Abstract
One-third of the lipid A found in the Escherichia coli outer membrane contains an unsubstituted diphosphate unit at position 1 (lipid A 1-diphosphate). We now report an inner membrane enzyme, LpxT (YeiU), which specifically transfers a phosphate group to lipid A, forming the 1-diphosphate species. 32P-labelled lipid A obtained from lpxT mutants do not produce lipid A 1-diphosphate. In vitro assays with Kdo2-[4′-32P]lipid A as the acceptor shows that LpxT uses undecaprenyl pyrophosphate as the substrate donor. Inhibition of lipid A 1-diphosphate formation in wild-type bacteria was demonstrated by sequestering undecaprenyl pyrophosphate with the cyclic polypeptide antibiotic bacitracin, providing evidence that undecaprenyl pyrophosphate serves as the donor substrate within whole bacteria. LpxT-catalysed phosphorylation is dependent upon transport of lipid A across the inner membrane by MsbA, a lipid A flippase, indicating a periplasmic active site. In conclusion, we demonstrate a novel pathway in the periplasmic modification of lipid A that is directly linked to the synthesis of undecaprenyl phosphate, an essential carrier lipid required for the synthesis of various bacterial polymers, such as peptidoglycan.
Introduction
The Gram-negative bacterial cell envelope consists of an inner membrane, an outer membrane and the periplasmic region (Nikaido, 2003). The outer membrane is an asymmetric lipid bilayer with phospholipids forming the inner leaflet and lipopolysaccharides (LPS) forming the outer leaflet (Nikaido, 2003), whereas the inner cytoplasmic membrane is composed of phospholipids. Within the periplasm resides a continuous cross-linked carbohydrate polymer that forms a homogeneous layer outside the cytoplasmic membrane, known as the peptidoglycan layer (Schleifer and Kandler, 1972; van Heijenoort, 2001a). Both LPS and peptidoglycan are essential for maintaining the structural integrity of the Gram-negative cell envelope, and are generally required for viability. LPS acts as an efficient permeability barrier against toxic compounds, such as antibacterial agents and detergents (Raetz and Whitfield, 2002), and peptidoglycan is important for both the shape of the bacterium and protection against internal osmotic pressure (Nanninga, 1998; van Heijenoort, 2001b).
Biosynthesis of LPS and peptidoglycan, as well as other bacterial cell wall polymers, requires an essential carrier lipid, undecaprenyl phosphate (C55-P) (Fig. 1). C55-P is generated initially from the dephosphorylation of undecaprenyl pyrophosphate (C55-PP), which is synthesized in the cytoplasm by UppS, a cis-prenyl-pyrophosphate synthase. UppS catalyses the addition of eight isoprene units to farnesyl pyrophosphate (FPP) to form C55-PP (Apfel et al., 1999; Kato et al., 1999). The C55-P carries various hydrophilic precursors in a pyrophosphate linkage (C55-PP-substrate) across the hydrophobic inner membrane serving as the donor substrate for various biosynthetic pathways within the periplasm. Following the polymerization reaction, the carrier lipid is released in its pyrophosphate form (C55-PP), requiring a second dephosphorylation event before the lipid can once again serve as a carrier molecule. An example of C55-P synthesis during the biosynthesis of peptidoglycan is shown in Fig. 1 (van Heijenoort, 2001a).
Fig. 1.

Phosphorylation of lipid A by LpxT is linked to the recycling of C55-P. The carrier lipid C55-P is first synthesized in its pyrophosphate form (C55-PP) by UppS synthase on the cytoplasmic side of the inner membrane. Prior to its use, the lipid must be dephosphorylated by an undecaprenyl pyrophosphatase. An example of the recycling of C55-P during the synthesis of the essential bacterial polymer peptidoglycan is shown. The MraY and MurG enzymes catalyse the successive transfers of the MurNAc-pentapeptide and GlcNAc motifs from the peptidoglycan nucleotide precursors onto C55-P, generating the lipid I and lipid II intermediates respectively. Following transport of lipid II across the inner membrane, reactions catalysed by the penicillin-binding proteins (PBPs) begin. This results in the generation of free C55-PP that must be dephosphorylated prior to its reuse for de novo peptidoglycan biosynthesis (van Heijenoort, 2001a, b). LpxT (formerly YeiU) dephosphorylates C55-PP and transfers the phosphate group to lipid A to form lipid A 1-diphosphate contributing to the recycling of C55-P. It cannot be excluded that LpxT may dephosphorylate C55-PP that has been directly transported across the inner membrane (indicated by dashed arrow) as part of the C55-P de novo synthesis pathway. All enzymes are indicated in blue.
Recently, several inner membrane proteins demonstrating undecaprenyl pryophosphate phosphatase activity were identified in Escherichia coli (El Ghachi et al., 2004; 2005): BacA and three members from the phosphatidic acid-phosphatase superfamily of phosphatases (PgpB, YbjG and YeiU). Overproduction of the first, BacA (UppP, C55-PP phosphatase), has been shown to increase resistance to the cyclic polypeptide antibiotic bacitracin (Bernard et al., 2005). Bacitracin strongly binds to C55-PP, inhibiting its dephosphorylation to C55-P, thereby preventing synthesis of the essential carrier lipid. Presumably, overexpression of BacA results in an increase in the cellular pool of C55-P, thus overcoming the effects of the antibiotic. The second, PgpB, was previously identified as a phosphatidylglycerol phosphate phosphatase (Icho and Raetz, 1983) in the synthesis of phosphatidylglycerol, but also displays significant UppP activity in vitro. YbjG and YeiU had not been previously characterized.
Overexpression of BacA, PgpB or YbjG results in bacitracin resistance in whole cells, and increases the level of UppP activity in cellular extracts (El Ghachi et al., 2005). Interestingly, deletion of both ybjG and pgpB is required to abolish the growth of a bacA mutant. Complementation of a temperature-sensitive triple mutant harbouring deletions in bacA, ybjG and pgpB can be achieved with an intact chromosomal copy of only one of the bacA, ybjG and pgpB genes (El Ghachi et al., 2005). YeiU also exhibits UppP activity in vitro; however, its expression was not able to complement the triple mutant (El Ghachi et al., 2005). This result suggested that YeiU may have other roles besides dephosphorylating C55-PP during carrier lipid synthesis.
