Abstract
We have further characterized at the single channel level the properties of epithelial sodium channels formed by coexpression of α with either wild-type β or γ subunits and α with carboxy-terminal truncated β (βT) or γ (γT) subunits in Xenopus laevis oocytes. αβ and αβT channels (9.6 and 8.7 pS, respectively, with 150 mM Li+) were found to be constitutively open. Only upon inclusion of 1 μM amiloride in the pipette solution could channel activity be resolved; both channel types had short open and closed times. Mean channel open probability (P o) for αβ was 0.54 and for αβT was 0.50. In comparison, αγ and αγT channels exhibited different kinetics: αγ channels (6.7 pS in Li+) had either long open times with short closings, resulting in a high P o (0.78), or short openings with long closed times, resulting in a low P o (0.16). The mean P o for all αγ channels was 0.48. αγT (6.6 pS in Li+) behaved as a single population of channels with distinct kinetics: mean open time of 1.2 s and closed time of 0.4 s, with a mean P o of 0.6, similar to that of αγ. Inclusion of 0.1 μM amiloride in the pipette solution reduced the mean open time of αγT to 151 ms without significantly altering the closed time. We also examined the kinetics of amiloride block of αβ, αβT (1 μM amiloride), and αγT (0.1 μM amiloride) channels. αβ and αβT had similar blocking and unblocking rate constants, whereas the unblocking rate constant for αγT was 10-fold slower than αβT. Our results indicate that subunit composition of ENaC is a main determinant of P o. In addition, channel kinetics and P o are not altered by carboxy-terminal deletion in the β subunit, whereas a similar deletion in the γ subunit affects channel kinetics but not P o.
Keywords: epithelial sodium channel, open probability, amiloride block, Xenopus oocyte
introduction
The epithelial sodium channel (ENaC)1 mediates sodium reabsorption from the distal nephron, colon, lungs, and other epithelia. The channel consists of three subunits: α, β, and γ that share ∼35% identity at the amino acid level (Canessa et al., 1993, 1994). Each subunit has two transmembrane domains linked by a large extracellular loop, and the amino and carboxy termini (COOH termini) in the cytoplasm. Biophysical properties of ENaC at the single channel level consist of a small conductance (∼5 pS), ionic selectivity of Li+ > Na+ >> K+, and high affinity (K i of 0.1 μM) for the open-channel blocker amiloride (reviewed extensively by Garty and Palmer, 1997).
Injection of the three subunits of ENaC in Xenopus laevis oocytes induces the expression of channels with properties similar to the ones exhibited by channels in native tissues (Canessa et al., 1994). α subunits alone can induce amiloride-sensitive currents, but the very low level of expression (1% of αβγ current) has precluded their characterization at the single channel level. Coexpression of α and β or α and γ, but not β and γ, subunits induces whole-cell amiloride-sensitive currents that reach 10–20% of the magnitude obtained with α, β, and γ together.
Previous characterization of whole-cell currents induced by αβ and αγ channels showed that these channels differ in many properties (McNicholas and Canessa, 1997). For instance, αβ channels have 10-fold higher K i for amiloride than αγ channels (1 and 0.1 μM, respectively); and the ion selectivity of αβ channels is Li+ ≈ Na+, whereas for αγ channels it is Li+ > Na+. These findings, together with the demonstration of differential subunit expression within epithelial tissues (Farman et al., 1997), may explain the variability in single channel properties of amiloride-sensitive ENaCs in native tissues (e.g., Palmer and Frindt, 1986; MacGregor et al., 1994) and suggest that alteration of subunit composition may be physiologically important.
In this study, we sought to define the single channel properties of αβ and αγ channels. We examined single channel conductances and amiloride kinetics of channels formed by α and wild-type or truncated β (βT) and γ (γT) subunits. Furthermore, we also examined the kinetics and open probability (P o) of αβ and αγ channels and compared them with those of COOH-terminally truncated channels αβT and αγT. The importance of the COOH termini of the β and γ subunits on the activity of ENaC was first shown by mutations that produce deletions of part or almost all the COOH termini of β or γ subunits in patients with Liddle's syndrome, a hereditary form of autosomal dominant hypertension (Shimkets et al., 1994; Hansson et al., 1995). Indeed, expression of COOH-terminally truncated β or γ subunits in Xenopus oocytes induces amiloride-sensitive whole-cell currents that are three- to fivefold larger than those observed with wild-type subunits (Schild et al., 1995). At least two mechanisms have been proposed to account for the increase in current observed with the truncated subunits. The first mechanism is an increase in channel number at the plasma membrane, and the second is an increase in channel P o. Using cell-attached single channel patches from oocytes expressing either wild-type (αβ or αγ) or truncated (αβT or αγT) subunits, we have examined whether the COOH terminus alters channel kinetics and P o.
The findings of this study demonstrate that subunit composition confers distinct kinetics of amiloride block to αβ and αγ channels and that amiloride block is not affected by deleting the COOH termini. We also show that subunit composition is a major determinant of channel P o. We found that αβ channels exhibited a very high P o that approximated 1, whereas αγ channels exhibited a mean P o of 0.5. Expression of α subunit with a γβ chimera (CH), which has only the M2 domain and short segment of amino acids preceding M2 from β, and the rest of the sequence from the γ subunit, induced channels with very high P o similar to αβ channels, indicating that this region of the β subunit confers the very high P o to the channels. The contribution of the COOH terminus of β and γ subunits to channel kinetics was assessed by examining αβ and αβT channels and αγ and αγT channels. Channel kinetics and P o of αβ and αβT channels were not affected by the COOH terminus of the β subunit; the P o of both types of channels remained close to 1. In contrast, COOH-terminal deletion of the γ subunit changed the gating kinetics without an overall effect on the mean P o. The main effect of deleting the COOH termini of β or γ on channel activity was an increase in the density of channels at the cell surface that could account for the three- to fivefold increase in whole-cell currents.
