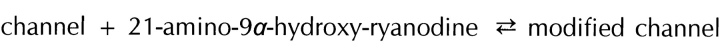Abstract
The binding of ryanodine to a high affinity site on the sarcoplasmic reticulum Ca2+-release channel results in a dramatic alteration in both gating and ion handling; the channel enters a high open probability, reduced-conductance state. Once bound, ryanodine does not dissociate from its site within the time frame of a single channel experiment. In this report, we describe the interactions of a synthetic ryanoid, 21-amino-9α-hydroxy-ryanodine, with the high affinity ryanodine binding site on the sheep cardiac sarcoplasmic reticulum Ca2+-release channel. The interaction of 21-amino-9α-hydroxy-ryanodine with the channel induces the occurrence of a characteristic high open probability, reduced-conductance state; however, in contrast to ryanodine, the interaction of this ryanoid with the channel is reversible under steady state conditions, with dwell times in the modified state lasting seconds. By monitoring the reversible interaction of this ryanoid with single channels under voltage clamp conditions, we have established a number of novel features of the ryanoid binding reaction. (a) Modification of channel function occurs when a single molecule of ryanoid binds to the channel protein. (b) The ryanoid has access to its binding site only from the cytosolic side of the channel and the site is available only when the channel is open. (c) The interaction of 21-amino-9α-hydroxy-ryanodine with its binding site is influenced strongly by transmembrane voltage. We suggest that this voltage dependence is derived from a voltage-driven conformational alteration of the channel protein that changes the affinity of the binding site, rather than the translocation of the ryanoid into the voltage drop across the channel.
Keywords: ryanodine, ryanodine receptor, calcium-release channel, sarcoplasmic reticulum, cardiac
introduction
Ryanodine is an alkaloid found in the wood of members of the genus Ryania. Ryanodine disrupts muscle function by binding to, and modifying the function of, an intracellular membrane Ca2+-release channel commonly referred to as the ryanodine receptor (RyR)1 (Sutko and Airey, 1996; Sutko et al., 1997).
The interaction of ryanodine with its receptor has been investigated using two experimental approaches: (a) directly, by monitoring the binding of [3H]ryanodine to isolated populations of membrane vesicles, or (b) indirectly, by monitoring the functional consequences of the interaction of the alkaloid with its receptor, either by determining the Ca2+ handling properties of isolated membrane vesicles or by monitoring the function of single RyR channels reconstituted into planar phospholipid bilayers.
The binding of [3H]ryanodine to isolated membrane vesicles has provided detailed information on the distribution and density of receptors in membrane populations, the affinity of the sites on the receptor, the rates of association and dissociation of ryanodine, and the number of sites on the receptor (Lai et al., 1989; Pessah and Zimanyi, 1991; McGrew et al., 1989; Wang et al., 1993; Coronado et al., 1994). Variations in the quantity of [3H]ryanodine bound to populations of receptor in the presence of ligands known to modify Ca2+-release channel function suggest that the interaction of the alkaloid with its receptor may be influenced by channel gating (Holmberg and Williams, 1990a ; Chu et al., 1990; Hawkes et al., 1992; Meissner and El-Hashem, 1992). Investigations in which the interaction with the receptor of various analogues and derivatives of ryanodine have been determined have provided information on the structural features of these ryanoids required for high affinity binding (Waterhouse et al., 1987; Jefferies et al., 1993; Gerzon et al., 1993; Welch et al., 1994, 1996; Sutko et al., 1997).
The interaction of ryanodine with the Ca2+-release channel modifies its function. At low concentrations (nano- to micromolar), ryanodine increases the Ca2+ permeability of isolated membrane vesicles; at high concentrations (high micro- to millimolar) ryanodine decreases Ca2+ permeability (Fairhurst and Hasselbach, 1970; Meissner, 1986; Fleischer et al., 1985; Lattanzio et al., 1987). The mechanisms underlying these concentration-dependent actions of ryanodine became apparent when the influence of ryanodine was examined on single Ca2+-release channels. Concentrations of the alkaloid that increase vesicle Ca2+ permeability modify single channel gating and ion handling; the channel enters a high open probability (P o), reduced-conductance state (Rousseau et al., 1987; Lindsay et al., 1994; Tinker et al., 1996). At higher concentrations of ryanodine, the Ca2+-release channel closes (Meissner, 1994; Tinker et al., 1996). Consistent with the extremely slow rates of association and dissociation of [3H]ryanodine determined in binding assays (Anderson et al., 1989; McGrew et al., 1989; Needleman and Hamilton, 1997; DiJulio et al., 1997), the onset of the modification of channel function by ryanodine is slow and, on the time scale of a single channel experiment, irreversible.
A study in which the influence, on single channel function, of a number of analogues and derivatives of ryanodine was examined has demonstrated that all of these ryanoids produce modifications of channel gating and ion handling. In all cases, the interaction of the ryanoid with the channel resulted in a dramatic increase in P o and a reduction in single channel conductance; however, the amplitude of the ryanoid-induced reduced conductance state is influenced by the structure of the ryanoid (Tinker et al., 1996; Welch et al., 1997). In addition, these investigations revealed that ryanoid structure also influenced the kinetics of the interaction of the alkaloid with its receptor. In particular, the time of residence of the channel in the ryanoid- induced modified conductance state varied. While the majority of the ryanoids examined, like ryanodine, modified conductance and gating irreversibly, some were reversible. With these ryanoids, under steady state conditions, channels alternated between periods of modified conductance and gating and periods of normal conductance and gating. Dwell times in the modified state lasted from tens of seconds to minutes (Tinker et al., 1996).
In this report, we provide a detailed description of the interaction of one particular reversible ryanoid, 21-amino-9α-hydroxy-ryanodine, with single sheep cardiac RyR channels. With this ryanoid, dwell times in the modified state last, in general, just a few seconds. This property makes it possible, for the first time, to obtain kinetic information on the interaction of a ryanoid with its receptor on a single channel.
materials and methods
Materials
Phosphatidylethanolamine was purchased from Avanti Polar Lipids (Alabaster, AL) and phosphatidylcholine from Sigma Ltd., (Poole, UK). [3H]Ryanodine was obtained from New England Nuclear Ltd. (Stevenage, UK). Aqueous counting scintillant was purchased from Packard (Groningen, The Netherlands). Standard chemicals were obtained as the best available grade from BDH Ltd. (Dagenham, UK) or Sigma Ltd. 21-amino-9α-hydroxy-ryanodine was synthesized as described earlier (Welch et al., 1997) and stored as a stock solution in 50% ethanol at −20°C.
Preparation of Sheep Cardiac Heavy Sarcoplasmic Reticulum Membrane Vesicles and Solubilization and Separation of the Ryanodine Receptor
Isolation of heavy sarcoplasmic reticulum (HSR) membrane vesicles was carried out using procedures described previously (Sitsapesan and Williams, 1990). Sheep hearts were collected from a local abattoir in ice-cold cardioplegic solution (Sitsapesan and Williams, 1990). A mixed membrane fraction was obtained by differential centrifugation after homogenization of the ventricular septum and left ventricle free wall. The mixed membrane vesicles were further fractionated by sucrose density gradient centrifugation and the HSR fraction collected at the 30/40% (wt/vol) interface. The HSR fraction was resuspended in 0.4 M KCl before sedimentation at 100,000 g. The resulting pellet was resuspended in 0.4 M sucrose, 5 mM HEPES, titrated to pH 7.2 with hydroxymethyl methylamine (Tris).
HSR membrane vesicles were solubilized with 3-[(3-cholamidopropyl)-dimethylammonio]-1-propane sulfonate (CHAPS) and RyR isolated and reconstituted into unilamellar liposomes for incorporation into planar phospholipid bilayers as described previously (Lindsay and Williams, 1991).
Planar Phospholipid Bilayers
Phospholipid bilayers were formed from suspensions of phosphatidylethanolamine in n-decane (35 mg/ml) across a 200-μm diameter hole in a polystyrene copolymer partition that separated two chambers referred to as cis (volume 0.5 ml) and trans (volume 1.0 ml). The trans chamber was held at virtual ground while the cis chamber could be clamped at holding potentials relative to ground. Current flow across the bilayer was monitored using an operational amplifier as a current–voltage converter (Miller, 1982). Bilayers were formed with solutions containing 600 mM KCl, 20 mM HEPES, titrated to pH 7.4 with KOH, resulting in a solution containing 610 mM K+ in both chambers. An osmotic gradient was created by the addition of an aliquot (50–100 μl) of 3 M KCl to the cis chamber. Proteoliposomes were added to the cis chamber and stirred. Under these conditions, channels usually incorporated into the bilayer within 2–3 min. If channels did not incorporate, a second aliquot of 3 M KCl could be added to the cis chamber. After channel incorporation, further fusion was prevented by perfusion of the cis chamber with 610 mM K+. Channel proteins incorporate into the bilayer in a fixed orientation so that the cytosolic face of the channel is exposed to the solution in the cis chamber and the luminal face of the channel to the solution in the trans chamber. The 610 mM K+ solution contained 10 μM Ca2+ as contaminant and, at this level of cytosolic Ca2+, P o is low. Unless stated otherwise, P o was increased by the addition of 100 μM EMD 41000 (McGarry and Williams, 1994) to the cytosolic face of the channel. With this combination of ligands, single channel P o was increased to ∼0.7 (see results), and under these conditions it was immediately apparent if more than one channel was present in the bilayer. Only bilayers containing a single channel were used in the experiments described in this communication. Experiments were carried out at room temperature (21 ± 2°C). Initially, the influence of 21-amino-9α-hydroxy-ryanodine on channel function was investigated by adding it to the solution bathing the cytosolic face of the channel.
Single Channel Data Acquisition
Single channel current fluctuations were displayed on an oscilloscope and stored on digital audio tape. For analysis, data were replayed, filtered at 1 kHz with an eight-pole Bessel filter and digitized at 4 kHz using Satori V3.2 (Intracel, Cambridge, UK). Single channel current amplitudes were monitored from digitized data. The representative traces shown in the figures were obtained from digitized data acquired with Satori V3.2 and transferred as an HPGL graphics file to a graphics software package (CorelDraw; Corel Systems Corporation, Ottawa, Ontario, Canada) for annotation and printing.