We now report that YeiU (renamed LpxT) is involved in the modification of the lipid A domain of Gram-negative LPS. Lipid A serves as the hydrophobic anchor of LPS, and is required to maintain the integrity of the outer membrane barrier (Raetz and Whitfield, 2002). In E. coli K-12, lipid A consists of a β(1′-6)-linked disaccharide of glucosamine that is acylated with R-3-hydroxymyristate at the 2, 3, 2′ and 3′ positions, and phosphorylated at the 1 and 4′ positions (Raetz and Whitfield, 2002; Trent et al., 2006) (Fig. 2). The majority of lipid A isolated from wild-type E. coli K-12 contains monophosphate moieties at positions 1 and 4′ (designated lipid A 1-, 4′-bis-phosphate). The genes encoding the enzymatic machinery required for the biosynthesis of bis-phosphorylated lipid A have been identified. Approximately one-third of the lipid A molecules in the E. coli K-12 outer membrane contain a diphosphate unit at the 1 position (termed lipid A 1-diphosphate) (Fig. 2) that originates by an unknown mechanism (Zhou et al., 1999). We demonstrate that deletion of lpxT results in the production of LPS containing only the bis-phosphorylated lipid A species. Purified LpxT uses C55-PP as its phosphate donor substrate catalysing the formation of 1-diphosphate lipid A (Figs 1 and 2). Removal and transfer of the phosphate group from C55-PP within the bacterial cell is dependent upon MsbA (Doerrler and Raetz, 2002), an essential LPS flippase that transfers the lipid A-core domain to the periplasmic side of the inner membrane prior to addition of O-antigen (Raetz and Whitfield, 2002). However, MsbA is not required for enzymatic function of LpxT during in vitro assay. Sequestration of C55-PP by exposure of E. coli K-12 to levels of bacitracin just below the minimal inhibitory concentration (MIC) inhibited lipid A 1-diphosphate formation in whole bacteria. Collectively, we have identified the last enzyme required for lipid A biosynthesis in E. coli K-12, and demonstrated a novel pathway in the synthesis of LPS that is directly linked to the synthesis of C55-PP.
Fig. 2.

LpxT transfers the distal phosphate from C55-PP to the 1-phosphate group of Kdo2-lipid A.
Results
Formation of lipid A 1-diphosphate in E. coli K-12 is catalysed by LpxT
LpxT is predicted to be 249 amino acids long and contains five putative membrane-spanning regions [see http://www.cbs.dtu.dk/services/TMHMM-2.0 (Sonnhammer et al., 1998)]. LpxT exhibits sequence similarity to members of the phosphatidic acid-phosphatase family (PAP2) and possesses the common conserved phosphatase motif KX6RP-(X12-54)-PSGH-(X31-54)-SRX5HX3D, previously identified by Stukey and Carman (1997). Further examination of LpxT using the COG (Clusters of Orthologous Groups) database (Wheeler et al., 2003) revealed that LpxT is a member of the same COG groups (COG0671) as Hp0021 (LpxEHP). LpxEHP was previously identified as a lipid A 1-phosphatase of Helicobacter pylori involved in the two-step modification of the 1 position of H. pylori lipid A (Tran et al., 2004). Like LpxT, LpxEHP is also a member of the PAP2 superfamily of phosphatases. As LpxT was not able to sustain cell growth in the triple mutant BWTsbacA, we wanted to examine another possible role for LpxT involving lipid A modification.
Thin-layer chromatography (TLC) analysis of 32P-labelled lipid A species isolated from wild-type E. coli strain BW25113 and its isogenic bacA, pgpB and ybjG mutants, previously generated (El Ghachi et al., 2005), revealed the two typical lipid A species; hexa-acylated lipid A 1,4′-bis-phosphate (lipid A) and hexa-acylated lipid A 1-diphosphate (lipid A 1-diphosphate), which contains a monophosphate group at position 4′ and an unsubstituted diphosphate unit at position 1 (Fig. 3A, lanes 1–4). The ratio of lipid A to lipid A 1-diphosphate was not altered in these mutants when compared with wild type (Fig. 3A, lanes 2–4), thus clearly demonstrating that both BacA and YbjG are not involved in lipid A 1-diphosphate formation or in lipid A modification (Fig. 3A, lanes 2 and 4). Our result corroborates the previous findings of Zhou et al. (1999) in that pgpB, E. coli phosphatidylglycerophosphate phosphatase, does not alter the ratio of lipid A to lipid A 1-diphosphate when compared with wild-type E. coli K-12 (Fig. 3A, lane 3). However, to our surprise, deletion of lpxT in BW25113 resulted in the total loss of the lipid A 1-diphosphate species (Fig. 3A, lane 5). This result indicates that in E. coli K-12 strains lipid A 1-diphosphate formation is dependent on the proper function of LpxT. Deletion of lpxT in BW25113 also revealed the presence of a minor lipid species. Based upon the migration of the lipid species and previous studies by Zhou et al. (1999), the minor lipid observed in the ΔlpxT mutant is the addition of a single phosphoethanolamine (pEtN) unit to lipid A (Fig. 3A, lane 5). Given that LpxT is a member of a larger family of phosphatases shown to play a role in the synthesis of bacterial lipids, the 32P-labelled phospholipids of each mutant were compared with that of wild type. Based upon TLC analysis, inactivation of lpxT had no effect on synthesis of the major phospholipids in E. coli (Fig. 3B).
Fig. 3.
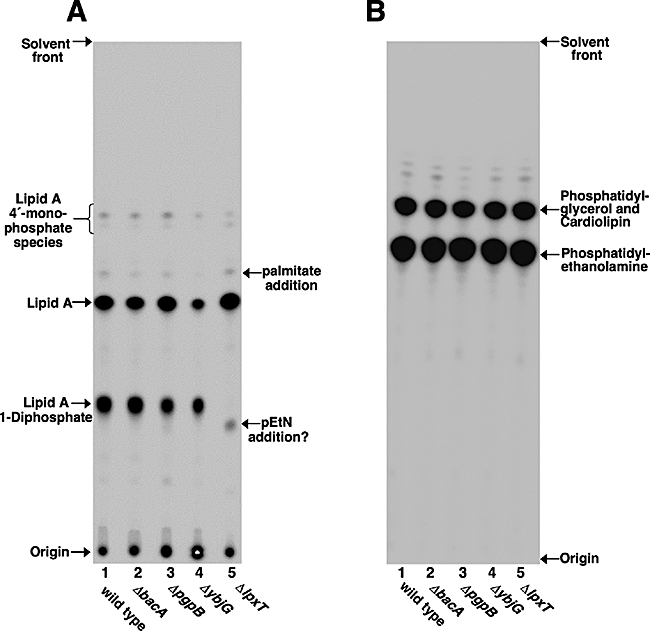
Comparison of 32P-labelled lipid A (A) and phospholipid (B) fractions isolated from wild-type BW25113 and UppP-deficient mutants. 32P-labelled lipids were isolated as described in Experimental procedures section, separated by TLC and visualized by PhosphorImaging. The identities of the major species of lipid A and phospholipids (Zhou et al., 1999) are indicated. Minor lipid A species are also indicated. The 4′-monophosphate species arise by the loss of the 1-phosphate group during pH 4.5 hydrolysis at 100°C.
LpxT restores 1-phosphotransferase activities in ΔlpxT-null mutants
To demonstrate the formation of lipid A 1-diphosphate is dependent upon LpxT; the lpxT gene was cloned into the plasmid pTrc99A and transformed into the lpxT mutant, as previously described (El Ghachi et al., 2005). TLC analysis of 32P-labelled lipid A species isolated from wild-type E. coli strain BW25113, BW25113 ΔlpxT and BW25113 ΔlpxT carrying the plasmid pTrcLpxT demonstrated that formation of lipid A 1-diphosphate is dependent upon a functional LpxT (Fig. 4A). Introduction of the vector control, pTrc99A, into BW25113 ΔlpxT had no effect upon the level of 1-diphosphate species (data not shown).
