Abstract
Basic electrophysiological properties of the KcsA K+ channel were examined in planar lipid bilayer membranes. The channel displays open-state rectification and weakly voltage-dependent gating. Tetraethylammonium blocking affinity depends on the side of the bilayer to which the blocker is added. Addition of Na+ to the trans chamber causes block of open-channel current, while addition to the cis side has no effect. Most striking is the activation of KcsA by protons; channel activity is observed only when the trans bilayer chamber is at low pH. To ascertain which side of the channel faces which chamber, residues with structurally known locations were mapped to defined sides of the bilayer. Mutation of Y82, an external residue, results in changes in tetraethylammonium affinity exclusively from the cis side. Channels with cysteine residues substituted at externally exposed Y82 or internally exposed Q119 are functionally modified by methanethiosulfonate reagents from the cis or trans chambers, respectively. Block by charybdotoxin, known to bind to the channel's external mouth, is observed only when the toxin is added to the cis side of channels mutated to be toxin sensitive. These results demonstrate unambiguously that the protonation sites linked to gating are on the intracellular portion of the KcsA protein.
Keywords: potassium channel, permeation, gating, block
INTRODUCTION
The high-resolution structure of the KcsA K+ channel has invigorated current approaches to the molecular foundations of cellular electrical excitability (Doyle et al. 1998). KcsA is a prokaryotic channel with little sequence similarity to eukaryotic K+ channels except in the pore-forming region. However, its structure provides compelling explanations for ion permeation and gating phenomena observed over many years in a multitude of K+ channels. Ironically, functional properties of KcsA have been described only in outline. Single-channel recording, flux measurements, and ligand-binding assays have shown KcsA to be a high-conductance, tetrameric, K+-selective channel with an externally located receptor site for charybdotoxin-family peptides (Schrempf et al. 1995; Cortes and Perozo 1997; Heginbotham et al. 1997, Heginbotham et al. 1998; MacKinnon et al. 1998). While its structure is largely in harmony with models of familiar K+ channels, an unexpected characteristic of KcsA is its gating by protons (Cuello et al. 1998; Perozo et al. 1998). The channel reconstituted into planar bilayer membranes opens significantly only at pH values lower than ∼5. Despite the fact that most of the protein's water-exposed mass, with fully 85% of its dissociable protons, is located on the cytoplasmic side of the membrane, proteolysis protection studies have led to the contention that activating protons are sensed on the channel's external side (Cuello et al. 1998; Perozo et al. 1999).
As a prelude to a full ion selectivity study of KcsA, we sought to establish a planar lipid bilayer system in which single purified KcsA channels may be recorded accurately and to survey several basic pore properties of the channel. Single KcsA channels can be observed at 5 kHz bandwidth in a low-noise planar bilayer system. We document functionally asymmetric characteristics of KcsA and use several of these to show that protons gate this channel from the cytoplasmic, not the external, side of the membrane.
MATERIALS AND METHODS
Materials
General chemicals were of reagent grade or higher. High-purity (>99.997%) KCl was obtained from Alfa Inorganics. [2-(trimethylammonium)ethyl]methanethiosulfonate (MTSET, Br salt)1 and 2-(sulfonatoethyl)methanethiosulfonate (MTSES, Na salt) were obtained from Anatrace. Dodecylmaltoside was from Calbiochem Corp. and CHAPS from Pierce Chemical Co. Lipids (Avanti Polar Lipids) were 1-palmitoyl-2-oleoyl phosphatidylethanolamine (POPE) and phosphatidylglycerol (POPG), stored in sealed ampules at −80°C. Charybdotoxin (CTX) was expressed in Escherichia coli and purified as described (Stampe et al. 1994).
Two slightly different constructs of KcsA were used. Most experiments employed a synthetic gene coding for the natural KcsA polypeptide sequence with a hexahistidine tag added to the NH2 terminus. This was derived by truncating the previously described “SliK” construct (Heginbotham et al. 1997) after residue R160, the natural COOH terminus. For charybdotoxin blocking experiments, we used a wild-type KcsA construct kindly provided by Dr. R. MacKinnon (The Rockefeller University, New York) and “KcsA-Tx,” a triple-mutant of this (Q58A/T61S/R64D) that binds CTX (MacKinnon et al. 1998).
Solutions used for planar bilayer recording are coded according to the convention: nKm, where n and m are numbers denoting the concentration (mM) of K+ ion, and the pH, respectively. The solutions also contained an appropriate anionic buffer. Thus, solution 200K7 consists of 195 mM KCl/5 mM KOH/10 mM HEPES, adjusted to pH 7.0 with HCl, and 20K4 consists of 15 mM KCl/5 mM KOH/10 mM succinic acid, adjusted to pH 4.0 with HCl.
Purification and reconstitution of KcsA.
KcsA was expressed in E. coli and purified on Ni2+ affinity columns as described (Heginbotham et al. 1997; MacKinnon et al. 1998). The purified channel was eluted in 400 mM imidazole at 1–5 mg/ml protein concentration quantified by the extinction coefficient at 280 nm (Heginbotham et al. 1998). Immediately after purification, KcsA was reconstituted into liposomes at room temperature as follows. A micellar solution of phospholipids (7.5 mg/ml POPE, 2.5 mg/ml POPG) and 34 mM CHAPS in reconstitution buffer (450 mM KCl/10 mM HEPES/4 mM N-methylglucamine, pH 7.0) was prepared as described (Heginbotham et al. 1998), and KcsA protein was added to final concentrations of 2.5–10 μg/ml, according to the number of channels per liposome desired. After 20–30 min incubation, 400 μl of the mixture was passed down a 20–ml Sephadex G-50 (fine) column equilibrated with reconstitution buffer. Liposomes eluted in the void volume with a dilution of approximately threefold and were stored in 75-μl aliquots at −80°C for up to 3 mo.
Single-channel recording in planar lipid bilayers.
Single-channel recordings of KcsA were performed in a horizontal planar lipid bilayer, with the following improvements over the system's previous description (Chen and Miller 1996). Partitions used to hold the bilayers were cut from 80-μm overhead transparency film (Primo™ Partitions), and holes (50–90-μm diameter) were handcrafted by the melt-and-shave method (Wonderlin et al. 1990). Electrodes were connected to the cis and trans chambers by salt bridges (2% agar, 200 mM KCl, 5 mM EGTA). Microphonic noise was greatly reduced by enclosing the bilayer chamber in a metal box soundproofed on all surfaces with “dB-Bloc” coating (Netwell). Electrophysiological data were collected with an Axopatch 200A amplifier and pCLAMP software (Axon Instruments). Data were sampled at 10–50 kHz and low-pass filtered at 2–5 kHz bandwidth (eight-pole Bessel filter).