methods
Oocyte Isolation and cRNA Injection
Xenopus laevis were anesthetized using 0.17% tricaine (3-aminobenzoic acid, methanesulfonate salt), stage V-VI oocytes were removed by partial ovariectomy and placed in hypotonic Ca2+-free ND96 containing (mM): 96 NaCl, 2 KCl, 1 MgCl2, 5 HEPES, pH 7.4. Subsequent treatment of the oocytes with collagenase type I (Worthington Biochemical Corp., Lakewood, NJ) at 2 mg/ml in Ca2+-free ND96 for 60–90 min essentially removed follicular membranes. After incubation in this solution for the allotted time, oocytes were washed several times in ND96 containing 1.8 mM CaCl2, and then further washed in supplemented ND96 (50 μg/ml Gentamycin [Life Technologies Inc., Grand Island, NY] and 2.5 mM sodium pyruvate was added).
cRNAs were in vitro transcribed from plasmid psD5 with SP6 RNA polymerase using Message Machine (Ambion Inc., Austin, TX) according to the supplier's instructions. Oocytes were injected 24 h after isolation and defolliculation. Equal amounts of subunit cRNA were used at all times (1–3 ng) in a constant injectate volume of 50 nl. After injection, oocytes were kept in the supplemented ND96 medium with 1 μM amiloride was added to prevent sodium loading of oocytes upon expression of ENaC. Recordings were made from oocytes 24–48 h after injection, depending on the subunit composition of the channels.
Electrophysiology
Before patch clamping, the vitelline membrane was removed manually using fine forceps after allowing the cell to shrink for ∼5 min in a hypertonic solution containing (mM): 220 N-methyl- d-glucamine, 220 aspartic acid, 2 MgCl2, 10 EGTA, 10 HEPES, pH 7.4.
Two standard solutions were used throughout this study. They were a Li+ pipette solution containing (mM): 150 LiCl, 1 CaCl2, 1 MgCl2, 5 HEPES, pH 7.4, and a K+ bath solution containing (mM): 150 KCl, 5 EDTA, 5 HEPES, pH 7.4. Amiloride was included in the pipette solution where indicated. All experiments described herein were performed at ambient room temperature.
Patch pipettes were pulled from fine borosilicate capillary glass (LG16 glass; Dagan Corp., Minneapolis, MN) in two stages, using a microelectrode puller (PP-83; Narishige International USA Inc., East Meadow, NY). They were not fire polished and typically had tip resistances between 3 and 5 MΩ when partially filled with pipette solution.
Channel currents were recorded from oocytes using the cell- attached configuration of the patch clamp technique (Methfessel et al., 1986). Recordings were made, lasting anywhere between several minutes (for αβ) to up to 1 h (for αγ), using an Axopatch 200A amplifier (Axon Instruments, Foster City, CA) interfaced, using a DigiData 1200 interface (Axon Instruments), to an IBM-compatible 166 MHz Pentium PC (Gateway 2000 Inc., N. Sioux City, SD). Data was acquired at 5 kHz, filtered at 250 Hz during acquisition using an eight-pole low pass Bessel Filter (Frequency Devices Inc., Haverhill, MA), and stored directly as data files on the hard drive of a personal computer. The pClamp 6 (Axon Instruments) suite of software was used for data acquisition and its subsequent analysis.
Data was further filtered digitally at 50 kHz during analysis and display. In all channel current traces, a downward deflection represents channel opening. Open and closed time histograms were generated using Pstat within pClamp. P o, and in some cases nP o for patches with more than one channel, was calculated using a specialized nP o program that was written by Jinliang Sui (Mount Sinai School of Medicine, New York) and is available online (http://www.axonet.com/pub/userware/popen). nP o was converted to mean channel P o in patches where the number of active channels could accurately be determined; in most cases, only single channel patches were used or patches with at most two active channels, based upon the number of current transitions observed using channel recordings lasting from several minutes to 1 h. Statistical analysis, where appropriate, was performed using the Student's paired t test.
results
Single Channel Currents and Conductance
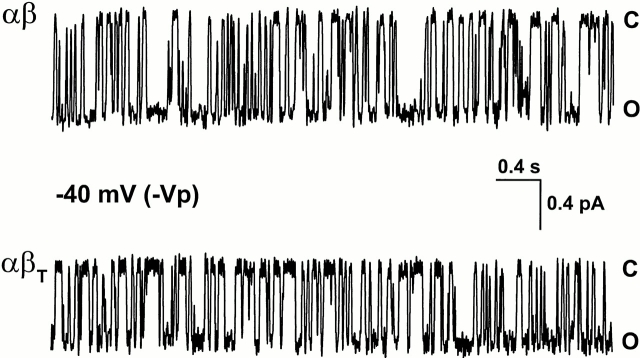
Oocytes injected with αβ- or αβT-expressed large amiloride-sensitive whole-cell currents measured with two-electrode voltage clamp (between 1–3 μA for αβ and ≥10 μA for αβT, not shown), but we could not discern channel activity when these oocytes were patch clamped. Upon inclusion of 1 μM amiloride in the pipette solution, which is the K i for αβ channels (McNicholas and Canessa, 1997), we detected channel currents. Fig. 1 shows representative examples of the currents observed for αβ and αβT at −40 mV; at this potential, both channel types displayed fast, flickery transitions between an open and a closed level. A common observation from all αβ and αβT single channel records was the absence of long closed states.
Figure 1.
Single channel records from oocytes expressing αβ or αβT channels. The pipette solution contained 150 mM LiCl plus 1 μM amiloride for reasons given in the text. The closed, or more correctly blocked, state is indicated (C). Inward current is represented by downward deflections (O). −Vp refers to the negative value of the pipette holding potential. The time scale and current amplitude are indicated on the scale bar. Data in this and all subsequent figures were obtained in the cell-attached configuration.