Fig. 4.

A. Complementation of lpxT-deficient mutants with a plasmid expressing LpxT. 32P-labelled lipid A species from wild-type BW25113, ΔlpxT mutant and ΔlpxT mutants expressing the plasmids pTrcLpxT, pTrcLpxT-EHEC or pTrcLpxT-ST were separated by TLC and visualized by PhosphorImaging. The presence of lipid A 1-diphosphate was restored in ΔlpxT mutants expressing the plasmids pTrcLpxT, pTrcLpxT-EHEC or pTrcLpxT-ST. B. Enzymatic confirmation of LpxT. Membranes from E. coli K-12 strains, BW25113, ΔlpxT and ΔlpxT expressing pTrcLpxT were assayed for 1-phosphotransferase activity using Kdo2-[4′-32P]lipid A. The protein concentration was 0.5 mg ml−1, and assays were carried out for 3 h at 30°C with 5 μM Kdo2-[4′-32P]lipid A and 100 μM C55-PP as the substrates. The reaction products were separated by TLC and detected with PhosphorImager analysis.
Modification of the phosphate groups of lipid A with 4-amino-4-deoxy-L-arabinose (L-Ara4N) or pEtN in E. coli and Salmonella typhimurium is regulated by the two-component regulatory system, PmrA/PmrB (Gunn et al., 1998). In both wild-type strains of E. coli, O157:H7 and S. typhimurium, the phosphate group at the 1 position is predominately modified with a pEtN residue (Zhou et al., 2001; Kim et al., 2006) and not with an additional phosphate group as previously seen with E. coli K-12. Therefore, we wanted to investigate whether the lpxT genes from both E. coli O157:H7 and S. typhimurium are functional by heterologously expressing the LpxT protein in an E. coli K-12 lpxT-null mutant strain, BW25113 ΔlpxT. TLC analysis of lipid A species isolated from BW25113 ΔlpxT carrying either pTrcLpxT-EHEC or pTrcLpxT-ST demonstrated that LpxT from either E. coli O157:H7 or S. typhimurium can successfully complement an E. coli K-12 lpxT mutant (Fig. 4A, lanes 4 and 5). Although LpxT is overexpressed by the inducible trc promoter in these constructs, it is interesting that no more than one-third of the lipid A is found in the diphosphate form.
To confirm the enzymatic function of LpxT in vitro, membranes of wild-type E. coli K-12 strain BW25113 and BW25113 ΔlpxT were isolated and assayed with Kdo2-[4′-32P]lipid A for 1-phosphotransferase activity. The Kdo sugars are linked to lipid A at the 6′-position and serve as bridge between the lipid A domain and the core oligosaccharide of LPS. Wild-type membranes catalysed the formation of two reaction products (Fig. 4B, lane 2). Product B results from the addition of palmitate by the outer membrane enzyme PagP, which has been thoroughly characterized (Bishop et al., 2000). Based upon its migration, Product A arises from conversion of the Kdo2-[4′-32P]lipid A substrate to a more hydrophilic reaction product. Addition of a phosphate group to the free hydroxyl on the 1-phosphate group of Kdo2-[4′-32P]lipid A would decrease the hydrophobicity of the lipid substrate, resulting in a slower-migrating lipid species when separated by the employed TLC system (see Experimental procedures). Membranes isolated from BW25113 ΔlpxT were unable to catalyse the formation of Product A (Fig. 4B, lane 3), but complementation with the covering plasmid (pTrcLpxT) restored this activity (lane 4). These data suggest that Product A results from the addition of a phosphate group to lipid A by LpxT. Treatment of the reaction mixture with LpxE, a lipid A 1-phosphatase, resulted in the formation of only 1-dephosphorylated Kdo2-[4′-32P]lipid A. This demonstrates that phosphate addition by LpxT occurs at the 1 position (Fig. S1). Also, as no exogenous source of phosphate was added to the assay milieu, it suggests that a membrane component serves as the donor substrate. Phosphotransferase activity was also observed when the tetra-acylated lipid A precursor Kdo2-[4′-32P]lipid IVA was employed as the lipid acceptor, but not with substrates lacking the Kdo sugars (data not shown). The Kdo region has been shown to be required by enzymes involved in the latter steps of lipid A biosynthesis (Clementz et al., 1996; Trent et al., 2001a; Reynolds et al., 2006). Also, it has been shown that mutants of E. coli unable to glycosylate their lipid A with Kdo produce a lipid A structure that is tetra-acylated lacking the 1-diphosphate modification (Meredith et al., 2006). Divalent cations are not required for LpxT activity, but inhibition can be seen with high concentrations of either CaCl2 or MgCl2 (≥ 10 mM) (data not shown). The pH optimum of the reaction is 7.0, but significant activity is observed from pH 5.5–7.5 (data not shown).
LpxT uses C55-PP as the phosphate donor
Previously, El Ghachi et al. (2005) demonstrated that membranes isolated from E. coli overexpressing LpxT (YeiU) showed a 10-fold increase in the level of C55-PP phosphatase activity. Therefore, we wanted to examine if purified LpxT uses C55-PP as the phosphate donor during the formation of lipid A 1-diphosphate. To facilitate purification, LpxT was expressed with an N-terminal His6-tag in E. coli strain C43(DE3) and, as expected, was found to localize to the membrane fraction (Fig. 5, lane 3). Following extraction of the enzyme from membranes using the detergent n-dodecyl-β-D-maltoside (DDM) (lane 4), the His6-LpxT was purified (lane 9) by affinity chromatography on Ni2+-NTA-agarose (see Experimental procedures). The purified LpxT was first tested for its ability to dephosphorylate C55-PP (Fig. 6A). Pure LpxT clearly exhibited significant C55-PP phosphatase activity, with a specific activity at 100 μM C55-PP of 0.2 μmol min−1 mg−1. In contrast, LpxT was not able to dephosphorylate the resulting C55-P into C55-OH, whatever the amount of C55-P formed during the reaction. Importantly, the addition of lipid A acceptor (Kdo2-lipid A) did not enhance C55-PP phosphatase activity during in vitro assay, suggesting that the dephosphorylation and phosphate transfer reactions are uncoupled (data not shown). Next, pure enzyme was assayed with Kdo2-[4′-32P]lipid A (5 μM) along with either C55-P or C55-PP (100 μM). As shown in Fig. 6B, lipid A 1-phosphotransferase activity was not detected with purified LpxT alone (lane 2) or with the addition of C55-P (lane 3). However, the production of Kdo2-[4′-32P]lipid A 1-diphosphate was observed upon the addition of C55-PP (lane 4). Within the linear range of phosphate transfer, purified LpxT showed a specific activity of 13.7 μmol min−1 mg−1. The proposed reaction catalysed by LpxT is shown in Fig. 2.
Fig. 5.

