Abstract
We investigated the modulation of cGMP-gated ion channels in single cone photoreceptors isolated from a fish retina. A new method allowed us to record currents from an intact outer segment while controlling its cytoplasmic composition by superfusion of the electropermeabilized inner segment. The sensitivity of the channels to agonists in the intact outer segment differs from that measured in membrane patches detached from the same cell. This sensitivity, measured as the ligand concentration necessary to activate half-maximal currents, K 1/2, also increases as Ca2+ concentration decreases. In electropermeabilized cones, K 1/2 for cGMP is 335.5 ± 64.4 μM in the presence of 20 μM Ca2+, and 84.3 ± 12.6 μM in its absence. For 8Br-cGMP, K 1/2 is 72.7 ± 11.3 μM in the presence of 20 μM Ca2+ and 15.3 ± 4.5 μM in its absence. The Ca2+-dependent change in agonist sensitivity is larger in extent than that measured in rods. In electropermeabilized tiger salamander rods, K 1/2 for 8Br-cGMP is 17.9 ± 3.8 μM in the presence of 20 μM Ca2+ and 7.2 ± 1.2 μM in its absence. The Ca2+-dependent modulation is reversible in intact cone outer segments, but is progressively lost in the absence of divalent cations, suggesting that it is mediated by a diffusible factor. Comparison of data in intact cells and detached membrane fragments from cones indicates that this factor is not calmodulin. At 40 μM 8Br-cGMP, the Ca2+-dependent change in sensitivity in cones is half-maximal at K Ca = 286 ± 66 nM Ca2+. In rods, by contrast, K Ca is ∼50 nM Ca2+. The difference in magnitude and Ca2+ dependence of channel modulation between photoreceptor types suggests that this modulation may play a more significant role in the regulation of photocurrent gain in cones than in rods.
Keywords: retina, phototransduction, teleost, cyclic nucleotides, light adaptation
introduction
In complete darkness, rod photoreceptors respond to single photons, and cones are 10–100-fold less sensitive (Baylor et al., 1979; reviewed by Miller et al., 1994). However, the absolute sensitivity of the photoreceptor response is continuously adjusted to the ambient illumination, a process known as adaptation. In general, absolute sensitivity to light decreases in proportion to the increase in background illumination, and photoreceptors, therefore, convey information on the contrast between an object and its background, rather than on the absolute brightness of the object (Shapley and Enroth-Cugell, 1984). Retinal cones adapt over a much wider range of background intensities than do rods in the same species (Normann and Werblin, 1974; Malchow and Yazulla, 1986; Miller and Korenbrot, 1993b ). Indeed, mechanisms of adaptation in cones are so effective that the sensitivity of the photoresponse can be adjusted to maintain cell responsiveness even when background intensities change over seven orders of magnitude (Normann and Perlman, 1979; Burkhardt, 1994). Cytoplasmic Ca2+ in the outer segment plays a role in adaptation in both rods and cones (reviewed by Fain and Matthews, 1990). While it is now established that light causes a fall in intracellular Ca2+ in the photoreceptor outer segment (McNaughton et al., 1986; Gray-Keller and Detwiler, 1994; McCarthy et al., 1996), the mechanisms of the action of Ca2+ are not fully understood. Ca2+ modulates the physiological activity of several of the biochemical events underlying phototransduction. A reduction in Ca2+ concentration activates guanylyl cyclase (Lolley and Racz, 1982; Pepe et al., 1986; Koch and Stryer, 1988), accelerates rhodopsin phosphorylation (Kawamura and Murakami, 1991), and reduces the light-induced catalytic activity of rhodopsin (Lagnado and Baylor, 1994).
Ca2+ in the cytoplasm of the photoreceptor outer segment also modulates the sensitivity of the cGMP-gated ion channels to their agonist. Sensitivity is measured by the value of K 1/2, the ligand concentration necessary to activate half maximal current. In rod outer segment membrane vesicles, a soluble factor, well mimicked by calmodulin, lowers the value of K 1/2 by ∼1.5-fold as Ca2+ concentration decreases (Hsu and Molday, 1993; Bauer, 1996). In membrane patches detached from outer segments of either rods or cones, exposure to a solution free of divalent cations also reduces the K 1/2 for cGMP by ∼1.5-fold (Gordon et al., 1995; Hackos and Korenbrot, 1997). Since in the membrane patches the modulation is irreversibly lost after exposure to solutions free of divalent cations, the modulation has also been presumed to arise from the action of a soluble, endogenous modulator (Gordon et al., 1995; Bauer, 1996; Hackos and Korenbrot, 1997). This irreversible loss of modulation poses two serious experimental limitations. First, it is possible that the endogenous modulator is partially lost upon membrane isolation and, therefore, that modulation in the intact cell differs from that in the membrane patch. Second, the equilibrium Ca2+ dependence of the modulation cannot be investigated in membrane patches because stationary conditions never exist once Ca2+ concentration begins to be lowered. For rods, these limitations have been overcome in studies of channel modulation in truncated rod outer segments (Nakatani et al., 1995; Sagoo and Lagnado, 1996), a preparation in which the cytoplasmic content of a single, intact outer segment can be rapidly controlled while measuring currents through the cGMP-gated channels. We report here on the development of a functionally comparable preparation to study transduction in cones: an intact outer segment with an electropermeabilized inner segment (ep-cones).
In truncated rod outer segments, the maximum Ca2+-dependent shift in K 1/2 for cGMP activation is ∼1.5-fold, the same as that observed in detached membrane patches from the same cell. Half-maximal shift occurs at ∼50 nM free Ca2+ (Nakatani et al., 1995; Sagoo and Lagnado, 1996). We report here that the Ca2+-dependent modulation of ligand sensitivity in the intact cone outer segments is larger in extent than that observed in intact rod outer segments or in detached membrane patches of either receptor type. Moreover, in cones, unlike rods, this modulation occurs over the full physiological range of Ca2+ concentrations expected in the intact cell. The extent of Ca2+ modulation in intact cones and its Ca2+ dependence provides a mechanism that may contribute to the light adaptation characteristics of cones and explains, in part, the significant difference in transduction and adaptation between the two photoreceptor types.
materials and methods
Materials
Striped bass were obtained from Professional Aquaculture Services (Chico, CA). Fish were maintained in an aquarium in the laboratory for up to 4 wk under 10:14 h dark:light cycles. Tiger salamanders were obtained from Charles Sullivan (Memphis, TN). They were maintained in the laboratory in an aquarium at 8°C under 12:12 h dark:light cycles. The UCSF Committee on Animal Research approved protocols for the maintenance and killing of the animals.
Cell Isolation and Solutions
Single cones were isolated from the dark-adapted retina of striped bass (Morone saxitilis) and rods from that of tiger salamander (Ambystoma tigrinum). Retinas were isolated under infrared illumination, and retinas and photoreceptors were dissociated as previously described (Miller and Korenbrot, 1993a , 1994). Dissociated cones were maintained in Ringer's solution consisting of (mM): 136 NaCl, 2.4 KCl, 5 NaHCO3, 1 NaH2PO4, 1 CaCl2, 1 MgCl2, 10 glucose, 10 HEPES, pH 7.5, 0.1 mg/ml BSA supplemented with MEM vitamins and amino acids. Dissociated rods were maintained in Ringer's solution consisting of (mM): 100 NaCl, 2 KCl, 5 NaHCO3, 1 NaH2PO4, 1 CaCl2, 1 MgCl2, 10 glucose, 10 HEPES, pH 7.4, 0.1 mg/ml BSA supplemented with MEM vitamins and amino acids.