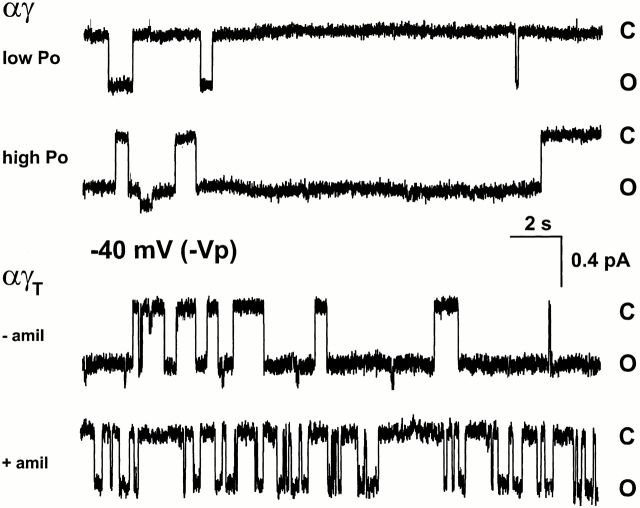
Fig. 2 shows that αγ channels were found to exist in two modes. One gating mode had long closed states with shorter less frequent openings and had a low channel P o. Conversely, the other gating mode had long open states with infrequent transitions to shorter closed states and had a high channel P o. No αγ channels with intermediate P o were found. All αγT channels, on the other hand, existed in only one state and had shorter and more frequent openings and closings when compared with αγ. However, the frequency of the open states exceeded that of the closed states. When 0.1 μM amiloride was included in the pipette, fewer open states were observed and their length was decreased.
Figure 2.
Single channel records from oocytes expressing αγ or αγT channels. Pipette solution was 150 mM LiCl, and 0.1 μM amiloride was present where indicated (+amil ). Low P o, P o < 0.25; high P o, P o > 0.65. −Vp has the same meaning as Fig. 1. The time scale and current amplitude are indicated on the scale bar.
In oocytes injected with either αβT or αγT, the incidence of channel activity was greater than in oocytes injected with αβ or αγ. The observation percentage (number of patches with channel activity/number of patches tested) was 40.7% (24/59) for αβT, 34.7% (108/309) for αγT, 12.8% for αβ (10/78) with 1 μM amiloride (0/24 without amiloride), and 17.3% (21/ 121) for the combined populations of αγ channels. Multiple channel-containing patches (between four and eight channels per patch) were very frequent with αβT - or αγT -expressing oocytes, whereas most patches from oocytes injected with αβ or αγ contained usually one or at most two channels. Therefore, the analysis of P o and kinetics in the following sections primarily came from patches that contained only one (kinetics) or at most two electrically active channels (P o).
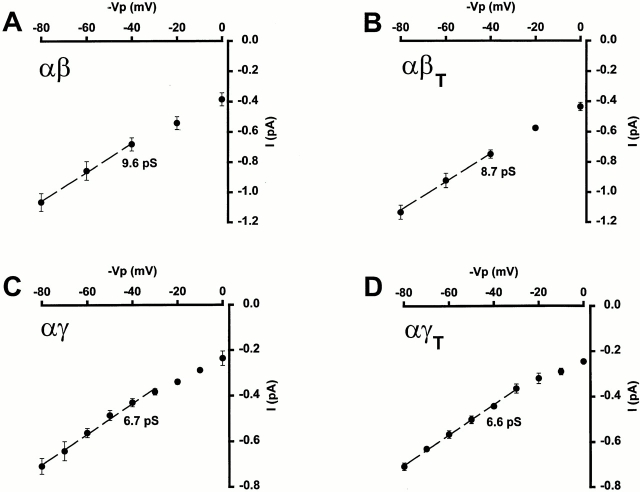
Single channel conductance (slope conductance) was estimated by measuring currents at a range of voltages with 150 mM Li+ in the pipette. Fig. 3 shows the conductance–voltage relationships for the various channel types. There was no difference between αβ and αβT (9.6 vs. 8.7 pS, P > 0.4) or αγ and αγT (6.7 vs. 6.6 pS, P > 0.4), although the channels containing β and βT subunits had slightly larger conductances than those with the corresponding γ subunits.
Figure 3.
Current (I )–voltage (V ) relationships for αβ (A), αβT (B), αγ (C), and αγT (D). Single channel conductance was estimated by linear regression either between −40 and −80 mV (A and B) or −30 and −80 mV (C and D) and is represented by the dashed line. The pipette solution contained 150 mM Li+. Each point is the mean ± SEM of three experiments. In this and all subsequent figures, where no error bars are shown, the error was within the symbol.
Channel Po
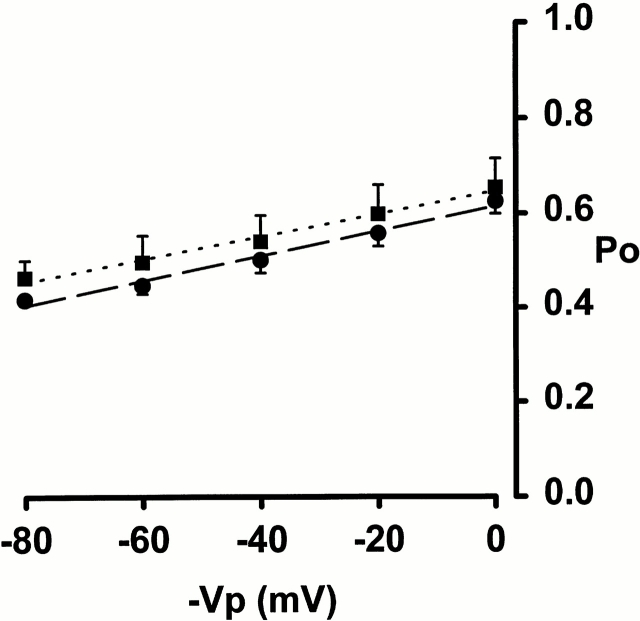
Fig. 4 shows the effect of voltage on channel P o for αβ and αβT channels. These measurements were made with 1.0 μM amiloride in the pipette. αβ channel P o was ∼0.65 at 0 mV and 0.5 at −80 mV. As can be seen, the plots of P o against membrane voltage for αβ and αβT were almost identical and were not greatly affected by voltage. The high channel P o in the presence of an amiloride concentration equal to the K i, and the absence of channel gating without amiloride, suggests that when amiloride is absent, channel P o would be close, if not equal, to 1.