After sealing the partition between cis and trans chambers with a worm of Vaseline or silicone grease, the hole was pretreated with ∼0.5 μl of phospholipid solution (15 mg/ml POPE, 5 mg/ml POPG in n-decane) and was allowed to air dry for ∼30 min. The trans chamber was then filled with 20K4 solution and the cis with 200K7 or 100K7 solution. A bilayer was spread on the hole with a glass or plastic rod wetted with phospholipid solution kept at room temperature. Capacitances were typically 25–40 pF, and resistances were in the 1 TΩ range.
For channel insertion, a day's supply of reconstituted vesicles was prepared by thawing an aliquot, transferring the suspension to a glass test tube, and sonicating in a cylindrical bath sonicator for ∼10 s. Vesicles were kept at room temperature throughout the day's use, and then discarded. A newly formed bilayer was ruptured by physical violence, ∼1 μl of reconstituted vesicles were added to the cis solution directly above the open hole and a new bilayer was immediately spread. Current was monitored at 100–200-mV holding voltages, and if channels failed to appear within 5 min, the bilayer was ruptured and the procedure was repeated. Typically, channels were observed in ∼50% of such attempts. After channel insertion, recording conditions were established by perfusion with desired solutions or by dilution of stock solutions into the bilayer chamber with mixing. In all experiments reported here, 100 mM K+ was present on both sides of the membrane. All data reported and all standard errors displayed are based on three to seven independent experiments.
RESULTS
An essential requirement for structure–function analysis of any ion channel is a firm knowledge of its orientation in the membrane under study. Most ion channels are studied in their native cellular membranes, where orientation is obvious. However, the KcsA channel is best investigated as a purified protein reconstituted in biochemically defined membranes. In such a system, transmembrane orientation of the channel is not assured and must be empirically established. This study proceeds towards a single goal: to assign specific functions to defined sides of the KcsA protein. We approach this goal in two steps. First, we show that several of the channel's fundamental properties of gating, permeation, and blockade are asymmetric with respect to the “cis” and “trans” sides of the reconstituted membrane. Then, the channel's absolute orientation is established by assigning specific residues in the KcsA structure to the corresponding sides of the bilayer.
The planar bilayer system consists of two experimentally accessible aqueous phases: the cis solution to which KcsA-reconstituted liposomes are added, and the opposing trans solution. According to the electrical polarity convention used here, the cis chamber is the zero-voltage reference. Single KcsA channels were inserted into planar lipid bilayers under asymmetric salt conditions, and both sides of the bilayer were then flushed with the desired recording solutions.
Asymmetry of Gating by Voltage and pH
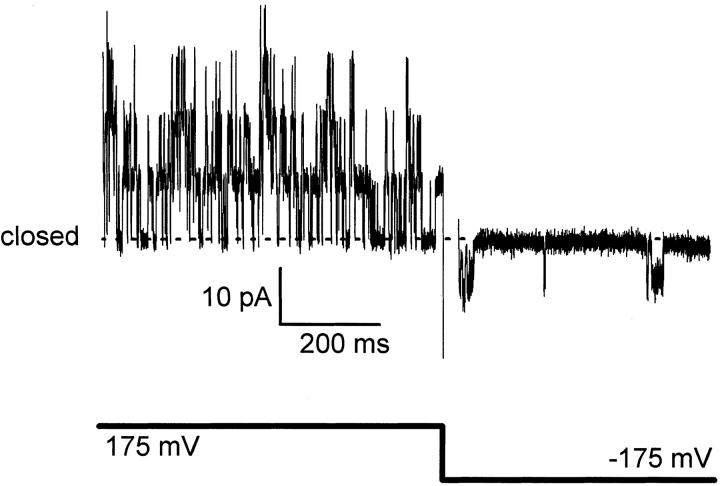
Like other two-transmembrane helix K+ channels, KcsA lacks an S4 voltage sensor. However, its gating shows a definite, though weak, voltage dependence. Fig. 1 compares KcsA channel activity at opposite voltage polarities in a multiple-channel membrane. At high positive voltage (175 mV), channel activity is marked by frequent openings throughout the duration of the record, a pattern that changes dramatically when voltage is reversed in polarity. At high negative voltage (−175 mV), the channel open probability is much lower. Most channels (>80%) insert into the planar bilayer with this orientation; a minority show reversed orientation, with frequent openings at negative voltages. Thus, KcsA channels preferentially orient in the reconstituted membrane, but the results do not even hint at their absolute orientation; i.e., which side of the bilayer is equivalent to the cytoplasmic or external face of the channel protein.
Figure 1.
Voltage-dependent gating of KcsA. A membrane containing at least five KcsA channels was held at 175 mV, with 100K7//100K4 solutions (cis//trans). Voltage was reversed in polarity where indicated. The current record was blanked during amplifier saturation after the voltage change and was subsequently corrected by fitting blank capacitative transients. Dashed lines represent closed-state level in all figures.
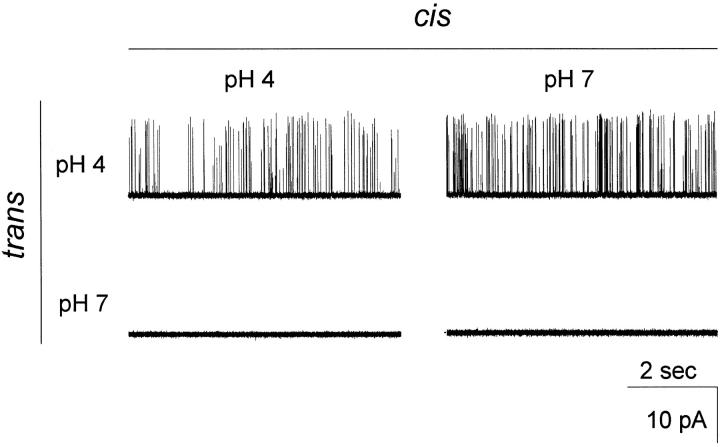
KcsA is a proton-activated channel. As described by Cuello et al. 1998, the channel opens significantly in planar bilayers only at pH values below ∼5. Fig. 2 shows, as in the original observations, that proton binding is linked to gating in a strictly sided manner. KcsA activity is responsive only to the pH of the trans solution; cis pH has no effect. These pH-dependent changes in gating are immediate upon perfusion of the trans side of the bilayer and are fully reversible.