A suspension of dissociated photoreceptor cells was added to a recording chamber held on the fixed stage of an inverted microscope. Under microscopic observation, using infrared illumination, the outer segment of a single photoreceptor was drawn into a suction electrode. The suction electrode was filled with a solution in all respects identical to the Ringer's solution, except that the free Ca2+ concentration was 1 μM. The cell held within the suction electrode was superfused with a solution delivered through a 75-μm diameter capillary positioned within 50 μm of the cell. The 75-μm capillary was the single output from a micromanifold that allowed the superfusing solution to be rapidly changed (<30 ms) with the use of electronically controlled valves (DAD-12; ALA Scientific Instruments, Inc., Westbury, NY). The superfusion solutions for single cones always consisted of (mM): 140 cholineCl, 10 HEPES, pH 7.5, 5 glucose, 0.4 Zaprinast. The solutions could contain divalent cations (20 μM Ca2+ and 0.5 mM Mg2+) or not (no added cations, with 2 mM HEDTA). Solutions of known Ca2+ concentration were prepared using EGTA in the absence of Mg2+, as described (Hackos and Korenbrot, 1997). Cyclic guanine monophosphate nucleotides were added to the solutions as needed. The same superfusion solutions were used with rods, but with 110 cholineCl. After electropermeabilization, the superfusion solution became the intracellular solution. Because the cGMP-gated channels are impermeable to choline, their activation under these ionic conditions and at 0-mV holding voltage generated an inward current.
Electropermeabilization
Two sharp tungsten microelectrodes, electrically insulated except at their tip (1 μm) (A-M Systems, Everett, WA), were mounted on separate micromanipulators. Under microscopic observation, they were positioned opposite each other and pressed against the surface of the inner segment of a photoreceptor cell; the outer segment of the cell was held within a suction electrode (see Fig. 1). Single, brief (1–10 ms) voltage pulses 1–5 V in amplitude for cones (1–5 kV/cm) and up to 8 V for rods (1–5 kV/cm) were applied between the metal electrodes. Successful electropermeabilization was apparent by a sudden and subtle change in the visual appearance of the inner segment membrane (see Fig. 1). Experimental results with electropermeabilized rods are similar to those obtained in truncated rod outer segments (see Fig. 4), indicating that outer segments are not harmed by electropermeabilization any more than by truncation.
Figure 1.
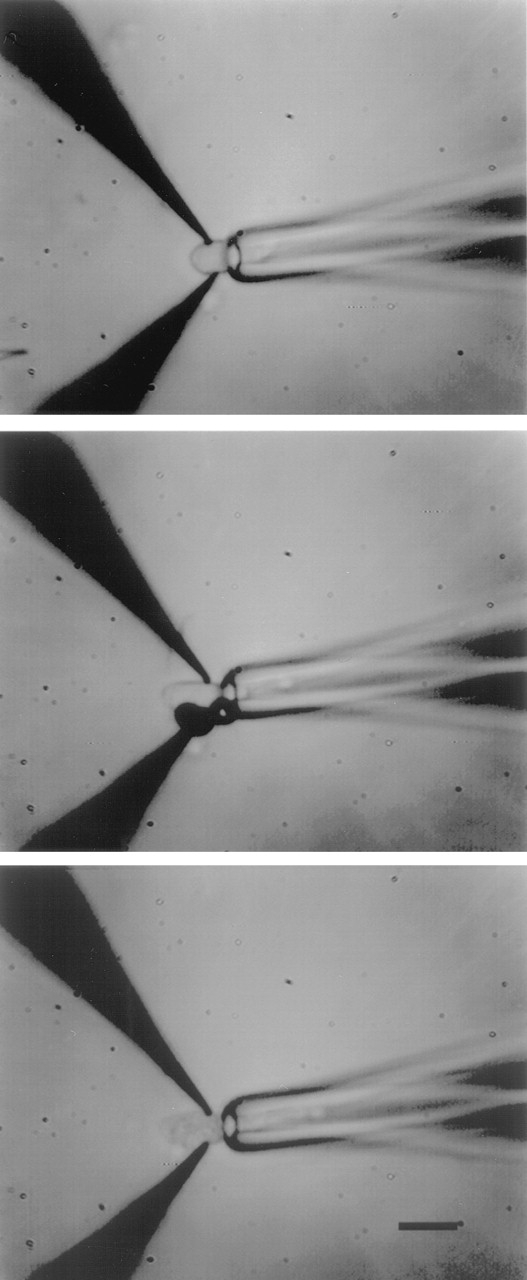
Photomicrograph illustrating the method to record outer segment currents and electropermeabilize the inner segment of a single cone isolated from the retina of striped bass. The outer segment is drawn into a suction electrode and two tungsten microelectrodes are pressed against the surface of the inner segment (top). A brief voltage pulse is applied between the metal electrodes causing electrolysis and local dielectric breakdown of the inner segment membrane (middle). Superfusion of the electropermeabilized inner segment permits continuous control of the cytoplasmic composition of the outer segment (bottom, calibration bar is 10 μm).
Figure 4.
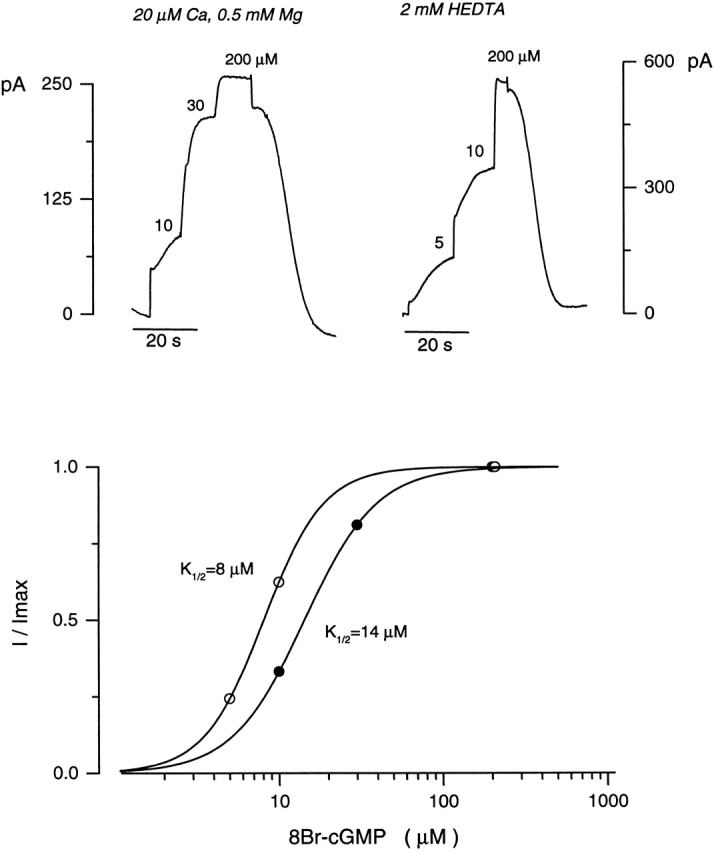
Ca2+-dependent modulation of activation by 8Br-cGMP of the cGMP-gated channels in electropermeabilized tiger salamander rods. (top left) Outer segment currents measured in the presence of 20 μM Ca2+ and 0.5 mM Mg2+ in response to increasing nucleotide concentrations. (top right) Currents activated by 8Br-cGMP in the absence of divalent cations. (bottom) The continuous curves illustrate the Hill equation that best fits the experimental data. For the data shown, K 1/2 = 14 μM cGMP and n = 2 in the presence of divalent cations (•), and K 1/2 = 8 μM cGMP and n = 2.3 in their absence (○).
Electrophysiological Recording and Data Analysis
Suction electrodes were produced from 1752 capillary glass (1.5 × 1.0 mm, o.d. × i.d.; Corning Glass Works, Corning, NY) and were rendered hydrophobic by coating with bis-dimethyl-amino dimethyl-silane. Individual cone outer segments were drawn by gentle suction and the electrode sealed against the surface of the slightly wider inner segment. Seal resistances were between 0.5 and 3 MΩ. Membrane currents were recorded at zero holding voltage with a patch-clamp amplifier (EPC-7; List Electronic, Darmstadt, Germany). Analogue signals were low pass filtered (50 Hz) through an eight-pole Bessel filter and digitized online (100 Hz; Axon Instruments, Foster City, CA) with a computer-based data acquisition system (FastLab; Indec Instruments, Capitola, CA).
