Abstract
To study the mechanism by which Ca2+, which enters during the odor response, is extruded during response recovery, recordings were made from isolated frog olfactory receptor cells using the suction pipette technique, while superfusing the olfactory cilia with solutions of modified ionic composition. When external Na+ was substituted with another cation, the response to odor was greatly prolonged. This prolongation of the response was similar irrespective of whether Na+ was replaced with Li+, which permeates the cyclic nucleotide-gated conductance, or choline, which does not. The prolonged current was greatly reduced by exposure to 300 μM niflumic acid, a blocker of the calcium-activated chloride channel, indicating that it is carried by this conductance, and abolished if Ca2+ was omitted from the external solution, demonstrating that Ca2+ influx is required for its generation. When the cilia were exposed to Na+-free solution after odor stimulation, the recovery of the response to a second stimulus from the adaptation induced by the first was greatly reduced. We conclude that a Na+-dependent Ca2+ extrusion mechanism is present in frog olfactory cilia and that it serves as the main mechanism that returns cytoplasmic Ca2+ concentration to basal levels after stimulation and mediates the normally rapid recovery of the odor response and the restoration of sensitivity after adaptation.
Keywords: olfactory receptor, calcium, adaptation
introduction
Amphibian olfactory receptor cells respond to odor stimulation with an inward receptor current (Firestein and Werblin, 1989; Kurahashi, 1989), and the mechanisms underlying their generation are now quite well understood. Binding of an odor molecule to a receptor in the ciliary membrane activates adenylyl cyclase via a G-protein-coupled cascade (Reed, 1992; Breer, 1994; Dionne and Dubin, 1994; Ache and Zhainazarov, 1995). The ensuing increase in intracellular cAMP opens cyclic nucleotide-gated channels (Nakamura and Gold, 1987; Firestein et al., 1991) through which Ca2+ enters (Zufall and Firestein, 1993; Frings et al., 1995; Leinders-Zufall et al., 1997), leading to additional inward current (Kurahashi and Yau, 1993; Lowe and Gold, 1993) through a Ca2+-activated Cl− conductance (Kleene and Gesteland, 1991). However, the subsequent processes that cause termination of the response remain largely unclear. In particular, the means by which the intracellular Ca2+ concentration is reduced to prestimulus levels, which allow the Ca2+-activated Cl− conductance to close, is not known.
We have used the suction pipette technique combined with rapid external solution changes to study the role of Na+ in response termination and adaptation in isolated frog olfactory receptor cells. The results obtained demonstrate the presence of a Na+-dependent Ca2+ extrusion mechanism in olfactory cilia and indicate that it is responsible for returning intracellular Ca2+ to resting levels after odor stimulation.
methods
Preparation
Frogs (Rana temporaria) were killed by rostral and caudal pithing. The olfactory epithelium was dissected and placed receptor side up on a layer of cured silicone rubber (Sylgard 184; Dow Corning, Wiesbaden, Germany) in a petri dish filled with Ringer solution. Olfactory receptor cells were mechanically isolated by lightly cutting the olfactory epithelium with a piece of razor blade. The dissociated cells were collected with a 200-μl pipette and transferred to the recording chamber on the stage of an inverted microscope with phase contrast optics (TMS; Nikon, Kingston, UK). Cells were allowed to settle on the floor of the recording chamber for 30 min before bath perfusion commenced.
Electrical Recording
The suction pipette technique was used to record odor-induced electrical responses (Baylor et al., 1979; Lowe and Gold, 1991). The cell body of an isolated olfactory receptor cell was drawn into a suction pipette, leaving the cilia exposed to the superfusing solution. After their isolation, olfactory receptor cells rounded progressively and the dendrite retracted, as has also been observed by others (Dubin and Dionne, 1994). Consequently, virtually the entire cell could be sucked into the suction pipette so that only the cilia were accessible to solution changes in the bath. The current signal was recorded with a patch clamp amplifier (Warner PC501; Warner Instruments, Hamden, CT) and low-pass filtered at 20 Hz to record only the receptor current without the fast biphasic current spikes corresponding to action potentials, which are also collected by the suction pipette. The low-pass filtered current signal was digitized continuously for subsequent analysis at a sampling rate of 100 Hz using an IBM-compatible microcomputer equipped with an intelligent interface card (Cambridge Research Systems, Rochester, UK).
