Abstract
To examine a possible relation between the swelling-induced ATP release pathway and the volume-sensitive Cl− channel, we measured the extracellular concentration of ATP released upon osmotic swelling and whole-cell volume-sensitive Cl− currents in a human epithelial cell line, Intestine 407, which lacks expression of cystic fibrosis transmembrane conductance regulator (CFTR). Significant release of ATP was observed within several minutes after a hypotonic challenge (56–80% osmolality) by the luciferin/luciferase assay. A carboxylate analogue Cl− channel blocker, 5-nitro-2-(3-phenylpropylamino)-benzoate, suppressed ATP release in a concentration-dependent manner with a half-maximal inhibition concentration of 6.3 μM. However, swelling-induced ATP release was not affected by a stilbene-derivative Cl− channel blocker, 4-acetamido-4′-isothiocyanostilbene at 100 μM. Glibenclamide (500 μM) and arachidonic acid (100 μM), which are known to block volume-sensitive outwardly rectifying (VSOR) Cl− channels, were also ineffective in inhibiting the swelling-induced ATP release. Gd3+, a putative blocker of stretch-activated channels, inhibited swelling-induced ATP release in a concentration-dependent manner, whereas the trivalent lanthanide failed to inhibit VSOR Cl− currents. Upon osmotic swelling, the local ATP concentration in the immediate vicinity of the cell surface was found to reach ∼13 μM by a biosensor technique using P2X2 receptors expressed in PC12 cells. We have raised antibodies that inhibit swelling-induced ATP release from Intestine 407 cells. Earlier treatment with the antibodies almost completely suppressed swelling-induced ATP release, whereas the activity of VSOR Cl− channel was not affected by pretreatment with the antibodies. Taking the above results together, the following conclusions were reached: first, in a CFTR-lacking human epithelial cell line, osmotic swelling induces ATP release and increases the cell surface ATP concentration over 10 μM, which is high enough to stimulate purinergic receptors; second, the pathway of ATP release is distinct from the pore of the volume-sensitive outwardly rectifying Cl− channel; and third, the ATP release is not a prerequisite to activation of the Cl− channel.
Keywords: adenosine triphosphate, anion channel, osmotic swelling, cell volume regulation
INTRODUCTION
Osmotic cell swelling leads to extrusion of a number of intracellular inorganic and organic osmolytes, thereby accomplishing the regulatory volume decrease (RVD)1 (Lang 1998; Okada 1998). Recently, release of intracellular ATP was found to be induced by osmotic swelling in human (Hazama et al. 1998b; Taylor et al. 1998), bovine (Mitchell et al. 1998), and mouse (Hazama et al. 1998b) epithelial cells, as well as in rat hepatoma cells (Wang et al. 1996; Roman et al. 1997). Since ATP exists in the cytoplasm at millimolar concentrations, opening of the large pore may induce a passive efflux of ATP. Actually, it is known that a mitochondrial outer membrane large-conductance voltage-dependent anion channel is permeable to anionic ATP (Rostovtseva and Colombini 1997). However, the plasma membrane ATP channel has not been identified.
Several studies have suggested that the cystic fibrosis transmembrane conductance regulator (CFTR) Cl− channel can act as an ATP-permeable channel (Reisin et al. 1994; Schwiebert et al. 1995; Cantiello et al. 1998). The CFTR-mediated ATP release hypothesis has now been challenged by many contradictory reports (Grygorczyk et al. 1996; Li et al. 1996; Reddy et al. 1996; Grygorczyk and Hanrahan 1997; Sugita et al. 1998). On the other hand, swelling-activated Cl− channels are known to show considerable permeability to large organic anions (Strange et al. 1996; Nilius et al. 1997; Okada 1997). Thus, it is possible that the volume-sensitive Cl− channel may serve as a pathway for ATP release. As proposed by Wang et al. 1996 and Roman et al. 1997, there is the possibility that activation of the volume-sensitive Cl− channel is induced by released ATP through stimulation of P2 receptors. In the present study, therefore, we addressed the question of whether the volume-sensitive Cl− channel serves the pathway of swelling-induced ATP release or its activity is a prerequisite to swelling-induced ATP release, and whether activation of the volume-sensitive Cl− channel is dependent on swelling-induced ATP release in a human epithelial cell line that lacks CFTR expression.
MATERIALS AND METHODS
Cells
Human epithelial Intestine 407 cells were cultured in Fischers' medium supplemented with 10% newborn calf serum, as described previously (Hazama and Okada 1988). For the luminometric ATP measurements, the cells were plated on 48-well plastic plates. The ATP assay was made at the density of 4 × 105 cells per well. For the biosensor ATP measurements, Intestine 407 cells were cultured on glass coverslips, and then transferred to the experimental chamber. For the patch-clamp whole-cell recordings, Intestine 407 cells were cultured in suspension with agitation for 10–120 min, and then plated on the experimental chamber. PC12 cells that express the P2X2 receptor channel were cultured, as reported previously (Hazama et al. 1998a).
Luciferin/Luciferase ATP Assay
The extracellular bulk ATP concentration was measured by luciferin-luciferase luminometry using an AF-100 ATP analyzer and AF-2L1 reagents (TOA Electronics). The culture medium was totally replaced by 300 μl of isotonic PBS containing (mM): 137 NaCl, 2.7 KCl, 1 CaCl2, 1 MgCl2, 8.1 Na2HPO4, and 1.5 KH2PO4 (pH 7.4, 300 mosmol/kg H2O). It is known that ATP release is dramatically stimulated by mechanical stimulation, such as a medium displacement and tilting the monolayer cells or touching to the cells (Grygorczyk and Hanrahan 1997; Watt et al. 1998). Preliminary experiments showed that this is the case for Intestine 407 cells. However, ATP release induced by replacement of the entire bathing solution subsided to the background level after 30–60 min. Also, mechanical ATP release was found to be avoided by gentle replacement of one third to one half of the solution by a new solution without touching and tilting the monolayer cells. Thus, after a 60-min equilibration, 100 μl of the extracellular isotonic solution (300 μl) was collected as a control sample and the ATP concentration was measured. Then, 200 μl of hypotonic solution was gently added to the remaining 200 μl of isotonic solution to reduce the extracellular osmolality down to the desired level (56% osmolality, unless indicated). The hypotonic solutions were prepared by mixing the isotonic PBS with a solution containing 5 mM KCl, 1 mM MgCl2, 1 mM CaCl2, and 10 mM HEPES/NaOH (pH 7.4, 35 mosmol/kg H2O). The test samples (100 μl) were collected after incubation for the indicated times (usually 15 min) at 37°C and the ATP concentration was measured at room temperature (24–26°C). When necessary, extracellular CaCl2 was removed and 0.1 mM EGTA was added during a hypotonic challenge. The effects of Cl− channel blockers were observed by adding the drug to the hypotonic solution. Only glibenclamide (500 μM) among the employed drugs was found to suppress (by 20–25%) the luciferase detection of ATP, as reported previously (Taylor et al. 1998). Thus, the data of glibenclamide effect were corrected by the calibration curve obtained by reactions with known amounts of ATP added to cell-free isotonic or hypotonic solution with or without 500 μM glibenclamide. The effect of pretreatment with antibodies was observed after incubating in the isotonic solution supplemented with 12.5 μg/ml antibodies for 60 min, and then applying the hypotonic solution in the absence of antibodies.