Figure 2.
pH dependence of KcsA. Currents were recorded at 200 mV in symmetrical 100-mM K+ solutions, with pH variation on cis and trans sides, as indicated. Channels were first recorded with pH 4 on both sides. The cis chamber was brought to pH 7, and more records were collected. After perfusion to pH 7 trans, where no openings were observed, the trans chamber was returned to pH 4 to confirm reversibility.
The majority of channels incorporate into the membrane with sensitivity to trans pH, only a minority appearing with reversed sensitivity. (The channels sensitive to cis pH also show reversed voltage sensitivity.) We exploited this asymmetric pH sensitivity to ensure that all channels observed have a single orientation; all subsequent experiments were performed with trans pH 4 and cis pH 7, a maneuver that enforces a perfectly oriented set of active channels by silencing any channel inserting in the “minority” direction.
Asymmetry of Ion Permeation
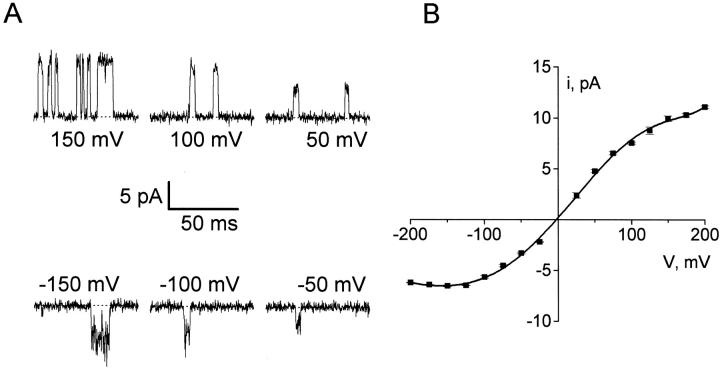
In symmetric 100-mM K+ solutions, KcsA shows open-channel rectification, as seen in the raw recordings and the open-channel current–voltage (I–V) curve of Fig. 3. Channels are well defined in amplitude and do not display the substate behavior reported previously (Schrempf et al. 1995; Cuello et al. 1998). Under these conditions, chord conductances are 56 and 31 pS at 200 and −200 mV, respectively, and zero-voltage slope conductance is 83 pS. The open channel is substantially noisier at negative potentials than at positive. At all potentials, the open state displays noise in excess of the inherent instrumental noise seen during closed intervals; this observation suggests that the open KcsA channel undergoes rapid, unresolved transitions. These transitions do not arise from endogenous blockers since the only cation present in this chemically defined system, aside from K+, is H+ and since the excess noise is not noticeably altered by changes of cis pH (in the range 4–7), by cis addition of 1 mM EDTA, or by the use of “purissimum grade” KCl in the recording solutions (data not shown).
Figure 3.
Currents through single KcsA channels. Recordings of single KcsA channels were made under conditions of Fig. 1. (A) Representative channel openings at the indicated voltages. (B) Open-channel current–voltage relation. Standard errors are smaller than the width of the points. Solid curve has no theoretical meaning.
Asymmetry of Tetraethylammonium Block
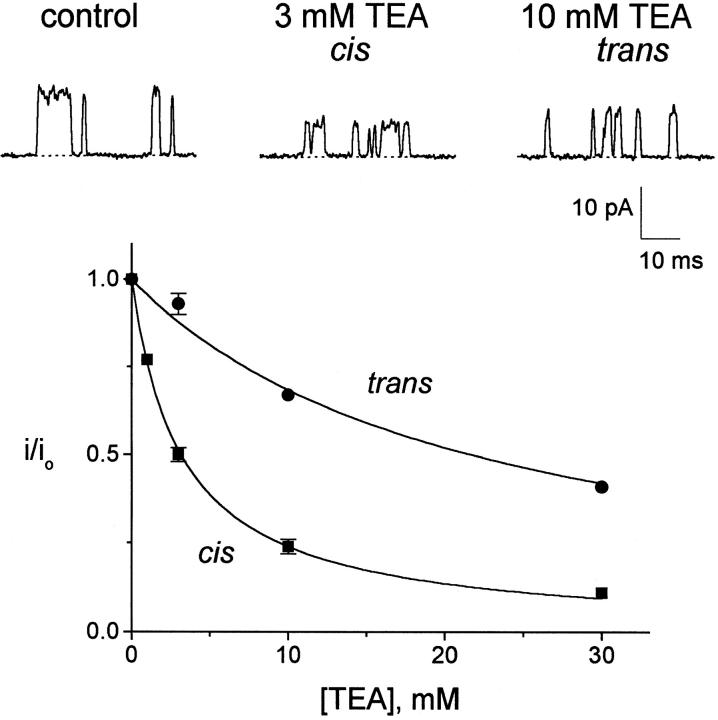
Many eukaryotic K+ channels are reversibly blocked by tetraethylammonium (TEA), which can bind to two distinct sites located near the two ends of the narrow selectivity filter (MacKinnon and Yellen 1990; Yellen et al. 1991; Newland et al. 1992). KcsA is also sensitive to TEA applied to either side of the membrane (Fig. 4). TEA reduces the open-channel amplitude in a concentration-dependent fashion, an effect expected for a reversible, low-affinity blocker with kinetics too rapid to be resolved by the recording electronics (Coronado and Miller 1979). At 200 mV, inhibition constants of 3 and 23 mM are seen for TEA added to the cis and trans solutions, respectively. The difference in affinity from the two sides must arise from intrinsic chemical differences of the binding sites involved, since the voltage polarity promotes blocker binding from the trans side and hinders it from the cis.
Figure 4.
TEA blockade of KcsA. (Top) KcsA channels were recorded at 200 mV with indicated TEA concentrations added to the 100-mM K+ solutions; these data were filtered at 1 kHz. (Bottom) Open-channel current, i, normalized to the value without TEA, i0, is shown with single-site inhibition curves, with K i = 3.2 and 22 mM for cis and trans, respectively.
Na+ Block: A Hint of Absolute Orientation
A biological imperative of K+ channels is to prohibit permeation by Na+. However, far from being inert to K+ channels, Na+ is known to interact with them in a sided fashion, blocking K+ currents in nerve and muscle membranes exclusively from the intracellular side (Bezanilla and Armstrong 1972; French and Wells 1977; Yellen 1984). This Na+ blocking asymmetry is satisfyingly rationalized in terms of the KcsA structure, which shows a widening of the pore in the center of the membrane (Doyle et al. 1998). Small cations should be able to gain ready admittance to this region from the internal solution, but would be unable to pass further through the narrow K+ selectivity filter. A Na+ ion in this region would thus block outward, but not inward, K+ current. Being rigorously excluded from the externally disposed selectivity filter, Na+ would be a functionally inert cation from the extracellular side.
