Cabl). Subsequent removal of apical Ca2+ (−Caap) substantially increases the rate of TER decrease. TER fully recovers upon addition of 1 mM Ca2+ to the basolateral solution. (B) In this bladder, the presence of 10 mM Ca2+ in the apical solution (+Caap = 10) completely abolished the decrease of TER that normally follows the removal of Ca2+ from the basolateral solution (−Cabl). The subsequent withdrawal of apical Ca2+ (−Caap) then triggers a drop of TER that starts with an extremely short delay after the Ca2+ removal. In the absence of basolateral Ca2+, the introduction of 10 mM Ca2+ into the apical solution (+Caap = 10) leads to an almost complete recovery of TER. A full recovery of TER is attained when 1 mM Ca2+ is added to the basolateral fluid. The vertical deflections of SCC, caused by pulses of ±1 mV in the clamping potential, are proportional to the overall tissue electrical conductance (G), where G = 1/TER.
Mg2+ Competes with Ca2+ for the Binding Sites
These experiments use the fact just described that open TJs allow access of apical ions to the binding sites that affect the TJs. The presence of Mg2+ in the apical solution causes a concentration-dependent inhibition of TER recovery in response to Ca2+. The effect of apical Mg2+ starts to be noticed at apical concentrations at or above 5 mM. Fig. 6 shows an example in which 20 mM Mg2+ in the apical bathing medium practically abolishes the recovery of TER that occurs in response to the reintroduction of basolateral Ca2+. The subsequent withdrawal of Mg2+ from the apical solution triggers the TJ recovery process. These results show that Mg2+ reversibly competes with Ca2+ for the binding sites that control the TJ. It is interesting to observe that a high apical Mg2+ concentration inhibits the Ca2+-induced recovery of TER, but not the ability Ca2+ has to halt the decrease of TER (Fig. 6).
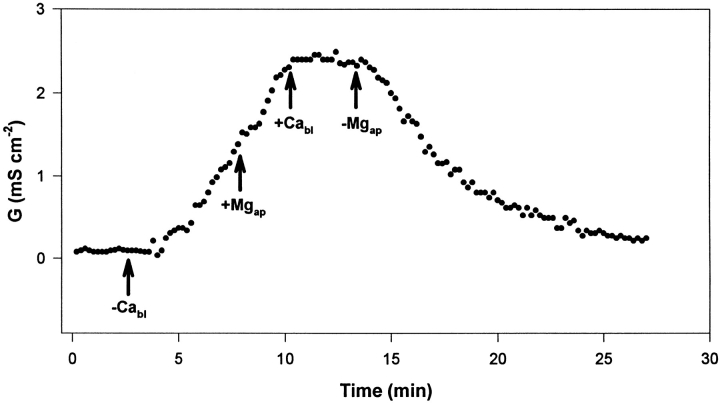
Figure 6.
Representative experiment (of a group of six) of the effect of apical Mg2+ on tissue electrical conductance (G) response to basolateral Ca2+ removal (−Cabl) and reintroduction (+Cabl). The urinary bladders were short-circuited and bathed on the apical side by 75 mM KCl and on the basolateral side by NaCl Ringer's solution. During the phase of G increase, the apical solution was replaced by a solution of 55 mM KCl plus 20 mM MgCl2 (+Mgap). Removal of apical Mg2+ was obtained by returning to a solution of 75 mM KCl (−Mgap).
La3+ Causes TJ Opening in the Presence of Ca2+
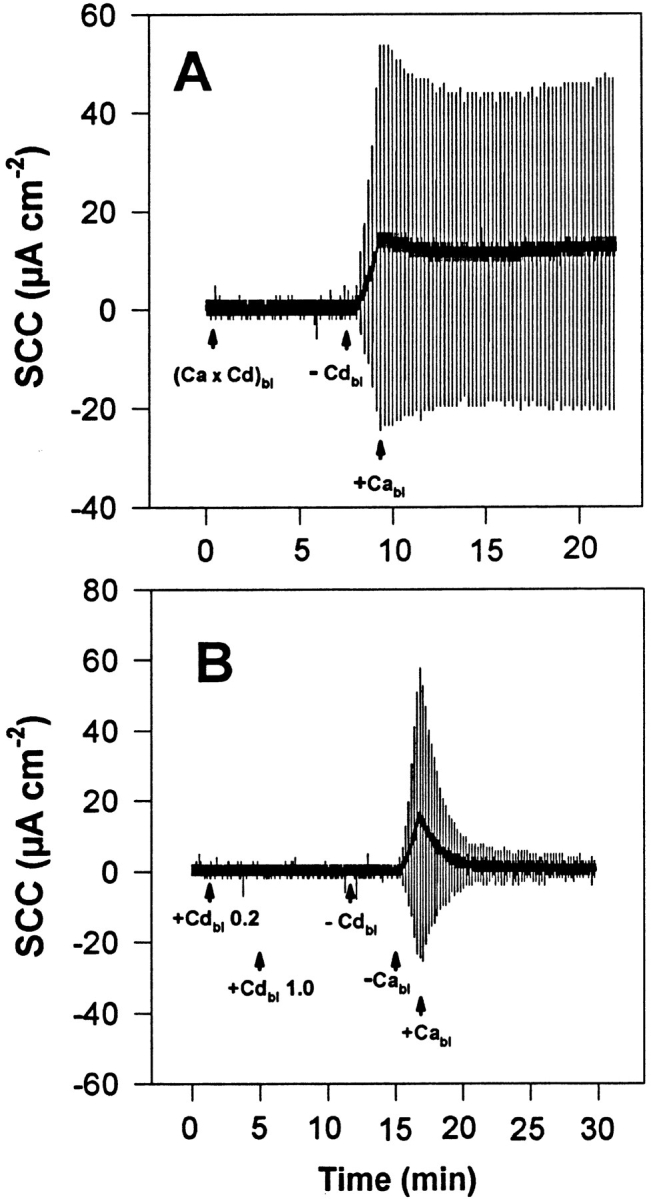
These experiments were carried out in NaCl HEPES Ringer's solution to prevent precipitation of La3+ in bicarbonate Ringer's solution due to the formation of poorly soluble lanthanum bicarbonate. Addition of La3+ (2 μM, as La(NO3)3) to the basolateral Ringer's solution (in the presence of Ca2+) causes a drop of TER (Fig. 7 A) with lag phases and time courses similar to those observed in response to basolateral Ca2+ withdrawal (Fig. 7 B). A subsequent removal of La3+ from the basolateral solution promotes a recovery of TER similar to what is observed when Ca2+ is readmitted to the basolateral solution after being previously removed. Higher concentrations of La3+ (>1 mM) may slow the quick recovery that follows its removal. This effect of basolateral La3+ on TER, observed in the presence of a normal Ca2+ concentration, characterizes a Ca2+ antagonistic effect of La3+.