Cell Viability Assay
After incubating Intestine 407 cells in the above isotonic or hypotonic solution for 30 min at 37°C, cell viability was assessed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay (Mossman 1983) using the Cell Counting Kit (Dojindo) according to the manufacturer's instructions. This method is based on the ability of viable (but not dead) cells to cleave the tetrazolium ring of the MTT into a dark blue formazan reaction product.
Biosensor ATP Assay
The local concentration of released ATP at the single cell surface was measured by a biosensor technique on single Intestine 407 cells, using PC12 cells expressing ligand-gated cation channels, P2X2 receptors, as described previously (Hazama et al. 1998a). In brief, after attaining the whole-cell configuration with a PC12 cell, the cell was lightly attached to the surface of an Intestine 407 cell under a microscope. The whole-cell currents were recorded at a holding potential of −50 mV using an Axopatch 200A amplifier (Axon Instruments) at room temperature. From the whole-cell current density thus recorded, the local ATP concentration was estimated by the calibration curve obtained by the response of PC12 cells alone to known amounts of ATP, as described previously (Hazama et al. 1998a). To apply hypotonic stimulation, the bathing solution was changed from isotonic to hypotonic solution by perfusion. The isotonic solution contained (mM): 100 NaCl, 4.2 KCl, 100 mannitol, 8 HEPES, 6 HEPES-Na, 1 CaCl2, and 1 MgCl2 (320 mosml/kg H2O, pH 7.4). The hypotonic solution (220 mosml/kg H2O, pH 7.4) was prepared by removing mannitol from the isotonic solution. The pipette (intracellular) solution was composed of (mM): 150 CsCl, 1 MgCl2, 10 EGTA, and 10 HEPES (280 mosml/kg H2O, pH 7.4 with CsOH).
Whole-Cell Cl− Current Measurements
Volume-sensitive Cl− channel currents were recorded by the whole-cell patch clamp technique, as reported previously (Liu et al. 1998). The time course of current activation and recovery was monitored by repetitively applying alternating step pulses of ±40 mV (2-s duration) from a holding potential of 0 mV. To monitor the voltage dependence of the current, stepping pulses (2-s duration) were sometimes applied from a prepotential at −100 mV (0.6-s duration) to test potentials of −80 to +100 mV in 20-mV increments. Currents were recorded using an Axopatch 200A amplifier, filtered at 1 kHz and digitized at 4 kHz. Cs-rich solutions were employed. Isotonic CsCl bathing solution contained (mM): 110 CsCl, 10 HEPES, 8 Tris, 5 MgSO4, and 100 mannitol (320 mosml/kg H2O, pH 7.5). Cell swelling was induced by hypotonic (260 mosml/kg H2O) CsCl solution in which mannitol was reduced to 40 mM. The pipette (intracellular) CsCl solution contained (mM): 110 CsCl, 2 MgSO4, 1 Na2ATP, 15 Na-HEPES, 10 HEPES, 1 EGTA, and 50 mannitol (290 mosml/kg H2O, pH 7.3).
Antibody Production
Since the molecular pathway for swelling-induced ATP release has not as yet been identified, we attempted to produce antibodies against unidentified extracellular epitopes of plasma membrane–associated protein involved in swelling-induced ATP release. Monoclonal antibodies were obtained according to the method described elsewhere (Tsukita et al. 1989). In brief, BALB/c mice were immunized with 106 Intestine 407 cells. Peripheral lymphocytes were fused with myeloma NS-1 cells using 50% polyethylene glycol (PEG 4000). The supernatant from the resulting hybridoma cells was screened to find colonies that had inhibitory effects on ATP release from swollen Intestine 407 cells. The wells that showed positive activity (15 of 288 wells) were immediately expanded and plated in 98-well plates. Positive colonies were found in 12 of 1,470 wells. The positive hybridoma cells were injected to the mice to produce ascites culture. One of these, termed H2D2-5, was employed for the present experiments after purification by using the E-Z-SEP™ antibody purification reagent (Pharmacia Fine Chemicals). By Western blot analysis using the anti–ATP release antibodies, no specific antigen was detected as a single band (data not shown). Affinity-purified mouse IgG (Zymed Laboratories) was used as a negative control.
Chemicals
The following agents were added to bathing solutions: 0.1 mg/ml apyrase, 10–30 μM 5-nitro-2-(3-phenylpropylamino)-benzoate (NPPB), 100 μM 4-acetamido-4′-isothiocyanostilbene (SITS), 500 μM glibenclamide, 100 μM arachidonic acid, and 5–30 μM GdCl3.
Data are given as means ± SEM of observations (n). Statistical differences of the data were evaluated by paired or unpaired Student's t test and considered significant at P < 0.05.
RESULTS
Swelling-induced ATP Release
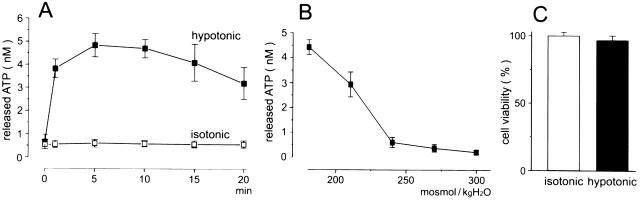
Luminometric ATP assay showed that Intestine 407 cells respond to a hypotonic challenge not only with cell swelling but also with significant release of ATP. The swelling-induced ATP release was found to start within a minute and continued for over 10 min (Fig. 1 A). From the results collected at 1 min after a hypotonic challenge, the initial rate of swelling-induced ATP release from a single Intestine 407 is estimated to be ∼2,500 ATP molecules/s under the assumption that degradation or reuptake of released ATP was negligible.
Figure 1.
Swelling-induced ATP release without cell lysis. (A) The time course of ATP release detected by the luminometric assay from 4 × 105 Intestine 407 cells after exposure to hypotonic solution (56%, ▪) or isotonic solution (□). The symbols represent the mean values of six observations with the SEM (bars). Hypotonic data are statistically different (P < 0.01) from isotonic data (except at zero time). (B) Dependence of ATP release on extracellular osmolality. The data obtained 15 min after exposure to solutions with given osmolality. The data represent the mean values of five observations with the SEM (bars). Data at 210 and 180 mosmol/kg H2O are statistically different (P < 0.01) from those at 300 mosmol/kg H2O. (C) Cell viability assessed at 30 min after exposure to hypotonic (black column) and isotonic (white column) solution. The data are the mean values of 10 observations with the SEM (bars).
ATP release increased with decreasing extracellular osmolality (Fig. 1 B).
The cell viability was not essentially affected by the hypotonic challenge (Fig. 1 C).