Analysis of Single Channel Data in the Presence of 21-Amino-9α-Hydroxy-Ryanodine
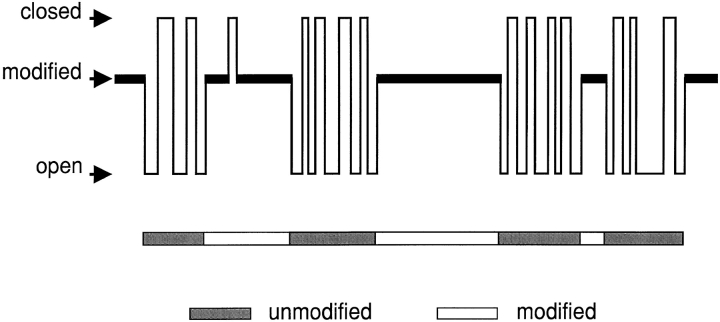
The basic observation reported in this communication is that 21-amino-9α-hydroxy-ryanodine induces the occurrence of subconductance events in the sheep cardiac muscle RyR. This is shown schematically in Fig. 1. Under steady state conditions, in the presence of 21-amino-9α-hydroxy-ryanodine, the channel oscillates between periods of normal gating and periods in a noisy subconductance state characterized by a high P o. We assume that the occurrence of the subconductance state results from the interaction of 21-amino-9α-hydroxy-ryanodine with the channel and that when the ryanoid is bound the channel can close but cannot enter the normal open state. To uncover the mechanisms underlying this behavior we have monitored the following parameters (Fig. 1).
Figure 1.
Schematic representation of the reversible interaction of 21-amino-9α-hydroxy-ryanodine with its site on the channel. On binding, the ryanoid induces a modified conductance state with high open probability. With the ryanoid bound, the channel can close but cannot enter the normal open state. When 21-amino-9α-hydroxy-ryanodine dissociates from its site, channel gating and ion handling return to normal. Dwell times in the unmodified and modified states are monitored using the pattern recognition program described in materials and methods. The probability of the channel being in the modified state (P mod) is then calculated for the entire 6-min recording as described in the text.
Fractional conductance.
Fractional conductance is the amplitude of the subconductance state expressed as a proportion of the normal full amplitude of the channel (Lindsay et al., 1994; Tinker et al., 1996). Amplitudes were monitored by placing cursors at levels corresponding to the center of the noise for each of the closed, modified, and open conductance states.
Dwell times in the unmodified and modified conductance states, and the probability of the channel occurring in the modified state (Pmod).
These parameters were determined by using Satori V3.2 to assign digitized events to one of the three possible states (open, closed, and modified). Sections of data were then defined as unmodified (periods in which the channel displayed transitions only between the open and closed levels) or modified (periods in which the channel displayed transitions only between the modified and closed levels) using a pattern recognition program with the minimum duration of an unmodified event set at 2 ms and that of a modified event set at 15 ms. The accuracy of this procedure was verified by inspecting the data and counting the number of modified events. P mod was calculated from the dwell times in the unmodified and modified events as P mod = total time in modified state ÷ (total time in modified state + total time in unmodified state). In all experiments reported here, P mod was determined from steady state runs lasting at least 6 min.
The probability of the channel being open (Po).
The open probability of single channels in the presence of 21-amino-9α-hydroxy- ryanodine was determined by monitoring this parameter in the sections of the recorded data during which the channel was unmodified; i.e., displaying transitions only between the open and closed levels (Fig. 1). P o in these periods was determined by 50% threshold analysis (Sitsapesan and Williams, 1994).
results
Initial Observations
Ryanodine alters both the gating and ion handling properties of single RyR channels, inducing modified conductance states of high P o (Rousseau et al., 1987; Lindsay et al., 1994; Tinker et al., 1996). The same basic pattern of modification of channel function is seen with a wide range of ryanodine analogs; however, specific features such as the amplitude of the modified conductance state and the dwell time in the modified conductance state have been shown to be dependent upon the structure of the ryanoid (Tinker et al., 1996; Welch et al., 1997).
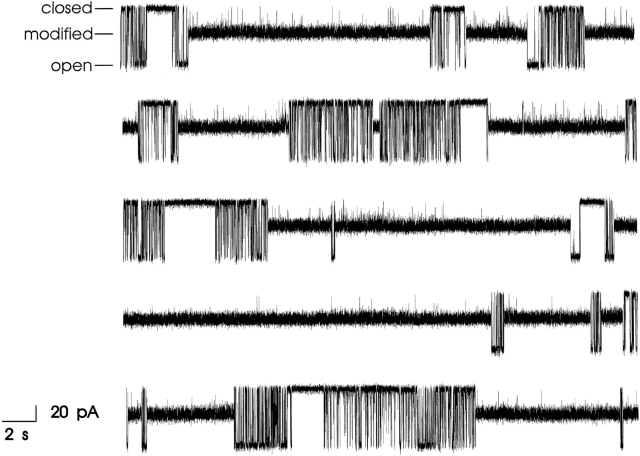
Consistent with this, in the presence of 21-amino-9α-hydroxy-ryanodine, the RyR channel enters a modified conductance state as shown in Fig. 2. Reduced conductance states induced by ryanoids are often referred to as “noisy.” In other words, the modified conductance state displays excess noise when compared with the other gating states of the channel. This can be seen clearly in Fig. 2 where the amplitude of noise in the modified state is approximately three times larger than that of the closed state. If current amplitude is monitored by placing cursors at the center of the noise associated with each state, the modified conductance induced by 21-amino-9α-hydroxy-ryanodine is 354 ± 6 pS (SEM, n = 14), while the conductance of the unmodified state is 813 ± 10 pS (SEM, n = 14); a fractional conductance of 0.44.
Figure 2.
Spontaneous transitions of a single sheep cardiac RyR channel from the unmodified to the modified conductance state under steady state conditions; the five traces are contiguous. Holding potential was +40 mV with 700 nM 21-amino-9α-hydroxy-ryanodine in the solution at the cytosolic face of the channel.
Unlike ryanodine, the modification of channel function induced by this ryanoid is reversible. Under steady state conditions, we observe spontaneous transitions between unmodified and modified conductance states. In this example, dwell times in the modified state vary from <1 to >10 s. In contrast to the situation with ryanodine, washing 21-amino-9α-hydroxy-ryanodine out of the solution bathing the channel rapidly restores normal, unmodified channel function (not shown).
The simplest mechanism that could account for these observations is one in which the interaction of a single molecule of 21-amino-9α-hydroxy-ryanodine with its binding site on the channel protein results in the occurrence of the modified state (i.e., Scheme I).
If a bimolecular scheme of this kind is to provide a valid description of the interaction of 21-amino-9α-hydroxy-ryanodine with its receptor, certain testable criteria should be fulfilled. The distribution of dwell times in both the unmodified and modified states should be described by single exponentials. The relationship between 21-amino-9α-hydroxy-ryanodine concentration and P mod should be described by Michaelis-Menten kinetics (i.e., P mod should be saturable with respect to ryanoid concentration). The rate of association should be dependent upon the first power of the concentration of 21-amino-9α-hydroxy-ryanodine, while the rate of dissociation should be independent of 21-amino-9α-hydroxy-ryanodine concentration.
Dwell Times in the Unmodified and Modified Conductance States
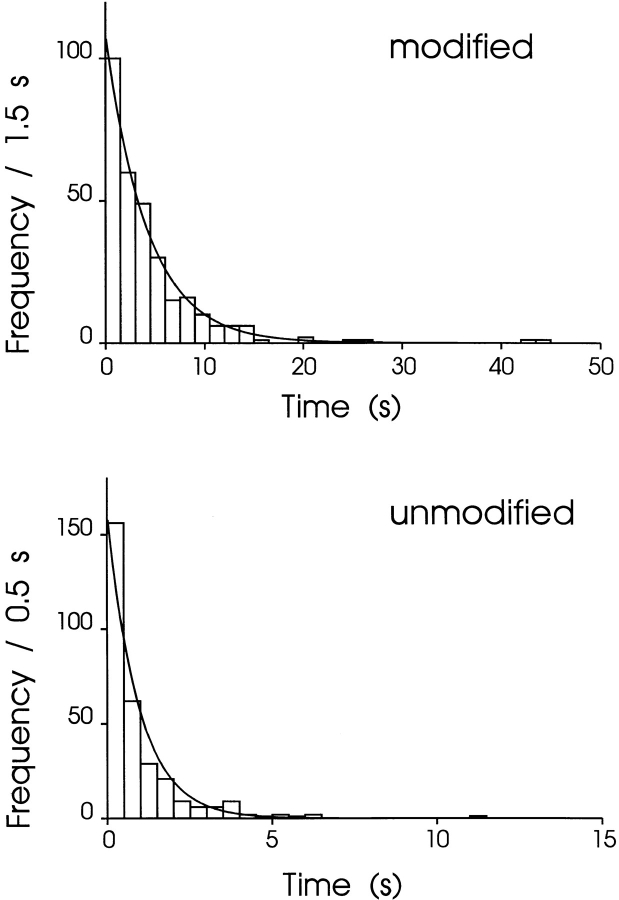
A bimolecular reaction scheme (Scheme I) predicts that, in the presence of 21-amino-9α-hydroxy-ryanodine, the dwell times in both the unmodified and modified states should be distributed exponentially. Fig. 3 shows noncumulative histograms of dwell times in the unmodified and modified states of a channel monitored over a period of 20 min at +40 mV with 500 nM 21-amino-9α-hydroxy-ryanodine in the solution at the cytosolic face of the channel. In both plots, the solid line is a probability density function with a single exponential term determined by maximum likelihood fitting. The mean dwell time in the unmodified state (τunmod) is 0.95 s, while that of the modified state (τmod) is 4.21 s. As both sets of dwell times can be described by single exponentials, the apparent rate constants for the association (k on) and dissociation (k off) of 21-amino-9α-hydroxy-ryanodine can be determined from the mean dwell times in the unmodified and modified conductance states as:
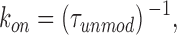 |
1 |
Figure 3.