Figure 7.
Representative experiment (of a group of nine tissues) of the action of basolateral La3+ (2 μM as La(NO3)3) on the TJ seal, as evaluated by TER. The urinary bladders were short-circuited and bathed on the apical side by 75 mM KCl and on the basolateral side by NaCl HEPES Ringer's solution. (A) TER decreases in response to addition of basolateral La3+ (+Labl) and fully recovers upon La3+ removal (−Labl). (B) Control experiment performed in the same tissue of the effect of basolateral Ca2+ withdrawal and subsequent reintroduction, showing the similarity between the responses to La3+ addition and Ca2+ removal. The vertical deflections of SCC, caused by pulses of ±1 mV in the clamping potential, are proportional to the overall tissue electrical conductance (G), where G = 1/TER.
Addition of La3+ (2 μM as La(NO3)3) to the apical solution (75 mM KCl) causes no effect on TER as it does when added to the basolateral solution. This indicates that the normally closed TJs of the urinary bladder are sufficiently restrictive to hamper the movement of the trivalent La3+ ion, preventing it from reaching the Ca2+ binding sites that, as previously seen, are readily accessible to La3+ from the basolateral aspect of the tissue. After La3+ removal from the apical bathing fluid (Fig. 8), tissues respond to the withdrawal of basolateral Ca2+ as a fresh tissue. Conversely, the addition of La3+ (2 μM) to the apical solution after the permeability of the TJs had been increased in response to basolateral Ca2+ removal has two distinct effects (Fig. 8 B): (a) apical La3+ promptly terminates the process of TER decrease, and (b) its presence in the apical compartment blocks TER recovery that takes place in response to basolateral Ca2+ addition. The first effect resembles that of Ca2+, Cd2+, and Mn2+. The second indicates that open TJs permit La3+ to enter from the apical side and reach the Ca2+ binding sites that control TJ function, acting as if La3+ had been added to the basolateral fluid.
Figure 8.
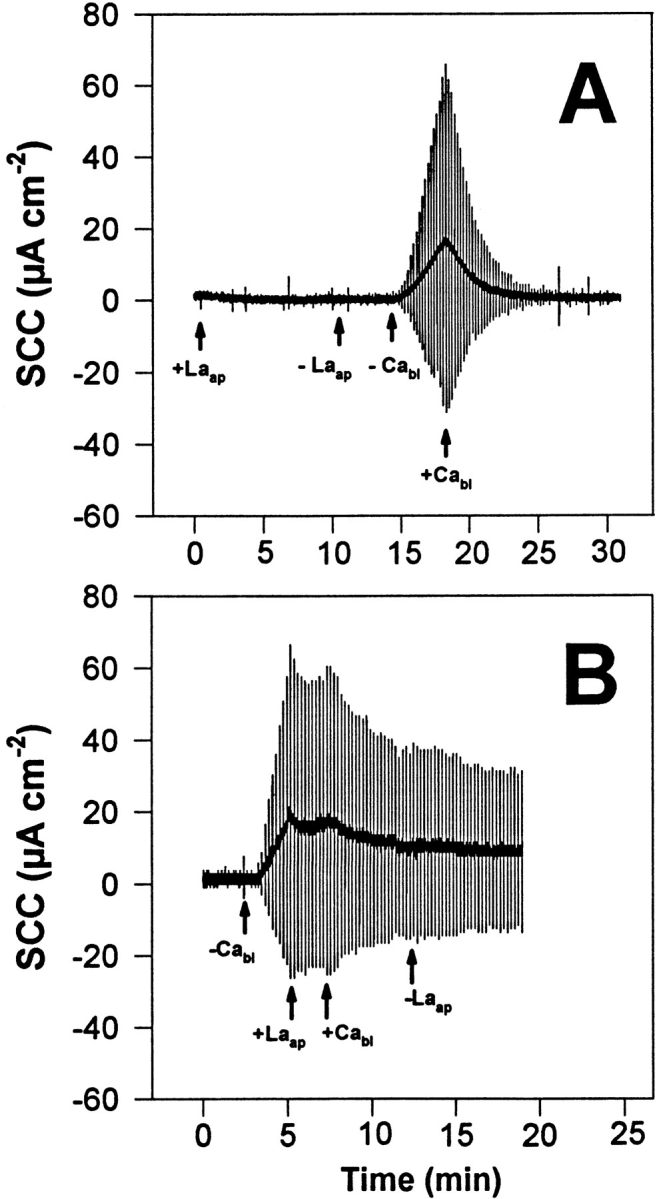
Representative experiments (in two groups of six tissues) of the action of apical La3+ on tissue response to manipulation of basolateral Ca2+. (A) Addition of La3+ (2 μM as La(NO3)3) to the apical solution (+Laap) has no effect whatever upon TER. Its subsequent removal (−Laap) renders the tissue responsive to the removal (−Cabl) and reintroduction (+Cabl) of basolateral Ca2+, as in control tissues not exposed to apical La3+. (B) Addition of La3+ (2 μM as La(NO3)3) to the apical solution (+Laap) during TER decrease caused by removal of basolateral Ca2+ (−Cabl) terminates the process of TER decrease and blocks TER recovery induced by reintroduction of basolateral Ca2+ (+Cabl). The vertical deflections of the SCC, caused by pulses of ±10 mV in the clamping potential, are proportional to the overall tissue electrical conductance (G), where G = 1/TER.
discussion
The present study deals with the interactions of metal ions with the extracellular Ca2+-binding sites that modulate the TJs in the frog urinary bladder. Focus was addressed to the early events associated with TJ opening and closing.