Figure 4.
Plot of channel open probability (Po) against pipette holding potential (−Vp) for αβ (▪) and αβT (•). Pipette solution contained 1 μM amiloride. Channel P o was measured from single channel patches recorded at each of the indicated voltages for several minutes. Each point is the mean ± SEM of three experiments.
Table I shows a comparison of the mean P o values obtained for the various channel types at −40 mV. The P o values of αβ (0.54 ± 0.03) and αβT (0.50 ± 0.03) were almost identical, suggesting that truncation of the COOH terminus of the β subunit did not alter channel P o (P > 0.15). We were unable to obtain similar plots of P o vs. voltage for the αγ and αγT channels due to the channel kinetics. For example, to obtain an accurate and meaningful plot for αγ, we would have had to record from a cell-attached patch for at least 90 min (e.g., 0, −40, −80 mV for at least 30 min each), but found that oocytes became difficult to patch after 1 h in the bath. Therefore, we chose to measure channel P o (and single channel kinetics) for αγ and αγT at −40 mV. This voltage was selected to minimize the activity of a variety of channels endogenous to oocytes (Stühmer and Parekh, 1995). A mean P o of 0.16 ± 0.04 was calculated for the low P o and 0.78 ± 0.05 for the high P o αγ channels, with the mean P o for the entire αγ channel types being 0.47. Preliminary experiments indicate that after excision, patches containing high P o αγ channels adopt a low P o mode instantaneously (Fyfe, G.K., and C.M. Canessa, unpublished observations). The mean P o of αγT was found to be 0.60 ± 0.01, a value that is not significantly different to the mean value for the αγ channels (P > 0.19). Inclusion of 0.1 μM amiloride in the pipette (the K i value of amiloride for αγT channels determined by two-electrode voltage clamp; McNicholas and Canessa, 1997) reduced the mean P o of the αγT channels to 0.30 ± 0.02.
Table I.
Channel Po
| Channel type | Mean P o | n | ||
|---|---|---|---|---|
| αβ (+ 1.0 μM amil) | 0.54 ± 0.03 | 6 | ||
| αβ T(+ 1.0 μM amil) | 0.50 ± 0.03 | 3 | ||
| αγ high P o | 0.78 ± 0.05 | 4 | ||
| αγ low P o | 0.16 ± 0.04 | 5 | ||
| αγT | 0.60 ± 0.10 | 5 | ||
| αγT+ 0.1 μM amil | 0.30 ± 0.02 | 4 |
Summary of mean P o at −40 mV for the various channel types. Values were calculated using a specialized nP o program as described in methods. All values are mean ± SEM. The number of patches, most of which were single channel, used to generate the mean P o are indicated in the far right column.
We have also measured the P o of channels formed by the α subunit and a γβ chimera. This CH combined the amino terminus, the first transmembrane region (M1), and the extracellular domain of the γ subunit with the second transmembrane (M2) region and COOH terminus of the β subunit. With 1.0 μM amiloride in the pipette, the mean P o of these αγβ CH channels was 0.62 ± 0.06 (n = 3).
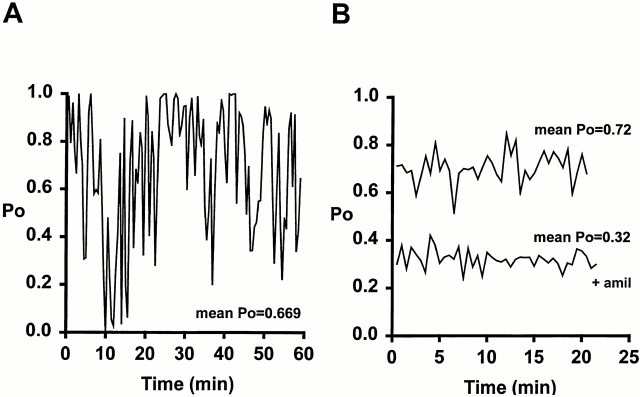
Fig. 5 shows two plots of P o vs. time. Fig. 5 A shows a representative example of a single channel αγ high P o patch that was recorded for 1 h. Note that although the mean P o for this example was 0.67, the P o of the channel (measured every 30 s) varied greatly from close to 1 to near 0 constantly throughout the entire recording. In Fig. 5 B, a representative example of an αγT patch in the absence and presence of 0.1 μM amiloride is shown. Although the length of these recordings is shorter, it appears truncation of the COOH terminus has given the αγT channel a more stable P o; i.e., a variation of 0.5–0.8, as opposed to that of 0–1 for αγ.
Figure 5.
Plot of channel P o against time for αγ (A) and αγT with and without 0.1 μM amiloride (B). The data used to generate A was from a patch that contained a single high P o αγ channel. B was generated from data from two separate patches, both containing single αγT channels. P o was measured at 30-s intervals in all three of these representative examples. Mean P o refers to P o measurements averaged over the entire life of the patch.
Single Channel and Amiloride Blocking Kinetics
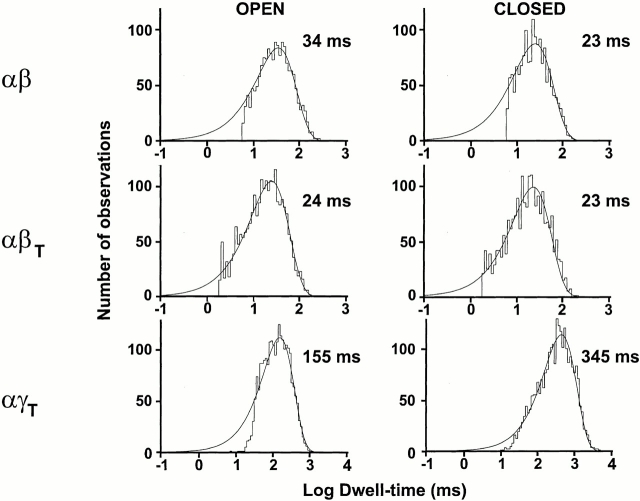
Fig. 6 shows open- and closed-time histograms for αβ, αβT, and αγT in the presence of amiloride. The kinetics for αβ and αβT were very similar. On the other hand, αγT had relatively longer open and closed times.