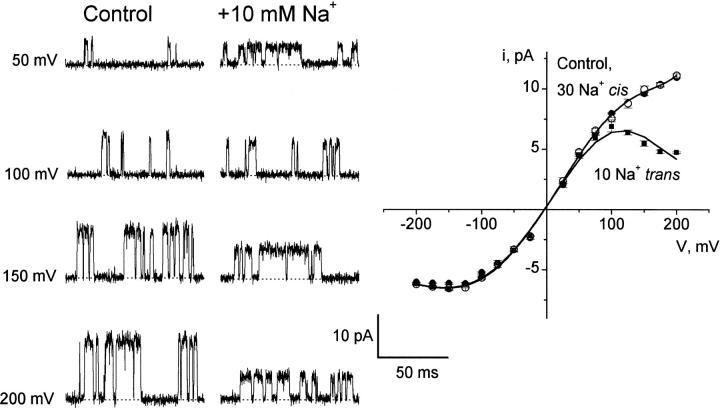
This Na+ blocking behavior seen in eukaryotic K+ channels is echoed in KcsA (Fig. 5). Addition of 30 mM Na+ to the cis side has no effect on the open-channel I–V relation in symmetrical K+. In contrast, 10 mM Na+ added to the trans side reduces the channel amplitude with voltage dependence strong enough to produce a negative conductance, as seen originally in squid axon K+ channels (Bezanilla and Armstrong 1972). The sidedness of Na+ action intimates, by precedent from excitable membrane channels, that the trans-facing side of KcsA is intracellular, and the cis side extracellular.
Figure 5.
Block of single KcsA channels by trans Na+. Single KcsA openings were recorded in 100 mM K+ solutions with or without Na+ added to either side. (Left) Raw current traces in the absence of Na+ (control) or with 10 mM Na+ added to the trans side. (Right) Open-channel I–V curves. ○, no Na+ added; •, 30 mM Na+ cis; ▪, 10 mM Na+ trans. Solid curve through the control points is a spline fit with no theoretical significance. Solid curve through the trans Na+ points is the prediction of a single-site Woodhull 1973 blocking mechanism, with an effective valence of 0.5 and a zero-voltage dissociation constant for Na+ of 322 mM.
The Absolute Orientation of KcsA
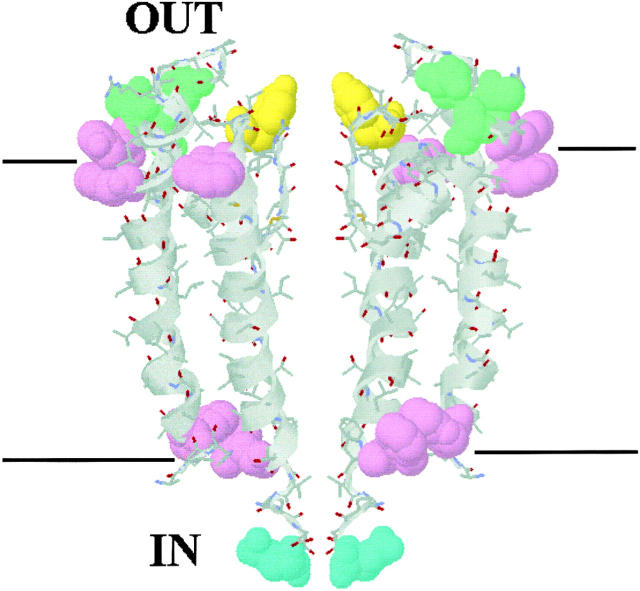
This conclusion is uncertain, however, because of its reliance on an analogy to only a few carefully studied K+ channels. We therefore scrutinized the absolute sidedness of KcsA by combining measurements of the functional influences of specific residues with the channel's known structure (Fig. 6).
Figure 6.
Locations of selected residues on KcsA structure. For clarity, only two KcsA subunits are shown in this RASMOL image. Space-filled residues are as follows. Violet, W26-Y45-W87-W113: markers of the lipid polar headgroup region. Yellow, Y82: position influencing cis TEA block. Green, Q58-T61-R64: positions mutated to strengthen CTX binding. Cyan, Q119: position modified by trans MTSES when mutated to cysteine.
Location of the external TEA binding site.
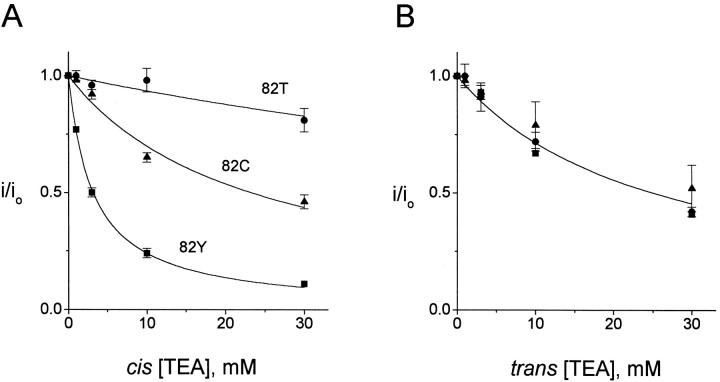
It is well established in eukaryotic K+ channels that the affinity of external TEA block is enhanced by an aromatic residue at the position equivalent to Y82 in KcsA (MacKinnon and Yellen 1990; Heginbotham and MacKinnon 1992; Kavanaugh et al. 1992). Accordingly, we examined the effects of substitutions at Y82 on TEA block (Fig. 7). The affinity of TEA blockade from the cis side is responsive to substitution here, with tyrosine producing the strongest block (K i = 3.2 mM) and the nonaromatic substitutions showing weaker blocking affinities (Y82C, K i = 23 mM; Y82T, K i = 143 mM). In contrast, TEA inhibition from the trans side is insensitive to these replacements. These results argue that position 82, known from the structure to be externally exposed, faces the cis aqueous solution.
Figure 7.
Sidedness of aromatic TEA blocking site. TEA inhibition of KcsA channels substituted at position 82 was assessed as in Fig. 4. (A) cis TEA block. Solid curves are Langmuir fits with K i of 3.2, 23, and 143 for 82Y (▪), 82C (▴), and 82T (•) channels, respectively. (B) Trans TEA block. Solid curve is Langmuir function with K i = 25 mM.