Rapid Solution Changes
Rapid solution changes for odor stimulation or exchange of the external solution were effected by translating the interface between two flowing streams of solution across the exposed cilia using a computer-controlled stepper motor coupled to the microscope stage (Hodgkin et al., 1985; Matthews, 1995). Streams of solution emerged from up to four parallel tubes built into the recording chamber. Solutions were delivered by gravity, and selected by inert six-way rotary valves (Rheodyne, Cotati, CA). Recordings have been corrected for the junction currents arising between solutions of different ionic composition by subtraction of records obtained in the absence of odor stimulation.
External Solutions
Ringer solution contained (mM) 111 NaCl, 2.5 KCl, 1.6 MgCl2, 1 CaCl2, 0.01 EDTA, 3 HEPES, pH 7.7 with NaOH. Li+- and choline+-substituted solutions contained 111 mM LiCl or choline-Cl instead of NaCl; ∼2 mM NaOH was added to adjust the pH to 7.7. 0 Na+, 1 μM Ca2+ solution contained 2 mM EDTA to buffer Ca2+, NaCl was replaced by choline-Cl, and pH was adjusted to 7.7 with choline hydroxide. All solutions also contained 10 mM glucose. 300 μM niflumic acid (Sigma Chemical Co., Poole, UK) was added to the choline+-substituted solution when required, and the pH was readjusted to 7.7. Odor solutions were made daily by a single dilution from a stock solution of appropriate ionic composition containing 1 mM cineole.
results
Fig. 1 shows the effect of replacing external Na+ with another cation in the solution bathing the cilia of an isolated olfactory receptor cell immediately after stimulation at three different odor concentrations. When the cell was stimulated with odor for 1 s in Ringer solution, the receptor current rose after a short delay and returned to zero rapidly after stimulation (Fig. 1, Control). But when the cell was instead exposed immediately after the odor stimulus to solutions in which Na+ had been replaced by another cation (Fig. 1, Li + and Cho +), the response did not terminate after stimulation. Instead, the receptor current remained elevated for an extended period, declining only slowly during the 5-s exposure to low Na+ solution and not falling to baseline levels until after the cell was returned to Na+-containing Ringer solution. In contrast, exposing the cell to Na+-free solution for 5 s immediately before stimulation in Ringer solution did not affect the subsequent odor-induced response (not shown). The contribution of the cyclic nucleotide-gated conductance to this prolonged current was probed by replacing Na+ with either Li+, which permeates the amphibian cyclic nucleotide-gated channel, or choline+, which does not (Kurahashi, 1990). The prolonged currents recorded in these two solutions were remarkably similar in time course and magnitude (Fig. 1, Li + and Cho +). Furthermore, at all three odor concentrations, the initial value of the receptor current in Li+- and choline+-substituted solutions was the same as that in Ringer solution at the time of the solution change, indicating that the current through the cyclic nucleotide-gated conductance must have declined nearly to zero by that time. Similar results were observed in a total of 13 cells.
Figure 1.