Representative lifetime histograms of unmodified and modified events obtained in the presence of 500 nM cytosolic 21-amino-9α-hydroxy-ryanodine at +40 mV, together with probability density functions with a single exponential term determined by maximum likelihood fitting. Parameters are given in the text.
and
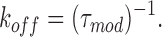 |
2 |
The Influence of 21-Amino-9α-Hydroxy-Ryanodine Concentration on Pmod
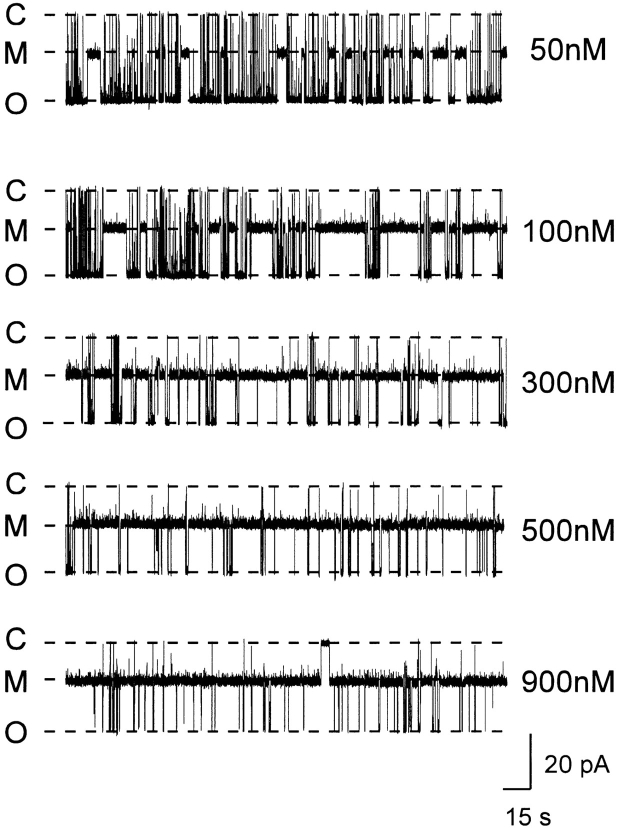
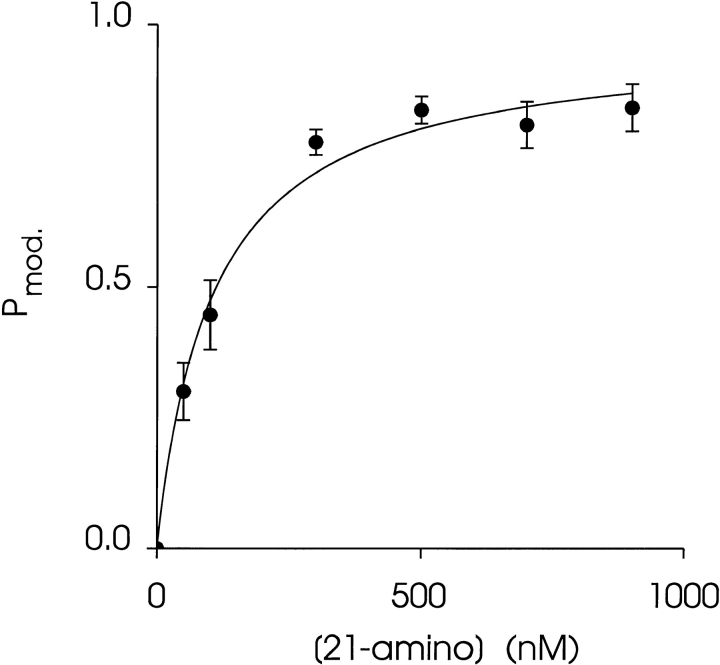
Fig. 4 shows an experiment in which a single RyR channel was exposed to increasing concentrations of 21-amino-9α-hydroxy-ryanodine at the cytosolic face of the channel. The holding potential was +40 mV. As the concentration of the ryanoid is increased from 50 to 900 nM, the likelihood of the channel being in the modified state increases. The relationship between 21-amino-9α-hydroxy-ryanodine concentration and P mod for a number of channels is shown in Fig. 5. P mod was determined at a holding potential of +40 mV by monitoring dwell times in the unmodified and modified conductance states as described in materials and methods. Each point is the mean ± SEM of at least four experiments. The curve is drawn according to a single-site binding scheme of the form:
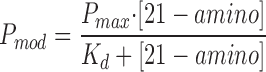 |
3 |
Figure 4.
Raising the concentration of 21-amino-9α-hydroxy- ryanodine in the solution at the cytosolic face of the channel increases the probability of modification. Current fluctuations of a single sheep cardiac RyR channel at a holding potential of +40 mV. C, closed; M, modified; O, open.
Figure 5.
The relationship between P mod, the concentration of 21-amino-9α-hydroxy-ryanodine in the solution at the cytosolic face of the channel. P mod was determined by monitoring dwell times in the unmodified and modified conductance states in 6-min recordings at +40 mV. Each point is the mean ± SEM of at least four experiments. The curve is drawn according to a single-site binding scheme (Eq. 3), with the parameters P max = 0.97 and K d = 105 nM obtained by nonlinear regression.
with values of P max = 0.97 and K d = 105 nM determined by nonlinear regression. The Hill slope for this data is 0.99. These observations are consistent with a simple bimolecular scheme in which modification of channel function results from the interaction of a single molecule of 21-amino-9α-hydroxy-ryanodine with a channel.
Variations of Association and Dissociation Rate with 21-Amino-9α-Hydroxy-Ryanodine Concentration
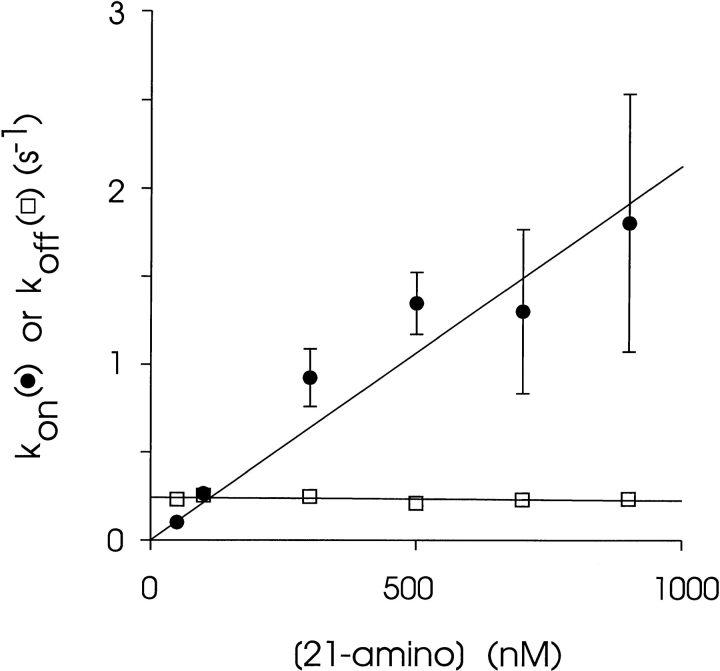
Consistent with a bimolecular reaction scheme, the apparent rate constant for the association of 21-amino-9α-hydroxy-ryanodine increases linearly as its concentration is raised with a slope of 2.16 ± 0.25 × 106 s−1 M−1, while the apparent rate constant for dissociation is independent of concentration with a mean value of 0.24 ± 0.01 s−1 (Fig. 6). The apparent K d derived from the rate constants is 111 nM, in excellent agreement with the apparent K d measured directly from ligand-induced changes in P mod (Fig. 5) and indicates self-consistency of the measurements. A plot of ln(rate constant) vs. ln([21-amino-9α-hydroxy-ryanodine]) is linear with a slope of 0.975 (not shown), supporting the contention that the rate of association of the ryanoid with its binding site is first order with respect to ligand concentration. A plot of ln(K a) vs. ln([21-amino-9α-hydroxy-ryanodine]) is linear with a slope of −0.006 (not shown), supporting the implicit contention that the association constant is independent of the ryanoid concentration.
Figure 6.
Concentration dependence of association and dissociation rates of 21-amino-9α-hydroxy-ryanodine at +40 mV. Each point is the mean ± SEM of at least four experiments. Where not visible, error bars are included within the symbol. Rates were determined as described in the text. The solid lines were obtained by linear regression with the parameters quoted in the text.
The experiments outlined above indicate that we can describe the modification of RyR channel function by 21-amino-9α-hydroxy-ryanodine by a simple bimolecular association. The observed alterations in gating and ion handling are the result of the interaction of a single molecule of ryanoid with a binding site on the channel protein. We can now use this basic model to investigate other factors that might influence the interaction of 21-amino-9α-hydroxy-ryanodine with its receptor.
The Influence of Po on the Interaction of 21-Amino-9α-Hydroxy-Ryanodine
It is well established that the interaction of [3H]ryanodine with its high affinity receptor site on the SR Ca2+-release channel is influenced by factors that are known to alter single channel P o. Ligands such as caffeine and ATP, which increase P o, increase binding, while ligands that decrease P o, such as ruthenium red or Mg2+, reduce [3H]ryanodine binding (Holmberg and Williams, 1990a ; Chu et al., 1990; Hawkes et al., 1992; Meissner and El-Hashem, 1992). As a consequence, the level of binding of [3H]ryanodine is often used as an indicator of channel function.
The availability of a ryanoid that interacts reversibly with its receptor provides us with the opportunity to monitor ryanoid binding at the single channel level. Variations in P mod are equivalent to variations in the quantity of ryanoid bound to a population of receptors in an SR membrane vesicle preparation; i.e., P mod is equivalent to the fractional saturation of the high affinity site. In addition, the apparent rates of association and dissociation can be determined from dwell times in the unmodified and modified conductance states as described above. Therefore, we can now make a direct determination of the influence of channel P o on the binding of a ryanoid to its receptor on the SR Ca2+-release channel.