Covalent modification of Y82C.
The introduction of cysteine at position 82 offers an independent means of assessing the channel's orientation. Devoid of cysteine, KcsA is an ideal target for analysis by site-specific modification. In eukaryotic K+ channels, ion permeation is affected by substitutions at this position (MacKinnon and Yellen 1990), as may be easily understood from the KcsA structure, which places Y82 close to the external opening of the narrow pore (Fig. 6). We therefore sought functional evidence of chemical modification of Y82C by MTSET, a cationic, water-soluble sulfhydryl-modifying reagent.
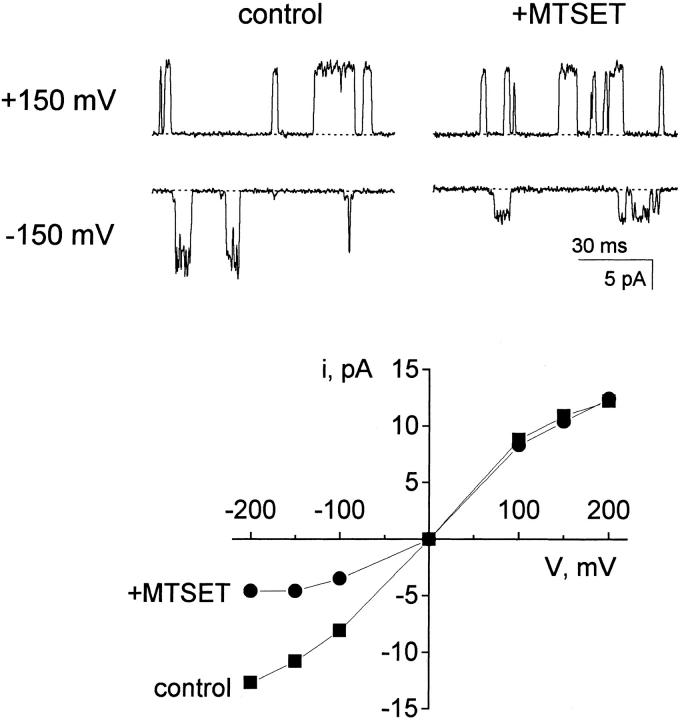
The Y82C substitution preserves the basic KcsA properties of trans pH sensitivity and voltage-dependent gating, but it alters the shape of the open-channel I–V curve, nearly eliminating the rectification normally observed (Fig. 8). The I–V curve was unchanged immediately following addition of 70 μM MTSET to the cis compartment, but ∼3 min of exposure to the reagent led to a distinct asymmetry. This rectification resulted from a decrease in cis-to-trans current while leaving current in the reverse direction unchanged, as expected from an electrostatic influence of a positive charge near the channel's cis entryway. This effect persisted after removal of the MTSET by perfusion, and it was not reversed by several minutes of exposure to 5 mM dithiothreitol. Since MTSET is membrane impermeant (Holmgren et al. 1996), and since the acidic pH of the trans side greatly disfavors formation of the thiolate nucleophile, the reagent-induced alteration in I–V curve further supports a cis-facing location of residue 82. We have not attempted to determine the number of subunits modified by MTSET under these conditions. However, prolonged application of the reagent leads to the disappearance of the channel, and so we suspect that channels like the one shown in Fig. 8 have not been modified on all four subunits. In unsurprising control experiments without the cysteine substitution at Y82, MTSET had no effect (data not shown).
Figure 8.
MTSET modification of KcsA-Y82C. Single Y82C channel openings were recorded in symmetrical 100 mM K+. After recording under control conditions, MTSET (70 μM) was added to the cis chamber. Currents were monitored at negative potentials until an obvious change in conductance was observed (∼7 min). Unreacted MTSET was then removed by perfusion, and the “+MTSET” recordings were collected. (Top) Raw channel records. (Bottom) Open-channel I–V curves.
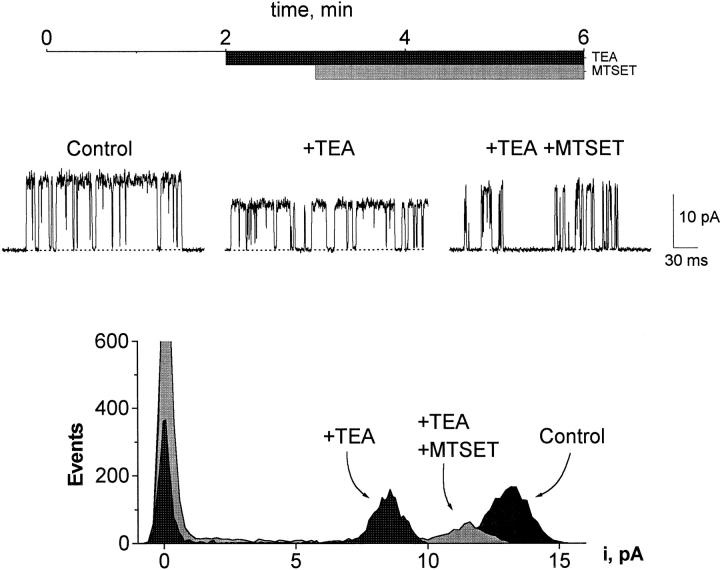
The modification of Y82C provoked a supplementary experiment. We have already seen that mutations at Y82 affect external TEA block, and we anticipate from experiments on the equivalent position in Shaker channels (MacKinnon and Yellen 1990) that modification of this residue by MTSET would greatly reduce TEA affinity. Single Y82C channels were held at 200 mV (K+ current towards the cis side), and upon cis addition of 10 mM TEA, open-channel current was reduced by ∼35% (Fig. 9). MTSET was then added to the cis side. Initially, there was no effect of the reagent, but after ∼1 min, an increase of open-channel current occurred (concurrently with a shortening of open times). This increase reflects the expected decrease of TEA affinity arising from the introduction of a trimethylammonium group near the blocking site. This result corroborates the cis exposure of position 82.
Figure 9.
Effect of MTSET on TEA sensitivity of KcsA-Y82C. Time line (top) indicates the sequence of addition of TEA (10 mM) and MTSET (350 μM). Currents were recorded from Y82C at 200 mV. All-points amplitude histograms were constructed from 30–40 s of continuous data. The data shown are from a single experiment; the experiment was repeated two more times with similar results.
Charybdotoxin sensitivity of KcsA.