SDS-PAGE of the purified E. coli LpxT protein. N-terminal His6-tagged LpxT was overproduced in E. coli strain CD43(DE3), and purified by affinity chromatography using Ni2+-NTA-agarose. Aliquots were loaded on SDS-PAGE and the gel stained with Coomassie Blue. Lane 1: total cell extract; Lane 2: soluble cytosolic fraction; Lane 3: membrane fraction; Lane 4: detergent (DDM) solubilized membrane fraction; Lane 5: molecular weight standards; Lane 6: Ni2+-NTA flow through; Lane 7: 10 mM imidazole wash; Lane 8: 30 mM imidazole wash and Lane 9: 400 mM imidazole elution.
Fig. 6.
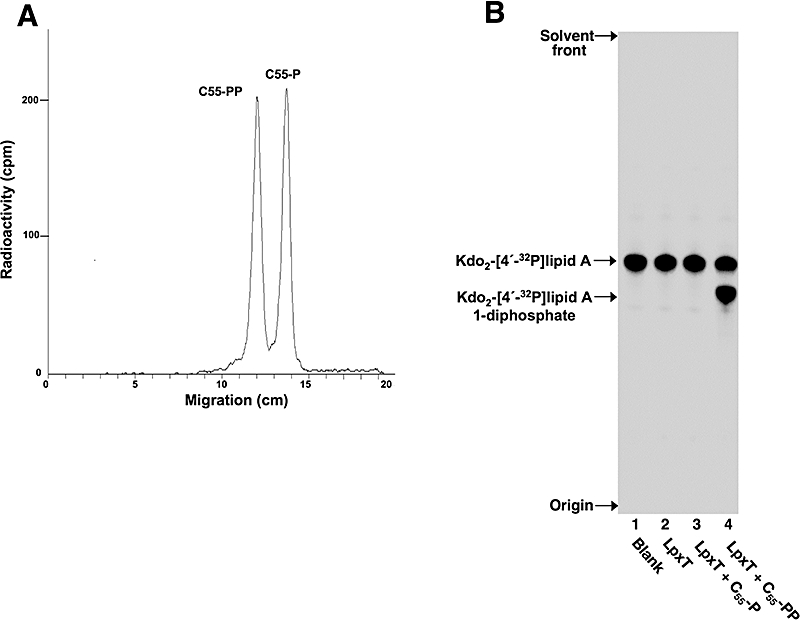
Purified LpxT transfers a phosphate group from C55-PP to lipid A. A. Purified LpxT was assayed for phosphatase activity in the presence of [14C]C55-PP as described in the text. Following separation by TLC, the lipids were located and quantified with a radioactivity scanner. B. Purified LpxT was assayed for 1-phosphotransferase activity using Kdo2-[4′-32P]lipid A. The protein concentration was 0.001 mg ml−1 and assays were carried out for 3 h at 30°C with 5 μM Kdo2-[4′-32P]lipid A and 100 μM of C55-P or C55-PP as the substrates. The products were separated by TLC and detected with PhosphorImager analysis.
To determine whether other phosphate donors could replace C55-PP in vitro, we tested several potential high-energy phosphate donors: adenosine 5′-triphosphate (ATP), diacylglycerol pyrophosphate (DGPP), FPP and isopentenyl pyrophosphate (IPP), at various concentrations (0.1–500 μM) for phosphotransferase activity (data not shown). Each potential phosphate donor was assayed under optimal conditions for phosphotransferase activity using 0.001 mg ml−1 of purified LpxT and 5 μM of Kdo2-[4′-32P]lipid A. As shown in Table 1, both ATP and IPP, which are precursors of C55-PP, assayed at 100 μM, did not stimulate phosphotransferase activity. Minor phosphotransferase activity was detected with FPP (100 μM), another precursor of C55-PP (Table 1), at 5000 times less than that seen with C55-PP.
Table 1.
Phosphotransferase activity of purified LpxT with various phosphate donor substrates (100 μM).
| Substrate | Specific activitya (nmol min−1 mg−1) |
|---|---|
| ATP | Not detectable |
| DGPP | 11.9 |
| FPP | 0.003 |
| IPP | Not detectable |
| C55-P | Not detectable |
| C55-PP | 13.7 |
Kdo2-[4′-32P]lipid A (5 μM) was used as the acceptor substrate. Assays were performed within the linear range of time and enzyme concentration.
Conversely, DGPP (100 μM) served as an efficient donor substrate for LpxT in the phosphorylation of Kdo2-[4′-32P]lipid A (Table 1). DGPP contains a pyrophosphate group attached to diacylglycerol (Wu et al., 1996), and was previously shown in vitro to serve as a substrate for E. coli PgpB. Although DGPP served as an efficient phosphate donor for LpxT, there is no conclusive evidence that DGPP is found within the E. coli membrane (Dillon et al., 1996).
In vivo formation of Lipid A 1-diphosphate is dependent upon the lipid a transporter, MsbA
C55-PP is synthesized on the cytoplasmic side of the inner membrane, but it is also regenerated on the periplasmic side of the inner membrane following various polymeriza tion reactions (e.g. polymerization of peptidoglycan) (Fig. 1). Dephosphorylation of C55-PP is required before it can be used for peptidoglycan biosynthesis (Fig. 1). Although several E. coli proteins showing UppP activity have been identified, it is still unclear how many phosphatases are involved in the metabolism of C55-PP and on what side of the inner membrane dephosphorylation of C55-PP occur.
We have demonstrated that LpxT employs both C55-PP and Kdo2-lipid A as its substrates to form lipid A 1-diphosphate (Fig. 6B); however, we were uncertain if phosphorylation of lipid A takes place on the cytoplasmic or periplasmic side of the inner membrane. Previous work from our laboratory and that of others have shown that lipid A modifications generally occur after lipid A transport across the inner membrane by MsbA (Tran et al., 2004; Boon Hinckley et al., 2005; Wang et al., 2006a). MsbA-dependent transport of lipid A is lost in the E. coli temperature-sensitive mutant WD2 by shifting the cells from 30°C to 44°C for 30 min during mid-log phase (Doerrler et al., 2001). To determine whether the active site of LpxT is oriented towards the cytoplasmic or periplasmic surface of the inner membrane in vivo, derivatives of BW25113 harbouring a temperature-sensitive msbA mutation (aroA::Tn10 msbA2) (Doerrler et al., 2001) were constructed by P1vir transduction (Table 2). These strains, designated BW2 and BW2 ΔlpxT, and the isogenic control strain BW25113A were grown at 30°C until the A600 reached 0.6–0.8. After 30 min at 44°C, the cells were labelled with 4 μCi ml−1 of 32Pi for 20 min. The 32P-labelled lipid A species were isolated and separated by TLC. At 44°C, the control strain BW25113A synthesized the normal E. coli 1,4′-bis-phosphorylated lipid A and its 1-diphosphate derivative (Fig. 7, lane 2), whereas BW2 synthesized mainly the 1,4′-bis-phosphorylated lipid A species (lane 4). BW2 ΔlpxT at both temperatures synthesized only 1,4′-bis-phosphorylated lipid A (lanes 5 and 6). These results demonstrate that LpxT transfers a phosphate group from C55-PP to the lipid A domain of LPS within the periplasmic region of the cell. In support of our findings, Tatar et al. (2007) recently demonstrated by analysis of PhoA and GFP fusion proteins that the conserved acid phosphatase motifs of YbjG and LpxT (YeiU) face the periplasmic region of the cell.