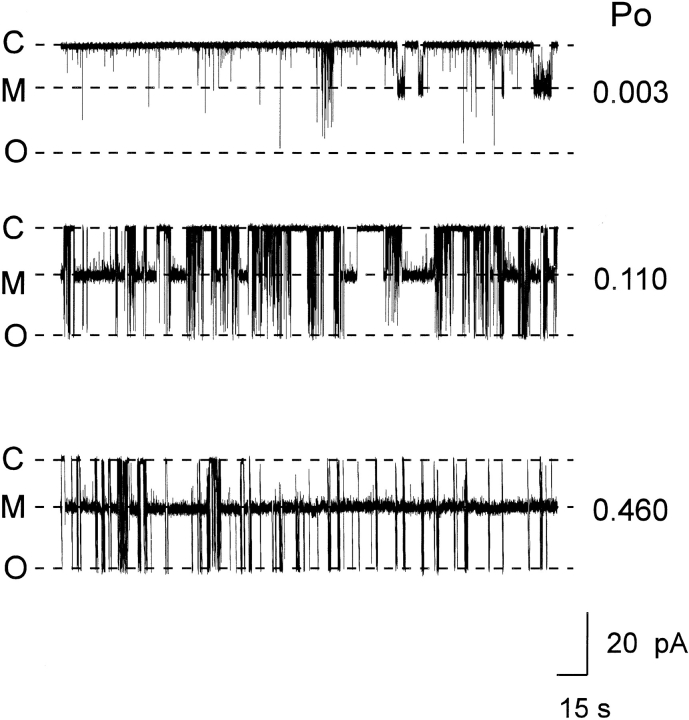
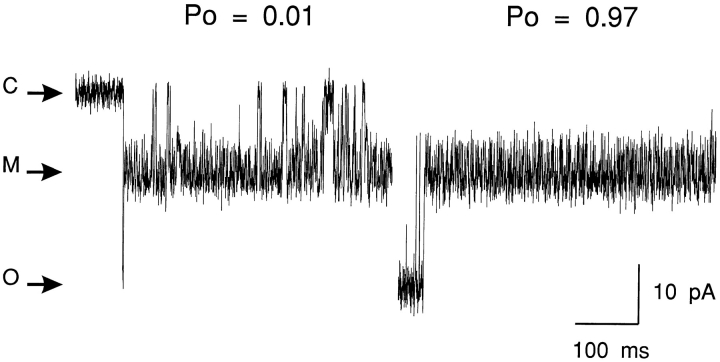
We have investigated this by monitoring the interaction of 21-amino-9α-hydroxy-ryanodine (700 nM in the solution at the cytosolic face of the channel) at +40 mV under conditions in which P o was varied by the addition of increasing concentrations of EMD 41000 (McGarry and Williams, 1994). An example of such an experiment is given in Fig. 7. The trace in Fig. 7 (top) shows spontaneous channel activity in the absence of any added EMD 41000. Under these conditions, P o is very low (0.003) and we observe only very occasional modifications by the ryanoid. As channel P o is increased by the addition of EMD 41000 to the solution bathing the cytosolic face of the channel (Fig. 7, center : 50 μM EMD 41000, giving a P o of 0.11; and bottom: 75 μM EMD 41000, giving a P o of 0.46), the likelihood of the channel residing in the modified conductance state increases. It is also noticeable that the structure of the modified conductance state changes as P o is increased; the excess noise of this state is considerably greater when channel P o is low. An inspection of modified conductance events at enhanced temporal resolution reveals that the excess noise observed at low P o results from the occurrence of closing events. This is demonstrated in Fig. 8, in which typical modified conductance events are shown for a single channel in the presence of 700 nM 21-amino-9α-hydroxy-ryanodine at a P o of 0.01 and, after the addition of EMD 41000, 0.97. At low P o, the modified conductance event contains numerous, well resolved, closing events; these are not seen at high P o.
Figure 7.
Variation in P mod with increasing P o. The figure shows current fluctuations of a single sheep cardiac RyR channel at a holding potential of +40 mV with 700 nM 21-amino-9α-hydroxy- ryanodine in the solution at the cytosolic face of the channel. Channel P o was increased by the addition of EMD 41000 to the solution at the cytosolic face of the channel (see text for details). Note the excess noise of the modified conductance state decreases as channel P o increases. C, closed; M, modified; O, open.
Figure 8.
The probability of closings within the 21-amino-9α- hydroxy-ryanodine-induced conductance state is influenced by channel P o. The figure shows representative modified conductance states from a single sheep cardiac SR RyR channel at a holding potential of +40 mV with 700 nM 21-amino-9α-hydroxy-ryanodine in the solution at the cytosolic face of the channel. While the noise of the modified conductance state is the same under both conditions, numerous transitions to the closed state occur at low P o. C, closed; M, modified; O, open.
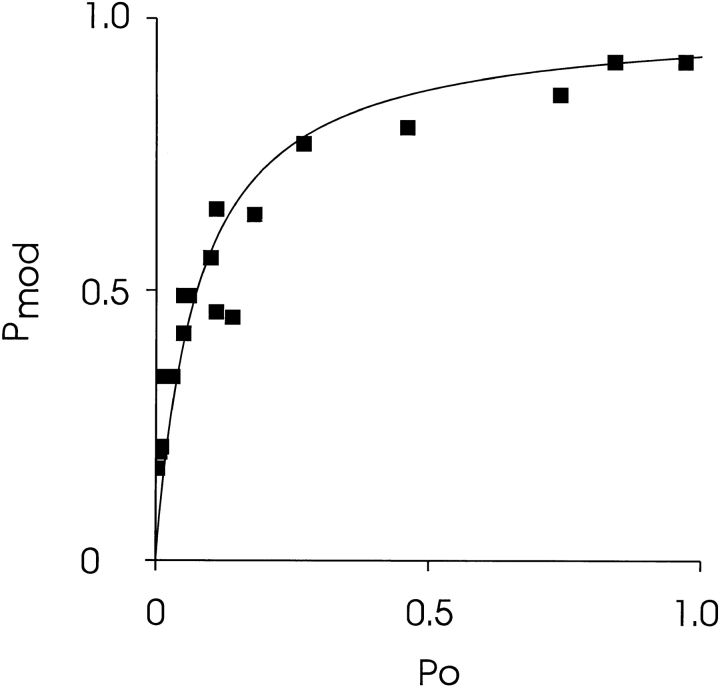
The relationship between channel P o and P mod is shown in Fig. 9. Values of P mod were monitored for 18 six-min recordings obtained from six single channels at P o varying from 0.003 to 0.97. Under these conditions, P mod rises steeply as P o increases with a P mod of 0.5 occurring at a P o of ∼0.05. At this concentration of 21-amino-9α-hydroxy-ryanodine, P mod tends to saturate at P o values in excess of 0.5.
Figure 9.
The relationship between P mod and P o. P mod was determined by monitoring dwell times in the unmodified and modified conductance states in 18 six-min recordings, and P o was determined during the unmodified sections of each recording. P o was altered by varying the concentration of EMD 41000 at the cytosolic face of the channel. 700 nM 21-amino-9α-hydroxy-ryanodine was present in the solution at the cytosolic face of the channel and the holding potential was +40 mV. The curve has no theoretical significance.
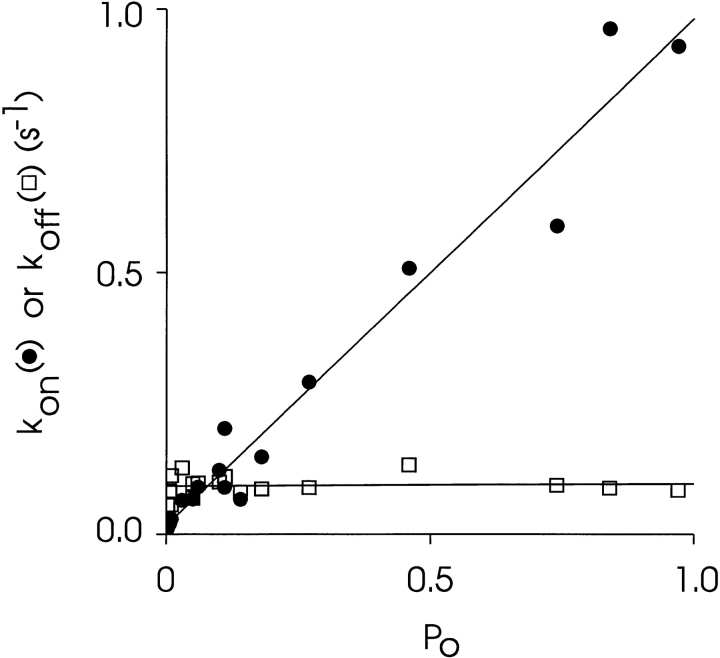
An inspection of the apparent rates of association and dissociation over this range of P o reveals that the increase in P mod associated with an increase in P o results from an increase in the rate of association (Fig. 10). k on is linearly dependent on P o with a slope of 0.97 ± 0.05 s−1 over the range P o = 0–1. The intercept of the regression line is effectively zero (0.02 ± 0.02 s−1). k off is independent of P o with a mean value of 0.09 ± 0.01 s−1.
Figure 10.
The dependence of the association and dissociation rates of 21-amino-9α-hydroxy-ryanodine on P o at a holding potential of +40 mV. Rates were determined for the experiments shown in Fig. 9 as described in the text. The solid lines were obtained by linear regression with the parameters quoted in the text.
Clearly, the P o of the RyR channel has a marked effect on the probability of 21-amino-9α-hydroxy-ryanodine interacting with its binding site on the channel and inducing a modification of ion handling and gating. These findings indicate that the ryanoid binding site is only accessible when the channel is open.
Given this observation, it is important to monitor and, if possible, control channel P o when investigating the effects of other factors on the probability of channel modification by 21-amino-9α-hydroxy-ryanodine. For example, in the studies reported in an earlier section of this communication, in which we determined the influence of 21-amino-9α-hydroxy-ryanodine concentration on P mod and the rates of association and dissociation, P o values were as follows (21-amino-9α-hydroxy-ryanodine [nM], mean P o ± SEM [n]): 50, 0.62 ± 0.15 (5); 100, 0.71 ± 0.16 (5); 300, 0.70 ± 0.16 (5); 500, 0.56 ± 0.16 (5); 700, 0.73 ± 0.15 (4); 900, 0.73 ± 0.15 (4). Therefore, variations in P o will not have made a major contribution to the relationships determined in these experiments.