Scorpion venom peptides of the charybdotoxin family block eukaryotic K+ channels by binding to a receptor site in the outer vestibule, thereby occluding the conduction pathway (Miller 1995). Wild-type KcsA fails to bind CTX, but a triple mutant in the CTX-receptor region, KcsA-Tx, reveals a binding site for radiolabeled CTX (MacKinnon et al. 1998), albeit with rather low affinity (0.1–1 μM).
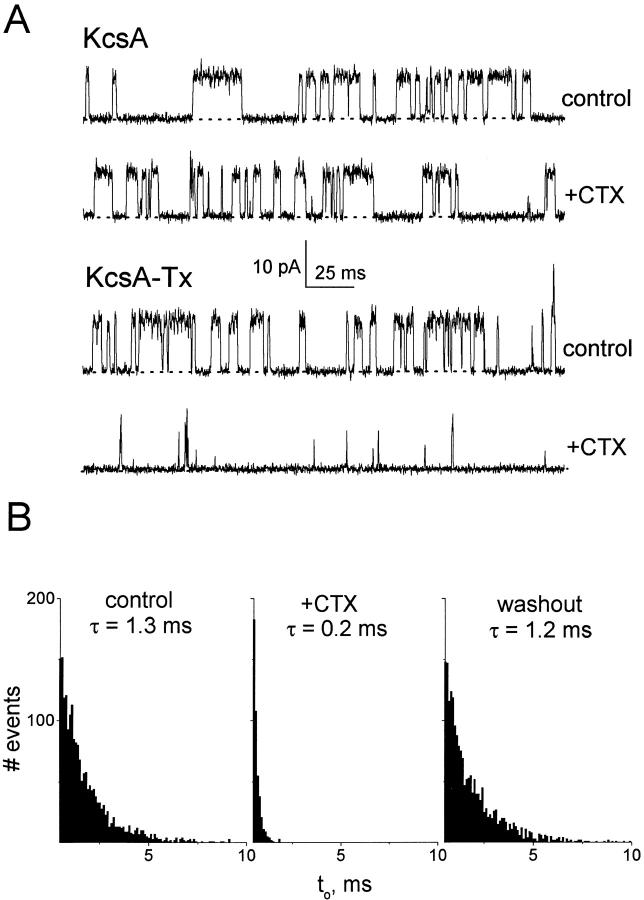
Fig. 10 demonstrates block of single KcsA-Tx channels by CTX added to the cis side. As expected (MacKinnon et al. 1998), CTX is without effect on the wild-type channel and is profoundly inhibitory on KcsA-Tx. The toxin acts by shortening open times and by modestly lengthening closed times, as expected for a bimolecular process slow enough to resolve individual toxin-block events. CTX action is fully reversed upon perfusion with toxin-free solutions, as illustrated by the open-time histograms of Fig. 10 B. It is not our purpose to carry out a detailed analysis of toxin block, which would minimally require dissecting a four-state model (Anderson et al. 1988). Qualitatively, however, the toxin dwell-time appears to be close to the normal closed times in the absence of toxin (10–100 ms). Furthermore, analysis of the shortening of open times leads to an estimated bimolecular association rate constant of ∼4 × 109 M−1 s−1, 60-fold higher than that seen for Shaker channels at a similar ionic strength (Goldstein and Miller 1993). In additional experiments, CTX-K27H, a toxin variant with greatly reduced affinity for Shaker K+ channels (Goldstein et al. 1994), had a minimal effect on KcsA-Tx (data not shown). Thus, CTX block of KcsA mirrors its action in eukaryotic systems and buttresses the argument (MacKinnon et al. 1998) for fundamentally similar toxin receptor sites in eukaryotic and prokaryotic K+ channels. The results dictate a cis-facing location for this externally exposed receptor site in KcsA. We did not perform experiments with trans addition of CTX, since the acid conditions on the trans side would make the expected negative results meaningless; the conclusion of a cis-facing CTX receptor stands without these controls.
Figure 10.
CTX block of KcsA. KcsA wild-type or KcsA-Tx triple mutant channels were held at 150 mV in 100 mM K+ solutions; bovine serum albumin (50 μg/ml) was also included in the cis solution to suppress nonspecific binding of CTX to the chamber walls. (A) Records are displayed before (control) or after (+CTX) addition of 1 μM CTX to the cis chamber. (Top) Wild-type KcsA; (bottom) KcsA-Tx. (B) Representative open-time histograms of KcsA-Tx collected from 30 s of continuous data for each condition indicated. Time constants were determined from single-exponential fits to the histograms.
Covalent modification of Q119C.
The above experiments all involved manipulations of residues exposed to the cis solution. For completeness, it was desirable to test a residue exposed on the opposite side of the membrane. We substituted cysteine at Q119 and monitored changes in channel conductance in response to covalent modification. While the experiment is identical in motivation to those with Y82C above, the low pH of the trans solution requires two special considerations. First, the reaction rate of cysteine with thiosulfonate reagents is negligible at pH 4, and so MTSES was applied in a pH 7 solution. Since under these conditions the channels were closed, after several minutes of exposure to the reagent, the trans chamber was returned to pH 4 to gauge the effect of the modification. Second, two criteria guided the selection of a target residue: (a) susceptibility to closed-state modification by MTSES, and (b) proximity to the conduction pathway, maximizing the likelihood of an electrostatic influence on conduction.
On the basis of these requirements, we selected Q119 as the target residue. This residue is located in the COOH-terminal domain close to the cytoplasmic opening of the pore (Fig. 6). The equivalent residue in the Shaker K+ channel, H486, reacts with MTS reagents in both open and closed states (Liu et al. 1997). Fig. 11 shows that under control conditions, Q119C displays wild-type behavior, with normal rectification of the open-channel I–V curve. Several minutes of exposure to MTSES from the trans side led to an increase in single-channel current at positive voltages, with no discernible effect at negative voltages. This is as expected for the MTSES reaction, which places a negatively charged ethylsulfonate group near the channel's trans entryway. No effects were observed in control experiments in which the reagent was added to the cis side or to bilayers containing wild-type KcsA channels (data not shown).
Figure 11.