The mean values of bulk ATP concentration in 400 μl ambient solutions of 4 × 105 cells were 0.47 ± 0.08 nM (n = 39) after a 60-min exposure to isotonic solution and 2.63 ± 0.44 nM (n = 53) after a 15-min exposure to hypotonic (56% osmolality) solution. The mean rate of ATP release from a single Intestine 407 cell during a 15-min exposure to a hypotonic solution is estimated to be ∼1,700 ATP molecules/s, and that during a 60-min exposure to isotonic solution is ∼60/s.
A biosensor ATP assay method (Hazama et al. 1998a) was employed to assess the ATP concentration at the outer surface of swollen Intestine 407 cells. Hypotonic stimulation failed to evoke currents in PC12 cell alone (data not shown, n = 11). However, when a voltage-clamped PC12 cell was placed close to an Intestine 407 cell, a series of spiky and sustained inward currents were induced within several minutes after exposure to hypotonic solution (69%) at room temperature (Fig. 2 A). ATP release was found to be always coupled to cell swelling under a microscope. In the presence of an ATP-hydrolyzing enzyme, apyrase, in contrast, the inward current response to hypotonic challenge was virtually abolished, as shown in Fig. 2 B. In the absence of apyrase, the average peak response was 7.9 ± 1.2 pA/pF (n = 20), which corresponds to 13.1 μM ATP on a calibration curve of ATP-induced PC12 responses (Fig. 2 C).
Figure 2.
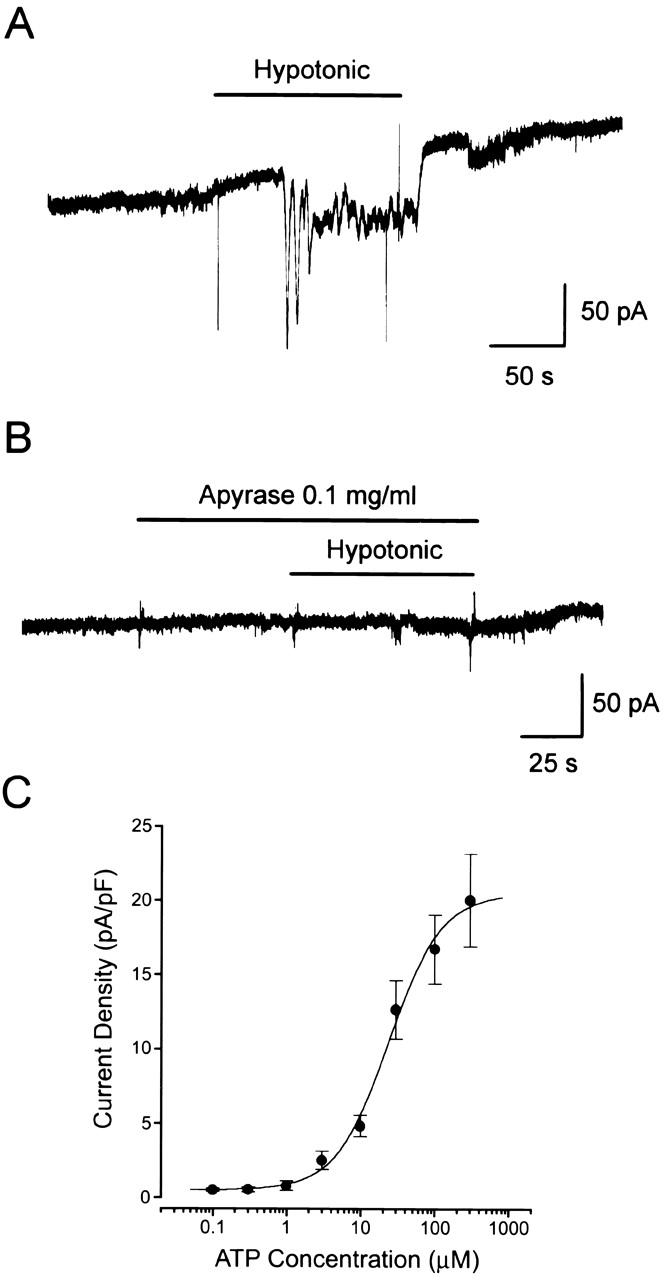
Cell surface release of ATP upon osmotic swelling detected by the biosensor technique. (A) Representative trace of control records from PC12 cells placed close to an Intestine 407 cell in response to a hypotonic challenge. (B) Representative trace of the current response to a hypotonic challenge in the presence of 0.1 mg/ml apyrase in the bath solution. A similar result was obtained from four cells. (C) Concentration–response curve of the ATP-induced currents in PC12 cells. Each symbol represents the mean peak current density of 4–13 observations (with SEM, vertical bar) in response to application of a single puff of ATP. The mean input capacitance of PC12 cells employed in these experiments was 14.5 ± 0.99 pF (n = 32).
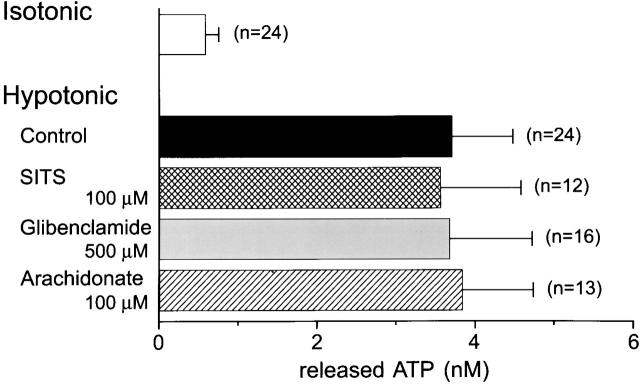
Effects of Cl− Channel Blockers on Swelling-induced ATP Release and Cl− Currents
A possible relation of swelling-induced ATP release with the activity of volume-sensitive Cl− channel was investigated by applying a high concentration of Cl− channel blockers. Luminometric ATP assay showed that swelling-induced ATP release was not blocked by 100 μM SITS (Fig. 3), as found previously (Hazama et al. 1998b). Glibenclamide and arachidonic acid were also ineffective at the concentrations by which volume-sensitive Cl− channel currents are known to be almost completely abolished in Intestine 407 cells (Kubo and Okada 1992; Liu et al. 1998). Glibenclamide insensitivity is in good agreement with a previous observation of swelling-induced ATP release from rabbit ciliary epithelial cells (Mitchell et al. 1998), but is in contrast to those of mechanical stress–induced ATP release from rabbit red blood cells (Sprague et al. 1998) or Ehrlich ascites tumor cells (Pedersen et al. 1999) and cAMP-activated ATP release from CFTR-expressing human epithelial cells (Schwiebert et al. 1995).
Figure 3.
Effects of blockers for volume-sensitive Cl− channels on swelling-induced ATP release detected by the luminometric technique. Swelling-induced ATP release in the absence of blockers is not statistically different from those in the presence of SITS (P = 0.90), glibenclamide (P = 0.98), and arachidonic acid (P = 0.91).