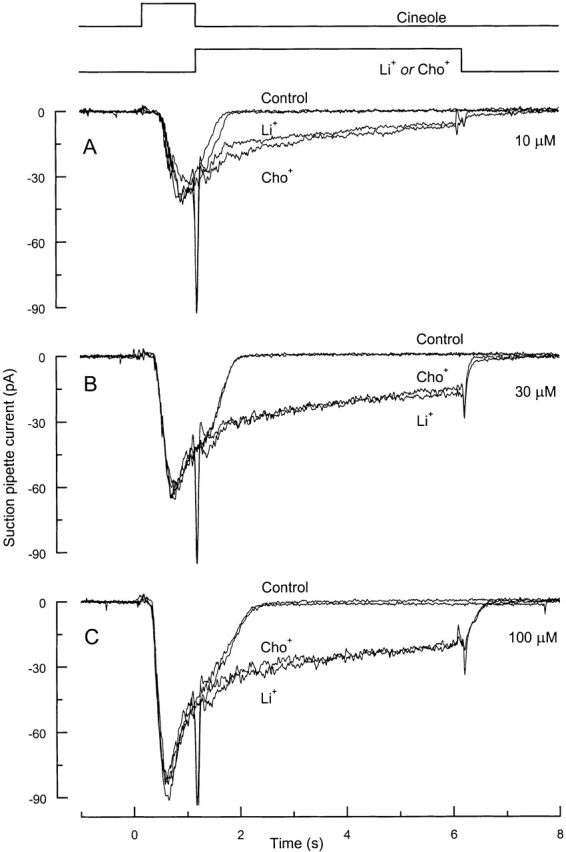
The odor-induced response can only terminate in the presence of external Na+. Control responses were generated by stimulating an olfactory receptor cell with (A) 10, (B) 30, or (C) 100 μM cineole in Ringer solution for 1 s. Traces Li+ and Cho+ were obtained by exposing the cell immediately after odor stimulation to solutions that contained LiCl or choline-Cl instead of NaCl. The two control responses were recorded before and after those in Li+- and choline+-substituted solutions.
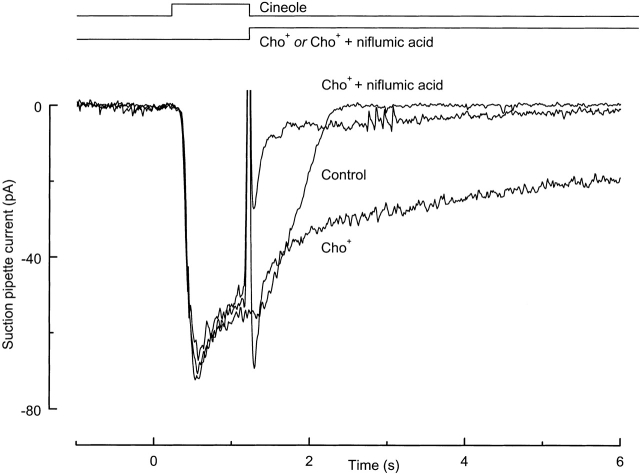
The persistence of the prolonged current in the absence of external cations that permeate the cyclic nucleotide-gated channel indicates that most of the prolonged current must be carried not by this channel but through some other conductance. An obvious candidate for this conductance is the Ca2+-activated Cl− channel. Its contribution to the prolonged current is investigated in Fig. 2 by exposing an isolated olfactory receptor cell to niflumic acid, which blocks the Ca2+-activated Cl− conductance but not the cAMP-gated conductance in these cells (Kleene, 1993). The cell was first stimulated with odor for 1 s in Ringer solution, yielding a response that terminated rapidly after the end of stimulation (Fig. 2, Control). When the cell was exposed after stimulation to choline+-substituted solution instead of Ringer solution, a prolonged current resulted, as in Fig. 1 (Cho +). But if 300 μM niflumic acid was included in the choline+-substituted solution, the amplitude of the prolonged current was greatly reduced (Fig. 2, Cho + + niflumic acid). Similar results were obtained from a total of 10 cells for which niflumic acid reduced the prolonged current after 1 s in choline+-substituted solution to 25 ± 4% (mean ± SEM) of its value in the absence of the blocker. Therefore, most of the prolonged current that was observed under Na+-free conditions must have flowed through the Ca2+-activated Cl− conductance. Since this conductance can only remain open while the intracellular Ca2+ concentration remains elevated, these results indicate that the intracellular Ca2+ concentration, which increases during stimulation (Kurahashi and Yau, 1993; Lowe and Gold, 1993), must have largely been prevented from falling during exposure to the low-Na+ solution.