The Influence of Transmembrane Voltage on the Interaction of 21-Amino-9α-Hydroxy-Ryanodine
In a previous study, we have demonstrated that the interaction of ryanoids with the sheep cardiac SR Ca2+- release channel can be influenced by the transmembrane voltage (Tinker et al., 1996). The interaction of ryanodol with the channel results in the occurrence of a reduced conductance state with an amplitude of ∼70% of the normal unmodified conductance. As is the case with 21-amino-9α-hydroxy-ryanodine, the interaction of ryanodol with the channel is reversible under steady state conditions; however, the dwell times in the modified state are very much longer than those observed with 21-amino-9α-hydroxy-ryanodine and make it impractical to obtain quantitative estimates of the kinetics of the interaction. However, we were able to make a qualitative statistical demonstration of the voltage dependence of the interaction of ryanodol by comparing the likelihood of the transition from the unmodified to the modified state and the transition from the modified to the unmodified state at two extreme voltages (+60 and −60 mV). These studies demonstrated that modifications of channel function by ryanodol are more likely to occur and are longer lasting at a holding potential of +60 than −60 mV.
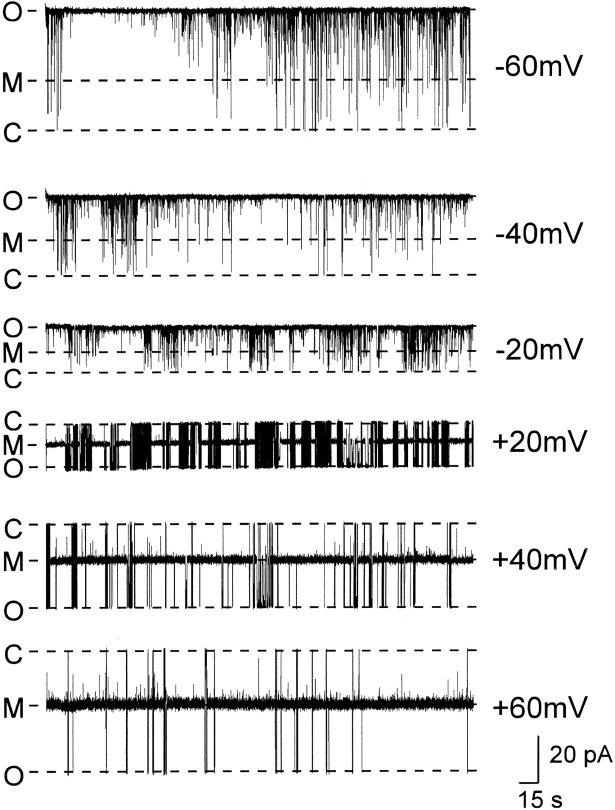
We have been able to carry out a more detailed investigation of the influence of transmembrane voltage on the interaction of 21-amino-9α-hydroxy-ryanodine with the sheep cardiac SR Ca2+-release channel. Transmembrane voltage has a dramatic influence on the probability of the channel occurring in the modified conductance state. Fig. 11 shows traces obtained from a single channel in the presence of 500 nM 21-amino-9α-hydroxy- ryanodine in the solution at the cytosolic side of the channel. At −60 mV, we see no evidence of interaction of the ryanoid with the channel; there are no discernible modified-conductance events. As the transmembrane potential is made more positive, P mod increases. As we have demonstrated previously with ryanodine (Lindsay et al., 1994), the fractional conductance of the modified state induced by 21-amino-9α-hydroxy-ryanodine does not vary as transmembrane potential is altered (holding potential [mV], mean fractional conductance ± SEM [n]): 60, 0.462 ± 0.003 (10); 40, 0.440 ± 0.003 (10); 20, 0.438 ± 0.004 (10); −20, 0.437 ± 0.005 (10); −40, 0.442 ± 0.002 (6).
Figure 11.
Recordings from a single sheep cardiac RyR channel with 500 nM 21-amino-9α-hydroxy-ryanodine in the solution at the cytosolic face of the channel at holding potentials ranging from +60 to −60 mV. The occurrence of the modified conductance state increases as holding potential is made more positive. The fractional conductance of the modified state does not vary with holding potential (see text). C, closed; M, modified; O, open.
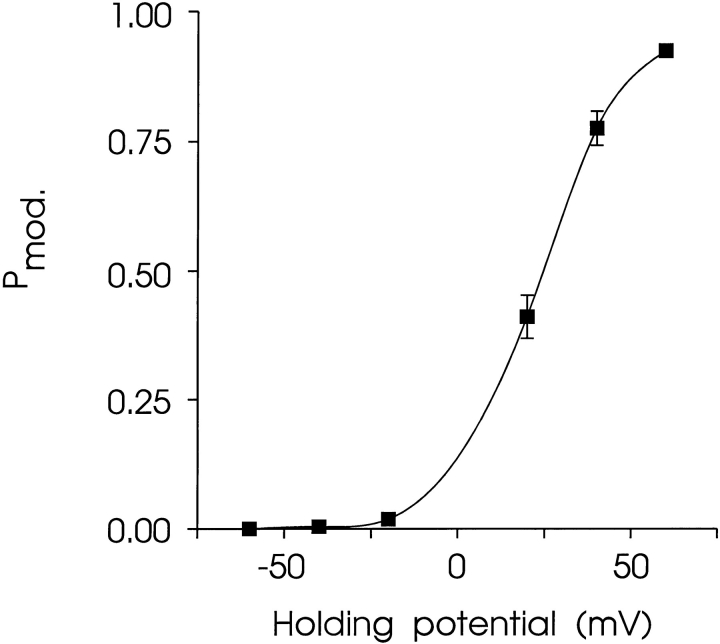
The relationship between P mod and transmembrane holding potential is shown in Fig. 12. The solid line is the best fit Boltzmann distribution obtained by nonlinear regression:
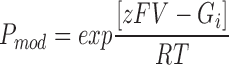 |
4 |
Figure 12.
The relationship between P mod and holding potential. The probability of occurrence of the channel in the modified conductance state was determined as described in materials and methods in 6-min recordings with 500 nM 21-amino-9α-hydroxy-ryanodine in the solution at the cytosolic face of the channel. Each point is the mean ± SEM of five to seven experiments. Where not visible, error bars are included within the symbol. The solid line is the best fit Boltzmann distribution obtained by nonlinear regression with the parameters quoted in the text.
where F is the Faraday constant, V is the transmembrane voltage, z is the voltage dependence of the occurrence of the modified conductance state and G i/RT is an expression of the equilibrium of the reaction at a holding potential of 0 mV. The value of z derived from this plot is 2.16.
If the association and dissociation rates of 21-amino-9α-hydroxy-ryanodine are described by the Boltzmann relationship, then the rate constants at a given voltage will be described by
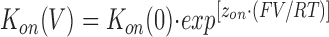 |
5 |
and
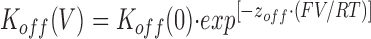 |
6 |
where K(V) and K(0) are the rate constants at a particular voltage and at 0 mV, respectively, and z is the valence of the appropriate reaction. z may then be determined as the slope of the plot of the natural logarithm of the rate constant against holding potential and K(0) may be determined from the intercept. The total voltage dependence of the reaction is then given by z on + z off.
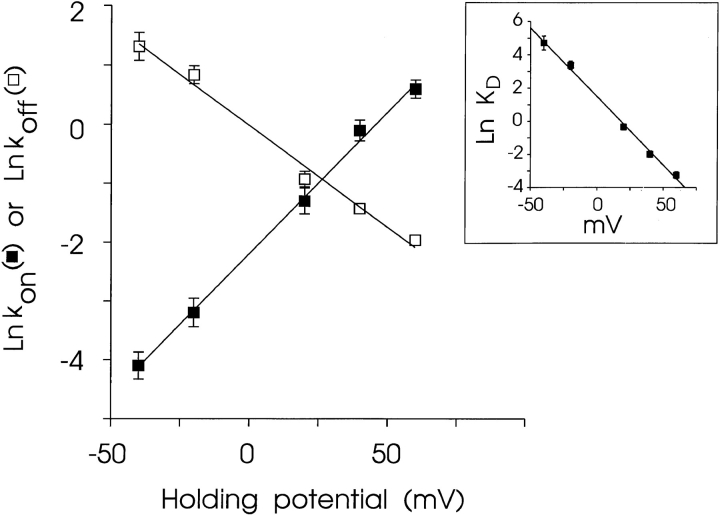
A plot of this form is shown in Fig. 13. The rates of association and dissociation of 21-amino-9α-hydroxy-ryanodine both vary with the applied holding potential. K on increases as the holding potential is made more positive; at the same time, K off decreases. The solid lines drawn through the points in Fig. 13 were obtained by linear regression and the values of z on and z off obtained from the slopes of these lines are 1.11 and 0.87, respectively, giving a total valency of 1.98. Fig. 13 (inset) shows the relationship of the K d of 21-amino-9α-hydroxy-ryanodine (calculated from the data in Fig. 13 as K d = K off (s−1)/K on (μM−1 · s−1) to holding potential. This plot gives a value of K d at +40 mV of 137 nM, which is in good agreement with the values determined from the ligand-induced changes in P mod (Fig. 5) and from the rate constants monitored with varying 21-amino-9α-hydroxy-ryanodine concentration (Fig. 6) at this holding potential. The extrapolated value of K d at 0 mV is 4.41 μM.
Figure 13.
Variation of association and dissociation rates of 21-amino-9α-hydroxy-ryanodine with holding potential. Rates were determined from the experiments depicted in Fig. 12 with 500 nM 21-amino-9α-hydroxy-ryanodine in the solution at the cytosolic face of the channel. Each point is the mean ± SEM of five to seven experiments. Where not visible, error bars are included within the symbol. The solid lines were obtained by linear regression with parameters quoted in the text. The inset shows the variation of the K d of 21-amino-9α-hydroxy-ryanodine (calculated from the data in Fig. 13 as K d = K off (s−1)/K on (μM−1 · s−1) with changing holding potential.
Is the voltage dependence determined in these experiments real or does it reflect a voltage-dependent shift in channel P o? We have tested this hypothesis by monitoring P o values for these channels during the experiments that provided the data shown in Figs. 12 and 13. The results of this study are as follows (holding potential [mV], mean P o ± SEM [n]): −60, 0.78 ± 0.17 (5); −40, 0.73 ± 0.13 (5); −20, 0.62 ± 0.15 (6); +20, 0.65 ± 0.09 (7); +40, 0.69 ± 0.09 (7); +60, 0.45 ± 0.06 (7). These data indicate that, under these experimental conditions, there is no marked variation in P o with holding potential. Therefore, the variations in the parameters that we have reported in Figs. 12 and 13 reflect true voltage-dependent changes in the interaction of 21-amino-9α-hydroxy-ryanodine with its receptor on the cardiac SR Ca2+-release channel.