The dependence of TER, which reflects the degree of permeability of the TJs on basolateral Ca2+ concentration was evaluated to characterize the dependence of the TJ regulatory system of the frog urinary bladder on the external Ca2+ concentration. The steep dependence of TER on [Ca2+]bl, with a K m of 62 ± 28 μM and a Hill coefficient of 8.6 ± 0.9 (n = 5), indicates a high Ca2+ affinity of the extracellular Ca2+ sites and is in agreement with other tissues, such as MDCK (González-Mariscal et al., 1990) and A6 (Jovov et al., 1994) cell monolayers.
The results with the intracellular Ca2+ chelator indicates that a rise of intracellular Ca2+ concentration is not a critical step in the resealing of TJs induced by raising the basolateral Ca2+ concentration. Nonetheless, it cannot be ruled out that a sudden increase of cytosolic Ca2+ concentration may activate, at least transiently, the mechanism of TJ sealing. However, a complete and stable recovery of the TJ seal was only obtained upon addition of Ca2+ to the basolateral solution. Our findings are in agreement with observations in monolayers of human cervical cell line CaSki, where the effects of extracellular Ca2+ on TJ permeability were found not to be mediated by mobilization of cytosolic Ca2+ (Wild et al., 1997). On the other hand, the observations with the Ca2+ ionophore recalls the findings in A6 cell monolayers, where an increase of cytosolic Ca2+ concentration induced by the ionophore A-23187 caused recovery of the TJ seal (Jovov et al., 1994).
The result of a prolonged extracellular Ca2+ withdrawal has been described as causing a progressive disarray of the TJ structure in natural epithelia or in cell-cultured monolayers. In short-term experiments, however, Ca2+ removal is not accompanied by gross distortions of freeze-fracture images (Martinez-Palomo and Erlij, 1975; Martinez-Palomo et al., 1980; Lacaz-Vieira and Kachar, 1996), indicating that the rapid phase of TER drop after Ca2+ withdrawal might result from subtle alterations of the TJs structure not detectable by conventional methods.
The finding that the alkaline earth metals, Mg2+ and Ba2+, used as controls, were ineffective both in keeping the TJs closed and inducing the resealing of previously opened TJs is in consonance with findings in MDCK cell monolayers where Mg2+ and Ba2+ were also ineffective in promoting junction resealing (Martinez-Palomo et al., 1980; Contreras et al., 1992).
The transition elements, Mn2+ and Cd2+, behave as Ca2+ agonists since, in the absence of basolateral Ca2+, they promote stability of the TJs and halt almost instantly the TJ opening process triggered by basolateral Ca2+ removal. These effects of Mn2+ and Cd2+, which resemble the action of Ca2+, might result from their interaction with E-cadherin molecules that are mostly concentrated in the zonula adhaerens (Boller et al., 1985) and are known to be involved in the assembly of the junctional complex (Gumbiner et al., 1988). In addition, Mn2+ promotes an almost complete recovery of TER, closely resembling the effect of extracellular Ca2+. This effect of Mn2+ is in harmony with the observation in the toad urinary bladder, where Mn2+ and Sr2+ were able to revert the rapid fall of TER that takes place when Ca2+ was withdrawn from the medium (Lipson et al., 1965). In contrast, in the bullfrog gastric mucosa, Sr2+ does not promote recovery of the junctional seal (Forte and Nauss, 1963); in MDCK cell monolayers, only Ca2+ was effective in triggering TJ formation during a Ca2+ switch; Mg2+, Ba2+, Mn2+, and Cd2+ were ineffective (Contreras et al., 1992). These results show that major differences can be found among tissues and, for the sake of consistency, one given tissue must be thoroughly studied.
E-cadherin, in addition to its affinity for Ca2+ (Ringwald et al., 1987), also binds Cd2+, as can be inferred from binding experiments with E-CAD1, a recombinant 145-residue polypeptide that corresponds to one of the extracellular Ca2+-binding regions of E-cadherin (Prozialeck et al., 1996). In contrast to our experiments, where Cd2+ stabilizes the TJs in the closed state and interrupts the TJ opening process that is triggered by basolateral Ca2+ removal, recent studies show that Cd2+ can selectively damage the TJs between LLC-PK1 cells (Prozialeck et al., 1995) and human proximal tubule cells (Hazen Martin et al., 1993) and causes disruption of the TJ-associated microfilaments in rat Sertoli cells (Hew et al., 1993). It is conceivable that this discrepancy might result from the duration of Cd2+ contact with the preparation. In our case, brief tissue exposures to Cd2+ might have prevented the onset of major toxic effect, which might have been the cause of TJ disruption in other structures. Consequently, our short-term experiments apparently permit us to dissociate an agonistic effect of Cd2+ on the TJs from a less specific toxic effect.
How could Cd2+ stabilize the TJs in closed state, halt the TJ opening process but, at the same time, be unable to promote recovery to the TJ seal? A reasonable interpretation would be that Cd2+ acts as a Ca2+ agonist but, in addition, it presents toxic side effects that develop at a slower pace, preventing the recovery of the TJ seal. The possibility cannot be discarded that Cd2+ (as well as Ca2+ and Mn2+), in addition to interacting with E-cadherin, acts by bridging junctional sites at the level of the TJs themselves. This interpretation is supported by experiments in MDCK cell monolayers prefixed with glutaraldehyde, where Ca2+ removal still caused a pronounced drop of TER (Martinez-Palomo et al., 1980). In disharmony, however, is the observation that in junctional complex–enriched fractions from mouse liver, Ca2+ chelation with EGTA does not disrupt the negative-stained images of zonulae occludentes (Stevenson and Goodenough, 1984).
The fact that Cd2+ does not leave a residual, apparently toxic, effect when its contact with the tissue takes place in the presence of Ca2+ may result from a competitive interaction with Ca2+ for a common binding site, which most probably is E-cadherin. In support of this interpretation are the observations in LLC-PK1 cell monolayers that Cd2+ shows a higher binding affinity at low (0.1 mM) than at high (10 mM) Ca2+ concentrations (Prozialeck and Lamar, 1993), and also the experiments of Cd2+ binding to E-CAD1, a Ca2+ binding polypeptide analog of E-cadherin (Prozialeck et al., 1996).