Table 2.
Bacterial strains and plasmids used in this study.
| Strain | Genotype or description | Source or reference |
|---|---|---|
| CD43(DE3) | Avidis | |
| W3110 | Wild type, F-, λ- | E. coli Genetic Stock Center (Yale) |
| W3110A | Wild type, F-, λ-, aroA::Tn10 | Doerrler et al. (2001) |
| WD2 | W3110, aroA::Tn10 msbA (A270T) | Doerrler et al. (2001) |
| BW25113 | lacIqrrnBT14ΔlacZWJ16hsdR514ΔaraBADAH33ΔrhaBADLD78 | Datsenko and Wanner (2000) |
| BW25113A | BW25113 aroA::Tn10 | This work |
| BW2 | BW25113 aroA::Tn10 msbA (A270T) | This work |
| DMEG1 | BW25113 ΔbacA::CamR | El Ghachi et al. (2005) |
| DMEG2 | BW25113 ΔybjG::CamR | El Ghachi et al. (2005) |
| DMEG3 | BW25113 ΔlpxT::CamR | El Ghachi et al. (2005) |
| DMEG4 | BW25113 ΔpgpB::KanR | El Ghachi et al. (2005) |
| DMEG3/pTrcLpxT | BW25113 ΔlpxT::CamR/pTrcLpxT | This work |
| DMEG3/pTrcLpxT-EHEC | BW25113 ΔlpxT::CamR/pTrcLpxT-EHEC | This work |
| DMEG3/pTrcLpxT-ST | BW25113 ΔlpxT::CamR/pTrcLpxT-ST | This work |
| CD43/pLpxTHIS | CD43(DE3)/pLpxTHIS | This work |
| Plasmids | ||
| pTrc99A | Expression Vector containing a T7 promoter, AmpR | Amersham |
| pTrcLpxTEC | pTrc99A containing lpxT | El Ghachi et al. (2005) |
| pTrcLpxTEHEC | pTrc99A containing lpxT of E. coli O157:H7 | This work |
| pTrcLpxTST | pTrc99A containing lpxT of S. typhimurium LT2 | This work |
| pET2130 | Derivative of pET21d (Novagen) – expression vector, AmpR | El Ghachi et al. (2004) |
| pLpxTHIS | pET2130 containing lpxT | This work |
CamR and KanR represent inserted resistance genes to chloramphenicol and kanamycin respectively.
Fig. 7.
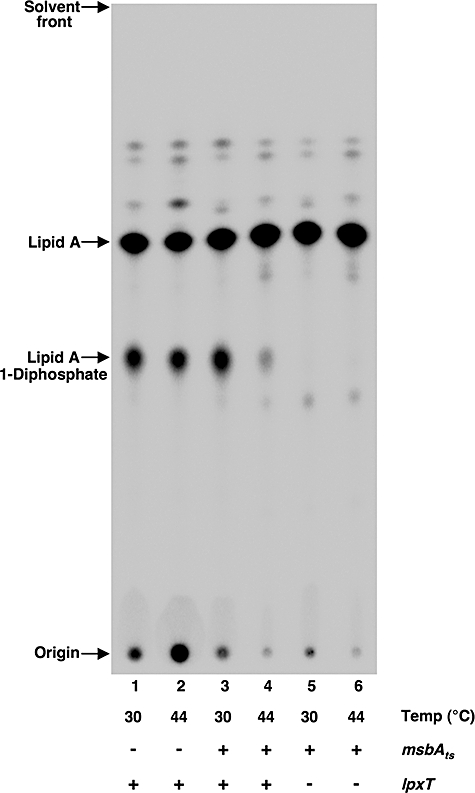
In vivo formation of lipid A 1-diphosphate is dependent upon MsbA. E. coli strains, BW25113A, BW2 and BW2 ΔlpxT, were temperature-shifted to 44°C for 30 min. Newly synthesized lipids were 32P-labelled for 20 min following the temperature shift as described previously (Tran et al., 2004), and the lipid A was isolated as described under Experimental procedures. Lipid A species from indicated strains were separated by TLC as described in the text and visualized by PhosphorImaging.
Sequestering C55-PP with bacitracin prevents lipid A 1-diphosphate formation
The decapeptide antibiotic bacitracin interdicts the recycling of the undecaprenyl carrier lipid by binding to the pyrophosphate domain of C55-PP (Storm and Strominger, 1973). To determine whether depletion of C55-PP in whole bacteria with bacitracin would prevent lipid A 1-diphosphate formation, E. coli wild-type strain BW25113 was grown at 37°C until the A600 reached ∼0.1. The cells were exposed to various antibiotics, as indicated, at levels just below the MIC, and labelled immediately with 2.5 μCi ml−1 of 32Pi for 2.5 h. The 32P-labelled lipid A species were isolated and separated by TLC. Cells grown in the absence of antibiotics synthesized both lipid A and lipid A 1-diphosphate (Fig. 8A, lane 1). Similar results were seen when cells were exposed to ampicillin (10 μg ml−1), a β-lactam antibiotic that inhibits the formation of peptidoglycan cross-links in the bacterial cell wall, and to rifampicin (40 μg ml−1), an antibiotic that prevents transcription of messenger RNA (Fig. 8, lanes 3 and 4). However, cells exposed to bacitracin (60 units ml−1) displayed a reduction in the level of lipid A 1-diphosphate formation (lane 2) when compared with cells grown in the absence of any antibiotics (lane 1).
Fig. 8.

Bacitracin reduces formation of lipid A 1-diphosphate in whole cells. A. E. coli K-12 strain, BW25113, was exposed to various antibiotics at levels just below the MIC and labelled immediately with 2.5 μCi ml−1 of 32Pi for 2.5 h. The antibiotic concentrations were as follows: ampicillin (10 μg ml−1), rifampicin (40 μg ml−1) and bacitracin (60 units ml−1). B. Complete inhibition of lipid A 1-diphosphate formation with high concentrations of bacitracin. E. coli K-12 strain, BW25113, was exposed to 150 units ml−1 of bacitracin for 50 min prior to labelling the cells with 32Pi for 20 min. 32P-labelled lipid A species from (A) and (B) were isolated as described under Experimental procedures and visualized by PhosphorImaging.
To examine if we can further reduce the formation of lipid A 1-diphosphate with bacitracin, cells were exposed to 150 units ml−1 of bacitracin for 50 min prior to the addition of 32Pi. Presumably, this allowed for the complete sequestering of free C55-PP within the cell prior to pulse labelling with 32Pi. As shown in Fig. 8B, depleting the available pool of free C55-PP completely prevented the formation of lipid A 1-diphosphate. These results clearly demonstrate that, in vivo, LpxT phosphotransferase activity is dependent upon C55-PP. Given there are approximately 106 lipid A molecules within an E. coli cell with one-third of these residues modified with an additional phosphate (Raetz et al., 2007), LpxT must contribute significantly to the available pool of C55-P.