Since swelling-induced ATP release was reported to be largely inhibited by a Cl− channel blocker, NPPB, in rabbit ocular ciliary epithelial cells at 100 μM (Mitchell et al. 1998), we then examined the NPPB effect. As shown in Fig. 4 A, NPPB was found to inhibit ATP release from swollen Intestine 407 cells at lower concentrations. The concentration required for 50% inhibition (IC50) was 6.3 ± 0.3 μM (Fig. 4 B, □).
Figure 4.
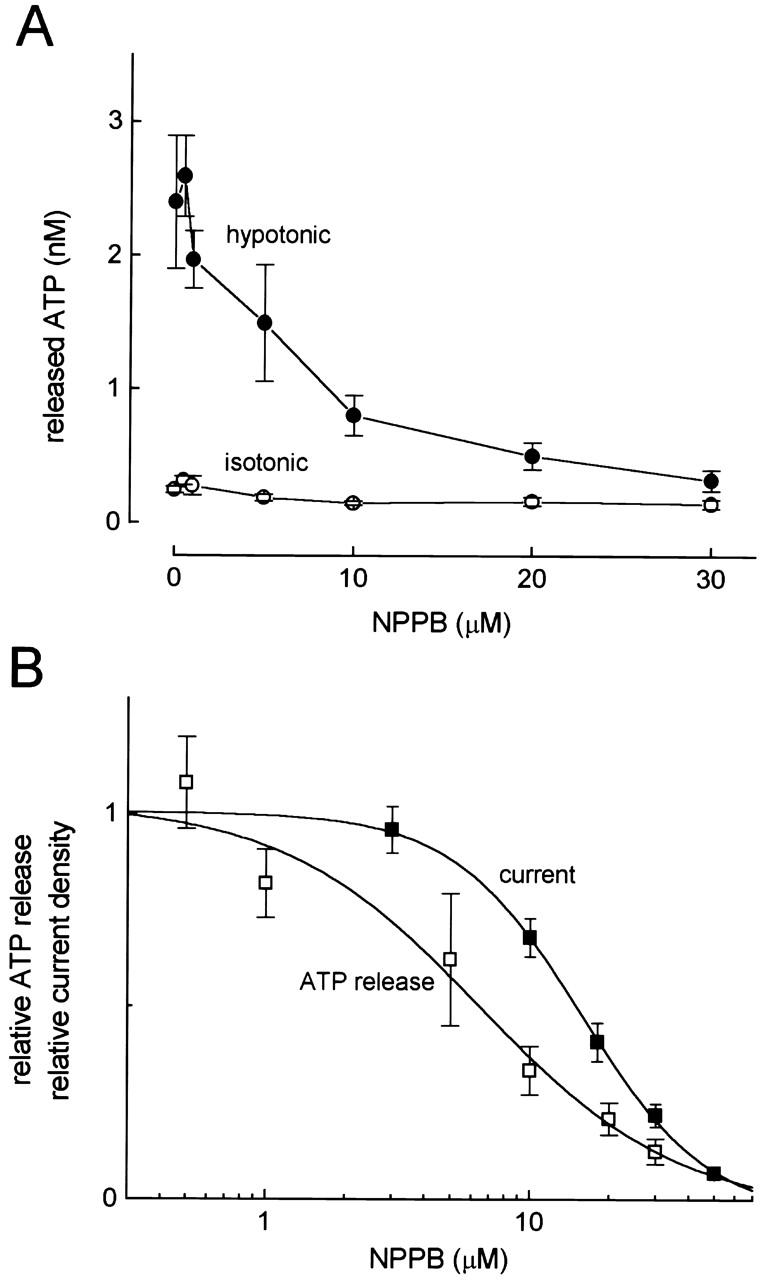
Concentration-dependent effects of NPPB on swelling-induced ATP release detected by the luminometric assay and on whole-cell volume-sensitive outwardly rectifying Cl− currents. (A) Concentration–inhibition curves of NPPB for ATP release under hypotonic (•) and isotonic (○) conditions. (Symbols) Means ± SEM; n = 10–26 experiments. Inhibitory effects of 10, 20, and 30 μM NPPB on hypotonic ATP release were statistically significant at P = 0.0038, 0.0006, and 0.0003, respectively, and those of 10 and 30 μM NPPB on isotonic ATP release were at P = 0.0017 and 0.0195, respectively. (B) Logarithmic concentration–inhibition curves of NPPB for swelling-induced ATP release (□) and VSOR Cl− currents at −60 mV (▪). (Symbols) Means ± SEM; n = 4–11 experiments.
Cell swelling activated outwardly rectifying whole-cell Cl− currents in Intestine 407 cells, and this volume-sensitive outwardly rectifying (VSOR) Cl− current was inhibited by NPPB, as reported previously (Kubo and Okada 1992). The IC50 value for Cl− current inhibition was 16.0 ± 0.2 μM (Fig. 4 B, ▪), which is significantly different from that for ATP release inhibition (0.01 < P < 0.05).
These results suggest that swelling-induced ATP release is independent of the activity of volume-sensitive outwardly rectifying Cl− channels in Intestine 407 cells.
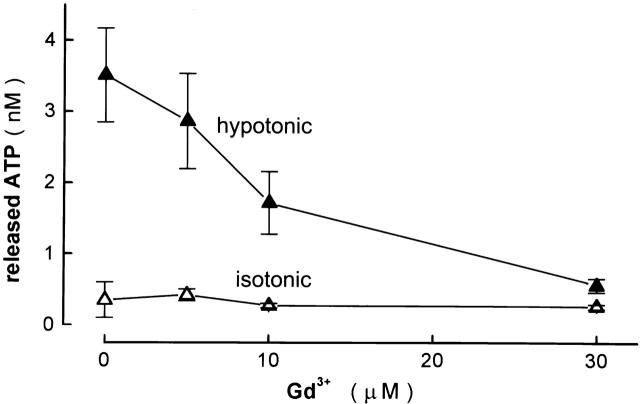
Effects of Gadolinium on Swelling-induced ATP Release and Cl− Currents
Recently, Taylor et al. 1998 showed that swelling-induced ATP release from human airway epithelial cells was inhibited by a trivalent lanthanide, Gd3+, which is the most commonly used blocker of mechanogated ion channels (Hamill and McBride 1996). Luciferin/luciferase ATP assay showed that ATP release from swollen human epithelial Intestine 407 cells was also sensitive to Gd3+ (Fig. 5). The Gd3+ effect was concentration dependent, with IC50 of ∼10 μM. Since Intestine 407 cells are known to have Gd3+-sensitive, stretch-activated, Ca2+-permeable cation channels (Okada et al. 1990), there is a possibility that the Gd3+ effect on ATP release had been mediated by the inhibiting effect on Ca2+ influx through this stretch-activated cation channel. However, swelling-activated ATP release was not inhibited, but rather enhanced [from 1.18 ± 0.12 nM (n = 11) to 3.02 ± 0.27 nM (n = 20); P = 0.000002], by Ca2+ removal from the extracellular solution. A similar enhancing effect of Ca2+ removal was previously observed on cAMP-activated ATP release from CFTR-expressing cells and was explained by a cofactor role of extracellular Ca2+ for ecto-ATPase activation (Prat et al. 1996). Gd3+ (30 μM) also inhibited swelling-activated ATP release in the Ca2+-free conditions [to 1.80 ± 0.39 nM (n = 19); P = 0.014].