Previous studies in frog skin (Castro et al., 1993) and urinary bladder (Lacaz-Vieira and Kachar, 1996) have shown that apical Ca2+ may reach the sites that control the TJs when the permeability of the TJs was increased. As Ca2+ channel blockers in the apical solution did not block the effect of apical Ca2+ (Lacaz-Vieira and Kachar, 1996), it can be inferred that apical Ca2+, passing through partially opened TJs, may reach the sites that affect the TJs located at the zonula adhaerens. In the present study, we explored in more detail this subject and showed that at concentrations higher than those of the Ringer's solution, apical Ca2+ is able to effectively fulfill the role of basolateral Ca2+, maintaining the TJs closed or even causing the resealing of open TJs in the absence of basolateral Ca2+. The fact that to be effective apical Ca2+ concentrations must be higher than that needed in the basolateral solution is reasonable if we take into consideration the diffusion barrier imposed by the TJs before Ca2+ reaches the zonula adhaerens.
The fact of Mg2+ competitively inhibiting the Ca2+-induced recovery of open TJs means that Mg2+ interacts with the Ca2+ binding sites, despite the fact that this interaction, in the absence of Ca2+ has no effect whatsoever on the stability of the TJs, the halting of the opening process, or recovery of the TJ seal. This competitive inhibition is in agreement with findings in A6 cell monolayers (Jovov et al., 1994).
Another aspect of the interaction of Mg2+ with the TJ regulatory system is the dissociation between the ability of basolateral Ca2+ to stop almost instantly the process of TJ opening, which is preserved, and the ability to induce the recovery of TER, which is abolished by Mg2+. This dualistic behavior may be regarded as an argument in favor of a dual effect of Ca2+. The preserved effect could be due to the formation of ionic bridges between components in adjacent cells or, most likely, Ca2+- induced changes in E-cadherin adhesiveness (Ringwald et al., 1987). The other, slower, abolished by Mg2+, is the recovery of the TJ seal, presumably resulting from a rearrangement of the TJ molecular organization mediated by cell signaling triggered by the interaction of Ca2+ with E-cadherin.
The fact that 200 μM La3+ causes a reversible TJ opening in the presence of 1 mM Ca2+ characterizes an antagonistic effect for Ca2+ in an apparent competitive interaction. Interaction of La3+ with Ca2+ binding sites has been studied in different structures (Weiss, 1974). La3+ is a modulator of gating activity of ionic channels (Takata et al., 1966; Vogel, 1974; Hille et al., 1975; Armstrong and Cota, 1990; Watkins and Mathie, 1994), a potent Ca2+ channel blocker (Nelson, 1987; Poncet et al., 1992; Clarke et al., 1994), and may also act as a Ca2+ agonist (Powis et al., 1994). These effects may result from the fact that La3+, by virtue of an effective ionic radius (1.10 Å) similar to that of Ca2+ (1.06 Å) (Snyder et al., 1990) and a valence higher than Ca2+, is expected to bind at Ca2+ sites more tightly than does Ca2+. The action of La3+ on the TJs is a complicated matter since different effects have been described. Our present finding that La3+ promotes TJ opening in the presence of Ca2+ contrasts with those in MDCK cells, where La3+ was used as a Ca2+ channel blocker and found not to interfere with junction sealing (Contreras et al., 1992), with neurophysiological experiments (where La3+ was used as a TJ blocker) (Sostman and Simon, 1991; Simon, 1992; Bryant and Moore, 1995; Wang et al., 1993), and with experiments in A6 cell monolayers, where a toxic effect was reported (Jovov et al., 1994). In the frog skin, however, the reversible opening of the TJs by an apical hypertonicity (Ussing, 1965) was made irreversible by the presence of La3+ in the apical solution (Erlij and Martinez-Palomo, 1972), indicating that La3+ interacted with open TJs, preventing their resealing. Transient effects of La3+ on TER and changes of ion selectivity have been described in rabbit gallbladder and ileum (Machen et al., 1972). Studies of protein Ca2+ sites have indicated that La3+ is often able to effectively replace bound Ca2+ because of the proximity of their effective ionic radius and because many Ca2+ sites bind both divalent and trivalent metal ions with high affinity (Brittain et al., 1976; Horrocks, 1984). Comparison of the dissociation constants for the binding of spherical metal ions from groups IA, IIA, IIIA, and lantanides indicates that both charge and size are important parameters in determining the specificity of the protein binding sites (Snyder et al., 1990).
The ability of La3+ to open TJs in the presence of Ca2+ is a relevant matter to the general use of La3+ in the evaluation of the permeability of TJs in epithelial and endothelial membranes (Arendt, 1991; Unakar et al., 1991; Vu et al., 1992; Shirai and Ikemoto, 1992; Caldwell and Slapnick, 1992; Morales and Cavicchia, 1993; Adamson and Michel, 1993; Zhong et al., 1994; Pelletier, 1994; Hochman et al., 1994; Hara et al., 1994; Devalia et al., 1994), particularly during in vivo perfusions or in the absence of a simultaneously present fixative such as glutaraldehyde or formaldehyde (Martinez-Palomo et al., 1971; Whittembury and Rawlins, 1971; Machen et al., 1972; Martinez-Palomo and Erlij, 1973; Tisher and Yarger, 1973). A comparative study of the permeability of TJs of blood barriers of the epididymis, vas deferens, and testis in the mink, using horseradish peroxidase and lanthanum nitrate, showing that lanthanum deposits were found at the microvilli despite the impermeability of the TJs to horseradish peroxidase, permitted the authors to suggest that the lanthanum technique yielded false positive results (Pelletier, 1994). It is conceivable that in this case some TJs could have been opened by the effect of La3+ before the action of fixatives could have taken place. Supporting this interpretation are studies (with high resolution electron micrographs of TJs in different structures in which La3+ was used during fixation) that have given no evidence that these junctions were permeable to colloidal lanthanum (Overton, 1968; Brightman and Reese, 1969; Goodenough and Revel, 1970), in contrast to experiments in which La3+ was perfused in living tissue (Schatzki, 1969, 1971), where there are evidences of lanthanum passage through the TJs.