Discussion
Both Gram-negative and Gram-positive bacteria have lipid-linked intermediary stages in their biosynthesis of various cell wall polysaccharides that are dependent on the carrier lipid C55-P (Wright et al., 1967; Scher et al., 1968; Watkinson et al., 1971; Johnson and Wilson, 1977; Rick et al., 1998; van Heijenoort, 2001b; Raetz and Whitfield, 2002). For example, in peptidoglycan biosynthesis, C55-P is required for the synthesis and transport of the hydrophilic GlcNAc-MurNAc-peptide monomeric motifs across the cytoplasmic membrane to the external sites of polymerization (Fig. 1). Another example is that C55-P can serve as acceptor for oligosaccharides repeat units as in the biosynthesis of O-antigen (Whitfield, 1995). Biosynthesis of C55-P is initiated from the dephosphorylation of C55-PP either on the cytosolic face of the inner membrane or on the periplasmic side during the late polymerization steps of peptidoglycan biosynthesis (Fig. 1) (van Heijenoort, 2001a).
Recently, several inner membrane proteins, BacA, PgpB, YbjG and YeiU, were identified in E. coli as having UppP activity (El Ghachi et al., 2004; 2005). Overexpression of these proteins in E. coli K-12 results in increased resistance to the cyclic polypeptide bacitracin, presumably by directly competing with the antibiotic for the available pool of free C55-PP. Multiple UppPs are thought to be involved in the metabolism of C55-P, because inactivation of at least three UppPs (BacA, YbjG and PgpB) enzymes are required to block cell wall synthesis and provoke cell lysis (El Ghachi et al., 2005). Interestingly, only one of the following genes, bacA, ybjG or pgpB, is necessary to support the growth of a temperature-sensitive UppP triple mutant (El Ghachi et al., 2005). Although YeiU was shown to exhibit UppP activity in vitro, inactivation experiments (El Ghachi et al., 2005) demonstrated that yeiU alone was not able to sustain growth of the temperature-sensitive UppP triple mutant. This result suggests that YeiU may have another role besides dephosphorylating C55-PP for peptidoglycan biosynthesis.
Lipid A isolated from wild-type E. coli K-12 is typically a hexa-acylated disaccharide of glucosamine with a monophosphate unit at positions 1 and 4′ (lipid A). However, one-third of the lipid A also contains an unsubstituted diphosphate unit at the 1 position (lipid A 1-diphosphate). All of the enzymes required for making lipid A in E. coli K-12 have been described, with the exception of the reaction that generates the 1-diphosphate unit. We now provide concrete evidence that YeiU (renamed LpxT) is responsible for the 1-diphosphate moiety found at the C-1 position of E. coli K-12 lipid A. Mutants lacking a functional copy of lpxT were deficient in synthesizing lipid A 1-diphosphate (Fig. 3). Using an in vitro assay, we determined that membranes of E. coli K-12 lpxT-deficient mutant were unable to convert Kdo2-lipid A to Kdo2-lipid A 1-diphosphate (Fig. 4B).
Our data demonstrate that LpxT specifically uses the carrier lipid C55-PP for phosphotransferase activity (Fig. 5), and that formation of lipid A 1-diphosphate in vivo is dependent upon the transfer of lipid A across the inner membrane by MsbA (Fig. 6). The fact that LpxT requires MsbA in vivo provides compelling evidence to support the periplasmic orientation (Tatar et al., 2007) of its active site (Fig. 1). This work provides the first biochemical evidence that dephosphorylation of C55-PP occurs on the periplasmic side of the inner membrane, and also corroborates the UppP activity of LpxT previously reported by El Ghachi et al. (2005). However, it is yet to be determined on which side of the inner membrane dephosphorylation of C55-PP occurs with the other UppPs during peptidoglycan biosynthesis.
Modification of negatively charged phosphate groups of lipid A with positively charged amine-containing substituents is an important strategy employed by a wide variety of Gram-negative bacteria to increase resistance to cationic antimicrobial peptides (Trent et al., 2006; Raetz et al., 2007). In some organisms, these modifications are under the control of the PhoP/PhoQ and the PmrA/PmrB two-component regulatory systems (Guo et al., 1997; Gunn et al., 1998). Pathogenic E. coli or E. coli K12 in which PmrA is constitutively active produces lipid A species modified with pEtN and/or L-Ara4N (Zhou et al., 1999; Kim et al., 2006), but not species containing the 1-diphosphate moiety. However, expression of LpxT from either E. coli O157:H7 or S. typhimurium LT2 can successfully complement an E. coli K-12 lpxT mutant (Fig. 5). Additionally, it has been shown that Salmonella pmrA-null mutants that are unable to modify their lipid A with pEtN or L-Ara4N synthesize the 1-diphosphate species (Zhou et al., 2001). Taken together, these data suggest that PmrA may play a role in the regulation of the 1-diphosphate species. Further studies are under way to determine if this regulation is occurring at the transcriptional level of lpxT, or possibly at the post-translational level within the bacterial membrane.
This work also provides another example of how undecaprenyl carrier lipids are not only involved in the biosynthesis of the O-antigen domain of LPS, but also in the modification of its hydrophobic anchor. For example, the periplasmic modification of lipid A by the aminoarabinose transferase requires undecaprenyl-phospho-L-Ara4N (Trent et al., 2001b). Similarly, the attachment of galacturonic acid residues to the lipid A and core domains of Rhizobium leguminosarum LPS (Kanjilal-Kolar and Raetz, 2006), and the incorporation of galactosamine units into lipid A in Francisella tularensis subsp. novicida (Wang et al., 2006b) are dependent upon undecaprenyl-linked intermediates. The biological function of the diphosphate moiety on lipid A in E. coli K-12 is still unclear as LpxT was not essential for cell growth on nutrient broth. Of interest was that loss of LpxT function resulted in the formation of a minor lipid A species that resembled the addition of a single pEtN unit (Fig. 3A). The presence of a diphosphate group or pyrophosphoethanolamine in the lipid A structure may provide some benefit to the bacterium by increasing the stability of the outer membrane under different environmental conditions. Perhaps the diphosphate moieties of lipid A are used as an energy source within the extracytoplasmic compartment of the bacterial cell as ATP is not available. Given that LpxT function is directly linked to the synthesis of C55-P, and that modification of the lipid A domain of LPS has been shown important for bacterial pathogenesis (Guo et al., 1997; van Velkinburgh and Gunn, 1999; Gunn et al., 2000; Gunn, 2001; Gibbons et al., 2005), further investigation of the function and regulation of LpxT is warranted.