Because the suction electrode/cell combination is low in impedance, there is an unavoidable drift in the current data recorded experimentally. To minimize uncertainties due to this drift, we used a track-and-hold circuit before data digitization. The data acquisition computer controlled both this circuit and the valves in the superfusion system, such that current was zeroed immediately before superfusion of the ep-cone commenced. In addition, the superfusing solutions were changed at fixed intervals, 6–8-s long. These intervals were sufficiently long for current to reach a nearly unvarying value, but we did not always obtain an absolute steady state. To asses the error that may arise from this experimental compromise, we measured the current drift in the presence of constant cyclic nucleotide (a “typical bad” drift is illustrated in Fig. 3). Current drift was no worse than ±10%/min. Considering that superfusing solutions were exchanged at intervals of 6–8 s, in any given data set the error in the value of K 1/2 due to drift can be calculated to be no worse than 1%. This error is negligible when compared with the variance from cell to cell, which is the limiting source of error in our data. Mathematical functions were fit to experimental data with the use of a nonlinear, least square minimization algorithm (N-Fit, Galveston, TX). Experimental error is presented throughout as mean ± SD.
Figure 3.
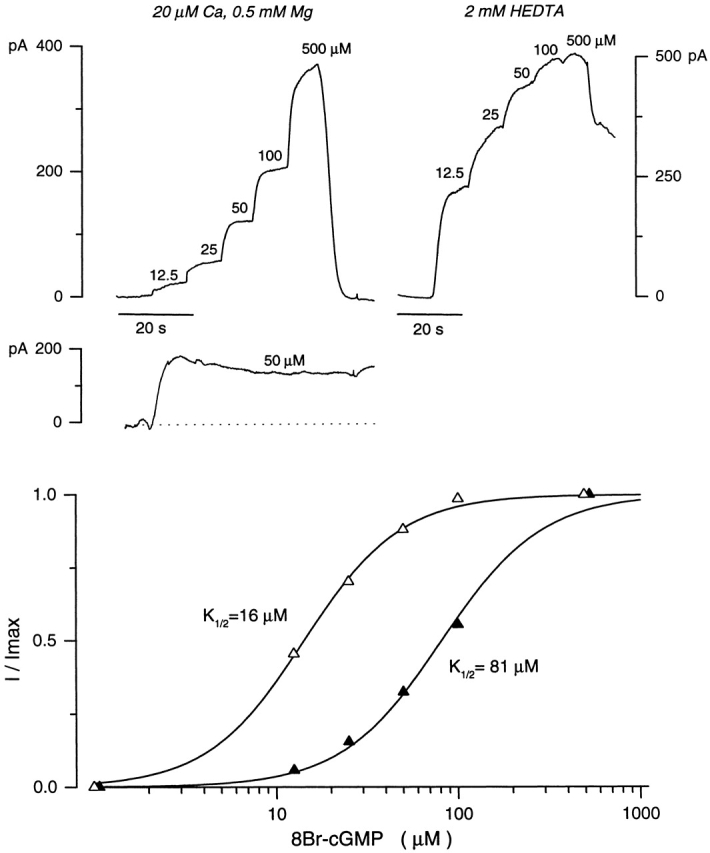
Ca2+-dependent modulation of activation by 8Br-cGMP of the cyclic nucleotide-gated channels in electropermeabilized striped bass single cones. (top left) Outer segment currents measured in the presence of 20 μM Ca2+ and 0.5 mM Mg2+ in response to increasing nucleotide concentrations. Also shown is the continuous response of a different ep-cone to a single step of subsaturating 8Br-cGMP (50 μM). The example shown is atypical, but was selected to illustrate extreme drift in current amplitude and make evident its small contribution to experimental error in the determination of the values of K 1/2 and n in the Hill equation (Eq. 1). (top right) Currents activated by 8Br-cGMP in the absence of divalent cations. (bottom) The continuous curves illustrate the Hill equation that best fits the experimental data. For the data shown, K 1/2 = 81 μM cGMP and n = 1.3 in the presence of divalent cations (▴), and K 1/2 = 16 μM cGMP and n = 1.1 in their absence (▵).
results
We investigated the modulation of the cGMP-gated channels in single cones isolated from a fish retina. We measured outer segment currents with suction electrodes in cones whose inner segment was rendered freely permeable by electropermeabilization (ep-cones) (Fig. 1). The cytoplasmic composition of the outer segment was controlled by continuous superfusion of the electropermeabilized inner segment. Activation of the cGMP-gated ion channels (the only channels in the outer segment membrane; Miller and Korenbrot, 1993a ) by superfusion with varying concentrations of cGMP caused an inward current. The dependence on cGMP of the steady state amplitude of the current was well described by the Hill equation (Fig. 2).
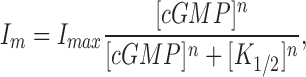 |
1 |
Figure 2.
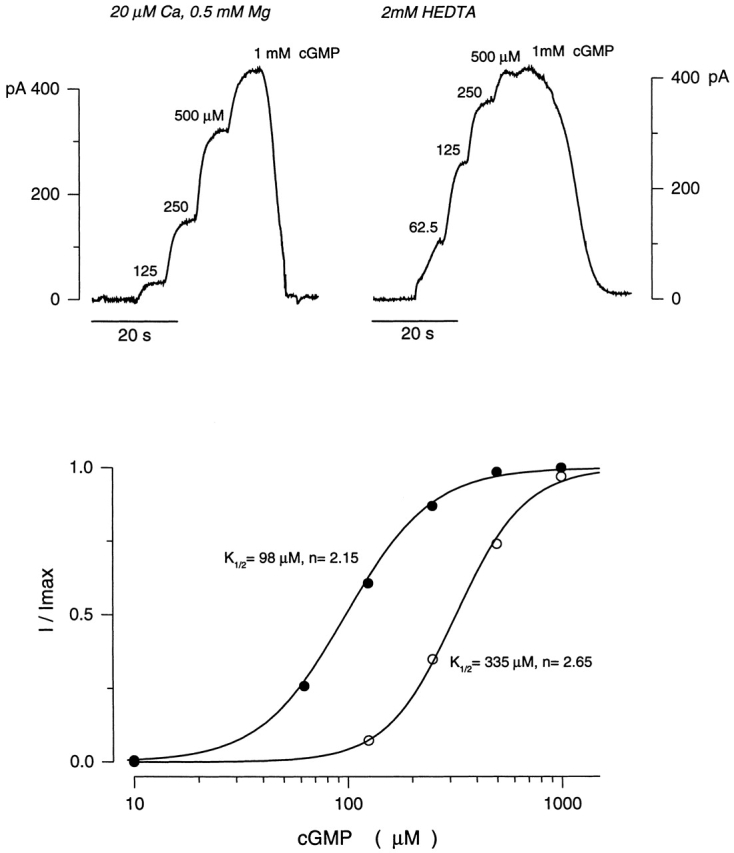
Activation of cGMP-dependent currents in the intact outer segment of electropermeabilized cones. (top left) Inward outer segment currents activated by increasing concentrations of cGMP in the presence of 20 μM Ca2+ and 0.5 mM Mg2+. (top right) Currents measured in response to cGMP, but in the absence of divalent cations (with 2 mM HEDTA). (bottom) The dependence of normalized current amplitude on cGMP concentration. The continuous curves illustrate an optimized fit of the Hill equation (Eq. 1) to the experimental. For the data shown, K 1/2 = 335 μM cGMP and n = 2.65 in the presence of divalent cations (○), and K 1/2 = 98 μM cGMP and n = 2.15 in their absence (•).
where I m is the membrane current, I max is the maximum, saturated amplitude, [cGMP] is the concentration of the agonist, K 1/2 is the cGMP concentration that activates half maximal current, and n is an adjustable parameter that measures a cooperative action of cGMP molecules. I max varied widely among electropermeabilized cones, from a few hundred picoamperes up to one nanoampere. This range likely reflects varying lengths of functional outer segment within the suction electrode.