To conclude, it is rewarding to compare (Table I) the effects of Ca2+ and metal ions upon uvomorulin (E-cadherin) in a study of early embryogenesis (Hyafil et al., 1981), where the authors concluded that uvomorulin undergoes a Ca2+ (or Mn2+- or Cd2+-)-dependent transition from a trypsin-sensitive to a trypsin-resistant conformation that favors recognition of uvomorulin by a monoclonal antibody and triggers cell compaction in early embryogenesis, and the findings of the present study. The close similarity of behavior observed in those two systems in response to similar treatments is a strong indication that the modulation Ca2+ and other metal ions exert on the TJs is most importantly mediated through their interaction with E-cadherin molecules.
Table I.
Comparative Effects of Metal Ions on the Dynamics of TJs, as Observed in the Present Study, and on the Uvomorulin Molecule (Hyafil et al., 1981)
| TJs | Uvomorulin | |
|---|---|---|
| • Ca2+, Mn2+, and Cd2+ confer stability to the TJs, but not to Mg2+ or Ba2+. | • Ca2+, Mn2+, and Cd2+ confer trypsin resistance to Umt (an 84-kD fragment of uvomorulin) and trigger the recognition of Umt by a monoclonal antibody. Mg2+ and Ba2+ are ineffective. | |
| • Ca2+ and Mn2+ are able to trigger the resealing of TJs previously opened by Ca2+ removal. Cd2+ is ineffective and apparently has a toxic effect upon the epithelial cells. Mg2+ and Ba2+ were ineffective. | • Ca2+ and Mn2+ (but not Cd2+, which is toxic to cells) prevent EC cells or morulae from decompacting in Ca2+-free medium. Mg2+ and Ba2+ were ineffective. | |
| • La3+ did not confer stability to the TJs. In addition, La3+ in the presence of Ca2+ reverts the Ca2+-dependent stability of the TJs, thus behaving as an inhibitor of the Ca2+ action. | • La3+ did not protect Umt against trypsin digestion. In addition, La3+ in the presence of Ca2+ reverts the Ca2+-induced protection, thus behaving as an inhibitor of Ca2+ action. | |
| • The inhibitory action of La3+ upon the Ca2+ effect is reversible upon La3+ removal. | • The inhibitory action of La3+ upon the Ca2+ effect is reversible upon La3+ removal. |
Footnotes
This project was supported by grants 96/3367-5 from Fundação de Amparo à Pesquisa do Estado de São Paulo, and 521869/94-3 and 303633/85-9 from Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil.
Abbreviations used in this paper: SCC, short-circuit current; TER, transepithelial electrical resistance; TJ, tight junction.
references
- Adamson RH, Michel CC. Pathways through the intercellular clefts of frog mesenteric capillaries. J Physiol (Camb) 1993;466:303–327. [PMC free article] [PubMed] [Google Scholar]
- Arendt T. Penetration of lanthanum through the main pancreatic duct epithelium in cats following exposure to infected human bile. Dig Dis Sci. 1991;36:75–81. doi: 10.1007/BF01300091. [DOI] [PubMed] [Google Scholar]
- Armstrong CM, Cota G. Modification of sodium channel gating by lanthanum. Some effects that cannot be explained by surface charge theory. J Gen Physiol. 1990;96:1129–1140. doi: 10.1085/jgp.96.6.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balda MS, González-Mariscal L, Contreras RG, Macias-Silva M, Torres-Marquez ME, García JA, Sáinz, Cereijido M. Assembly and sealing of tight junctions: possible participation of G-proteins, phospholipase C, protein kinase C and calmodulin. J Membr Biol. 1991;122:193–202. doi: 10.1007/BF01871420. [DOI] [PubMed] [Google Scholar]
- Balda MS, González-Mariscal L, Matter K, Cereijido M, Anderson JM. Assembly of the tight junction: the role of diacylglycerol. J Cell Biol. 1993;123:293–302. doi: 10.1083/jcb.123.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat M, Toledo D, Velasquez, Wang L, Malanga CJ, Ma JK, Rojanasakul Y. Regulation of tight junction permeability by calcium mediators and cell cytoskeleton in rabbit tracheal epithelium. Pharm Res. 1993;10:991–997. doi: 10.1023/a:1018906504944. [DOI] [PubMed] [Google Scholar]
- Boller K, Vestweber D, Kemler R. Cell-adhesion molecule uvomorulin is localized in the intermediate junction of adult intestinal epithelial cells. J Cell Biol. 1985;100:327–332. doi: 10.1083/jcb.100.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brightman MW, Reese TS. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol. 1969;40:648–677. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittain HG, Richardson FS, Martin RB. Terbium(III) emission as a probe of calcium(II) binding sites in proteins. J Am Chem Soc. 1976;98:8255–8260. doi: 10.1021/ja00441a060. [DOI] [PubMed] [Google Scholar]
- Bryant BP, Moore PA. Factors affecting the sensitivity of the lingual trigeminal nerve to acids. Am J Physiol. 1995;268:R58–R65. doi: 10.1152/ajpregu.1995.268.1.R58. [DOI] [PubMed] [Google Scholar]
- Caldwell RB, Slapnick SM. Freeze-fracture and lanthanum studies of the retinal microvasculature in diabetic rats. Invest Ophthalmol Vis Sci. 1992;33:1610–1619. [PubMed] [Google Scholar]
- Castro JA, Sesso A, Lacaz-Vieira F. Deposition of BaSO4 in the tight junctions of amphibian epithelia causes their opening; apical Ca2+ reverses this effect. J Membr Biol. 1993;134:15–29. doi: 10.1007/BF00233472. [DOI] [PubMed] [Google Scholar]
- Cereijido M, Ehrenfeld J, Meza I, Martinez-Palomo A. Structural and functional membrane polarity in cultured monolayers of MDCK cells. J Membr Biol. 1980;52:147–159. doi: 10.1007/BF01869120. [DOI] [PubMed] [Google Scholar]
- Cereijido M, Meza I, Martinez-Palomo A. Occluding junctions in cultured epithelial monolayers. Am J Physiol. 1981;240:C96–C102. doi: 10.1152/ajpcell.1981.240.3.C96. [DOI] [PubMed] [Google Scholar]
- Clarke BL, Moore DR, Blalock JE. Adrenocorticotropic hormone stimulates a transient calcium uptake in rat lymphocytes. Endocrinology. 1994;135:1780–1786. doi: 10.1210/endo.135.5.7956901. [DOI] [PubMed] [Google Scholar]
- Colquhoun, D., and F.J. Sigworth. 1983. Fitting and statistical analysis of single-channel records. In Single Channel Recording. B. Sakmann and E. Neher, editors. Plenum Press Inc., New York.