Figure 5.
Concentration-dependent effects of Gd3+ on swelling-induced ATP release detected by the luminometric assay. (• and ○) Mean data under hypotonic and isotonic conditions, respectively, of 12–30 experiments. Bars represent the SEM. Inhibitory effects of 10 and 30 μM Gd3+ were statistically significant at P = 0.0276 and P = 0.0001, respectively.
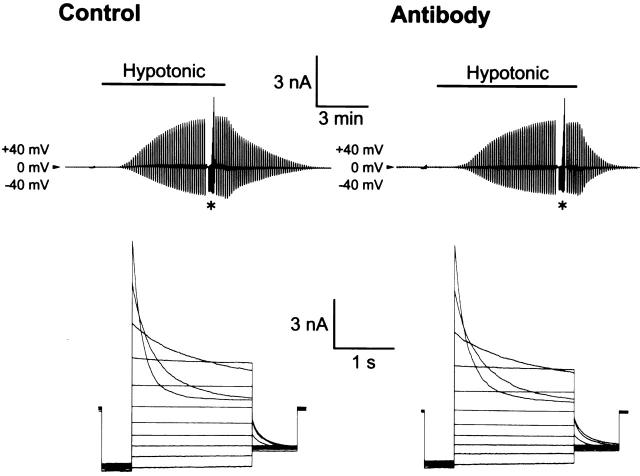
The effect of Gd3+ on the volume-sensitive Cl− current was then examined in Intestine 407 cells. Gd3+ (30 μM), which was applied 10–20 min before and during hypotonic challenge, failed to affect swelling-induced activation of the Cl− current (Fig. 6 A, left). Neither time- and voltage-dependent inactivation kinetics (Fig. 6 A, right) nor outwardly rectifying current–voltage relation (Fig. 6 B) was affected by Gd3+.
Figure 6.
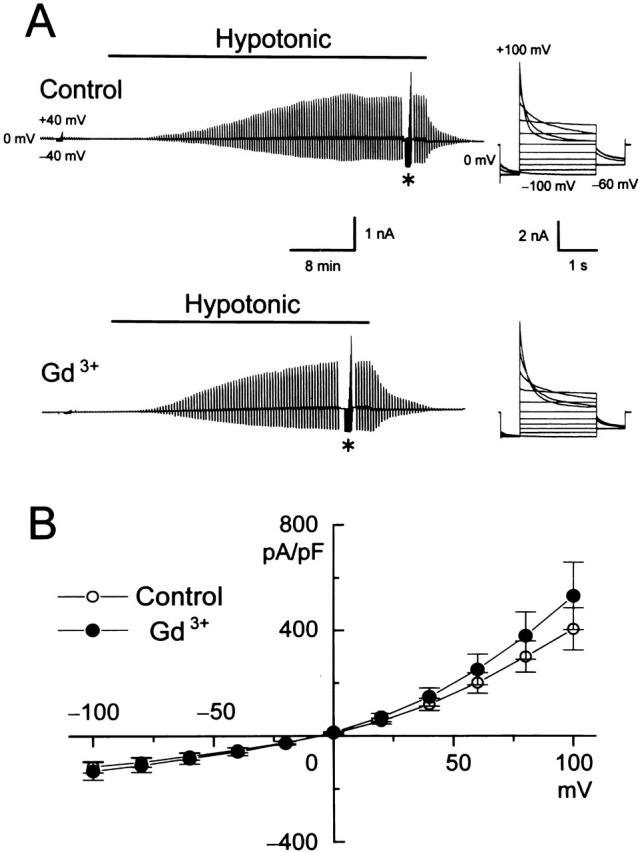
Effects of 30 μM Gd3+ on whole-cell volume-sensitive Cl− currents recorded from swollen Intestine 407 cells. (A) Representative records from a control cell (top) and a cell pretreated with Gd3+ (bottom) during application of alternating pulses from 0 ± 40 mV or of step pulses from −100 to +100 mV in 20-mV increments (*). *Gain of chart recorder was changed by 40%. (Right) Expanded traces of current responses to step pulses (at *). (B) Current–voltage relationships without (○) and with (•) Gd3+ pretreatment. (Symbols) Means ± SEM; n = 8. Effects of pretreatment with Gd3+ on current densities are not statistically significant (P > 0.05).
These results clearly indicate that the activity of volume-sensitive outwardly rectifying Cl− channels is totally independent of the swelling-induced ATP release in Intestine 407 cells.
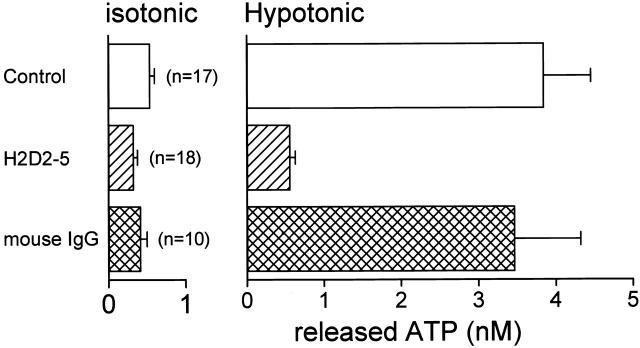
Effects of Antibodies on Swelling-induced ATP Release and Cl− Currents
To assess the molecular nature of swelling-induced ATP release pathway, we raised antibodies that can block ATP release upon osmotic swelling in Intestine 407 cells. As summarized in Fig. 7, pretreatment with the H2D2-5 antibodies at 12.5 μg/ml for 60 min markedly inhibited swelling-induced ATP release and slightly suppressed basal background ATP release. In contrast, mouse IgG failed to affect significantly the ATP release. Similar inhibiting effects were also obtained by pretreatment with the antibodies at 25 μg/ml for 30 min (data not shown, n = 13).
Figure 7.
Effects of 60-min pretreatment with anti–ATP release antibodies (H2D2-5) and mouse IgG on the basal (isotonic) ATP release and swelling-induced (hypotonic) ATP release detected by the luminometric assay. The concentration of antibodies or IgG was 12.5 μg/ml. The data with H2D2-5 were significantly different from the control data (P = 0.006 for isotonic release and P = 0.00006 for hypotonic release), whereas those with IgG were not (P = 0.59 and 0.37 for isotonic and hypotonic release, respectively).