The parameters of the equation, K 1/2 and n, provide a measurement of the sensitivity of the channels to their ligand. Manipulation of the concentration of divalent cations caused dramatic shifts in this sensitivity (Fig. 2). In the presence of 20 μM Ca2+ and 0.5 mM Mg2+, K 1/2 was 335.5 ± 64.4 μM cGMP (range 200–550, n = 28), and n was 2.58 ± 0.42 (range 1.66–3.52). However, in the absence of divalent cations, K 1/2 decreased to 84.3 ± 12.6 μM cGMP (range 67–103, n = 7), and n was 2.30 ± 0.37 (range 1.9–3). When Mg2+ was removed and Ca2+ maintained at 20 μM, we did not observe a shift in the cGMP dependence of current activation, though current amplitude increased due to the relief of channel block caused by Mg2+ at the concentration used. Thus, cytoplasmic Ca2+ specifically modulates the ligand sensitivity of the cGMP-gated channels in the intact cone outer segment.
The experiments with cGMP in electropermeabilized cones were executed in the presence of Zaprinast, an effective inhibitor of phosphodiesterase (PDE)1 in cones (Gillespie and Beavo, 1989). Nevertheless, to rule out the possibility that some component of channel modulation might reflect effects of residual PDE activity, we tested current activation by 8Br-cGMP in the presence of Zaprinast. 8Br-cGMP activates the channels but is poorly hydrolyzed by PDE (Zimmerman et al., 1985). Although the channel's absolute sensitivity to 8Br-cGMP is higher than to cGMP, as was previously known, the modulation by Ca2+ of this sensitivity is nearly the same for both cyclic nucleotides (Fig. 3). In the presence of 20 μM Ca2+ and 0.5 mM Mg2+, K 1/2 was 72.7 ± 11.3 μM 8Br-cGMP (range 46–100, n = 25), and n was 1.55 ± 0.22 (range 1–1.75). Removal of divalent cations shifted K 1/2 to 15.3 ± 4.5 μM 8Br-cGMP (range 6.7–19, n = 30), and n to 1.26 ± 0.37 (range 1–1.69). These results indicate that PDE is inactive under our experimental conditions, and does not influence channel sensitivity to cyclic nucleotides or its modulation by Ca2+.
As an additional control, we measured the response to a continuous step of subsaturating 8Br-cGMP concentration in the presence of Ca2+ and Mg2+. If there were a “spontaneous” increase in channel sensitivity over the time scale of our observations, then the current activated by low nucleotide concentrations would be expected to increase dramatically and systematically over the 60–100 s of a typical experimental trial. Fig. 3 illustrates the continuous response of an ep-cone to 50 μM 8Br-cGMP in the presence of 20 mM Ca2+ and 0.5 mM Mg2+. The current changed rapidly to a new value and, in this example, it then drifted at a rate of ∼−30 pA/min. Drift, of course, is random and we selected this as an example of large drift. On average, drift was no worse than ±10%/min. To within this accuracy there is no evidence of spontaneous changes, and K 1/2 is in error by <±1% due to drift.
Comparison with Channel Modulation in Detached Membrane Patches
It is instructive to compare the present results with those obtained in studies of membrane patches detached from the same outer segments and studied under similar experimental conditions. In the membrane patches at −40 mV and under 20 μM Ca2+ and 0.1 mM Mg2+, the average values for cGMP activation were K 1/2 = 86 μM and n = 2.57, and for 8Br-cGMP they were K 1/2 = 22 μM and n = 2.29 (Hackos and Korenbrot, 1997). The high values of K 1/2 characteristic of intact cone outer segment, but not of detached patches, suggests that modulation of the channel sensitivity depends on a molecular process that is lost upon membrane isolation. On the other hand, the K 1/2 for both cGMP and 8Br-cGMP measured in the absence of divalent cations is essentially the same in the electropermeabilized cone and the detached membrane patch. In membrane patches in the absence of divalent cations, the average values for cGMP activation were K 1/2 = 58 μM and n = 1.8, and for 8Br-cGMP they were K 1/2 = 16 μM and n = 1.54 (Hackos and Korenbrot, 1997). Thus, the channels in the absence of the endogenous modulator are functionally similar in the membrane patch and the intact outer segment. Moreover, the similarity in channel sensitivity measured in the absence of endogenous modulator in intact cells and detached membrane fragments confirms the suggestion that PDE is essentially inactive in the presence of Zaprinast in our experiments.
Channel Modulation in Intact Rod Outer Segments with an Electropermeabilized Inner Segment
To compare channel modulation in the intact cone outer segment with that in the intact rod outer segments, we studied current modulation in electropermeabilized rods isolated from the tiger salamander retina (Fig. 4). In rods, removal of divalent cations caused a modest shift in the channel sensitivity to cyclic nucleotides. We measured a K 1/2 for activation with 8Br-cGMP of 17.9 ± 3.8 μM and n of 2.06 ± 0.16 (n = 6) in the presence of 20 μM Ca2+ and 0.5 mM Mg2+ that shifted to 7.2 ± 1.2 and n to 2.1 ± 0.38 upon removal of the divalent cations. These results essentially reproduce those previously reported for truncated rod outer segments of tiger salamander (Sagoo and Lagnado, 1996) or frog (Nakatani et al., 1995) and confirm that the extent of Ca2+-dependent channel modulation in intact cones is larger than that in intact rods. These results also indicate that electropermeabilization does not damage the outer segment any more than truncation does.
Ca2+ Dependence of Channel Modulation
To investigate the Ca2+ dependence of the channel sensitivity to nucleotides, we measured the outer segment current in the presence of a constant cyclic nucleotide concentration and varying Ca2+ concentrations. We measured current activated by 40 μM 8Br-cGMP, a concentration at which we expected to observe currents at all Ca2+ concentrations of interest (see Fig. 3). We found that the current amplitude increased monotonically as Ca2+ decreased (Fig. 5). As Ca2+ was lowered, the current amplitude increased rapidly towards a new plateau value, but an absolute stationary state was not attained. Since Ca2+ dependence reflects the action of a diffusible factor (see below), the absence of an absolute steady state likely reflects the unavoidable and continuing loss of modulator from the ep-cone as Ca2+ concentration was lowered. There is an experimental compromise. We waited long enough to reach a reasonable plateau, but the plateau would never have been absolutely invariant. The dependence of the plateau on Ca2+ concentration was well described by an inverse Hill equation:
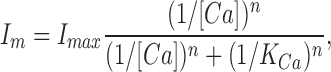 |
2 |
Figure 5.
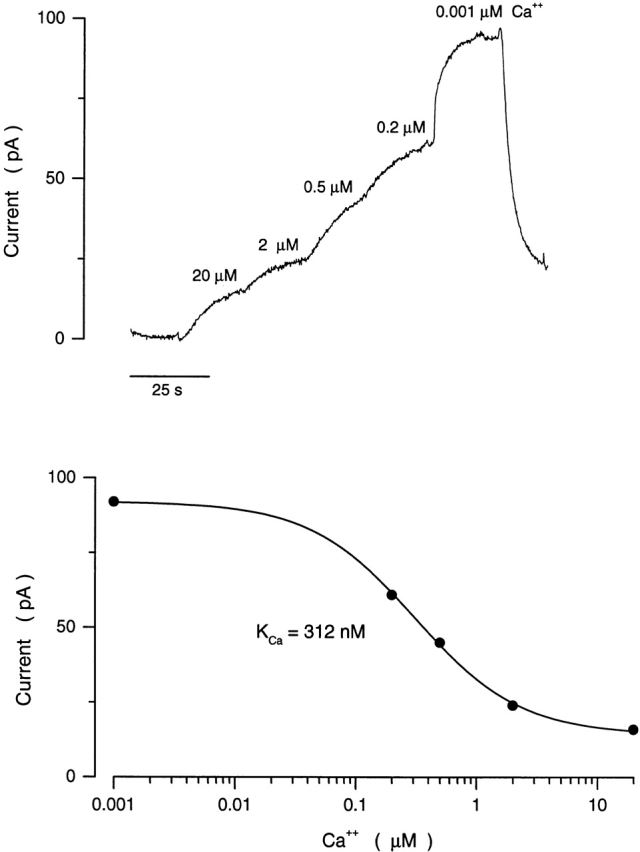
Ca2+ dependence of the 8Br-cGMP-activated current in an electropermeabilized single cone. The cell was electropermeabilized in the presence of 20 μM Ca2+ without Mg2+, current was then activated with 40 μM 8Br-cGMP and the free concentration of Ca2+ was progressively reduced in steps to 1 nM. (top) Currents measured with this experimental protocol. We consistently observed that the response time constant to changes in Ca2+ concentration was slower than that to changes in cyclic nucleotide concentration. (bottom) The dependence of current amplitude on Ca2+ concentration. The continuous curve illustrates an optimum fit to the experimental data of the inverse Hill function (Eq. 2). For the data shown, K Ca = 312 nM and n = 1.4.