Figure 2.
The prolonged current response generated in choline+-substituted solution is carried by the Ca2+-activated Cl− conductance. Control was recorded in response to a 1-s stimulation with 100 μM cineole. Trace Cho+ was obtained by exposure to choline+-substituted solution immediately after stimulation. The addition of 300 μM niflumic acid to the post-stimulus solution reduced the prolonged current after 1 s in the choline+-substituted solution to 15% of its value in the absence of the blocker. Current traces are the average of either two or three trials.
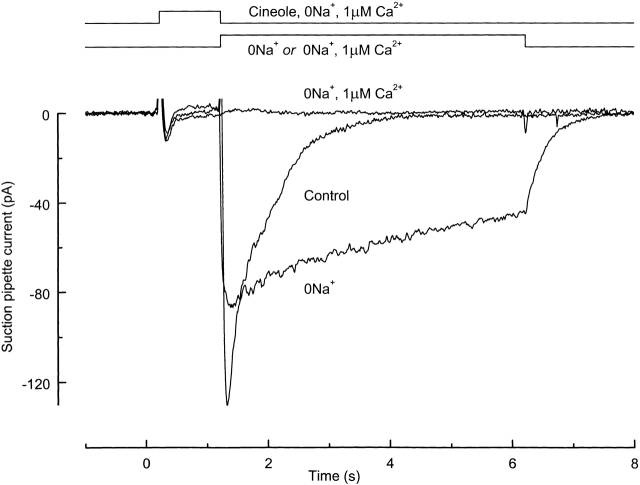
The involvement of Ca2+ in the activation of this prolonged current was substantiated by stimulating an olfactory receptor cell in a 0 Na+, 1 μM Ca2+ solution designed to prevent also the odor-induced influx of Ca2+ (Fig. 3). In this particular case, no current was evoked during the 1-s odor stimulus, presumably because no cations were present that could carry a significant inward current through the cyclic nucleotide-gated channel under these conditions (Kleene and Pun, 1996). If the cell was returned to Ringer solution thereafter (Fig. 3, Control), a large but rapidly decaying current was recorded, reflecting the transient influx through the cyclic nucleotide-gated conductance of both Na+ and Ca2+, and the opening of Ca2+-activated Cl− channels. But if the cell was instead exposed after stimulation to a solution in which choline+ had been substituted for Na+, a prolonged current was generated, which was presumably induced by influx of Ca2+ after the solution change, and which only terminated once Na+ was returned to the external solution (Fig. 3, 0Na +). However, if the concentration of Ca2+ in this Na+-free solution was reduced to 1 μM, as during stimulation, no current whatsoever was recorded (Fig. 3, 0Na + , 1μM Ca2 +). These observations are consistent with the notion that the prolonged current results from the opening of Ca2+-activated Cl− channels by the influx of Ca2+, whose efflux appears to be greatly reduced in the absence of external Na+. Interestingly, even higher odor concentrations elicited excitatory responses even in 0 Na+, 1 μM Ca2+ solution, which could only be abolished by the further removal of Mg2+ and the remaining Ca2+ from the external solution, suggesting that Mg2+ might also be capable of eliciting excitatory currents when the external Ca2+ concentration is greatly reduced. Similar results were obtained in a total of eight cells, from three of which no current could be evoked in 0 Na+, 1 μM Ca2+ solution by a 1-s odor stimulus of intermediate odor concentration.
Figure 3.
The prolonged current requires the influx of external Ca2+. An olfactory receptor cell was stimulated with 100 μM cineole in 0 Na+, 1 μM Ca2+ solution for 1 s. After stimulation, the cells were either returned to Ringer solution (Control) or exposed for 5 s to Na+-free solution containing 1 mM Ca2+ (0Na +) or 1 μM Ca2+ (0Na + , 1μM Ca2+), in which choline+ had been substituted for Na+. Current traces are the average of two trials.