Figure 6.
Examples of open and closed time histograms for αβ, αβT, and αγT in the presence of amiloride. Data was obtained from single channel patches. Fits were done using Pstat. All histograms were best fitted with one exponential. The y ordinate in all histograms corresponds to the number of observations and the x ordinate log dwell-time (milliseconds). Numbers to the right of each histogram represent the calculated closed or open times for the given example.
Table II shows a summary of the mean open and closed times at −40 mV for all channel types and, in the case of αγ, the high and low P o modes. High P o αγ channels had a relatively long mean open time of 6.9 s and a shorter mean closed time (1.9 s). Conversely, low P o αγ channels had a relatively short open time lasting <1 s and a longer closed time lasting almost 5 s. αγT channels exhibited different kinetics compared with αγ channels. They had a mean open time of 1.2 s and a relatively short mean closed time of 400 ms. Channel P o calculated from the mean open and closed times using the equation
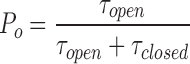 |
1 |
Table II.
Channel Open and Closed Times
| Channel type | Open time | Closed time | n | |||
|---|---|---|---|---|---|---|
| αβ (+ 1.0 μM amil) | 32 ± 2 ms | 23 ± 1 ms | 4 | |||
| αβT (+ 1.0 μM amil) | 26 ± 1 ms | 26 ± 3 ms | 3 | |||
| αγ high P o | 6.9 ± 1.3 s | 1.9 ± 0.9 s | 3 | |||
| αγ low P o | 0.7 ± 0.3 s | 4.7 ± 1.1 s | 3 | |||
| αγT | 1.2 ± 0.1 s | 400 ± 20 ms | 3 | |||
| αγT + 0.1 μM amil | 151 ± 1 ms | 365 ± 9 ms | 3 |
Summary of mean open and closed times for the channel types. Values were calculated using the Pstat program within pClamp 6. In all cases, only one exponential could be fitted to the open or closed time histograms. The number of single channel patches used to generate the values are indicated in the far right column.
yields values almost identical to that calculated using the nP o analysis program (Table I).
Having obtained the open and closed times for αβ and αβT at a range of voltages between 0 and −80 mV in the presence of 1 μM amiloride, we were able to calculate the blocking (K ON) and unblocking (K OFF) rate constants for amiloride at each of the voltages. K ON was calculated from the equation
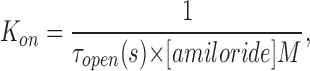 |
2 |
and likewise K OFF from
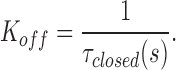 |
3 |
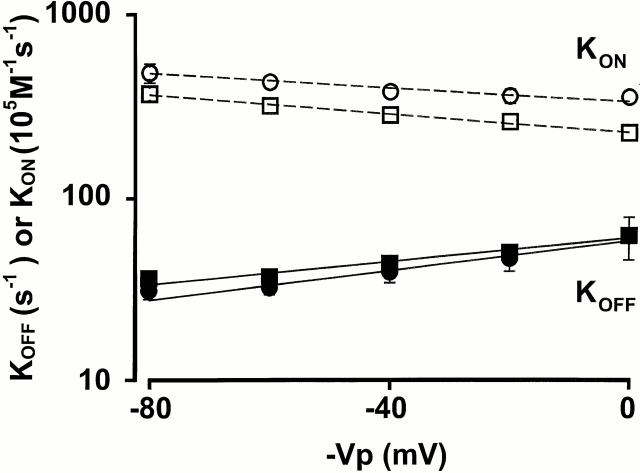
Fig. 7 shows the plot of K ON and K OFF vs. voltage for αβ and αβT. There was very little difference between the two channel types. The K ON and K OFF, dissociation constant at 0 mV (K d(0)), and dissociation constant at −40 mV (K d(−40)) values are shown in Table III. Amiloride block is very weakly voltage dependent because the K d(0) and K d(−40) for both αβ (2.7 and 1.5 μM) and αβT (1.7 and 1 μM) are close to each other. The difference in the amiloride blocking kinetics when comparing αβT (and αβ) to αγT is in the off rates: the off rate for αβT was calculated to be 39 s−1 and for αγT was 3 s−1, 1/13 of the αβT value. At −40 mV, the K d of amiloride for αγT was calculated to be 0.05 μM, a value similar to the amiloride K i (0.1 μM). We also estimated δ, the electrical distance sensed by amiloride, for αβ and αβT. Both values were found to be ∼0.3.
Figure 7.
Block of αβ and αβT by 1 μM amiloride: estimation of blocking (K ON) and unblocking (K OFF) rate constants. Each point is the mean ± SEM from three single channel patches and corresponds to 20-mV increments in the pipette holding potential. K ON and K OFF were estimated as described in the text. Note that the y axis is a logarithmic scale. □,▪ αβ; and ○,• αβT.
Table III.