Experimental procedures
Chemicals and other materials
[γ-32P]ATP and 32Pi were obtained from GE Healthcare Bio-Science AB. [14C]C55-PP was from Perkin Elmer. DDM was purchased from Anatrace. Silica gel 60 (0.25 mm) thin-layer plates were purchased from EM Separation Technology (Merck). Yeast extract and tryptone were from Difco. Triton X-100 and bicinchoninic acid (BCA) were from Pierce. ATP, IPP, FPP and bacitracin were purchased from Sigma. DGPP was obtained from Avanti Polar Lipids. C55-P and C55-PP were purchased from Institute of Biochemistry and Biophysics, Polish Academy of Sciences.
Bacterial strains and growth conditions
Bacterial strains and plasmids are summarized in Table 2. E. coli strain BW25113 harbouring mutations in genes encoding enzymes with C55-PP phosphatase activity were previously constructed (El Ghachi et al., 2004; 2005). To construct a strain of BW25113 harbouring a temperature-sensitive mutation [aroA::Tn10 msbA (A270T)] that results in loss of MsbA function at 44°C, a P1vir lysate of WD2 was used to transduce BW25113 to tetracycline resistance as described previously (Miller, 1972). Resulting colonies were repurified and tested for temperature sensitivity to give the strain BW2. The temperature-sensitive mutation was also introduced into the lpxT mutant of BW25113. A marker derivative of BW25113, designated BW25113A, was also prepared by transduction using a P1vir lysate of W3110A (see Table 2). Bacteria were routinely grown at 37°C in Luria–Bertani (LB) broth or on LB agar unless indicated otherwise. Cultures were supplemented with ampicillin (100 μg ml−1), chloramphenicol (30 μg ml−1) and/or kanamycin (30 μg ml−1) when appropriate.
Recombinant DNA techniques
Plasmid isolation, PCR clean-up and DNA gel-isolation kits were performed as per manufacturer's instructions (Qiagen). Custom primers were obtained from Integrated DNA Technologies. PCR reagents were purchased from Stratagene. Restriction endonucleases, T4 DNA ligase and antarctic phosphatase were purchased from New England Biolabs.
Isolation and analysis of lipid A and phospholipids species from 32Pi-labelled cells
Typically, a 7.5 ml cell culture was labelled uniformly with 2.5 μCi ml−1 32Pi until the cells reached late-log phase. Bacteria were collected using a clinical centrifuge, and washed with 5 ml of phosphate-buffered saline (pH 7.4). 32P-labelled lipid A and phospholipids were isolated using published protocols (Zhou et al., 1999) and spotted onto a Silica Gel 60 TLC plate (∼10 000 c.p.m. per lane). Lipids were separated using the solvent chloroform, pyridine, 88% formic acid, water (50:50:16:5, v/v), and visualized using a Bio-Rad Molecular Imager PhosphoImager equipped with Quantity One software.
Isolation of lipid A 32Pi-labelled cells exposed to antibiotics
Bacteria were grown at 37°C until an A600 of ∼0.1 was reached. Cells were then exposed separately to MICs of the following antibiotics: bacitracin (60 units ml−1), ampicillin (10 μg ml−1) or rifampicin (40 μg ml−1). 32Pi at 2.5 μCi ml−1 was added to the growth media and the labelling continued for 2.5 h. To completely sequester C55-PP within whole cells, bacteria were grown as described above, and then exposed to bacitracin (150 units ml−1) for 50 min prior to the addition of 2.5 μCi ml−1 of 32Pi. As this level of bacitracin was lethal to the bacteria, cells were only pulsed labelled for 20 min. The 32P-labelled lipid A species were isolated and separated by TLC as described above.
Construction of plasmids
The plasmid pTrcLpxT allowing expression of the lpxT gene under control of the isopropyl-β-D-thiogalactopyranoside (IPTG)-dependent trc promoter was generated as previously described (El Ghachi et al., 2005). The lpxT gene was amplified by PCR from E. coli O157:H7 and S. typhimurium LT2 genomic DNA using the following oligonucleotides: U-EHECLpxT and L-EHECLpxT, and U-STLpxT and L-STLpxT (Table 3) respectively. The resulting fragments were digested with BspHI and HindIII, gel-purified and cloned between the NcoI and HindIII sites of pTrc99A to generate the following plasmids: pTrcLpxT-EHEC and pTrcLpxT-ST. A His-tagged version of LpxT was constructed by using the following primers: LpxTBamHI and LpxTHindIII (Table 3), to PCR-amplify the gene lpxT from E. coli genomic DNA. The PCR product, engineered to contain a BamHI and a HindIII sites at the 5′ and at the 3′ end of the coding region respectively, was cloned into the corresponding sites of the T7 expression vector pET2130 (El Ghachi et al., 2004). The resulting plasmid, pLpxTHis, allowed the expression of a N-terminally His6-tagged form of LpxT protein under the control of the strong IPTG-inducible T7 promoter.
Table 3.
Oligonucleotides.
| Name | Sequence |
|---|---|
| LpxT1 | 5′-GAAATCATGATTAAAAATTTGCCGCAAATAGTGTTGTTG-3′ |
| LpxT2 | 5′-ATGAAAGCTTGGTGCGCATCATCAGGATTATCCTCAC-3′ |
| U-EHECLpxT | 5′-GCGCGCTCATGATTAAAAATTTGCCGCAAA-3′ |
| L-EHECLpxT | 5′-GCGCGCAAGCTTTTATTTGTTTTGGAAATG-3′ |
| U-STLT2LpxT | 5′-GCGCGCTCATGACGATGAAAACCCGCTATT-3′ |
| L-STLT2LpxT | 5′-GCGCGCAAGCTTTTATTTGTTTAAAATTTG-3′ |
| LpxTBamHI | 5′-GCGCGGATCCATGATTAAAAATTTGCCGCAAATAG-3′ |
| LpxTHindIII | 5′-GCGCATCATCAAGCTTATCCTCACTATTTT-3′ |
Expression and purification of LpxT
Escherichia coli C43(DE3) (Avidis) cells carrying the plasmid pLpxTHis were grown at 37°C in 2× tryptone-yeast extract medium (1 l) containing 100 mg ml−1 of ampicillin. When the optical density (A600) of the culture reached 1.0, IPTG was added at the final concentration of 1 mM, and the growth was continued for 3 h. Cells were then harvested (4000 g, 10 min) and resuspended in 40 ml of 20 mM Tris-HCl, pH 7.5, 1 mM MgCl2, 20 mM β-mercaptoethanol, 0.5 M NaCl and 15% glycerol (buffer A). Cells were disrupted by three successive passages through a French press; membranes and soluble proteins were segregated by ultracentrifugation (100 000 g, 1 h). The resulting pellet containing membrane proteins was washed three times in 20 ml of buffer A. The membrane proteins were solubilized by incubation in 20 ml of buffer A supplemented with 2% (w/v) DDM (buffer B) for 2 h at 4°C. The solution was centrifuged (100 000 g, 1 h), and the supernatant was incubated with 2 ml of nickel-nitrilotriacetate-agarose (Ni2+-NTA) and 10 mM imidazole at 4°C overnight. The resin was washed successively with 20 vols of 10 mM, 20 mM and 30 mM imidazole solutions prepared in buffer B, and the proteins were eluted with buffer B supplemented with 400 mM imidazole. The purified protein at 0.5 mg ml−1 was either stored in state at −20°C, or thoroughly dialysed against buffer A supplemented with 0.04% DDM before being stored at −20°C. In the latter case, more than 50% of the protein was lost as a result of precipitation. Protein concentration was determined by the BCA method (Smith et al., 1985), using bovine serum albumin as the standard.