- Contreras RG, Miller JH, Zamora M, González-Mariscal L, Cereijido M. Interaction of calcium with plasma membrane of epithelial (MDCK) cells during junction formation. Am J Physiol. 1992;263:C313–C318. doi: 10.1152/ajpcell.1992.263.2.C313. [DOI] [PubMed] [Google Scholar]
- Devalia JL, Godfrey RW, Sapsford RJ, Severs NJ, Jeffery PK, Davies RJ. No effect of histamine on human bronchial epithelial cell permeability and tight junctional integrity in vitro. Eur Respir J. 1994;7:1958–1965. [PubMed] [Google Scholar]
- Einspahar H, Bugg CE. Cristal structure studies of calcium complexes and implications for biological systems. Metal Ions Biol Syst. 1984;17:51–97. [Google Scholar]
- Erlij D, Martinez-Palomo A. Opening of tight junctions in frog skin by hypertonic urea solutions. J Membr Biol. 1972;9:229–240. [PubMed] [Google Scholar]
- Forte JG, Nauss AH. Effect of calcium removal on bullfrog gastric mucosa. Am J Physiol. 1963;205:631–637. doi: 10.1152/ajplegacy.1963.205.4.631. [DOI] [PubMed] [Google Scholar]
- Galli P, Camilli BP, Meldolesi J. Extracellular calcium and the organization of tight junctions in pancreatic acinar cells. Exp Cell Res. 1976;99:178–183. doi: 10.1016/0014-4827(76)90694-7. [DOI] [PubMed] [Google Scholar]
- González-Mariscal L, Chávez de Ramirez B, Cereijido M. Tight junction formation in cultured epithelial cells (MDCK) J Membr Biol. 1985;86:113–125. doi: 10.1007/BF01870778. [DOI] [PubMed] [Google Scholar]
- González-Mariscal L, Contreras RG, Bolivar JJ, Ponce A, Chávez de Ramirez B, Cereijido M. Role of calcium in tight junction formation between epithelial cells. Am J Physiol. 1990;259:C978–C986. doi: 10.1152/ajpcell.1990.259.6.C978. [DOI] [PubMed] [Google Scholar]
- Goodenough DA, Revel JP. A fine structural analysis of the intercellular junctions in the mouse liver. J Cell Biol. 1970;45:272–290. doi: 10.1083/jcb.45.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorodeski GI, Jin WW, Hopfer U. Extracellular Ca2+directly regulates tight junctional permeability in the human cervical cell line CaSki. Am J Physiol. 1997;272:C511–C524. doi: 10.1152/ajpcell.1997.272.2.C511. [DOI] [PubMed] [Google Scholar]
- Gumbiner B, Stevenson BR, Grimaldi A. The role of the cell adhesion molecule uvomorulin in the formation and maintenance of epithelial junctional complex. J Cell Biol. 1988;107:1575–1587. doi: 10.1083/jcb.107.4.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K, Yoshizuka M, Doi Y, Fujimoto S. Effect of bis (tributyl tin) oxide on permeability of the blood-brain barrier: a transient increase. Occup Environ Med. 1994;51:735–738. doi: 10.1136/oem.51.11.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays RM, Singer B, Malamed S. The effect of calcium withdrawal on the structure and function of the toad bladder. J Cell Biol. 1965;25:195–208. doi: 10.1083/jcb.25.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazen Martin DJ, Todd JH, Sens MA, Khan W, Bylander JE, Smyth BJ, Sens DA. Electrical and freeze-fracture analysis of the effects of ionic cadmium on cell membranes of human proximal tubule cells. Environ Health Perspect. 1993;101:510–516. doi: 10.1289/ehp.93101510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hew KW, Heath GL, Jiwa AH, Welsh MJ. Cadmium in vivo causes disruption of tight junction-associated microfilaments in rat Sertoli cells. Biol Reprod. 1993;49:840–849. doi: 10.1095/biolreprod49.4.840. [DOI] [PubMed] [Google Scholar]
- Hille B, Woodhull AM, Shapiro BI. Negative surface charge near sodium channels of nerve: divalent ions, monovalent ions and pH. Philos Trans R Soc Lond B Biol Sci. 1975;270:301–318. doi: 10.1098/rstb.1975.0011. [DOI] [PubMed] [Google Scholar]
- Hochman JH, Fix JA, LeCluyse EL. In vitro and in vivo analysis of the mechanism of absorption enhancement by palmitoylcarnitine. J Pharmacol Exp Ther. 1994;269:813–822. [PubMed] [Google Scholar]
- Horrocks WDJ. Lanthanide ion luminescence in coordination chemistry and biochemistry. Prog Org Chem. 1984;31:1–104. [Google Scholar]
- Hyafil F, Babinet C, Jacob F. Cell–cell interactions in early embryogenesis: a molecular approach to the role of calcium. Cell. 1981;26:447–454. doi: 10.1016/0092-8674(81)90214-2. [DOI] [PubMed] [Google Scholar]
- Jovov B, Lewis SA, Crowe WE, Berg JR, Wills NK. Role of intracellular Ca2+ in modulation of tight junction resistance in A6 cells. Am J Physiol. 1994;266:F775–F784. doi: 10.1152/ajprenal.1994.266.5.F775. [DOI] [PubMed] [Google Scholar]
- Lacaz-Vieira F. Sodium flux in the apical membrane of the toad skin: aspects of its regulation and the importance of the ionic strength of the outer solution upon the reversibility of amiloride inhibition. J Membr Biol. 1986;92:27–36. doi: 10.1007/BF01869013. [DOI] [PubMed] [Google Scholar]
- Lacaz-Vieira F, Kachar B. Tight junction dynamics in the frog urinary bladder. Cell Adhes Commun. 1996;4:53–68. doi: 10.3109/15419069609010763. [DOI] [PubMed] [Google Scholar]
- Lipson S, Dodelson R, Hays R. Multivalent cations and cell adhesion. Clin Res. 1965;13:237. [Google Scholar]