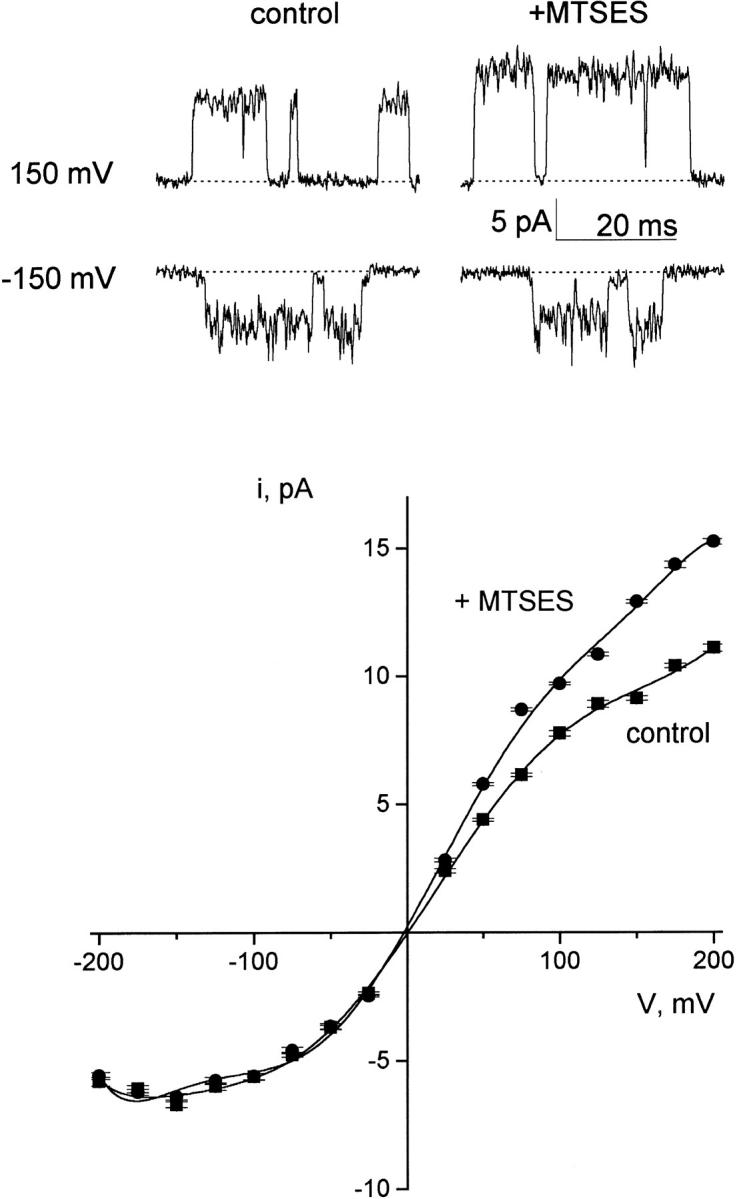
MTSES modification of KcsA-Q119C. Open-channel I–V curves were determined on the Q119C mutant before and after addition to MTSES to the trans chamber. After collecting control records, the trans chamber was perfused with 100K7 solution containing 200 μM MTSES, and the reaction was allowed to proceed for 2 min. Fresh 100K4 solution without MTSES was then reintroduced, and the I–V curve was recorded. Data shown in the figure are from a single experiment; similar results were obtained in three separate bilayers.
DISCUSSION
These experiments exploit a fully oriented reconstituted system in which single KcsA channels, purified after high-level expression in E. coli, can be studied electrophysiologically. We have taken pains to document functional asymmetries in KcsA to assign absolute sidedness to the system. Eight separate asymmetries were examined: voltage-dependent gating, proton activation, open-channel rectification, block by Na+, TEA, and CTX, and covalent modification of channels with cysteines substituted on either side of the membrane. Three independent lines of evidence establish the channel's orientation in the bilayer. The cis solution bathes the extracellular face of the channel protein containing the CTX receptor and the aromatic TEA blocking site. The trans solution bathes the intracellular face containing Q119.
These assignments unambiguously demonstrate that the protonation sites linked to KcsA gating face the intracellular solution. This orientation makes sense from a statistical standpoint: of the 56 carboxylate groups in KcsA (16 asp, 36 glu, 4 COOH termini), 44 are located at intracellular positions; all 44 histidine residues in the His-tagged protein are also intracellular. Previously, Cuello et al. 1998, arguing from protection of liposome-reconstituted KcsA against proteolysis, had concluded the reverse: that extracellular protons gate the channel. Our own results, in contrast, show robust proteolysis of KcsA under similar conditions (data not shown).
Our results are perplexing from a biological perspective. Indeed, they suggest strongly that this channel is not gated by low pH in its native membrane, since Streptomyces, like most bacteria, tightly regulates cytoplasmic pH near neutrality. If the physiological role of KcsA is in fact to gate the K+ conductance of the bacterial membrane, then it is likely that some factor other than pH, perhaps an as yet unrecognized partner protein, provides control of gating. Questions of this kind must remain unresolved until the physiological purposes of prokaryotic K+ channels are clarified.
In the course of assessing KcsA orientation, we have also shown that pore characteristics of this prokaryotic channel are remarkably similar to those of many well-studied eukaryotic K+ channels. The functional familiarity of KcsA is not surprising, given how closely the structural features proposed for eukaryotic K+ channels match those actually observed with KcsA (Doyle et al. 1998; Yellen 1998). But it is nevertheless important to confirm this functional similarity by experiment, since KcsA provides the first opportunity for a direct structure–function assault on an ion-selective channel.
Acknowledgments
We are grateful to Drs. Rob Blaustein, Merritt Maduke, and Irwin Levitan for suggestions on the manuscript.
Supported by National Institutes of Health grant GM-31768 and training grant GM-07596 (M. LeMasurier).
Footnotes
1used in this paper: CTX, charybdotoxin; I–V, current–voltage; MTSES, 2-(sulfonatoethyl)methanethiosulfonate; MTSET, [2-(trimethylammonium)ethyl]methanethiosulfonate; POPE, 1-palmitoyl-2-oleoyl phosphatidylethanolamine; POPG, 1-palmitoyl-2-oleoyl phosphatidylglycerol; TEA, tetraethylammonium
Dr. Heginbotham's present address is Department of Molecular Biophysics and Biochemistry, Yale University, New Haven, Connecticut 06520.