Amiloride Blocking and Unblocking Rate Constants for αβ, αβT and αγT
| Channel | [amil] | K ON (−40) | K OFF (−40) | K d (−40) | K d (0) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| αβ | 1.0 μM | 29 μM−1 s−1 | 44 s−1 | 1.5 μM | 2.7 μM | |||||
| αβT | 1.0 μM | 39 μM−1 s−1 | 39 s−1 | 1.0 μM | 1.7 μM | |||||
| αγT | 0.1 μM | 66 μM−1 s−1 | 3 s−1 | 0.05 μM | ND |
K ON, K OFF, and K d values for amiloride block of αβ, αβT, and αγT channels. Note that the units for the K ON values have been converted to μM−1 s−1 from 105 M−1 s−1 as given in Fig. 7.
discussion
Subunit Composition and Channel Kinetics
The results presented in this work indicate that subunit composition is a major determinant of channel kinetics. Combination of α with β subunits favored a conformation in the open state, giving rise to αβ channels with a very high P o. In the presence of 1 μM amiloride in the pipette (concentration that corresponds to the amiloride K i of αβ channels), the P o was 0.5, indicating that without amiloride the P o of αβ channels is close to one. These channels do not exhibit the characteristic long closed states as seen in oocytes injected with αγ, αβγ, and in cells from native tissues. αβ channels seem to be constitutively open, no discernible channel transitions were observed in seals obtained in the absence of amiloride, suggesting that αβ channels do not have a closed state.
Channels formed by the α subunit and a γβ CH when patched with 1.0 μM amiloride in the pipette also exhibited a very high P o similar to αβ channels (amiloride K i of αCH channels is 1.0 μM). However, in recordings from αCH channels, we occasionally detected very brief closed states that were never seen in αβ channels. The CH comprises the first amino-terminal 2/3 of the γ subunit and the last COOH-terminal 1/3 of the β subunit that includes the intracellular COOH terminus, the M2 domain, and a short segment of amino acids preceding M2 that has been implicated in amiloride binding (Waldmann et al., 1995; Schild et al., 1997). Similarity in channel kinetics observed in αβ and αCH indicates that the 1/3 of the β subunit is responsible for conferring the high P o on these channels. As we discuss later, the intracellular COOH terminus of the β subunit does not affect the P o of αβ channels; therefore, the high P o is a property of M2 and/or the region preceding it.
In contrast to αβ channels, αγ channels exhibited kinetics that were characteristic of wild-type αβγ with closed states that varied from a few milliseconds to several seconds. Two populations of αγ channels were clearly distinguishable: one consistently had a low P o (0.16 ± 0.04) with long closed states, and the other had a high P o (0.78 ± 0.05) with long open states. The mean P o for the whole population of αγ channels was 0.47. Similar findings have been documented for ENaC expressed in cortical collecting tubules where channels exhibited either predominately low or high P o; but, when all channels were averaged, the mean P o was 0.5 (Palmer and Frindt, 1996).
Although the low and high P o αγ channels may represent the extremes of continuous open probabilities, this does not seem to be the case because all the channels we observed fitted well into the low or high P o modes, no intermediate P o values were seen. In spite of the long recording times, we did not see conversion of channels from a low to a high P o mode or vice versa when cell attached. Furthermore, αγ channels in the two gating modes can coexist; occasionally, we obtained patches with two channels: one with high and the other low P o. Although not shown here, our observation that high P o channels, when excised, adopt a low P o state instantaneously before running down several minutes later suggests that some intracellular factor or process may be required to maintain a high P o. It has been reported that hyperpolarization of the membrane potential and low concentration of extracellular Na+ can shift channels to a high P o state (Palmer et al., 1998). For reasons explained in the results, we did not attempt these maneuvers; all of our experiments were performed at −40 mV with 150 mM Li+ in the pipette.
The COOH terminus of the γ subunit may contain elements that allow channels to adopt a high or low P o state. Phosphorylation/dephosphorylation of residues in γ may induce conformational changes that stabilize either high or low P o channels. Indeed, we have recently demonstrated that ENaC is phosphorylated in the γ subunit (Shimkets et al., 1998). Alternatively, binding of accessory proteins could be another way of inducing conformational changes.
Effect of COOH Terminal Deletion on Po and on Channel Density at the Plasma Membrane
Previous experiments have shown that oocytes expressing ENaC containing β and γ subunits truncated at their COOH termini had larger whole-cell currents than oocytes expressing wild-type β or γ. There are at least three mechanisms by which an increase in current could arise in cells expressing truncated subunits: increased single channel conductance, increased channel P o, or increased expression of conducting channels at the plasma membrane. Single channel conductance was found to be unaltered (Schild et al., 1995; Snyder et al., 1995), and the number of channels expressed at the cell surface was found to be increased, but an effect on channel P o has remained controversial. For example, Firsov et al. (1997), using a flag epitope inserted into the extracellular domain of the β subunit, demonstrated that cells expressing truncated subunits had an increased number of channels in the plasma membrane when compared with oocytes expressing wild-type β subunits. The calculated number of channels at the cell surface did not account for the total increase in amiloride-sensitive current measured in the same cells, leading to the inference that channel P o had also been altered to further increase whole-cell currents. Awayda et al. (1997), using a similar approach (fluorescent antibodies to detect surface expression of channels), arrived at the same conclusion because they found only a small rise in the fluorescent signal when comparing oocytes expressing wild-type β and βT subunits.
We have addressed this problem by measuring directly the single channel conductance and P o of αβ, αβT, αγ, and αγT channels using the cell-attached configuration of the patch clamp technique. Oocytes injected with αβT or αγT channels expressed three- to fivefold larger whole-cell currents than oocytes injected with αβ or αγ. The single channel conductances of αβ and αγ were unaltered by COOH-terminal truncations (Fig. 3). Therefore, with αβ or αγ, changes in single channel conductance cannot explain the increase in whole-cell currents. A slight difference in the channel conductances of αβ and αβT vs. αγ and αγT may reflect differences in the channel pore structure.
Fig. 4 and Table I show that the P o of αβ and αβT channels in the presence of 1 μM amiloride in the pipette were 0.54 and 0.50, respectively. These results indicate that both types of channels have a very high endogenous P o, ∼1, and that it is not affected by deletion of the COOH terminus of the β subunit.