It is widely accepted that an increase in intracellular Ca2+ concentration mediates the onset of olfactory adaptation (Kurahashi and Shibuya, 1990; Kurahashi and Menini, 1997; Leinders-Zufall et al., 1998). Since the removal of external Na+ appears to retard the subsequent decline in Ca2+ concentration, we have examined the effect of the removal of external Na+ on the recovery from adaptation after odor stimulation. Adaptation was investigated by exposing an olfactory receptor cell to two successive odor stimuli and varying the recovery interval between them (Kurahashi and Shibuya, 1990; Kurahashi and Menini, 1997; Leinders-Zufall et al., 1998). Fig. 4, A and C, shows an example of such a procedure under control conditions in Ringer solution for two different odor concentrations. When the interval between the two stimuli was ∼10 s, the response to the second pulse was of nearly the same amplitude as that evoked by the first. However, as the recovery interval was reduced, the magnitude of the second response became progressively smaller, indicating that a greater proportion of the adaptation induced by the first stimulus remained at the time of the second. The higher odor concentration (Fig. 4 C) yielded a qualitatively similar effect to the lower (Fig. 4 A), but with a smaller relative reduction in the amplitude of the second response.
Figure 4.
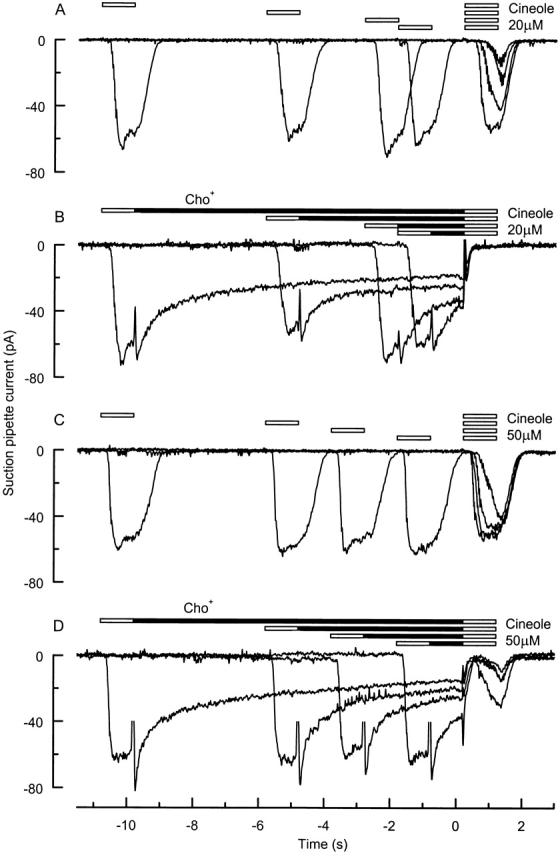
Removal of external Na+ prevents recovery from adaptation. The recovery of an olfactory receptor cell from adaptation was studied by varying the recovery interval between two successive 1-s odor stimuli of 20 (A and B) or 50 (C and D) μM cineole. The recovery interval between the two stimuli was either spent in Ringer solution (A and C) or in choline+-substituted solution (B and D). Current traces are the average of two trials. The largest response to the second stimulus in Fig. 4 D corresponds to the shortest recovery interval, possibly reflecting summation of the two stimuli.
When the same experiment was repeated but the cell exposed to choline+-substituted solution instead of Ringer solution during the recovery interval between the two-odor stimuli, a different picture emerged (Fig. 4, B and D). As was seen above, exposure to low-Na+ solution after stimulation prolonged the receptor current. However, when the cell was stimulated for the second time, either no response (Fig. 4 B, 20 μM cineole) or only a greatly reduced response (Fig. 4 D, 50 μM cineole) was generated, irrespective of the recovery interval. Similar results were obtained from a further seven cells, indicating that exposure to the choline+-substituted solution between the two stimuli prevented the normal recovery from adaptation.