where I m is current, I max is the maximum current, [Ca] is the Ca2+ concentration, K Ca is the Ca2+ concentration to reach half maximum current inhibition, and n is a parameter that indicates a cooperative interaction among Ca2+ ions. The average values of the adjustable parameters that best fit our data were K Ca = 286 ± 66 nM Ca2+ (range 176–456, n = 15) and n = 1.0 ± 0.3 (Fig. 5). Our findings in cones are in sharp contrast to the results of similar experiments in intact rod outer segments where K Ca is ∼50 nM (Nakatani et al., 1995; Sagoo and Lagnado, 1996).
The Channel Modulator Is a Factor that Diffuses Away from the Cone Outer Segment
Channel modulation in cones likely reflects the interaction of the membrane-bound channel protein with a soluble factor. In detached membrane patches, Ca2+-dependent modulation is irreversibly lost upon exposure to solutions free of divalent cations (Hackos and Korenbrot, 1997). The electropermeabilized cones, in contrast, could be exposed to divalent-free solutions, yet modulation recovered upon adding Ca2+ and Mg2+ (Fig. 6). Nonetheless, the extent of Ca2+-dependent modulation was progressively and irreversibly lost with continuing exposure to solutions free of divalent cations (Fig. 6). The time course of loss of modulation caused by exposure to solutions free of divalent cations varied from cell to cell. Thus, modulation is probably mediated by a factor that binds reversibly to the channel in a Ca2+-dependent manner. In the continuing absence of divalent cations, the factor is free and it slowly diffuses away from the electropermeabilized cell.
Figure 6.
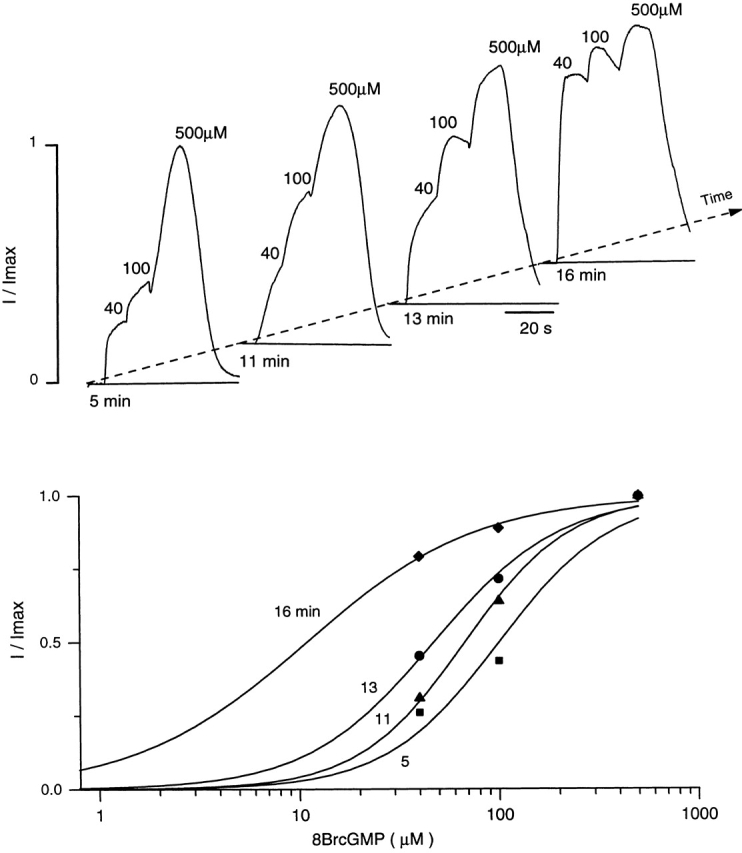
Modulation of the cGMP-gated current slowly washes out from the cone outer segment after intermittent exposure to Ca2+-free solutions. The dependence on 8Br-cGMP of the outer segment current in the presence of 20 μM Ca2+ and 0.5 mM Mg2+ was measured in successive trials in the same cells. In between trials, the electropermeabilized cone was superfused with a solution free of divalent cations for 1–3 min. (top) The currents measured in the same cell in trials conducted at 5, 11, 13, and 16 min after the electropermeabilization. The continuous curves in the bottom panel illustrate Hill functions optimally fit to the experimental data. The values of K 1/2 and n at each successive trial are: 5 min (▪) 100 μM, n = 1.52; 11 min (▴) 75 μM, n = 1.42; 13 min (•) 54 μM, n = 1.07; and 16 min (♦) 12 μM, n = 1.
discussion
We have investigated the modulation of cGMP-gated ion channels in intact cone outer segments and have found that the absolute sensitivity of the channels to activation by cyclic nucleotides is a function of cytoplasmic Ca2+ concentration. The affinity of the channels for cGMP or 8Br-cGMP decreases as Ca2+ concentration rises. Ca2+-dependent modulation of nucleotide sensitivity of cyclic nucleotide-gated ion channels has been previously observed in other sensory cells (reviewed in Molday, 1996). In olfactory sensory neurons, for example, the modulation is large, the sensitivity changes >100-fold between its extremes (Kramer and Siegelbaum, 1992; Chen and Yau, 1994; Lynch and Linderman, 1994; Liu et al., 1994; Balasubramanian et al., 1996), and the modulation plays an important role in adaptation to odors (Kurahashi and Menini, 1997). In rod photoreceptors, in contrast, the sensitivity changes by only ∼1.5–2-fold (Nakatani et al., 1995; Sagoo and Lagnado, 1996; this report). The extent of modulation in cones, four- to fivefold, is in between that of the other two cell types. The quantitative features of the modulation of chemical sensitivity, then, are not universal; rather, they are likely optimized for the physiological role of the channels in the various sensory cells.
The extent of channel modulation in intact cone outer segments is not only larger than that in rods, but is also larger than that measured in membrane patches detached from the same cell (Hackos and Korenbrot, 1997) (Table I). The K 1/2 for activation by cGMP in the presence of Ca2+ in the intact cone, ∼335 μM, is approximately five times larger than that measured in the membrane patches. Reassuringly, in the absence of Ca2+, the K 1/2 is similar in the intact cone and detached membrane patches (Picones and Korenbrot, 1992; Hackos and Korenbrot, 1997). Thus, membrane patches detached in the continuing presence of 20 μM Ca2+ exhibit some modulation of K 1/2, but much less than that of the intact cell. This result suggests that modulation is mediated by a factor that is lost upon membrane isolation, even in the presence of 20 μM Ca2+. The small extent of modulation observed in the detached patch could reflect the remaining presence of one modulator, but it is also possible that there exist two different modulators, each with a distinct membrane-binding behavior. Recent studies in cyclic nucleotide-gated channel from mammalian olfactory neurons indicate that Ca2+-dependent modulation of K 1/2 in these cells likely reflects the activity of two different modulators. One is calmodulin and the other, as yet unidentified, is characterized by its insensitivity to calmodulin blockers, such as mastoparan, and its irreversible loss upon exposure to high Mg2+ (3 mM) (Balasubramanian et al., 1996).
Table I.