αγ channels existed in two gating modes corresponding to low and high P o (Fig. 2 and Table I); each of these modes appeared with equal frequency such that the mean P o of the entire population was 0.47. Truncation of the COOH terminus of the γ subunit produced a single population of αγT channels with more homogenous kinetics, distinct from αγ channels. The mean open and closed times of αγT channels were shorter and less variable when compared with αγ channels (Table II). Also, the P o of αγ channels fluctuated less through time (Fig. 5, A and B). Although the mean P o of αγT channels (0.6) was slightly higher than that of αγ channels (0.48), the difference does not account for the observed increase in whole-cell current.
We think that our estimate of channel P o is accurate for the following reasons. Due to the nature of the kinetics of αβ and αβT channels (with amiloride in the pipette), multichannel patches were evident instantly and overestimation of channel P o was unlikely. Regarding αγ and αγT channels, the kinetics were slower and, in most cases, multichannel patches were not evident until 1–2 min after seal formation. Therefore, we recorded patches with αγ for longer periods to be confident of the number of electrically active channels present in the patch; only patches with one or two channels were used to calculate P o. Our results clearly demonstrate that truncations of the COOH termini of the β or γ subunits do not increase channel P o.
In contrast, the number of active channels at the plasma membrane was larger in oocytes injected with βT or γT subunits. The number of successful seals that contained channels and the frequency of patches having more than one channel was greater in oocytes expressing αβT or αγT, indicating that truncated channels are expressed at a higher density. These results are consistent with the presence of endocytosis signals in the COOH terminus of β and γ that, when deleted, increase the number of channels in the cell surface (Shimkets et al., 1997).
Staub et al. (1997) have proposed another mechanism to explain the increase in number of channels at the plasma membrane. According to these investigators, the protein Nedd4 binds to the COOH termini of the α, β, and γ subunits and subsequently ubiquitinates several conserved lysines in the amino terminus of α and γ, but not β. Only ubiquitinated channels could be removed from the plasma membrane; whereas ubiquitination of α had a modest effect on the rate of endocytosis, ubiquitination of γ appeared to be a prerequisite. Therefore, the hypothesis predicts that oocytes expressing αβ and αβT channels should express a similar number of channels at the cell surface. Instead, we found that the whole-cell currents and the density of channels in the plasma membrane were larger in cells expressing αβT channels.
Amiloride Block
Amiloride is an open channel blocker with high affinity for ENaC (K d of 0.1 μM). The binding site(s) for amiloride is located in the extracellular side of the channel protein. Previous work has shown that mutations of residues in M2 of the α subunit (Waldmann et al., 1995) and the region preceding M2 of α, β, and γ subunits (Schild et al., 1997) change the affinity for amiloride. The K d's for amiloride of αβ and αγ channels, measured by inhibition of whole-cell currents, are 1 and 0.1 μM, respectively (McNicholas and Canessa, 1997). Here, we have examined the kinetics of amiloride block of αβ and αγ channels. Direct measurements of K ON and K OFF for amiloride on αβ channels (Table II) showed that these channels have a high K d for amiloride, and that the decrease in affinity is due to a 10-fold larger K OFF when compared with values reported for αβγ channels (4 s−1; Palmer and Frindt, 1986) or αβTγ (2.5 s−1; Schild et al., 1995). Both the K ON and K OFF of amiloride for αβ channels showed a weak voltage dependence, with a calculated electrical distance sensed by amiloride of 30%. As expected, deletion of the COOH terminus, which is an intracellular domain of the β subunit, did not affect the kinetics of amiloride block as shown in Figs. 1 and 7. The K ON and K OFF values for αβ and αβT were almost identical (Table II). αCH channels resemble αβ channels in their amiloride affinity with a K d of 1 μM measured by inhibition of whole-cell currents. The kinetics of amiloride block of αCH channels were also indistinguishable from αβ channels, a result that agrees with the notion that amiloride interacts with M2 and the amino acids preceding it.
The kinetics of amiloride block of αγ channels were markedly different from αβ channels. Although the K ONs were similar for αβT and αγT when measured with 1 and 0.1 μM amiloride, respectively, in the patch pipette (Table III), the K OFF of amiloride for αγT channels was much slower, suggesting that amiloride may either occupy an inhibitory site on the channel (or reside in the channel pore) for longer times. The observation that the closed time for αγT in the presence of amiloride remained unaltered when compared with the absence of amiloride (365 and 400 ms, respectively, Table II), and that the corresponding histogram was described well by one exponential, suggests that the channel closed time and amiloride block time were similar or had the same order of magnitude. We are confident that the value attributed to the mean blocked time for amiloride is correct because the K d (0.05 μM) calculated from the K ON and K OFF is similar to the values obtained with two independent measurements: inhibition of whole-cell current (K i = 0.1 μM) and mean P o (a 50% reduction in P o with 0.1 μM amiloride).
The kinetics of amiloride block of αγ channels seemed very similar to those described for αβγ. Overall, except for the lower magnitude of whole-cell current due to the lower number of channels expressed at the cell surface, αγ channels exhibited similar properties to αβγ channels.
Acknowledgments
The authors thank Drs. Gordon Gregor MacGregor and Carmel McNicholas for critical discussion.
Abbreviations used in this paper
- CH
chimera
- ENaC
epithelial sodium channel
Footnotes
Portions of this work were previously published in abstract form (Fyfe, G.K., and C.M. Canessa. 1997. The Physiologist. 40:268. Fyfe, G.K., and C.M. Canessa. 1998. Biophys. J. 74:A403).