discussion
When Na+ was replaced by another cation in the solution bathing the olfactory cilia after odor stimulation in Ringer solution, the odor response was greatly prolonged. This result indicates that external Na+ is required for the normal rapid termination of the odor response, and that neither Li+ nor choline+ can substitute in this process. The presence of a prolonged response in the virtual absence of monovalent cations that permeate the cyclic nucleotide-gated conductance, and its sensitivity to niflumic acid, demonstrate that the prolonged current that underlies it is carried by the Ca2+-activated Cl− conductance. Since this prolonged current was only evoked when Ca2+ was included in the bathing solution during or immediately after odor stimulation, the activation of this conductance must have resulted from Ca2+ influx through the cyclic nucleotide-gated conductance, and the ensuing elevation of intracellular Ca2+ concentration. The persistent activation of the Ca2+-activated Cl− conductance during the exposure to the low-Na+ solution thus indicates that the intracellular Ca2+ concentration must have remained elevated for an extended period under these conditions. We therefore conclude that a Na+-dependent Ca2+ extrusion mechanism is present in frog olfactory cilia and that it normally serves as the main mechanism that returns the intracellular Ca2+ concentration to basal levels after odor stimulation.
Na+–Ca2+ exchange has been suggested to be present in the dendrite of Xenopus (Jung et al., 1994) and possibly in the cilia of rat (Noe et al., 1997) olfactory receptor cells. In other systems, Na+–Ca2+ exchange exhibits a strict requirement for Na+, which cannot be fulfilled by other cations (Reuter and Seitz, 1969; Blaustein and Russell, 1975; Yau and Nakatani, 1984). Our results thus provide the first functional demonstration of a role for Na+–Ca2+ exchange in shaping the odor response of olfactory receptor cells. The question as to whether K+ is also involved in Ca2+ extrusion in olfactory receptor cells, as has been shown in photoreceptors (Cervetto et al., 1989; Schnetkamp et al., 1989), remains to be investigated. The observation that the receptor current did nonetheless decline gradually during exposure to low-Na+ solutions implies that other quantitatively less significant mechanisms of Ca2+ removal are likely also to be present in the olfactory receptor. These might include diffusion of Ca2+ into the cell body (but see Leinders-Zufall et al., 1997) or a Ca2+-ATPase (Lo et al., 1994).
Exposure to low-Na+ solution also prevented recovery from olfactory adaptation after stimulation in Ringer solution. Since this low-Na+ solution will have prevented the extrusion by Na+–Ca2+ exchange of the Ca2+ that entered during the first response, it can therefore be concluded that Na+–Ca2+ exchange also plays a major role in restoring olfactory receptor cell sensitivity after stimulation by returning intracellular Ca2+ concentration to basal levels. It may also contribute to the oscillatory response pattern observed in the majority of frog olfactory receptor cells during prolonged odor stimulation, which is slowed by exposure to low-Na+ solution (Reisert and Matthews, 1997), and which may represent a coupled oscillation of cyclic nucleotide and Ca2+ concentrations (Cooper et al., 1995).
Acknowledgments
We are grateful to Dr. G.L. Fain for helpful comments on the manuscript.
This work was supported by the Wellcome Trust, and by a Medical Research Council Research Studentship (to J. Reisert).
Footnotes
Portions of this work were previously published in abstract form [Reisert, J., and H.R. Matthews. 1997. J. Physiol. (Camb.). 504:125P].