Agonist Sensitivity of Cyclic Nucleotide-gated Ion Channels in Intact Outer Segments or Detached Membrane Patches from Rods and Cones*
| Cone | Rod | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intact outer segment | Membrane patch | Intact outer segment | Membrane patch | |||||||||||||||||
| K1/2 | n | K1/2 | n | K1/2 | n | K1/2 | n | |||||||||||||
| cGMP | ||||||||||||||||||||
| High Ca2+‡ | 335.5 ± 64.4 | (1) | 2.58 ± 0.42 | 86.1 ± 18 | (2) | 2.57 ± 0.34 | 37.8 ± 8 | (3) | 2.3 ± 0.5 | 41.1 ± 7 | (2) | 2.58 ± 0.43 | ||||||||
| 40 | (4) | 2.4 | ||||||||||||||||||
| 0 Ca2+ | 84.3 ± 12.6 | 2.30 ± 0.37 | 58.8 ± 19.3 | 1.8 ± 0.23 | 28 ± 6 | 2.2 ± 0.6 | 27.5 ± 6.2 | 1.97 ± 0.28 | ||||||||||||
| 27 | 2 | |||||||||||||||||||
| 8BrcGMP | ||||||||||||||||||||
| High Ca2+‡ | 72.7 ± 11.3 | (1) | 1.55 ± 0.22 | 22.1 ± 3.1 | (2) | 2.29 ± 0.39 | 17.9 ± 3.8 | (1) | 2.06 ± 0.16 | |||||||||||
| 0 Ca2+ | 15.3 ± 4.5 | 1.26 ± 0.37 | 16.4 ± 3.8 | 1.54 ± 0.35 | 7.2 ± 1.2 | 2.1 ± 0.38 | 4 | (5) | 2.5 | |||||||||||
Data are taken from reports in which the modulatory effects of Ca2+ were investigated. Other thorough studies have reported the values of K 1/2 and n in the absence of divalent cations (reviewed in Zagotta and Siegelbaum, 1996). Data in those reports are in general agreement with those recapitulated in this table.
In the various reports quoted, High Ca2+ was anywhere between 1 and 20 μM, and Mg2+ was between 0.5 and 1 mM. 1. This report. 2. Hackos and Korenbrot (1997), striped bass, tiger salamander. 3. Sagoo and Lagnado (1996), tiger salamander. 4. Nakatani et al. (1995), bullfrog. 5. Gordon et al. (1995), frog.
The modulator in the intact cone outer segments is progressively lost, except when the Ca2+ and Mg2+ concentrations are kept high (20 μM and 0.5 mM, respectively). The extent of the loss can be limited by exposing the outer segment to Ca2+ and Mg2+ free solution for only brief periods of time (see Fig. 6). If outer segments are only briefly exposed to divalent free solutions, the K 1/2 for cGMP can be reversibly shifted from its high to its low value (Fig. 6). This reversibility observed in the absence of added triphosphate nucleotides suggests that the mechanism of modulation does not depend on the activity of a coupled system of phosphatase/kinase.
The molecular identity of the endogenous channel modulator is yet to be determined. It is likely to be a factor that binds reversibly to the channel in a Ca2+-dependent manner. Calmodulin is a candidate channel modulator in rods (Hsu and Molday, 1993; Gordon et al., 1995; Bauer, 1996). In detached cone membrane patches, adding calmodulin fails to restore Ca2+-dependent channel modulation with features that resemble those of the intact outer segments (Hackos and Korenbrot, 1997), or is altogether ineffective (Haynes and Stotz, 1997). This molecule, therefore, is almost certainly not the native modulator in cones. Nonetheless, because in cone membrane patches calmodulin modulates the channels to some extent (Hackos and Korenbrot, 1997), the authentic modulator probably shares structural homology with calmodulin. Other Ca2+-binding proteins found in photoreceptors, such as GCAP1, GCAP2, and recoverin, also fail to mimic the effects of the endogenous modulator (Hackos and Korenbrot, 1997).
Differences between Rods and Cones
Table I summarizes the values of K 1/2 and n in the Hill equation that define the sensitivity to cyclic nucleotides of the channels in striped bass cones and tiger salamander rods and the extent of their modulation by Ca2+. As was previously known, the absolute sensitivity to cyclic nucleotides is higher in rods than in cones, both in membrane patches and in the intact outer segment. In rods, there is a relatively small Ca2+-dependent modulation of this sensitivity and the extent of modulation is similar in intact outer segments and detached membrane patches. In cones, the modulation in detached patches is small, comparable to that in rods, but it is much larger in the intact outer segment. In the intact outer segments, the Ca2+ dependence of the modulation has very different midpoints in rods and cones. Because the modulator is continuously lost both from the ep-cones and the truncated or ep-rods, the value of this titration midpoint was measured only at a single cyclic nucleotide concentration and absolute equilibrium was not attained. This experimental caveat applies equally to measurements in rods (Nakatani et al., 1995; Sagoo and Lagnado, 1996), yet under similar experimental protocols the difference between rods and cones is striking.
Sagoo and Lagnado (1996) have suggested that modulation in rods may be initially larger than the 1.5–2.5-fold observed in a stationary state. They made this suggestion based on the observation that immediately after truncation of tiger salamander rods in low Ca2+, there is an initial large current that decays spontaneously. We note, however, that these rapid changes were observed after truncation in the presence of ATP, GTP, and cGMP and without added PDE inhibitor. We have not observed this rapid, spontaneous change in current amplitude under our experimental conditions in electropermeabilized rods or cones. Since we electropermeabilize in the presence of the standard intracellular solution, which lacks nucleotides and contains Zaprinast, we speculate that the transient changes observed by Sagoo and Lagnado (1996) reflect other biochemical events, in addition to specific changes in the agonist sensitivity of the channels.
The differences between rods and cones discussed here are based on data from only a few species: modulation in rods has been studied in cells from bovine (Hsu and Molday, 1993; Bauer, 1996), frog (Gordon et al., 1995; Nakatani et al., 1995), and tiger salamander (Sagoo and Lagnado, 1996), and cones have been studied in only two species of fish (this report; Hackos and Korenbrot, 1997; Haynes and Stotz, 1997). To be certain of the generality of our observations, additional species must be investigated. Nonetheless, the fundamental differences in the biophysical features of the cGMP-gated channels of rods and cones are not only consistently different among the various species studied, but also exist in cloned alpha subunits of the channels of bovine rods and cones (Frings et al., 1995).
Physiological Implications of the Results
The photoresponse in retinal cones is less sensitive to light, faster in time course, and adapts over a much wider range of intensities than does that of rods in the same species (reviewed in Miller et al., 1994). The mechanisms that explain the difference in the transduction signal between receptor types are not understood in detail. However, we now know that the relative Ca2+ permeability of the cGMP-gated ion channels is higher in cones than in rods (Picones and Korenbrot, 1995; Frings et al., 1995). Also, the rate of Ca2+ clearance from the outer segment is faster in the cone outer segment than in that of rods (Hestrin and Korenbrot, 1990; Perry and McNaughton, 1990). Since the cytoplasmic Ca2+ is controlled by the continuous balance between Ca2+ influx through cGMP-gated ion channels and its clearance through Na+/Ca2+,K exchangers (Yau and Nakatani, 1985), cones can be expected to exhibit light-dependent changes in cytoplasmic Ca2+ concentration in the outer segment that are larger and faster than those in rods (Miller and Korenbrot, 1994; Korenbrot, 1995).
The link between changes in cytoplasmic Ca2+ and control of gain, kinetics, and adaptation reflects the action of Ca2+ on several biochemical events that underlie the phototransduction signal, among them the agonist sensitivity of the cGMP-gated ion channels. In rods, modulation of the cGMP-gated channels has been deemed to be of relatively little physiological importance because the maximum shift in agonist sensitivity is small in extent and it occurs at Ca2+ concentrations expected only at bright light levels (Koutalos and Yau, 1996). The same line of thought suggests that channel modulation in cones, unlike rods, is of significant importance since it is larger in extent and, particularly, its Ca2+ sensitivity overlaps the cytoplasmic Ca2+ concentration expected in the cone outer segment in darkness.