- Machen TE, Erlij D, Wooding FBP. Permeability junctional complexes. The movement of lanthanum across rabbit gallbladder and intestine. J Cell Biol. 1972;54:302–312. doi: 10.1083/jcb.54.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RB. Bioinorganic chemistry of calcium. Metal Ions Biol Syst. 1984;17:1–49. [Google Scholar]
- Martinez-Palomo A, Erlij D, Bracho H. Localization of permeability barriers in the frog skin epithelium. J Cell Biol. 1971;50:277–287. doi: 10.1083/jcb.50.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Palomo A, Meza I, Beaty G, Cereijido M. Experimental modulation of occluding junctions in a cultured transporting monolayer. J Cell Biol. 1980;87:736–745. doi: 10.1083/jcb.87.3.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Palomo A, Erlij D. The distribution of lanthanum in tight junctions of the kidney tubule. Pflügers Archiv. 1973;343:267–272. doi: 10.1007/BF00586049. [DOI] [PubMed] [Google Scholar]
- Martinez-Palomo A, Erlij D. Structure of tight junctions in epithelia with different permeability. Proc Nat Acad Sci USA. 1975;72:4487–4491. doi: 10.1073/pnas.72.11.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldolesi J, Castiglioni G, Parma R, Nassivera N, Camilli P. Ca++-dependent disassembly and reassembly of occluding junctions in guinea pig pancreatic acinar cells. J Cell Biol. 1978;79:156–172. doi: 10.1083/jcb.79.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales A, Cavicchia JC. Seasonal changes of the blood-testis barrier in viscacha (Lagostomus maximus maximus): a freeze-fracture and lanthanum tracer study. Anat Rec. 1993;236:459–464. doi: 10.1002/ar.1092360306. [DOI] [PubMed] [Google Scholar]
- Nelson, M.T. 1987. Effects of permeant ions and blockers on properties of single calcium channels from brains. In Proteins of Excitable Membranes. B. Hille and D.M. Frambrough, editors. Society of General Physiologists and Wiley-Interscience, New York. 215–230. [PubMed]
- Neter, J., and W. Wasserman. 1974. Applied linear statistical models: regression analysis of variance and experimental designs. Richard D. Irwin, Inc. Homewood, Illinois. pp. 842.
- Overduin M, Harvey TS, Bagby S, Tong KI, Yau P, Takeichi M, Ikura M. Solution structure of the epithelial cadherin domain responsible for selective cell adhesion [see comments] Science (Wash DC) 1995;267:386–389. doi: 10.1126/science.7824937. [DOI] [PubMed] [Google Scholar]
- Overton J. Localized lanthanum staining of the intestinal brush border. J Cell Biol. 1968;38:447–452. doi: 10.1083/jcb.38.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palant CE, Duffey ME, Mookerjee BK, Ho S, Bentzel CJ. Ca2+ regulation of tight junction permeability and structure in Necturus gallbladder. Am J Physiol. 1983;245:C203–C212. doi: 10.1152/ajpcell.1983.245.3.C203. [DOI] [PubMed] [Google Scholar]
- Pelletier R-M. Blood barriers of the epididymis and vas deferens act asynchronously with the blood barrier of the testis in the mink (Mustela vison) . Microsc Res Tech. 1994;27:333–349. doi: 10.1002/jemt.1070270408. [DOI] [PubMed] [Google Scholar]
- Pitelka DR, Taggart BN, Hamamoto ST. Effects of extracellular calcium depletion on membrane topography and occluding junctions of mammary epithelial cells in culture. J Cell Biol. 1983;96:613–624. doi: 10.1083/jcb.96.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poncet V, Merot J, Poujeol P. A calcium-permeable channel in the apical membrane of primary cultures of the rabbit distal bright convoluted tubule. Pflügers Archiv. 1992;422:112–119. doi: 10.1007/BF00370410. [DOI] [PubMed] [Google Scholar]
- Powis DA, Clark CL, O'Brien KJ. Lanthanum can be transported by the sodium-calcium exchange pathway and directly triggers catecholamine release from bovine chromaffin cells. Cell Calcium. 1994;16:377–390. doi: 10.1016/0143-4160(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Prozialeck WC, Wellington DR, Mosher TL, Lamar PC, Laddaga RA. The cadmium-induced disruption of tight junctions in LLC-PK1 cells does not result from apoptosis. Life Sci. 1995;57:PL199–PL204. doi: 10.1016/0024-3205(95)02109-v. [DOI] [PubMed] [Google Scholar]
- Prozialeck WC, Lamar PC, Ikura M. Binding of cadmium (Cd2+) to E-CAD1, a calcium-binding polypeptide analog of E-cadherin. Life Sci. 1996;58:PL325–330. doi: 10.1016/0024-3205(96)00159-2. [DOI] [PubMed] [Google Scholar]
- Prozialeck WC, Lamar PC. Surface binding and uptake of cadmium (Cd2+) by LLC-PK1 cells on permeable membrane supports. Arch Toxicol. 1993;67:113–119. doi: 10.1007/BF01973681. [DOI] [PubMed] [Google Scholar]
- Ringwald M, Schuh R, Vestweber D, Eistetter H, Lottspeich F, Engel J, Dölz R, Jähnig F, Epplen J, Mayer S, et al. The structure of cell adhesion molecule uvomorulin. Insights into the molecular mechanism of Ca2+-dependent cell adhesion. EMBO (Eur Mol Biol Organ) J. 1987;6:3647–3653. doi: 10.1002/j.1460-2075.1987.tb02697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodwell, V.W. 1996. Enzymes: kinetics. In Harper's Biochemistry. R.K. Murray, D.K. Granner, P.A. Mayes, and V.W. Rodwell, editors. 75–90.