references
- Ache BW, Zhainazarov A. Dual second messenger pathways in olfactory transduction. Curr Opin Neurobiol. 1995;5:461–466. doi: 10.1016/0959-4388(95)80006-9. [DOI] [PubMed] [Google Scholar]
- Baylor DA, Lamb TD, Yau K-Y. The membrane current of single rod outer segments. J Physiol (Camb) 1979;288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Blaustein MP, Russell JM. Sodium–calcium exchange and calcium–calcium exchange in internally dialyzed squid giant axons. J Membr Biol. 1975;22:285–312. doi: 10.1007/BF01868176. [DOI] [PubMed] [Google Scholar]
- Breer H. Odor recognition and second messenger signaling in olfactory receptor neurons. Semin Cell Biol. 1994;5:25–32. doi: 10.1006/scel.1994.1004. [DOI] [PubMed] [Google Scholar]
- Cervetto L, Lagnado L, Perry RJ, Robinson DW, McNaughton PA. Extrusion of calcium from rod outer segments is driven by both sodium and potassium gradients. Nature. 1989;337:740–743. doi: 10.1038/337740a0. [DOI] [PubMed] [Google Scholar]
- Cooper DMF, Mons N, Karpen JW. Adenylyl cyclases and the interaction between calcium and cAMP signaling. Nature. 1995;374:421–424. doi: 10.1038/374421a0. [DOI] [PubMed] [Google Scholar]
- Dionne VE, Dubin AE. Transduction diversity in olfaction. J Exp Biol. 1994;194:1–21. doi: 10.1242/jeb.194.1.1. [DOI] [PubMed] [Google Scholar]
- Dubin AE, Dionne VE. Action potentials and chemosensitive conductances in the dendrites of olfactory neurons suggest new features for odor transduction. J Gen Physiol. 1994;103:181–201. doi: 10.1085/jgp.103.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein S, Werblin F. Odor-induced membrane currents in vertebrate olfactory receptor neurons. Science. 1989;244:79–82. doi: 10.1126/science.2704991. [DOI] [PubMed] [Google Scholar]
- Firestein S, Zufall F, Shepherd GM. Single odor-sensitive channels in olfactory receptor neurons are also gated by cyclic nucleotides. J Neurosci. 1991;11:3565–3572. doi: 10.1523/JNEUROSCI.11-11-03565.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frings S, Seifert R, Godde M, Kaupp UB. Profoundly different calcium permeation and blockage determine the specific function of distinct cyclic nucleotide-gated channels. Neuron. 1995;15:169–179. doi: 10.1016/0896-6273(95)90074-8. [DOI] [PubMed] [Google Scholar]
- Hodgkin AL, McNaughton PA, Nunn BJ. The ionic selectivity and calcium dependence of the light-sensitive pathway in toad rods. J Physiol (Camb) 1985;358:447–468. doi: 10.1113/jphysiol.1985.sp015561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung A, Lischka FW, Engel J, Schild D. Sodium/calcium exchanger in olfactory receptor neurons of Xenopus laevis. . Neuroreport. 1994;5:1741–1744. doi: 10.1097/00001756-199409080-00013. [DOI] [PubMed] [Google Scholar]
- Kleene SJ. Origin of the chloride current in olfactory transduction. Neuron. 1993;11:123–132. doi: 10.1016/0896-6273(93)90276-w. [DOI] [PubMed] [Google Scholar]
- Kleene SJ, Gesteland RC. Calcium-activated chloride conductance in frog olfactory cilia. J Neurosci. 1991;11:3624–3629. doi: 10.1523/JNEUROSCI.11-11-03624.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleene SJ, Pun RYK. Persistence of the olfactory receptor current in a wide variety of extracellular environments. J Neurophysiol. 1996;75:1386–1391. doi: 10.1152/jn.1996.75.4.1386. [DOI] [PubMed] [Google Scholar]
- Kurahashi T. Activation by odorants of cation-selective conductance in the olfactory receptor cell isolated from the newt. J Physiol (Camb) 1989;419:177–192. doi: 10.1113/jphysiol.1989.sp017868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurahashi T. The response induced by intracellular cyclic AMP in isolated olfactory receptor cells of the newt. J Physiol (Camb) 1990;430:355–371. doi: 10.1113/jphysiol.1990.sp018295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurahashi T, Menini A. Mechanism of odorant adaptation in the olfactory receptor cell. Nature. 1997;385:725–729. doi: 10.1038/385725a0. [DOI] [PubMed] [Google Scholar]