The difference in channel modulation between rods and cones may help explain the difference in the range of light intensities over which the photoreceptors adapt. In response to a step of light, the concentration of cGMP decreases to a new stationary value, the channels close and cytoplasmic Ca2+ declines. In cones, but not rods, the ligand sensitivity of the channels will rise. Consequently, it can then be expected that channels will reopen, in spite of the lowered cGMP level. That is, the standing outer segment current will fall at first, and then recover steadily as the channel sensitivity rises. The Ca2+ feedback on the channels opposes the direct action of light, thus recovering some of the operating range of the photocurrent. Constant intensity light flashes, when superimposed on increasing backgrounds of light, cause progressively smaller changes in cytoplasmic cGMP concentration. Because the channel sensitivity to cGMP increases as the background intensity rises, the constant flashes will close a proportionally larger number of channels in light (low Ca2+) than might have been predicted from their behavior in darkness (high Ca2+). This is the hallmark of light adaptation. Again, the feedback action of Ca2+ on channel sensitivity opposes the direct action of light and can cause the lowering of photocurrent gain characteristic of light adaptation.
The difference between rods and cones and the role that channel modulation by Ca2+ might play can be estimated through a simple calculation. This calculation does not attempt to predict differences on the comprehensive effects of Ca2+ in intact rods and cones, since such calculation requires the quantitative definition of all processes modulated by Ca2+. The simple calculation summarizes the observations we have made. If we assume that the modulation by Ca2+ on the channels shifts only K 1/2 and n, then it is possible to estimate the extent of the current amplitude enhancement caused by the given decrease in Ca2+ concentration. The magnitude of the current enhancement is a function of the cGMP concentration at which it is tested, and is given by Hackos and Korenbrot (1997):
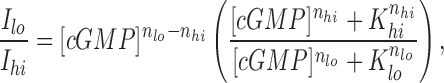 |
3 |
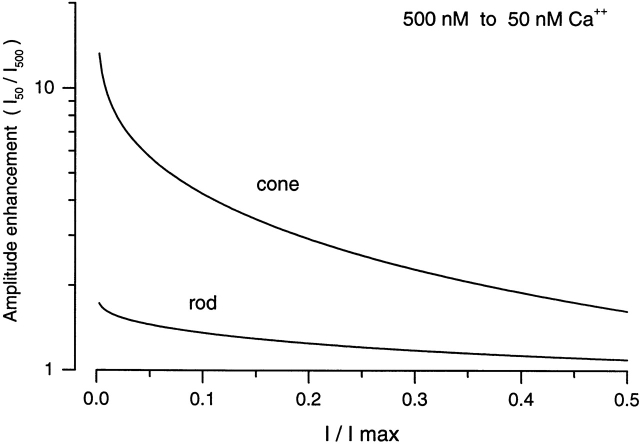
where I lo and I hi are the currents at low and high Ca2+, respectively, [cGMP] is the ligand concentration, K lo and K hi are the values for K 1/2, and nlo and nhi are the values for n at low and high Ca2+, respectively. Results of such calculations for rods and cones are illustrated in Fig. 7 for a change in Ca2+ from 500 to 50 nM. This is the range of decline in Ca2+ concentration between darkness and bright light measured in rod outer segments (Gray-Keller and Detwiler, 1994; McCarthy et al., 1996; Sampath et al., 1998). To compare rods and cones, we converted cGMP concentration to the fraction of the maximum, saturated current in the outer segment (using Eq. 1). This calculation makes quantitatively evident that the effect of channel modulation over the physiologically expected range of Ca2+ changes is much more significant in cones than in rods.
Figure 7.
Computation of the extent of enhancement in the steady state current amplitude as a function of cGMP concentration caused by a fixed fall in cytoplasmic Ca2+. Current enhancement is expressed as a ratio of the current at low over high Ca2+, where low is 50 and high is 500 nM. To compare data for rods and cones, the cGMP concentration, using Eq. 1, is expressed in dimensionless units that correspond to the fraction of the maximum possible current in the outer segment. In the dark and under normal physiological conditions, the standing outer segment current is 1–3% of the maximum possible current in the outer segment of both rods and cones. Shown are predictions for single fish cones and tiger salamander rods based on the data presented in this report. In a dark-adapted rod, 500–50 nM is the likely excursion of cytoplasmic Ca2+ in response to a bright flash (Grey-Keller and Detwiler, 1994; McCarthy et al., 1996; Sampath et al., 1998).
It is also interesting to note that the effectiveness of modulation increases as the probability of channel opening decreases. This observation may provide an evolutionary advantage to the puzzling fact that in photoreceptors the entire transduction signal operates by changing the probability of opening of the cGMP-gated channel from essentially 0 to only 3–5% at its highest value.
Acknowledgments
We thank D. Hackos, R. Hammer, A. Olson, T. Ohyama, A. Picones, D. Schneeweiss, and J. Schnapf for their valuable comments on this manuscript.
Abbreviation used in this paper
- PDE
phosphodiesterase
references
- Balasubramanian S, Lynch JW, Barry PH. Calcium-dependent modulation of the agonist affinity of the mammalian olfactory cyclic nucleotide-gated channel by calmodulin and a novel endogenous factor. J Membr Biol. 1996;152:13–23. doi: 10.1007/s002329900081. [DOI] [PubMed] [Google Scholar]
- Bauer PJ. Cyclic GMP-gated channels of bovine rod photoreceptors: affinity, density and stoichiometry of Ca2+-calmodulin binding sites. J Physiol (Camb) 1996;494:675–685. doi: 10.1113/jphysiol.1996.sp021523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor DA, Lamb TD, Yau KW. Responses of retinal rods to single photons. J Physiol (Camb) 1979;288:613–634. [PMC free article] [PubMed] [Google Scholar]
- Burkhardt DA. Light adaptation and photopigment bleaching in cone photoreceptors in situ in the retina of the turtle. J Neurosci. 1994;14:1091–1105. doi: 10.1523/JNEUROSCI.14-03-01091.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TY, Yau K-W. Direct modulation by Ca2+-calmodulin of cyclic nucleotide-activated channel of rat olfactory receptor neurons. Nature. 1994;368:545–548. doi: 10.1038/368545a0. [DOI] [PubMed] [Google Scholar]
- Fain GL, Matthews HR. Calcium and the mechanism of light adaptation in vertebrate photoreceptors. Trends Neurosci. 1990;13:378–384. doi: 10.1016/0166-2236(90)90023-4. [DOI] [PubMed] [Google Scholar]
- Frings S, Seifert R, Godde M, Kaupp UB. Profoundly different calcium permeation and blockage determine the specific function of distinct cyclic nucleotide-gated channels. Neuron. 1995;15:169–179. doi: 10.1016/0896-6273(95)90074-8. [DOI] [PubMed] [Google Scholar]
- Gillespie PG, Beavo JA. Inhibition and stimulation of photoreceptors phosphodiesterase by dipyridamole and M&B 22,948. Mol Pharmacol. 1989;36:773–781. [PubMed] [Google Scholar]
- Gordon SE, Downing-Park J, Zimmerman AL. Modulation of the cGMP-gated ion channel in frog rods by calmodulin and an endogenous inhibitory factor. J Physiol (Camb) 1995;486:533–546. doi: 10.1113/jphysiol.1995.sp020832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray-Keller MP, Detwiler PB. The calcium feedback signal in the phototransduction cascade of vertebrate rods. Neuron. 1994;13:849–861. doi: 10.1016/0896-6273(94)90251-8. [DOI] [PubMed] [Google Scholar]
- Hackos DH, Korenbrot JI. Calcium modulation of ligand affinity in the cyclic GMP-gated ion channels of cone photoreceptors. J Gen Physiol. 1997;110:515–528. doi: 10.1085/jgp.110.5.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes LW, Stotz SC. Modulation of rod, but not cone, cGMP-gated photoreceptor channel by calcium-calmodulin. Vis Neurosci. 1997;14:233–239. doi: 10.1017/s0952523800011378. [DOI] [PubMed] [Google Scholar]