Where Is the Site of Interaction of 21-Amino-9α-Hydroxy-Ryanodine?
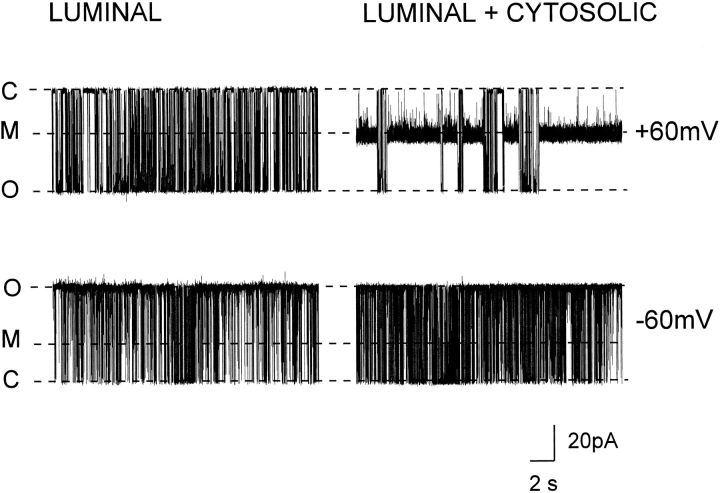
Ryanodine is membrane permeant and will induce modification of channel gating and conductance when added to the solution on either the cytosolic or luminal sides of the SR Ca2+-release channel. 21-amino-9α-hydroxy-ryanodine carries a net charge of +1 and is therefore likely to be considerably less membrane permeant. Fig. 14 shows records from a representative experiment (n = 6) in which a single Ca2+-release channel was incorporated into the bilayer. The traces in Fig. 14 (left) were obtained after the addition of 500 nM 21-amino-9α-hydroxy- ryanodine to the solution at the luminal face of the channel. Under these conditions, we saw no modification of gating or conductance at either +60 or −60 mV. Subsequent addition of the same concentration of 21-amino-9α-hydroxy-ryanodine to the solution at the cytosolic face of the channel (Fig. 14, right) led to the occurrence of modification events at +60 mV. In agreement with the voltage dependence reported above, modification of channel function was not seen at −60 mV. These experiments indicate that the site of interaction of 21-amino-9α-hydroxy-ryanodine on the SR Ca2+-release channel is only accessible from the cytosolic side of the channel.
Figure 14.
Representative traces from a single channel at holding potentials of +60 and −60 mV in the presence of 500 nM 21-amino-9α-hydroxy-ryanodine in the solution at the luminal face of the channel (left) or in the presence of 500 nM 21-amino-9α-hydroxy-ryanodine in both luminal and cytosolic solutions (right). C, closed; M, modified; O, open.
discussion
Before this study, the interaction of ryanoids, or more precisely ryanodine, with the receptors on the sarcoplasmic reticulum Ca2+-release channel has been monitored by measuring the binding of [3H]ryanodine to populations of receptors, either in native SR membrane vesicles or after purification of the channel protein. Such studies have revealed considerable information on the nature and properties of the binding sites. Equilibrium and kinetic analysis has established the existence of two classes of binding site on the release channel, high (K d in nanomolar range) and low (K d in micromolar range) affinity. Each functional channel molecule (homotetramer) has a single high affinity site and one or more low affinity sites (Lai et al., 1988; McGrew et al., 1989).
K d values determined for binding to the high affinity site vary over a wide range (∼1–200 nM) depending upon the experimental conditions, and it has been proposed that this value represents a weighted average of the K d values for the binding of [3H]ryanodine to different, interconvertible conformations of the receptor (Hawkes et al., 1992). It is generally assumed that the transition between these conformations involves the normal gating of the channel protein, with the binding site available when the channel is open. Consistent with this model, ligands that raise channel P o produce an increase in the affinity of the site for [3H]ryanodine as the result of an increase in the rate of association; ligands that reduce P o generally decrease the rate of association of [3H]ryanodine with the receptor (Chu et al., 1990). It should be noted that both the rate of association with the high affinity receptor and the rate of dissociation from this site are extremely slow; the association rate being below that predicted for a reaction limited by diffusion (Chu et al., 1990).
Interactions of ryanodine with the high affinity binding site result in increased permeability of SR vesicles to Ca2+, while interactions with the low affinity site correlate with decreased permeability to Ca2+ (Meissner, 1986; Humerickhouse et al., 1993). At the level of single channel resolution, these interactions appear to be equivalent, respectively, to modification of channel gating and conductance (the induction of a subconductance state with high P o) by nano- to micromolar concentrations of ryanodine, and the closure of the channel by high micro- to millimolar concentrations (Chu et al., 1990; Tinker et al., 1996).
The addition of ryanodine to the solutions bathing a single Ca2+-release channel reconstituted into a planar bilayer does not bring about an immediate alteration in function. Consistent with the extremely slow rates of association determined in binding studies, periods of tens of seconds or minutes elapse before normal channel function ceases and the channel enters a high P o, modified conductance state. It is assumed that this modification of function results from the interaction of ryanodine with the high affinity binding site of the channel (Tinker et al., 1996). At first sight, these may appear to be contradictory statements—how can an extremely slow rate of association correlate with a high affinity interaction? Rate constants considerably below the diffusion limit are commonly observed with tight binding ligands (Schloss, 1988). Two general mechanisms are offered for this observation. One postulates that the receptor must isomerize to an active conformation before binding can occur. Binding is slow because the concentration of the active conformer is low (the equilibrium favors the inactive conformation). This phenomenon is illustrated in Fig. 10, where increasing the amount of open conformer of the channel directly enhances the association rate constant (k on). In contrast, the other mechanism postulates that the receptor isomerizes at a slow rate after ligand binding occurs. Binding is slow because the rate of isomerization is slow. The overall affinity of an interaction is dependent upon both the rate of association and the rate of dissociation, and in the case of ryanodine the rate of dissociation dominates the equilibrium. On the time scale of a single channel experiment, ryanodine binds irreversibly to its receptor. After modification of channel function, ryanodine can be removed from the bulk solution and the channel will remain in the modified state for the duration of the experiment; often in excess of an hour. With these rates of association and dissociation, it is clearly not feasible to investigate the kinetics of the interaction of ryanodine with its receptor on a single channel. However, as described earlier (Tinker et al., 1996), alterations to the structure of ryanodine yields ryanoids that modify the gating and conductance of the Ca2+-release channel in a characteristic fashion but interact, on the time scale of a single channel experiment, reversibly with the receptor; one such ryanoid is 21-amino-9α-hydroxy-ryanodine.
The availability of a reversible ryanoid has allowed us to carry out the first characterization of the interaction of a ryanoid with its receptor on a single Ca2+-release channel. As is the case with all ryanoids that we have examined (Tinker et al., 1996), 21-amino-9α-hydroxy-ryanodine induces the occurrence of a modified conductance state and we assume that modified function results from the binding of the ryanoid to a ryanodine binding site on the channel protein. This is supported by the observation that the binding isotherm of 21-amino-9α-hydroxy-ryanodine demonstrates direct competition between ryanodine and 21-amino-9α-hydroxy-ryanodine with a 1:1 stoichiometry (Welch, unpublished observations). Furthermore, the K d of 21-amino-9α-hydroxy-ryanodine binding derived from the rate constants interpolated to 0 mV applied potential (∼4 μM; Fig. 13, inset) is in good agreement with the value of K d obtained from binding isotherms (1 μM).
How Many Molecules of 21-Amino-9α-Hydroxy-Ryanodine Interact with a Channel to Modify Function?
[3H]Ryanodine binding data indicates that each functional RyR channel contains a single high affinity binding site and in the past we have argued that it is probable that the binding of a ryanoid to this site results in the occurrence of the modified conductance state (Tinker et al., 1996). If this is the case, the spontaneous transitions between the unmodified and modified conductance states of a single channel in the presence of 21-amino-9α-hydroxy-ryanodine will be described by a simple bimolecular scheme (Scheme I). Consistent with such a scheme, our data demonstrate that (a) the distributions of dwell times in both the unmodified and modified conductance states can be described by single exponentials; (b) as the concentration of 21-amino- 9α-hydroxy-ryanodine is increased, the probability of the channel being in the modified state increases and saturates (this relationship can be described in terms of Michaelis-Menten kinetics); and (c) the rate of association of 21-amino-9α-hydroxy-ryanodine with its binding site varies linearly with ryanoid concentration, while its rate of dissociation is unaffected by the concentration of the ryanoid. Therefore, it would appear that ryanoid-induced modifications of channel gating and conductance result from the interaction of a single molecule of ryanoid per channel. Given the stoichiometry of binding of [3H]ryanodine to the high affinity site, it is most probable that the interaction of a single ryanoid molecule with this site results in the modification of function.
The Relationship between Channel Gating and the Binding of 21-Amino-9α-Hydroxy-Ryanodine
In these experiments, we have been able to make the first direct investigation of the influence of channel P o on the interaction of a ryanoid with its receptor on RyR. Our data demonstrate that the gating state of the channel has a marked influence on the ability of 21-amino-9α-hydroxy-ryanodine to bind to the channel and modify function; the ryanoid will only bind to the open channel. As channel P o is increased, the probability of a channel existing in the modified state increases as a direct result of a linear increase in the rate of association of the ryanoid with the channel. These findings are in agreement with the proposal that K d values determined by equilibrium [3H]ryanodine binding represent weighted average values for binding to different, inter-convertible conformations of the receptor (Hawkes et al., 1992) and confirm that these conformations are open and closed states of the channel.
Our observation that the level of noise of the 21-amino-9α-hydroxy-ryanodine-modified conductance state varies with channel P o is of interest. It would appear that on interaction with its binding site the ryanoid does not simply “lock” the channel into an open state; rather, the open probability of the modified conductance state reflects the P o of the channel in the absence of the ryanoid. If P o is high, closings of the modified channel are rare; if P o is low, closings of the modified channel are common.