This work was done under the tenure of an American Heart Association Postdoctoral Fellowship (G.K. Fyfe) and was supported by the Edward Mallinckrodt Jr. Foundation (C.M. Canessa).
references
- Awayda MS, Tousson A, Benos DJ. Regulation of a cloned epithelial Na+channel by its β- and γ-subunits. Am J Physiol. 1997;273:C1889–C1899. doi: 10.1152/ajpcell.1997.273.6.C1889. [DOI] [PubMed] [Google Scholar]
- Canessa CM, Horisberger J-D, Rossier BC. Epithelial sodium channel related to proteins involved in neurodegeneration. Nature. 1993;361:467–470. doi: 10.1038/361467a0. [DOI] [PubMed] [Google Scholar]
- Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger J-D, Rossier BC. Amiloride-sensitive epithelial Na+channel is made up of three homologous subunits. Nature. 1994;367:463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- Farman N, Talbot CR, Boucher RC, Canessa CM, Rossier BC, Bonvalet JP. Non-coordinated expression of α, β and γ subunit mRNAs of the epithelial sodium channel along the rat respiratory tract. Am J Physiol. 1997;272:C131–C141. doi: 10.1152/ajpcell.1997.272.1.C131. [DOI] [PubMed] [Google Scholar]
- Firsov D, Schild L, Gautschi I, Mérillat A-M, Scneeberger E, Rossier BC. Cell surface expression of the epithelial Na channel and a mutant causing Liddle syndrome: a quantitative approach. Proc Natl Acad Sci USA. 1997;93:15370–15373. doi: 10.1073/pnas.93.26.15370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garty H, Palmer LG. Epithelial sodium channels: function, structure and diversity. Physiol Rev. 1997;77:359–396. doi: 10.1152/physrev.1997.77.2.359. [DOI] [PubMed] [Google Scholar]
- Hansson JH, Nelson-Williams C, Suzuki H, Schild L, Shimkets RA, Lu Y, Canessa C, Iwasaki T, Rossier BC, Lifton RP. Hypertension caused by a truncated epithelial sodium channel subunit: genetic heterogeneity of Liddle syndrome. Nat Genet. 1995;11:76–82. doi: 10.1038/ng0995-76. [DOI] [PubMed] [Google Scholar]
- MacGregor GG, Olver RE, Kemp PJ. Amiloride-sensitive Na+channels in fetal type II pneumocytes are regulated by G proteins. Am J Physiol. 1994;267:L1–L8. doi: 10.1152/ajplung.1994.267.1.L1. [DOI] [PubMed] [Google Scholar]
- McNicholas CM, Canessa CM. Diversity of channels generated by different subunit combinations of epithelial sodium channel subunits. J Gen Physiol. 1997;109:681–692. doi: 10.1085/jgp.109.6.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Methfessel C, Witzemann V, Takahashi T, Mishina M, Numa S, Sakmann B. Patch clamp experiments on Xenopuslaevis oocytes: currents through endogenous channels and implanted acetylcholine receptor and sodium channels. Pflügers Arch. 1986;407:577–588. doi: 10.1007/BF00582635. [DOI] [PubMed] [Google Scholar]
- Palmer LG, Frindt G. Amiloride-sensitive Na channels from the apical membrane of the rat cortical collecting tubule. Proc Natl Acad Sci USA. 1986;83:2767–2770. doi: 10.1073/pnas.83.8.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer LG, Frindt G. Gating of Na channels in the rat cortical collecting tubule: effects of voltage and membrane stretch. J Gen Physiol. 1996;107:35–45. doi: 10.1085/jgp.107.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer LG, Sackin H, Frindt G. Regulation of Na+ channels by luminal Na+in rat cortical collecting tubule. J Physiol (Camb) 1998;509:151–162. doi: 10.1111/j.1469-7793.1998.151bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild L, Canessa CM, Shimkets RA, Gautschi I, Lifton RP, Rossier BC. A mutation in the epithelial sodium channel causing Liddle disease increases channel activity in the Xenopus laevisoocyte expression system. Proc Natl Acad Sci USA. 1995;92:5699–5703. doi: 10.1073/pnas.92.12.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild L, Schneeberger E, Gautschi I, Firsov D. Identification of amino acid residues in the α, β, and γ subunits of the epithelial sodium channel (ENaC) involved in amiloride block and ion permeation. J Gen Physiol. 1997;109:15–26. doi: 10.1085/jgp.109.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimkets RA, Warnock DG, Bostis CM, Nelson-Williams C, Hansson JH, Schambelan M, Gill JR, Ulick S, Milora RV, Findling JW, et al. Liddle's syndrome: heritable human hypertension caused by mutations in the β-subunit of the epithelial sodium channel. Cell. 1994;79:407–414. doi: 10.1016/0092-8674(94)90250-x. [DOI] [PubMed] [Google Scholar]
- Shimkets RA, Lifton RP, Canessa CM. The activity of the epithelial sodium channel is regulated by clathrin-mediated endocytosis. J Biol Chem. 1997;272:25537–25541. doi: 10.1074/jbc.272.41.25537. [DOI] [PubMed] [Google Scholar]
- Shimkets RA, Lifton RP, Canessa CM. In vivophosphorylation of the epithelial sodium channel. Proc Natl Acad Sci USA. 1998;95:3301–3305. doi: 10.1073/pnas.95.6.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder PM, Price MP, McDonald FJ, Adams CM, Volk KA, Zeiher BG, Stokes JB, Welsh MJ. Mechanism by which Liddle's Syndrome mutations increase activity or a human epithelial Na+channel. Cell. 1995;83:969–978. doi: 10.1016/0092-8674(95)90212-0. [DOI] [PubMed] [Google Scholar]
- Staub O, Gautschi I, Ishikawa T, Breitschopf K, Ciechanover A, Schild L, Rotin D. Regulation of stability and function of the epithelial Na+channel (ENaC) by ubiquitination. EMBO (Eur Mol Biol Organ) J. 1997;16:6325–6336. doi: 10.1093/emboj/16.21.6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stühmer, W., and A.B. Parekh. 1995. Electrophysiological recordings from Xenopus oocytes. In Single-Channel Recording. 2nd ed. B. Sakmann and E. Neher, editors. Plenum Publishing Corp., New York. 341–356.
- Waldmann R, Champigny G, Lazdunski M. Functional degenerin-containing chimeras identify residues essential for amiloride-sensitive Na+channel function. J Biol Chem. 1995;270:11735–11737. doi: 10.1074/jbc.270.20.11735. [DOI] [PubMed] [Google Scholar]