References
- Anderson C., MacKinnon R., Smith C., Miller C. Charybdotoxin inhibition of Ca2+-activated K+ channels. Effects of channel gating, voltage, and ionic strength. J. Gen. Physiol. 1988;91:317–333. doi: 10.1085/jgp.91.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F., Armstrong C.M. Negative conductance caused by entry of sodium and cesium ions into the potassium channels of squid axons. J. Gen. Physiol. 1972;60:588–608. doi: 10.1085/jgp.60.5.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T.Y., Miller C. Nonequilibrium gating and voltage dependence of the ClC-0 Cl− channel. J. Gen. Physiol. 1996;108:237–250. doi: 10.1085/jgp.108.4.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronado R., Miller C. Voltage dependent caesium blockade of a cation channel from fragmented sarcoplasmic reticulum. Nature. 1979;280:807–810. [Google Scholar]
- Cortes D.M., Perozo E. Structural dynamics of the Streptomyces lividans K+ channels (SCK1)oligomeric stoichiometry and stability. Biochemistry. 1997;36:10343–10352. doi: 10.1021/bi971018y. [DOI] [PubMed] [Google Scholar]
- Cuello L.G., Romero J.G., Cortes D.M., Perozo E. pH-dependent gating in the Streptomyces lividans K+ channel. Biochemistry. 1998;37:3229–3236. doi: 10.1021/bi972997x. [DOI] [PubMed] [Google Scholar]
- Doyle D.A., Cabral J.M., Pfuetzner A., Kuo J.M., Gulbis J.M., Cohen S.L., Chait B.T., MacKinnon R. The structure of the potassium channelmolecular basis of K+ conduction and selectivity. Science. 1998;280:69–76. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- French R.J., Wells J.B. Sodium ions as blocking agents and charge carriers in the potassium channel of the squid giant axon. J. Gen. Physiol. 1977;70:707–724. doi: 10.1085/jgp.70.6.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein S.A.N., Pheasant D.J., Miller C. The charybdotoxin receptor of a Shaker K+ channelpeptide and channel residues mediating molecular recognition. Neuron. 1994;12:1377–1388. doi: 10.1016/0896-6273(94)90452-9. [DOI] [PubMed] [Google Scholar]
- Goldstein S.A.N., Miller C. Mechanism of charybdotoxin block of a Shaker K+ channel. Biophys. J. 1993;65:1613–1619. doi: 10.1016/S0006-3495(93)81200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heginbotham L., Odessey E., Miller C. Tetrameric stoichiometry of a prokaryotic K+ channel. Biochemistry. 1997;36:10335–10342. doi: 10.1021/bi970988i. [DOI] [PubMed] [Google Scholar]
- Heginbotham L., Kolmakova-Partensky L., Miller C. Functional reconstitution of a prokaryotic K+ channel. J. Gen. Physiol. 1998;111:741–750. doi: 10.1085/jgp.111.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heginbotham L., MacKinnon R. The aromatic binding site for tetraethylammonium ion on potassium channels. Neuron. 1992;8:483–491. doi: 10.1016/0896-6273(92)90276-j. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes 2nd ed 1991. Sinauer Associates, Inc; Sunderland, MA: pp. 607 [Google Scholar]
- Holmgren M., Liu Y., Xu Y., Yellen G. On the use of thiol-modifying agents to determine channel topology. Neuropharmacology. 1996;35:797–804. doi: 10.1016/0028-3908(96)00129-3. [DOI] [PubMed] [Google Scholar]
- Kavanaugh M.P., Hurst R.S., Yakel J., Varnum M.D., Adelman J.P., North R.A. Multiple subunits of a voltage-dependent potassium channel contribute to the binding site for tetraethylammonium. Neuron. 1992;8:493–497. doi: 10.1016/0896-6273(92)90277-k. [DOI] [PubMed] [Google Scholar]
- Liu Y., Holmgren M., Yellen G. Gated access to the pore of a voltage-dependent K+ channel. Neuron. 1997;19:175–184. doi: 10.1016/s0896-6273(00)80357-8. [DOI] [PubMed] [Google Scholar]
- MacKinnon R., Cohen S.L., Kuo A., Lee A., Chait B. Structural conservation in prokaryotic and eukaryotic potassium channels. Science. 1998;280:106–109. doi: 10.1126/science.280.5360.106. [DOI] [PubMed] [Google Scholar]
- MacKinnon R., Yellen G. Mutations affecting TEA blockade and ion permeation in voltage-activated K+ channels. Science. 1990;250:276–279. doi: 10.1126/science.2218530. [DOI] [PubMed] [Google Scholar]
- Miller C. The charybdotoxin family of K+ channel-blocking peptides. Neuron. 1995;15:5–10. doi: 10.1016/0896-6273(95)90057-8. [DOI] [PubMed] [Google Scholar]
- Newland C.F., Adelman J.P., Tempel B.L., Almers W. Repulsion between tetraethylammonium ions in cloned voltage-gated potassium channels. Neuron. 1992;8:975–982. doi: 10.1016/0896-6273(92)90212-v. [DOI] [PubMed] [Google Scholar]
- Perozo E., Cortes D.M., Cuello L.G. Three-dimensional architecture and gating mechanism of a K+ channel studied by EPR spectroscopy. Nat. Struct. Biol. 1998;5:459–469. doi: 10.1038/nsb0698-459. [DOI] [PubMed] [Google Scholar]
- Perozo E., Cortes D.M., Cuello L.G. Structural rearrangements underlying K+-channel activation gating. Science. 1999;285:73–78. doi: 10.1126/science.285.5424.73. [DOI] [PubMed] [Google Scholar]
- Schrempf H., Schmidt O., Kummerlin R., Hinnah S., Muller D., Betzler M., Steinkamp T., Wagner R. A prokaryotic potassium ion channel with two predicted transmembrane segments from Streptomyces lividans . EMBO (Eur. Mol. Biol. Organ.) J. 1995;14:5170–5178. doi: 10.1002/j.1460-2075.1995.tb00201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stampe P., Kolmakova-Partensky L., Miller C. Intimations of K+ channel structure from a complete functional map of the molecular surface of charybdotoxin. Biochemistry. 1994;33:443–450. doi: 10.1021/bi00168a008. [DOI] [PubMed] [Google Scholar]
- Wonderlin W.F., Finkel A., French R.J. Optimizing planar lipid bilayer single-channel recordings for high resolution with rapid voltage steps. Biophys. J. 1990;58:289–297. doi: 10.1016/S0006-3495(90)82376-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhull A. Ionic blockage of sodium channels in nerve. J. Gen. Physiol. 1973;61:687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen G. Relief of Na+ block of Ca2+-activated K+ channels by external cations. J. Gen. Physiol. 1984;84:187–199. doi: 10.1085/jgp.84.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen G. The moving parts of voltage-gated ion channels. Q. Rev. Biophys. 1998;31:239–295. doi: 10.1017/s0033583598003448. [DOI] [PubMed] [Google Scholar]
- Yellen G., Jurman M., Abramson T., MacKinnon R. Mutations affecting internal TEA blockade identify the probable pore-forming region of a K+ channel. Science. 1991;251:939–942. doi: 10.1126/science.2000494. [DOI] [PubMed] [Google Scholar]