- Kurahashi T, Shibuya T. Ca2+-dependent adaptive properties in the solitary olfactory receptor cell of the newt. Brain Res. 1990;515:261–268. doi: 10.1016/0006-8993(90)90605-b. [DOI] [PubMed] [Google Scholar]
- Kurahashi T, Yau K-W. Co-existence of cationic and chloride components in odorant-induced current of vertebrate olfactory receptor cells. Nature. 1993;363:71–74. doi: 10.1038/363071a0. [DOI] [PubMed] [Google Scholar]
- Leinders-Zufall T, Rand MN, Shepherd GM, Greer CA, Zufall F. Calcium entry through cyclic nucleotide-gated channels in individual cilia of olfactory receptor cells: spatiotemporal dynamics. J Neurosci. 1997;17:4136–4148. doi: 10.1523/JNEUROSCI.17-11-04136.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinders-Zufall T, Greer CA, Shepherd GM, Zufall F. Imaging odor-induced calcium transients in single olfactory cilia: specificity of activation and role in transduction. J Neurosci. 1998;18:5630–5639. doi: 10.1523/JNEUROSCI.18-15-05630.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo YH, Bradley TM, Rhoads DE. High-Affinity Ca2+,Mg2+-ATPase in plasma membrane-rich preparations from olfactory epithelium of Atlantic salmon. Biochim Biophys Acta. 1994;1192:153–158. doi: 10.1016/0005-2736(94)90113-9. [DOI] [PubMed] [Google Scholar]
- Lowe G, Gold GH. The spatial distributions of odorant sensitivity and odorant-induced currents in salamander olfactory receptor cells. J Physiol (Camb) 1991;442:147–168. doi: 10.1113/jphysiol.1991.sp018787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe G, Gold GH. Nonlinear amplification by calcium-dependent chloride channels in olfactory receptor cells. Nature. 1993;366:283–286. doi: 10.1038/366283a0. [DOI] [PubMed] [Google Scholar]
- Matthews HR. Effects of lowered cytoplasmic calcium concentration and light on the responses of salamander rod photoreceptors. J Physiol (Camb) 1995;484:267–286. doi: 10.1113/jphysiol.1995.sp020664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Gold GH. A cyclic nucleotide-gated conductance in olfactory receptor cilia. Nature. 1987;325:442–444. doi: 10.1038/325442a0. [DOI] [PubMed] [Google Scholar]
- Noe J, Tareilus E, Boekhoff I, Breer H. Sodium/calcium exchanger in rat olfactory neurons. Neurochem Int. 1997;30:523–531. doi: 10.1016/s0197-0186(96)00090-3. [DOI] [PubMed] [Google Scholar]
- Reed RR. Signaling pathways in odorant detection. Neuron. 1992;8:205–209. doi: 10.1016/0896-6273(92)90287-n. [DOI] [PubMed] [Google Scholar]
- Reisert J, Matthews HR. Effects of sodium removal on the oscillatory response to prolonged stimulation in frog olfactory receptor cells. J Physiol (Camb) 1997;499:88P. [Google Scholar]
- Reuter H, Seitz N. The dependence of calcium efflux from cardiac muscle on temperature and external ion composition. J Physiol (Camb) 1969;195:451–470. doi: 10.1113/jphysiol.1968.sp008467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnetkamp PP, Basu DK, Szerencsei RT. Na+–Ca2+ exchange in bovine rod outer segments requires and transports K+ . Am J Physiol. 1989;257:C153–C157. doi: 10.1152/ajpcell.1989.257.1.C153. [DOI] [PubMed] [Google Scholar]
- Yau K-W, Nakatani K. Electrogenic Na-Ca exchange in retinal rod outer segment. Nature. 1984;311:661–663. doi: 10.1038/311661a0. [DOI] [PubMed] [Google Scholar]
- Zufall F, Firestein S. Divalent cations block the cyclic nucleotide-gated channel of olfactory receptor neurons. J Neurophysiol. 1993;69:1758–1768. doi: 10.1152/jn.1993.69.5.1758. [DOI] [PubMed] [Google Scholar]