- Hestrin S, Korenbrot JI. Activation kinetics of retinal cones and rods: response to intense flashes of light. J Neurosci. 1990;10:1967–1973. doi: 10.1523/JNEUROSCI.10-06-01967.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YT, Molday RS. Modulation of the cGMP-gated channel of rod photoreceptor cells by calmodulin. Nature. 1993;361:76–79. doi: 10.1038/361076a0. [DOI] [PubMed] [Google Scholar]
- Kawamura S, Murakami M. Calcium-dependent regulation of cyclic GMP phosphodiesterase by a protein from frog retinal rods. Nature. 1991;349:420–423. doi: 10.1038/349420a0. [DOI] [PubMed] [Google Scholar]
- Koch KW, Stryer L. Highly cooperative feedback control of retinal rod guanylate cyclase by calcium ions. Nature. 1988;334:64–66. doi: 10.1038/334064a0. [DOI] [PubMed] [Google Scholar]
- Korenbrot JI. Ca2+ flux in retinal rod and cone outer segments: differences in Ca2+ selectivity of the cGMP-gated ion channels and Ca2+clearance rates. Cell Calcium. 1995;18:285–300. doi: 10.1016/0143-4160(95)90025-x. [DOI] [PubMed] [Google Scholar]
- Koutalos Y, Yau K-W. Regulation of sensitivity in vertebrate rod photoreceptors by calcium. Trends Neurosci. 1996;19:73–81. doi: 10.1016/0166-2236(96)89624-x. [DOI] [PubMed] [Google Scholar]
- Kramer RH, Siegelbaum SA. Intracellular Ca2+regulates the sensitivity of cyclic nucleotide-gated channels in olfactory receptor neurons. Neuron. 1992;9:897–906. doi: 10.1016/0896-6273(92)90242-6. [DOI] [PubMed] [Google Scholar]
- Kurahashi T, Menini A. Mechanism of odorant adaptation in the olfactory receptor cell. Nature. 1997;385:725–729. doi: 10.1038/385725a0. [DOI] [PubMed] [Google Scholar]
- Lagnado L, Baylor DA. Calcium controls light-triggered formation of catalytically active rhodopsin. Nature. 1994;367:273–277. doi: 10.1038/367273a0. [DOI] [PubMed] [Google Scholar]
- Liu M, Chen TY, Ahamed B, Li J, Yau K-W. Calcium-calmodulin modulation of the olfactory cyclic nucleotide-gated cation channel. Science. 1994;266:1348–1354. doi: 10.1126/science.266.5189.1348. [DOI] [PubMed] [Google Scholar]
- Lolley RM, Racz E. Ca2+modulation of cyclic GMP synthesis in rat visual cells. Vision Res. 1982;22:1481–1486. doi: 10.1016/0042-6989(82)90213-9. [DOI] [PubMed] [Google Scholar]
- Lynch JW, Lindermann B. Cyclic nucleotide-gated channels of rat olfactory receptor cells: divalent cations control the sensitivity to cAMP. J Gen Physiol. 1994;103:87–106. doi: 10.1085/jgp.103.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malchow RP, Yazulla S. Separation and light adaptation of rod and cone signals in the retina of the goldfish. Vision Res. 1986;26:1655–1666. doi: 10.1016/0042-6989(86)90053-2. [DOI] [PubMed] [Google Scholar]
- McCarthy ST, Younger PJ, Owen WG. Dynamic, spatially nonuniform calcium regulation in frog rods exposed to light. J Neurophysiol. 1996;76:1991–2004. doi: 10.1152/jn.1996.76.3.1991. [DOI] [PubMed] [Google Scholar]
- McNaughton PA, Cervetto L, Nunn BJ. Measurements of the intracellular free calcium concentration in salamander rods. Nature. 1986;322:261–263. doi: 10.1038/322261a0. [DOI] [PubMed] [Google Scholar]
- Miller JL, Korenbrot JI. In retinal cones, membrane depolarization in darkness activates the cGMP-dependent conductance. A model of Ca homeostasis and the regulation of guanylate cyclase. J Gen Physiol. 1993a;101:933–960. doi: 10.1085/jgp.101.6.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JL, Korenbrot JI. Phototransduction and adaptation in rods, single cones, and twin cones of the striped bass retina: a comparative study. Vis Neurosci. 1993b;10:653–667. doi: 10.1017/s0952523800005356. [DOI] [PubMed] [Google Scholar]
- Miller JL, Korenbrot JI. Differences in calcium homeostasis between retinal rod and cone photoreceptors revealed by the effects of voltage on the cGMP-gated conductance in intact cells. J Gen Physiol. 1994;104:909–940. doi: 10.1085/jgp.104.5.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JL, Picones A, Korenbrot JI. Differences in transduction between rod and cone photoreceptors: an exploration of the role of calcium homeostasis. Curr Opin Neurobiol. 1994;4:488–495. doi: 10.1016/0959-4388(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Molday RS. Calmodulin regulation of cyclic-nucleotide-gated channels. Curr Opin Neurobiol. 1996;6:445–452. doi: 10.1016/s0959-4388(96)80048-1. [DOI] [PubMed] [Google Scholar]
- Nakatani K, Koutalos Y, Yau KW. Ca2+modulation of the cGMP-gated channel of bullfrog retinal rod photoreceptors. J Physiol (Camb) 1995;484:69–76. doi: 10.1113/jphysiol.1995.sp020648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normann RA, Perlman I. The effects of background illumination on the photoresponses of red and green cones. J Physiol (Camb) 1979;286:491–507. doi: 10.1113/jphysiol.1979.sp012633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normann RA, Werblin FS. Control of retinal sensitivity. I: Light and dark adaptation of vertebrate rods and cones. J Gen Physiol. 1974;63:37–61. doi: 10.1085/jgp.63.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepe IM, Panfoli I, Cugnoli C. Guanylate cyclase in rod outer segments of the toad retina. Effect of light and Ca2+ . FEBS Lett. 1986;203:73–76. doi: 10.1016/0014-5793(86)81439-9. [DOI] [PubMed] [Google Scholar]
- Perry RJ, McNaughton PA. Response properties of cones of the retina of the tiger salamander. J Physiol (Camb) 1991;433:561–587. doi: 10.1113/jphysiol.1991.sp018444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picones A, Korenbrot JI. Permeation and interaction of monovalent cations with the cGMP-gated channel of cone photoreceptors. J Gen Physiol. 1992;100:647–673. doi: 10.1085/jgp.100.4.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picones A, Korenbrot JI. Permeability and interaction of Ca2+with cGMP-gated ion channels differ in retinal rod and cone photoreceptors. Biophys J. 1995;69:120–127. doi: 10.1016/S0006-3495(95)79881-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagoo MS, Lagnado L. The action of cytoplasmic calcium on the cGMP-activated channel in salamander rod photoreceptors. J Physiol (Camb) 1996;497:309–319. doi: 10.1113/jphysiol.1996.sp021770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath AP, Matthews HR, Cornwall MC, Fain GL. Bleached pigment produces a maintained decrease in outer segment Ca2+in salamander rods. J Gen Physiol. 1998;111:53–64. doi: 10.1085/jgp.111.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapley, R., and C. Enroth-Cugell. 1984. Visual adaptation and retinal gain control. In Progress in Retinal Research. N.N. Osborne and C.T. Chader, editors. Pergamon Press, Oxford, UK. 263–346.
- Yau K-W, Nakatani K. Light-induced reduction of cytoplasmic free Ca2+in retinal rod outer segments. Nature. 1985;313:579–582. doi: 10.1038/313579a0. [DOI] [PubMed] [Google Scholar]
- Zagotta WN, Siegelbaum SA. Structure and function of cyclic nucleotide–gated channels. Annu Rev Neurosci. 1996;19:235–263. doi: 10.1146/annurev.ne.19.030196.001315. [DOI] [PubMed] [Google Scholar]
- Zimmerman AL, Yamanaka G, Eckstein F, Baylor DA, Stryer L. Interaction of hydrolysis-resistant analogs of cyclic GMP with the phosphodiesterase and light-sensitive channel of retinal rod outer segments. Proc Natl Acad Sci USA. 1985;82:8813–8817. doi: 10.1073/pnas.82.24.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]