As shown in Fig. 8, VSOR Cl− currents were not significantly affected by pretreatment with H2D2-5 antibodies (25 μg/ml, 30–50 min). The mean peak current densities with pretreatment with the antibodies (n = 9) and the negative control IgG (n = 10) were 274.3 ± 32.6 and 281.4 ± 46.1 pA/pF (P = 0.45) at +60 mV and 166.1 ± 20.7 and 165.8 ± 28.0 pA/pF (P = 0.25) at −60 mV, respectively.
Figure 8.
Effects of pretreatment with anti–ATP release antibodies on whole-cell volume-sensitive Cl− currents from swollen Intestine 407 cells. The representative records after 45-min treatment with 25 μg/ml mouse IgG (left) and H2D-25 antibodies (right). *The pulse protocols were the same as those in Fig. 6. Gain of chart recorder was changed by 40%. (Bottom) Expanded traces of current responses to step pulses (at *).
These results also show that the swelling-induced ATP release is not a prerequisite to activation of the volume-sensitive Cl− channel in Intestine 407 cells. Also, it is likely that the swelling-induced ATP release pathway is distinct from the pore of volume-sensitive outwardly rectifying Cl− channels in the human epithelial cell line.
DISCUSSION
Extracellular ATP at low micromolar concentrations is known to influence activities in a number of muscular and neuronal cells as well as platelets (Gordon 1986). Recently, ATP release has been found to be induced by stimulation with cAMP (Schwiebert et al. 1995; Prat et al. 1996; Jiang et al. 1998), by removing extracellular Cl− (Rotoli et al. 1996), by mechanical perturbation (Grygorczyk and Hanrahan 1997; Sprague et al. 1998; Watt et al. 1998; Pedersen et al. 1999), and by osmotic perturbation (Wang et al. 1996; Mitchell et al. 1998; Taylor et al. 1998) in nonneuronal, nonmuscular cells. CFTR was reported to be associated with ATP release induced by these maneuvers by some investigators (Schwiebert et al. 1995; Prat et al. 1996; Rotoli et al. 1996; Jiang et al. 1998; Sprague et al. 1998; Taylor et al. 1998). However, the pore properties of the CFTR Cl− channel and the ATP channel were found to be distinct (Sugita et al. 1998). Other investigators showed that the ATP release is not specifically associated with CFTR (Takahashi et al. 1994; Grygorczyk et al. 1996; Li et al. 1996; Reddy et al. 1996; Grygorczyk and Hanrahan 1997; Mitchell et al. 1998). The present study demonstrated that reduction of extracellular osmolality can induce ATP release in a dose-dependent manner (Fig. 1 B) in a human epithelial cell line that lacks CFTR expression (Hazama et al. 1998b). The possibility that ATP release induced by hypotonic perturbation was an artifact of cell lysis or damage could be ruled out because (a) a hypotonic challenge did not essentially affect the cell viability (Fig. 1 C), and moreover, (b) the swelling-induced ATP release could be inhibited by NPPB (Fig. 4), Gd3+ (Fig. 5), and anti–ATP-release antibodies (Fig. 7).
Upon swelling-induced ATP release, the extracellular ATP concentration in the immediate vicinity of the outer cell surface was found to reach 13 μM (Fig. 2). This value would be an underestimate because most cells express ecto-nucleotidases that rapidly hydrolyze ATP present at the cell surface (Plesner 1995). This concentration is much higher than that needed to stimulate P2-purinergic receptors (>10 nM; Dubyak and El-Moatassim 1993). Since Intestine 407 cells have purinergic receptors (Yada et al. 1989), it is possible that released ATP has some biological functions.
There have been two pieces of evidence that extracellular ATP is involved in the RVD after osmotic swelling: (a) addition of ATP to the bathing solution facilitates RVD in Intestine 407 cells (Tsumura et al. 1998), and (b) removal of released ATP with apyrase retards RVD in rat hepatoma cells (Wang et al. 1996; Roman et al. 1997) and Intestine 407 cells (Maeno, E., and Y. Okada, unpublished observations). This ATP action would be mediated by stimulation of P2-purinergic receptors because a blocker of the receptor, suramin, inhibited RVD in both cell species (Wang et al. 1996; Roman et al. 1997; Tsumura et al. 1998).
Roman et al. 1997 and Wang et al. 1996 proposed the hypothesis that the released ATP is involved in activation or upregulation of volume-sensitive Cl− channels, which are associated with Cl− efflux during RVD, in rat hepatoma cells. However, addition of extracellular ATP did not activate inward Cl− currents (Cl− efflux), but inhibited outward Cl− currents (Cl− influx) under hypotonic conditions in human epithelial Intestine 407 cells (Tsumura et al. 1996) and rat glioma C6 cells (Jackson and Strange 1995). Furthermore, the present study showed that Gd3+ (30 μM) virtually abolished swelling-induced ATP release (Fig. 5) without affecting activation of swelling-induced VSOR Cl− currents in Intestine 407 cells (Fig. 6). In addition, even when the ATP release had been blocked by anti–ATP release antibodies (Fig. 7), the activity of the VSOR Cl− channel was normally induced upon osmotic swelling (Fig. 8). In Intestine 407 cells, therefore, it appears that ATP released upon osmotic swelling facilitates RVD by a mechanism other than activation of volume-sensitive, outwardly rectifying Cl− channels or regulation of the volume-regulatory Cl− efflux. Intestine 407 cells are known to respond to extracellular ATP with an increase in cytosolic Ca2+ and Ca2+-activated K+ conductance (Yada et al. 1989). Ca2+-activated K+ conductance was shown to be responsible for volume-regulatory K+ efflux during RVD of Intestine 407 cells (Hazama and Okada 1988). Taken together, it can be speculated that swelling-induced ATP release brings about autocrine stimulation of Ca2+-activated K+ efflux by stimulation of purinergic receptors, thereby facilitating RVD.
The route of swelling-induced ATP release is not yet identified, although some investigators have suggested that CFTR (Reisin et al. 1994; Schwiebert et al. 1995; Cantiello et al. 1998) and MDR1 (Abraham et al. 1993) are involved in the route. The involvement of CFTR can be excluded in the present study because Intestine 407 cells lack expression of CFTR (Hazama et al. 1998b) and failed to respond with ATP release to stimulation with 1 mM dibutyryl cyclic AMP (Tanaka, S., and Okada, Y., unpublished observations). In fact, a blocker of CFTR Cl− channel, glibenclamide, failed to suppress swelling-induced ATP release in the present study (Fig. 3). Since Intestine 407 cells are known to express MDR1 (Tominaga et al. 1995), the involvement of MDR1 in swelling-induced ATP release seems possible. However, the transporter function of P-glycoprotein, the product of the MDR1 gene, has recently been shown to be inhibited by glibenclamide (Golstein et al. 1999). Also, our recent studies have demonstrated that osmotic swelling induced ATP release to an essentially similar degree in both human epidermoid KB cells lacking MDR1 expression (KB-3-1) and a MDR1-transfected KB cell line (KB-G2) (Hazama, A., and Y. Okada, unpublished observations).