[3H]Ryanodine binding is used routinely to monitor the effects of ligands on the P o of populations of RyR channels. While the data presented here indicate that this is valid, it should be noted that [3H]ryanodine binding will not be directly equivalent to P o over the full range of this parameter (0–1). In our experiments, P mod effectively saturates at values of P o in excess of 0.5; interventions that raise P o from this value to, say, 0.9 would produce only a very small increase in P mod. This observation is consistent with earlier studies in which we demonstrated that the stimulation of [3H]ryanodine binding by various ligands is not directly related to channel P o. For example, the addition of doxorubicin increases channel P o in the presence of 10 μM Ca2+, where the initial P o is low, and at 100 μM Ca2+, where initial P o is considerably higher. However, doxorubicin only produced a significant increase in [3H]ryanodine binding at the lower concentration of Ca2+ (Holmberg and Williams, 1990b ).
Where Is the Ryanoid Binding Site?
The channel has to be open for 21-amino-9α-hydroxy-ryanodine to have access to its binding site. This could mean that the site is within the conduction pathway; alternatively, the site could be located elsewhere on the protein but will only be available when the channel is in an open conformation. Do we have any other lines of evidence identifying the location of ryanoid binding sites on the channel protein?
The high affinity binding site for ryanodine has been localized to a 76-kD region of RyR extending from Arg-4475 to the COOH terminus of the protein in studies using proteolytic degradation and photoaffinity labeling (Callaway et al., 1994; Witcher et al., 1994); this region may be involved in pore formation (Callaway et al., 1994). Some indications on the location of the ryanoid binding site are also available from functional studies. As the interaction of a ryanoid with its receptor on the RyR channel produces a reduction in single channel conductance, it might seem reasonable to assume that this results from a partial block of the conduction pathway by the ryanoid. However, studies in which the ion handling properties of ryanodine-modified channels have been investigated indicate that reduced channel conductance cannot be explained in terms of a simple blocking reaction; many aspects of ion handling are modified by ryanodine binding (Lindsay et al., 1994). The amplitude of the ryanoid-induced modified conductance state is dependent upon the structure of the ryanoid (Tinker et al., 1996), and comparative molecular field analysis has revealed specific structural loci on the ryanoid that determine the conductance properties of the modified channel; however, these studies provide no evidence of a direct steric interaction between the bound ryanoid and the translocated ion (Welch et al., 1997). Taken together, these functional studies support a scheme in which modification of ion handling results from the binding of a ryanoid to a site located outside the strict confines of the conduction pathway, for example in the cytosolic vestibule of the channel (Tinker et al., 1996).
21-amino-9α-hydroxy-ryanodine has a net charge of +1; therefore, if its site of interaction is within the channel's conduction pathway, it would be expected that the interaction would be influenced by voltage. This is the case; the probability of the channel being in the modified conductance state is high at high positive holding potentials and low at high negative potential, and both association and dissociation rates are sensitive to voltage. Analysis of the voltage dependence of the interaction of 21-amino-9α-hydroxy-ryanodine with the receptor indicates that the reaction has a total voltage dependence of 2. In other words, if the voltage dependence of the interaction is derived from the movement of a charged ryanoid molecule into the applied electric field within the conduction pathway, an absolute minimum of two molecules of 21-amino-9α-hydroxy-ryanodine would need to be translocated across the entire voltage drop. As the binding site is only accessible from the cytosolic face of the channel, this would place the site at the luminal extreme of the voltage drop. Alternatively, several ryanoid molecules could interact with sites nearer the cytosolic mouth of the channel.
Neither of these alternatives is consistent with our earlier suggestion that the occurrence of the modified conductance state of the channel results from the interaction of a single molecule of 21-amino-9α-hydroxy-ryanodine with a high affinity site on the protein, nor with the observation that each channel (homotetramer) possesses a single high affinity binding site for [3H]ryanodine. Similarly, it is difficult to envisage a situation in which several ryanoid molecules could occupy sites within the conduction pathway of the channel and yet have no direct steric interaction with the translocated cation. How can we resolve this apparent anomaly? One possibility is that the measured voltage dependence of the interaction of 21-amino-9α-hydroxy-ryanodine with the channel does not result from the movement of the charged ryanoid into an electric field; rather, we could envisage a voltage-dependent conformational change in the channel protein that switches the ryanoid binding site between two states with different affinities. A similar mechanism has been proposed for the voltage-dependent block of Na+ channels by guanidinium toxins (Moczydlowski et al., 1984).
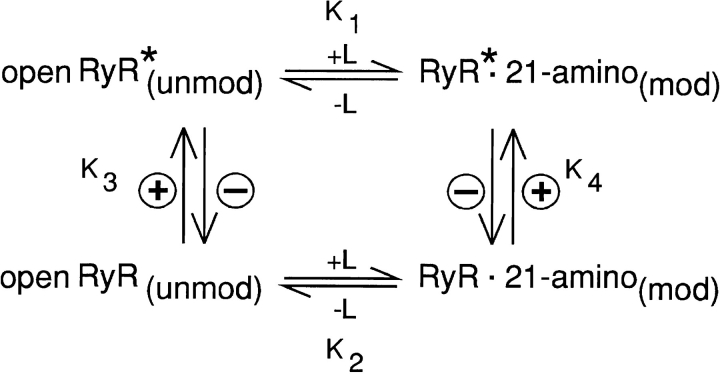
In Scheme SII, modification of channel function results from the binding of one molecule of 21-amino-9α-hydroxy-ryanodine to the channel. Fig. 13 demonstrates that the apparent dissociation constant of 21-amino-9α-hydroxy- ryanodine decreases with increasingly positive applied potential due to a decreasing dissociation rate constant and an increasing association rate constant. In the simplest terms, this can be expressed as an equilibrium between two states of RyR and is similar to the familiar T to R transition in allosteric proteins.
Scheme II.
L represents the ligand (21-amino-9α-hydroxy-ryanodine); + and − indicate, respectively, alterations in applied potential to positive and negative values.
In the case of RyR, we propose that the applied potential is the effector, shifting the equilibrium toward RyR*(unmod) with increasingly positive potential. The rates of association and dissociation of ligand are unaffected by the applied potential and the ryanoid binds preferentially to RyR*(unmod). This model deals with binding to what is generally termed the high affinity ryanodine binding site. Other experiments are required to determine what effect, if any, applied potential may exert on the low affinity sites.
Is this model anything more than a convenient explanation of a larger than expected voltage dependence? In support of the scheme, we have demonstrated previously that the interaction of a ryanoid that carries no net charge, ryanodol, with its receptor on the channel, shows the same qualitative dependence on voltage as 21-amino-9α-hydroxy-ryanodine. With ryanodol, entry into the modified conductance state is more likely to occur and dwell times in the modified state last longer at +60 than at −60 mV (Tinker et al., 1996). From this it would appear that the net charge on the ryanoid does not influence the basic voltage dependence of the interaction; this is consistent with Scheme SII. It should be noted that voltage-dependent influences on the interaction of the ryanoids with the RyR channel will not be detected in [3H]ryanodine binding assays to populations of receptors. Under these conditions, transmembrane potential will be 0 mV and will not change during the experiment.
Summary
In this report, we have described the first investigation of factors regulating the interaction of a ryanoid with the high affinity binding site on single RyR channels. These studies have identified a number of novel features. The modification of RyR channel function (residence in a high P o, reduced conductance state) results from the binding of one molecule of 21-amino-9α-hydroxy-ryanodine to a site on the channel. The binding site for this ryanoid is only accessible from the cytosolic side of the channel and the site is only available when the channel is open. The conformation of the open form of the channel is sensitive to the applied electrical field (Scheme SII). We propose a minimum of two forms of the open RyR channel (designated openRyR and openRyR*). Increasing positive potential favors openRyR* by increasing the value of the equilibrium constants K3 and K4. The openRyR* form has a higher affinity for ryanoid than the openRyR form (K1 > K2); therefore, increasing positive potential favors formation of the modified channel (RyR* · 21-amino). Thermodynamics requires that the equilibrium between openRyR(unmod) and RyR* · 21-amino must be independent of the path between the two forms. That is, the equilibrium constants connecting the various open forms are linked functions and K1 × K3 = K2 × K4. Since K1 > K2 and K4 > K3. In other words, the binding of 21-amino-9α-hydroxy-ryanodine to the low affinity, open form of the RyR channel favors the conversion of the low affinity form to the high affinity form just as the voltage-induced conversion of openRyR to openRyR* favors binding of the ryanoid.
Scheme I.
Acknowledgments
We are grateful to Andrew Griffin for preparing RyR channels, and to Dr. Rebecca Sitsapesan for many helpful discussions.
Supported by the Biotechnology and Biological Sciences Research Council, Wellcome Trust, British Heart Foundation, American Heart Association (93012790), and National Science Foundation (MCB-9317684).