- Schatzki PF. Bile canaliculus and space of Disse. Electron microscopic relationships as delineated by lanthanum. Lab Invest. 1969;20:87–93. [PubMed] [Google Scholar]
- Schatzki PF. The passage of radioactive lanthanum from the biliary to the vascular system. An electron microscopic and radioactive tracer study. Z Zellforsch Mikrosk Anat. 1971;119:451–459. doi: 10.1007/BF00455242. [DOI] [PubMed] [Google Scholar]
- Sedar AW, Forte JG. Effects of calcium depletion on the junctional complex between oxyntic cells of gastric glands. J Cell Biol. 1964;22:173–188. doi: 10.1083/jcb.22.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai T, Ikemoto I. Mechanism of alcoholic testicular damage. Nippon Hinyokika Gakkai Zasshi. 1992;83:305–314. doi: 10.5980/jpnjurol1989.83.305. [DOI] [PubMed] [Google Scholar]
- Simon SA. Influence of tight junctions on the interaction of salts with lingual epithelia: responses of chorda tympani and lingual nerves. Mol Cell Biochem. 1992;114:43–48. doi: 10.1007/BF00240296. [DOI] [PubMed] [Google Scholar]
- Snyder EE, Buoscio BW, Falke JJ. Calcium(II) site specificity: effect of size and charge on metal ion binding to an EF-hand-like site. Biochemistry. 1990;29:3937–3943. doi: 10.1021/bi00468a021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sostman AL, Simon SA. Trigeminal nerve responses in the rat elicited by chemical stimulation of the tongue. Arch Oral Biol. 1991;36:95–102. doi: 10.1016/0003-9969(91)90071-2. [DOI] [PubMed] [Google Scholar]
- Stevenson BR, Goodenough A. Zonulae occludentes in junctional complex-enriched fractions from mouse liver: preliminary morphological and biochemical characterization. J Cell Biol. 1984;98:1209–1221. doi: 10.1083/jcb.98.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strynadka NCJ, James MNG. Crystal structures of the helix-loop-helix calcium-binding proteins. Annu Rev Biochem. 1989;58:951–998. doi: 10.1146/annurev.bi.58.070189.004511. [DOI] [PubMed] [Google Scholar]
- Stuart RO, Sun A, Panichas M, Hebert SC, Brenner BM, Nigam SK. Critical role for intracellular calcium in tight junction biogenesis. J Cell Physiol. 1994;159:423–433. doi: 10.1002/jcp.1041590306. [DOI] [PubMed] [Google Scholar]
- Takata M, Pickard WF, Lettvin JY, Moore JW. Ionic conductance changes in lobster axon membranes when lanthanum is substituted for calcium. J Gen Physiol. 1966;50:461–471. doi: 10.1085/jgp.50.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisher CC, Yarger WE. Lanthanum permeability of the tight junction (zonula occludens) in the renal tubule of the rat. Kidney Int. 1973;3:238–250. doi: 10.1038/ki.1973.37. [DOI] [PubMed] [Google Scholar]
- Unakar NJ, Johnson MJ, Hynes K. Permeability studies in neonatal rat lens epithelium. Lens Eye Toxic Res. 1991;8:75–99. [PubMed] [Google Scholar]
- Ussing HH. Relationship between osmotic reactions and active sodium transport in the frog skin epithelium. Acta Physiol Scand. 1965;63:141–155. doi: 10.1111/j.1748-1716.1965.tb04052.x. [DOI] [PubMed] [Google Scholar]
- Van Driessche W, Zeiske W. Ba2+-induced conductance fluctuations of spontaneously fluctuating K+ channels in the apical membrane of frog skin (Rana pipiens) . J Membr Biol. 1980;56:31–42. doi: 10.1007/BF01869349. [DOI] [PubMed] [Google Scholar]
- Vogel W. Calcium and lanthanum effects at the nodal membrane. Pflügers Archiv. 1974;350:25–39. doi: 10.1007/BF00586736. [DOI] [PubMed] [Google Scholar]
- Vu DD, Tuchweber B, Raymond P, Yousef IM. Tight junction permeability and liver plasma membrane fluidity in lithocholate-induced cholestasis. Exp Mol Pathol. 1992;57:47–61. doi: 10.1016/0014-4800(92)90048-g. [DOI] [PubMed] [Google Scholar]
- Wang Y, Erickson RP, Simon SA. Selectivity of lingual nerve fibers to chemical stimuli. J Gen Physiol. 1993;101:843–866. doi: 10.1085/jgp.101.6.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins CS, Mathie A. Modulation of the gating of the transient outward potassium current of rat isolated cerebellar granule neurons by lanthanum. Pflügers Archiv. 1994;428:209–216. doi: 10.1007/BF00724499. [DOI] [PubMed] [Google Scholar]
- Weiss GB. Cellular pharmacology of lanthanum. Annu Rev Pharmacol. 1974;14:343–354. [Google Scholar]
- Whittembury G, Rawlins FA. Evidence of a paracellular pathway for ion flow in the kidney proximal tubule: electromicroscopic demonstration of lanthanum precipitate in the tight junction. Pflügers Archiv. 1971;330:302–309. doi: 10.1007/BF00588582. [DOI] [PubMed] [Google Scholar]
- Wild G, Madsen K, Thomson ABR. Intestinal tight junctions and their importance in health and disease: role of dietary lipids. J Nutr Biochem. 1997;8:2–12. [Google Scholar]
- Wills NK, Millinoff LP. Amiloride-sensitive Na+ transport across cultured renal (A6) epithelium: evidence for large currents and high Na:K selectivity. Pflügers Archiv. 1990;416:481–492. doi: 10.1007/BF00382680. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Enomoto K, Isomura H, Sawada N, Minase T, Oyamada M, Konishi Y, Mori M. Localization of the 7H6 antigen at tight junctions correlates with the paracellular barrier function of MDCK cells. Exp Cell Res. 1994;214:614–620. doi: 10.1006/excr.1994.1299. [DOI] [PubMed] [Google Scholar]