It seems unlikely that the pore of the volume-sensitive outwardly rectifying Cl− channel can provide a pathway of swelling-induced ATP release in human epithelial Intestine 407 cells because: (a) blockers of the Cl− channel, SITS, arachidonate and glibenclamide, did not abolish swelling-induced ATP release (Fig. 3), (b) a potent blocker of swelling-induced ATP release, Gd3+, did not inhibit the Cl− currents (Fig. 6), and (c) antibodies that inhibit swelling-induced ATP release never affected the Cl− currents (Fig. 8). However, the possibility remains that the ATP release represents an ATP efflux through ATP-permeable anion channels that can be activated by cell swelling, but is distinct from the volume-sensitive outwardly rectifying Cl− channel. Since the rate of swelling-induced ATP release seems very low (on the order of 103 s−1 per cell) compared with the transporting rate of many types of ion channels (106–108 s−1 per channel), it is also possible that the ATP release is mediated by transporter or exocytosis, but not by ATP-selective channels.
Acknowledgments
The authors are grateful to A.F. James (King's College, London) for reading the manuscript, to S. Morishima for discussion, and to A. Miwa, M. Ohara, A. Hattori, and K. Shigemoto for technical assistance.
This work was supported in part by a Grant-in-Aid for Scientific Research on Priority Areas of “ABC Proteins” from the Ministry of Education, Science and Culture of Japan.
Footnotes
1used in this paper: CFTR, cystic fibrosis transmembrane conductance regulator; RVD, regulatory volume decrease; VSOR, volume-sensitive outwardly rectifying
References
- Abraham E.H., Prat A.G., Gerveck L., Seneveratne T., Arceci R.J., Kramer R., Guidotti G., Cantiello H.F. The multidrug resistance (mdr1) gene product functions as an ATP channel. Proc. Natl. Acad. Sci. USA. 1993;90:312–316. doi: 10.1073/pnas.90.1.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantiello H.F., Jackson G.R., Jr., Grosman C.F., Prat A.G., Borkan S.C., Wang Y., Reisin I.L., O'Riordan C.R., Ausiello F.A. Electrodiffusional ATP movement through the cystic fibrosis transmembrane conductance regulator. Am. J. Physiol. 1998;274:C799–C809. doi: 10.1152/ajpcell.1998.274.3.C799. [DOI] [PubMed] [Google Scholar]
- Dubyak G.R., El-Moatassim C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am. J. Physiol. 1993;265:C577–C606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- Golstein P.E., Boom A., van Geffel J., Jacobs P., Masereel B., Beauwens R. P-glycoprotein inhibition by glibenclamide and related compounds. Pflügers Arch. 1999;437:652–660. doi: 10.1007/s004240050829. [DOI] [PubMed] [Google Scholar]
- Gordon J.L. Extracellular ATPeffects, sources and fate. Biochem. J. 1986;33:309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grygorczyk R., Hanrahan J.W. CFTR-independent ATP release from epithelial cells triggered by mechanical stimuli. Am. J. Physiol. 1997;272:C1058–C1066. doi: 10.1152/ajpcell.1997.272.3.C1058. [DOI] [PubMed] [Google Scholar]
- Grygorczyk R., Tabcharani J.A., Hanrahan J.W. CFTR channels expressed in CHO cells do not have detectable ATP conductance. J. Membr. Biol. 1996;151:139–148. doi: 10.1007/s002329900065. [DOI] [PubMed] [Google Scholar]
- Hamill O., McBride D.W. The pharmacology of mechanogated membrane ion channels. Pharmacol. Rev. 1996;48:231–252. [PubMed] [Google Scholar]
- Hazama A., Hayashi S., Okada Y. Cell surface measurements of ATP release from single pancreatic β cells using a novel biosensor technique Pflügers Arch. 437 1998. 31 35a [DOI] [PubMed] [Google Scholar]
- Hazama A., Miwa A., Miyoshi T., Shimizu T., Okada Y. ATP release from swollen or CFTR-expressing epithelial cells Okada Y. Cell Volume RegulationThe Molecular Mechanism and Volume Sensing Machinery 1998. 93 98 Elsevier; Amsterdam, The Netherlands: b [Google Scholar]
- Hazama A., Okada Y. Ca2+ sensitivity of volume-regulatory K+ and Cl− channels in cultured human epithelial cells. J. Physiol. 1988;402:687–702. doi: 10.1113/jphysiol.1988.sp017229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson P.S., Strange K. Characterization of the voltage-dependent properties of a volume-sensitive anion conductance. J. Gen. Physiol. 1995;105:661–677. doi: 10.1085/jgp.105.5.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q., Mak D., Devidas S., Schwiebert E.M., Bragin A., Zhang Y., Skach W.R., Guggino W.B., Foskett J.K., Engelhardt J.F. Cystic fibrosis transmembrane conductance regulator–associated ATP release is controlled by a chloride sensor. J. Cell Biol. 1998;143:645–657. doi: 10.1083/jcb.143.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo M., Okada Y. Volume-regulatory Cl− channel currents in cultured human epithelial cells. J. Physiol. 1992;456:351–371. doi: 10.1113/jphysiol.1992.sp019340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang F. Cell Volume Regulation 1998. S. Karger AG; Basel, Switzerland: pp. 264 [Google Scholar]
- Li C., Ramjeesingh M., Bear C.E. Purified cystic fibrosis transmembrane conductance regulator (CFTR) does not function as an ATP channel. J. Biol. Chem. 1996;271:11623–11626. doi: 10.1074/jbc.271.20.11623. [DOI] [PubMed] [Google Scholar]
- Liu Y., Oiki S., Tsumura T., Shimizu T., Okada Y. Glibenclamide blocks volume-sensitive Cl− channels by dual mechanisms. Am. J. Physiol. 1998;275:C343–C351. doi: 10.1152/ajpcell.1998.275.2.C343. [DOI] [PubMed] [Google Scholar]
- Mitchell C.H., Carre D.A., Mcglinn A.M., Stone R.A., Civan M.M. A release mechanism for stored ATP in ocular ciliary epithelial cells. Proc. Natl. Acad. Sci. USA. 1998;95:7174–7178. doi: 10.1073/pnas.95.12.7174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossman T. A rapid colorimetric assay for cellular growth and survivalapplication to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nilius B., Eggermont J., Voets T., Buyse G., Manolopoulos V., Droogmans G. Properties of volume-regulated anion channels in mammalian cells. Prog. Biophys. Mol. Biol. 1997;68:69–119. doi: 10.1016/s0079-6107(97)00021-7. [DOI] [PubMed] [Google Scholar]