Abbreviations used in this paper
- HSR
heavy sarcoplasmic reticulum
- RyR
ryanodine receptor
references
- Anderson K, Lai FA, Liu Q-Y, Rousseau E, Erickson HP, Meissner G. Structural and functional characterization of the purified cardiac ryanodine receptor-Ca2+release channel complex. J Biol Chem. 1989;264:1329–1335. [PubMed] [Google Scholar]
- Callaway C, Seryshev A, Wang J-P, Slavik KJ, Needleman DH, Cantu C, III, Wu Y, Jayaraman T, Marks AR, Hamilton SL. Localization of the high and low affinity [3H]ryanodine binding sites on the skeletal muscle Ca2+release channel. J Biol Chem. 1994;269:15876–15884. [PubMed] [Google Scholar]
- Chu A, Diaz-Munoz M, Hawkes MJ, Brush K, Hamilton SL. Ryanodine as a probe for the functional state of the skeletal muscle sarcoplasmic reticulum calcium release channel. Mol Pharmacol. 1990;37:735–741. [PubMed] [Google Scholar]
- Coronado R, Morrissette J, Sukhareva M, Vaughan DM. Structure and function of ryanodine receptors. Am J Physiol. 1994;266:C1485–C1504. doi: 10.1152/ajpcell.1994.266.6.C1485. [DOI] [PubMed] [Google Scholar]
- DiJulio DH, Watson EL, Pessah IN, Jacobson KL, Ott SM, Buck ED, Singh JC. Ryanodine receptor type III (RyR3) identification in mouse parotid acini: properties and modulation of [3H]ryanodine-binding sites. J Biol Chem. 1997;272:15687–15696. doi: 10.1074/jbc.272.25.15687. [DOI] [PubMed] [Google Scholar]
- Fairhurst AS, Hasselbach W. Calcium efflux from a heavy sarcotubular fraction. Effects of ryanodine, caffeine and magnesium. Eur J Biochem. 1970;13:504–509. doi: 10.1111/j.1432-1033.1970.tb00953.x. [DOI] [PubMed] [Google Scholar]
- Fleischer S, Ogunbunmi EM, Dixon MC, Fleer EAM. Localization of Ca2+release channels with ryanodine in junctional terminal cisternae of sarcoplasmic reticulum of fast skeletal muscle. Proc Natl Acad Sci USA. 1985;82:7256–7259. doi: 10.1073/pnas.82.21.7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerzon K, Humerickhouse RA, Besch HR, Jr, Bidasee KR, Emmick JT, Roeske RW, Tian Z, Ruest L, Sutko JL. Amino- and guanidinoacylryanodines: basic ryanodine esters with enhanced affinity for the sarcoplasmic reticulum Ca2+-release channel. J Med Chem. 1993;36:1319–1323. doi: 10.1021/jm00062a003. [DOI] [PubMed] [Google Scholar]
- Hawkes MJ, Nelson TE, Hamilton SL. [3H]Ryanodine as a probe of changes in the functional state of the Ca2+-release channel in malignant hyperthermia. J Biol Chem. 1992;267:6702–6709. [PubMed] [Google Scholar]
- Holmberg SRM, Williams AJ. The cardiac sarcoplasmic reticulum calcium-release channel: modulation of ryanodine binding and single-channel activity. Biochim Biophys Acta. 1990a;1022:187–193. doi: 10.1016/0005-2736(90)90113-3. [DOI] [PubMed] [Google Scholar]
- Holmberg SRM, Williams AJ. Patterns of interaction between anthraquinone drugs and the calcium release channel from cardiac sarcoplasmic reticulum. Circ Res. 1990b;67:272–283. doi: 10.1161/01.res.67.2.272. [DOI] [PubMed] [Google Scholar]
- Humerickhouse RA, Besch HR, Jr, Gerzon K, Ruest L, Sutko JL, Emmick JT. Differential activating and deactivating effects of natural ryanodine congeners on the calcium release channel of sarcoplasmic reticulum: evidence for separation of effects at functionally distinct sites. Mol Pharmacol. 1993;44:412–421. [PubMed] [Google Scholar]
- Jefferies PR, Lehmberg E, Lam W-W, Casida JE. Bioactive ryanoids from nucleophilic additions to 4,12-seco-4,12-dioxoryanodine. J Med Chem. 1993;36:1128–1135. doi: 10.1021/jm00061a003. [DOI] [PubMed] [Google Scholar]
- Lai FA, Erickson HP, Rousseau E, Liu Q-Y, Meissner G. Purification and reconstitution of the Ca release channel from skeletal muscle. Nature. 1988;331:315–319. doi: 10.1038/331315a0. [DOI] [PubMed] [Google Scholar]
- Lai FA, Misra M, Xu L, Smith HA, Meissner G. The ryanodine receptor-Ca2+release channel complex of skeletal muscle sarcoplasmic reticulum. Evidence for a cooperatively coupled, negatively charged homotetramer. J Biol Chem. 1989;264:16776–16785. [PubMed] [Google Scholar]
- Lattanzio FA, Schlatterer RG, Nicar M, Campbell KP, Sutko JL. The effects of ryanodine on passive calcium fluxes across sarcoplasmic reticulum membranes. J Biol Chem. 1987;262:2711–2718. [PubMed] [Google Scholar]
- Lindsay ARG, Tinker A, Williams AJ. How does ryanodine modify ion handling in the sheep cardiac sarcoplasmic reticulum Ca2+-release channel? . J Gen Physiol. 1994;104:425–447. doi: 10.1085/jgp.104.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay ARG, Williams AJ. Functional characterisation of the ryanodine receptor purified from sheep cardiac muscle sarcoplasmic reticulum. Biochim Biophys Acta. 1991;1064:89–102. doi: 10.1016/0005-2736(91)90415-5. [DOI] [PubMed] [Google Scholar]
- McGarry SJ, Williams AJ. Activation of the sheep cardiac sarcoplasmic reticulum Ca2+-release channel by analogues of sulmazole. Br J Pharmacol. 1994;111:1212–1220. doi: 10.1111/j.1476-5381.1994.tb14874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrew SG, Wolleben C, Siegl P, Inui M, Fleischer S. Positive cooperativity of ryanodine binding to the calcium release channel of sarcoplasmic reticulum from heart and skeletal muscle. Biochemistry. 1989;28:1686–1691. doi: 10.1021/bi00430a039. [DOI] [PubMed] [Google Scholar]
- Meissner G. Ryanodine activation and inhibition of the Ca2+release channel of sarcoplasmic reticulum. J Biol Chem. 1986;261:6300–6306. [PubMed] [Google Scholar]
- Meissner G. Ryanodine receptor/Ca2+release channels and their regulation by endogenous effectors. Annu Rev Physiol. 1994;56:485–508. doi: 10.1146/annurev.ph.56.030194.002413. [DOI] [PubMed] [Google Scholar]
- Meissner G, El-Hashem A. Ryanodine as a functional probe of the skeletal muscle sarcoplasmic reticulum Ca2+release channel. Mol Cell Biochem. 1992;114:119–123. doi: 10.1007/BF00240306. [DOI] [PubMed] [Google Scholar]
- Miller C. Open-state substructure of single chloride channels from Torpedo electroplax. Philos Trans R Soc Lond B Biol Sci. 1982;299:401–411. doi: 10.1098/rstb.1982.0140. [DOI] [PubMed] [Google Scholar]
- Moczydlowski E, Hall S, Garber SS, Strichartz GS, Miller C. Voltage-dependent blockade of muscle Na+channels by guanidinium toxins: effect of toxin charge. J Gen Physiol. 1984;84:687–704. doi: 10.1085/jgp.84.5.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman DH, Hamilton SL. Factors influencing [3H]ryanodine binding to the skeletal muscle Ca2+release channel. Anal Biochem. 1997;248:173–179. doi: 10.1006/abio.1997.2125. [DOI] [PubMed] [Google Scholar]
- Pessah IN, Zimanyi I. Characterization of multiple [3H]ryanodine binding sites on the Ca2+release channel of sarcoplasmic reticulum from skeletal and cardiac muscle: evidence for a sequential mechanism in ryanodine action. Mol Pharmacol. 1991;39:679–689. [PubMed] [Google Scholar]
- Rousseau E, Smith JS, Meissner G. Ryanodine modifies conductance and gating behavior of single Ca2+release channel. Am J Physiol. 1987;253:C364–C368. doi: 10.1152/ajpcell.1987.253.3.C364. [DOI] [PubMed] [Google Scholar]
- Schloss JV. Significance of slow-binding enzyme inhibition and its relationship to reaction-intermediate analogues. Accounts of Chemical Research. 1988;21:348–353. [Google Scholar]
- Sitsapesan R, Williams AJ. Mechanisms of caffeine activation of single calcium-release channels of sheep cardiac sarcoplasmic reticulum. J Physiol (Camb) 1990;423:425–439. doi: 10.1113/jphysiol.1990.sp018031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitsapesan R, Williams AJ. Gating of the native and purified cardiac SR Ca2+-release channel with monovalent cations as permeant species. Biophys J. 1994;67:1484–1494. doi: 10.1016/S0006-3495(94)80622-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutko JL, Airey JA, Welch W, Ruest L. The pharmacology of ryanodine and related compounds. Pharmacol Rev. 1997;49:53–98. [PubMed] [Google Scholar]
- Sutko JL, Airey JA. Ryanodine receptor Ca2+ release channels: does diversity in form equal diversity in function? . Physiol Rev. 1996;76:1027–1071. doi: 10.1152/physrev.1996.76.4.1027. [DOI] [PubMed] [Google Scholar]
- Tinker A, Sutko JL, Ruest L, Deslongchamps P, Welch W, Airey JA, Gerzon K, Bidasee KR, Besch HR, Jr, Williams AJ. Electrophysiological effects of ryanodine derivatives on the sheep cardiac sarcoplasmic reticulum calcium-release channel. Biophys J. 1996;70:2110–2119. doi: 10.1016/S0006-3495(96)79777-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JP, Needleman DH, Hamilton SL. Relationship of low affinity [3H]ryanodine binding sites to high affinity sites on the skeletal muscle Ca2+release channel. J Biol Chem. 1993;268:20974–20982. [PubMed] [Google Scholar]
- Waterhouse AL, Pessah IN, Francini AO, Casida JE. Structural aspects of ryanodine action and selectivity. J Med Chem. 1987;30:710–716. doi: 10.1021/jm00387a022. [DOI] [PubMed] [Google Scholar]
- Welch W, Ahmad S, Airey JA, Gerzon K, Humerickhouse RA, Besch HR, Jr, Ruest L, Deslongchamps P, Sutko JL. Structural determinants of high-affinity binding of ryanoids to the vertebrate skeletal muscle ryanodine receptor: a comparative molecular field analysis. Biochemistry. 1994;33:6074–6085. doi: 10.1021/bi00186a006. [DOI] [PubMed] [Google Scholar]
- Welch W, Sutko JL, Mitchell KE, Airey JA, Ruest L. The pyrrole locus is the major orienting factor in ryanodine binding. Biochemistry. 1996;35:7165–7173. doi: 10.1021/bi9527294. [DOI] [PubMed] [Google Scholar]
- Welch W, Williams AJ, Tinker A, Mitchell KE, Deslongchamps P, Lamothe J, Gerzon K, Bidasee KR, Besch HR, Jr, Airey JA, et al. Structural components of ryanodine responsible for modulation of sarcoplasmic reticulum calcium channel function. Biochemistry. 1997;36:2939–2950. doi: 10.1021/bi9623901. [DOI] [PubMed] [Google Scholar]
- Witcher DR, McPherson PS, Kahl SD, Lewis T, Bentley P, Mullinnix MJ, Windass JD, Campbell KP. Photoaffinity labeling of the ryanodine receptor/Ca2+release channel with an azido derivative of ryanodine. J Biol Chem. 1994;269:13076–13079. [PubMed] [Google Scholar]