- Okada Y. Volume expansion-sensing outward-rectifier Cl− channelfresh start to the molecular identity and volume sensor. Am. J. Physiol. 1997;273:C755–C789. doi: 10.1152/ajpcell.1997.273.3.C755. [DOI] [PubMed] [Google Scholar]
- Okada Y. Cell Volume RegulationThe Molecular Mechanism and Volume Sensing Machinery 1998. Elsevier, ; Amsterdam, The Netherlands: pp. 214 [Google Scholar]
- Okada Y., Hazama A., Yuan W.-L. Stretch-induced activation of Ca2+-permeable ion channels is involved in the volume regulation of hypotonically swollen epithelial cells. Neurosci. Res. 1990;12:S5–S13. doi: 10.1016/0921-8696(90)90004-m. [DOI] [PubMed] [Google Scholar]
- Pedersen S., Pedersen S.F., Nilius B., Lambert I.H., Hoffmann E.K. Mechanical stress induces release of ATP from Ehrlich ascites tumor cells. Biochim. Biophys. Acta. 1999;1416:271–284. doi: 10.1016/s0005-2736(98)00228-4. [DOI] [PubMed] [Google Scholar]
- Plesner L. Ecto-ATPaseidentities and functions. Int. Rev. Cytol. 1995;158:141–214. doi: 10.1016/s0074-7696(08)62487-0. [DOI] [PubMed] [Google Scholar]
- Prat A.G., Reisin I.L., Ausiello D.A., Cantiello H.F. Cellular ATP release by the cystic fibrosis transmembrane conductance regulator. Am. J. Physiol. 1996;270:C538–C545. doi: 10.1152/ajpcell.1996.270.2.C538. [DOI] [PubMed] [Google Scholar]
- Reddy M.M., Quinton P.M., Haws C., Wine J.J., Grygorczyk R., Tabcharani J.A., Hanrahan J.W., Gunderson K.L., Kopito R.R. Failure of the cystic fibrosis transmembrane conductance regulator to conduct ATP. Science. 1996;271:1876–1879. doi: 10.1126/science.271.5257.1876. [DOI] [PubMed] [Google Scholar]
- Reisin I.L., Prat A.G., Abraham E.H., Amara J.F., Grygory R.J., Ausiello D.A., Cantiello H.F. The cystic fibrosis transmembrane conductance regulator is a dual ATP and chloride channel. J. Biol. Chem. 1994;269:20584–20591. [PubMed] [Google Scholar]
- Roman R.M., Wang Y., Lidofsky S.D., Feranchak A.P., Lomri N., Scharschmidt B.F., Fitz J.G. Hepatocellular ATP-binding cassette protein expression enhances ATP release and autocrine regulation of cell volume. J. Biol. Chem. 1997;272:21970–21976. doi: 10.1074/jbc.272.35.21970. [DOI] [PubMed] [Google Scholar]
- Rostovtseva T., Colombini M. VDAC channels mediate and gate the flow of ATPimplications for the regulation of mitochondrial function. Biophys. J. 1997;72:1954–1962. doi: 10.1016/S0006-3495(97)78841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotoli B.M., Bussolati O., Dall'Asta V., Hoffmann E.K., Cabrini G., Gazzola G.C. CFTR expression in C127 cells is associated with enhanced cell shrinkage and ATP extrusion in Cl−-free medium. Biochem. Biophys. Res. Commun. 1996;227:755–761. doi: 10.1006/bbrc.1996.1581. [DOI] [PubMed] [Google Scholar]
- Schwiebert E.K., Egan M.E., Hwang T.-H., Fulmer S.B., Allen S.S., Cutting G.R., Guggino W.B. CFTR regulates outwardly rectifying chloride channels through an autocrine mechanism involving ATP. Cell. 1995;81:1063–1073. doi: 10.1016/s0092-8674(05)80011-x. [DOI] [PubMed] [Google Scholar]
- Sprague R.S., Ellsworth M.L., Stephenson A.H., Kleinhenz M.E., Lonigro A.J. Deformation-induced ATP release from red blood cells requires CFTR activity. Am. J. Physiol. 1998;275:H1726–H1732. doi: 10.1152/ajpheart.1998.275.5.H1726. [DOI] [PubMed] [Google Scholar]
- Strange K., Emma F., Jackson P.S. Cellular and molecular physiology of volume-sensitive anion channels. Am. J. Physiol. 1996;270:C711–C730. doi: 10.1152/ajpcell.1996.270.3.C711. [DOI] [PubMed] [Google Scholar]
- Sugita M., Yue Y., Foskett J.K. CFTR Cl− channel and CFTR-associated ATP channeldistinct pores regulated by common gates. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:898–908. doi: 10.1093/emboj/17.4.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Matsushita K., Welsh M.J., Stokes J.S. Effect of cAMP on intracellular and extracellular ATP content of Cl−-secreting epithelia and 3T3 fibroblasts. J. Biol. Chem. 1994;269:17853–17857. [PubMed] [Google Scholar]
- Taylor A.L., Kudlow B.A., Marrs K.L., Gruenert D.C., Guggino W.B., Schwiebert E.M. Bioluminescence detection of ATP release mechanisms in epithelia. Am. J. Physiol. 1998;275:C1391–C1406. doi: 10.1152/ajpcell.1998.275.5.C1391. [DOI] [PubMed] [Google Scholar]
- Tominaga M., Tominaga T., Miwa A., Okada Y. Volume-sensitive chloride channel activity does not depend on endogenous P-glycoprotein. J. Biol. Chem. 1995;270:27887–27893. doi: 10.1074/jbc.270.46.27887. [DOI] [PubMed] [Google Scholar]
- Tsukita S., Itoh M., Tsukita S. A new 400-kD protein from isolated adherens junctionsits localization at the undercoat of adherens junctions and at microfilament bundles such as stress fibers and circumferential bundles. J Cell Biol. 1989;108:31–41. doi: 10.1083/jcb.109.6.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsumura T., Oiki S., Ueda S., Okuma M., Okada Y. Sensitivity of volume-sensitive Cl− conductance in human epithelial cells to extracellular nucleotides. Am. J. Physiol. 1996;271:C1872–C1878. doi: 10.1152/ajpcell.1996.271.6.C1872. [DOI] [PubMed] [Google Scholar]
- Tsumura T., Hazama A., Ueda S., Okada Y. Extracellular ATP accelerates cell volume regulation after osmotic swelling in human intestinal epithelial cells Jpn. J. Physiol. 48 1998. S108(Abstr.) [Google Scholar]
- Wang Y., Roman R., Lidofsky S.D., Fitz J.G. Autocrine signaling through ATP release represents a novel mechanism for cell volume regulation. Proc. Natl. Acad. Sci. USA. 1996;93:12020–12025. doi: 10.1073/pnas.93.21.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt W.C., Lazarowski E.R., Boucher R.C. Cystic fibrosis transmembrane regulator–independent release of ATP. Its implications for the regulation of P2Y2 receptors in airway epithelia. J. Biol. Chem. 1998;273:14053–14058. doi: 10.1074/jbc.273.22.14053. [DOI] [PubMed] [Google Scholar]
- Yada T., Oiki S., Ueda S., Okada Y. Intestinal secretagogues increase cytosolic free Ca2+ concentration and K+ conductance in a human intestinal epithelial cell line. J. Membr. Biol. 1989;112:159–167. doi: 10.1007/BF01871277. [DOI] [PubMed] [Google Scholar]