Preparation of cell-free extracts, double-spun cytosol and washed membrane
Typically, 200 ml of E. coli cultures was grown to an A600 of 1.0 at 37°C and harvested by centrifugation at 10 000 g for 10 min. All samples were prepared at 4°C. Cell-free extract, double-spun cytosol and washed membranes were prepared as previously described (Trent et al., 2001c), and were stored in aliquots at −20°C. Protein concentration was determined by the BCA method.
Undecaprenyl pyrophosphate phosphatase assay
The radiolabelled [14C]C55-PP substrate was prepared as previously described (El Ghachi et al., 2004) by successive condensations of [14C]IPP to FPP (Sigma) catalysed by the purified UppS enzyme. The C55-PP phosphatase assay was performed in a 20 μl reaction mixture containing 20 mM Tris-HCl, pH 7.5, 20 mM MgCl2, 10 mM β-mercaptoethanol, 150 mM NaCl, 0.6% DDM, 100 μM [14C]C55-PP (2305 Bq) and purified LpxT enzyme. In order to examine the effect of lipid A on C55-PP phosphatase activity of LpxT, purified lipid A was added to the reaction mixture. The reaction mixture was incubated 30 min at 37°C, and terminated by heating at 100°C for 1 min. Assays were performed within the linear range of time and enzyme concentration. The sample was spotted onto a Silica Gel 60 TLC plate, and the substrate (C55-PP) and reaction product (C55-P) separated using the solvent di-isobutyl ketone, acetic acid and water (8:5:1, v/v) as a mobile phase (Rf values of C55-PP and C55-P were 0.36 and 0.5 respectively). The radioactive spots were located and quantified with a radioactivity scanner (model Multi-Tracermaster LB285; Berthold-France).
Assay of phosphate transfer to Kdo2-lipid A
The substrate Kdo2-[4′-32P]lipid A was synthesized as previously described (Tran et al., 2004). The 1-phosphotransferase activity of purified LpxT (0.001 mg ml−1) was assayed under optimized conditions in a 10 μl reaction mixture containing 50 mM HEPES, pH 7.0, 0.3% Triton X-100, 5 μM Kdo2-[4′-32P]lipid A (∼5000 c.p.m. nmol−1) and 100 μM of C55-PP as the donor substrate. For comparison, phosphate transfer to the Kdo2-[4′-32P]lipid A acceptor was also tested with the phosphate donors (100 μM) listed in Table 1. When washed membranes (0.5 mg ml−1) were employed as the enzyme source, assays contained 1.0% Triton X-100. Phosphotransferase reactions were incubated at 30°C for 180 min and terminated by spotting 4.5 μl portions of the mixtures onto silica gel 60 TLC plates. Reaction products were separated using the solvent chloroform, pyridine, 88% formic acid, water (30:70:16:10, v/v), and visualized as described above. The enzyme activity was calculated by determining the percentage of the substrate converted to product.
Acknowledgments
The authors thank Meriem El Ghachi, Ahmed Bouhss, Didier Blanot and Christopher Stead for helpful discussions. This work was supported by National Institutes of Health (NIH) Grant RO1-AI064184 to M.S.T.D. M.L. was supported by grants from the European Community (FP6, COBRA project, LSHM-CT-2003–503335) and from the Centre National de la Recherche Scientifique (UMR8619).
Supplementary material
This material is available as part of the online article from:
http://www.blackwell-synergy.com/doi/abs/10.1111/j.1365-2958.2007.06044.x
(This link will take you to the article abstract).
Please note: Blackwell Publishing is not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Apfel CM, Takacs B, Fountoulakis M, Stieger M, Keck W. Use of genomics to identify bacterial undecaprenyl pyrophosphate synthetase: cloning, expression, and characterization of the essential uppS gene. J Bacteriol. 1999;181:483–492. doi: 10.1128/jb.181.2.483-492.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard R, El Ghachi M, Mengin-Lecreulx D, Chippaux M, Denizot F. BcrC from Bacillus subtilis acts as an undecaprenyl pyrophosphate phosphatase in bacitracin resistance. J Biol Chem. 2005;280:28852–28857. doi: 10.1074/jbc.M413750200. [DOI] [PubMed] [Google Scholar]
- Bishop RE, Gibbons HS, Guina T, Trent MS, Miller SI, Raetz CR. Transfer of palmitate from phospholipids to lipid A in outer membranes of gram-negative bacteria. EMBO J. 2000;19:5071–5080. doi: 10.1093/emboj/cdd507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon Hinckley M, Reynolds CM, Ribeiro AA, McGrath SC, Cotter RJ, Lauw FN, et al. A Leptospira interrogans enzyme with similarity to yeast Ste14p that methylates the 1-phosphate group of lipid A. J Biol Chem. 2005;280:30214–30224. doi: 10.1074/jbc.M506103200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementz T, Bednarski JJ, Raetz CR. Function of the htrB high temperature requirement gene of Escherchia coli in the acylation of lipid A: HtrB catalyzed incorporation of laurate. J Biol Chem. 1996;271:12095–12102. doi: 10.1074/jbc.271.20.12095. [DOI] [PubMed] [Google Scholar]
- Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon DA, Wu WI, Riedel B, Wissing JB, Dowhan W, Carman GM. The Escherichia coli pgpB gene encodes for a diacylglycerol pyrophosphate phosphatase activity. J Biol Chem. 1996;271:30548–30553. doi: 10.1074/jbc.271.48.30548. [DOI] [PubMed] [Google Scholar]
- Doerrler WT, Raetz CR. ATPase activity of the MsbA lipid flippase of Escherichia coli. J Biol Chem. 2002;277:36697–36705. doi: 10.1074/jbc.M205857200. [DOI] [PubMed] [Google Scholar]
- Doerrler WT, Reedy MC, Raetz CR. An Escherichia coli mutant defective in lipid export. J Biol Chem. 2001;276:11461–11464. doi: 10.1074/jbc.C100091200. [DOI] [PubMed] [Google Scholar]
- El Ghachi M, Bouhss A, Blanot D, Mengin-Lecreulx D. The bacA gene of Escherichia coli encodes an undecaprenyl pyrophosphate phosphatase activity. J Biol Chem. 2004;279:30106–30113. doi: 10.1074/jbc.M401701200. [DOI] [PubMed] [Google Scholar]
- El Ghachi M, Derbise A, Bouhss A, Mengin-Lecreulx D. Identification of multiple genes encoding membrane proteins with undecaprenyl pyrophosphate phosphatase (UppP) activity in Escherichia coli. J Biol Chem. 2005;280:18689–18695. doi: 10.1074/jbc.M412277200. [DOI] [PubMed] [Google Scholar]
- Gibbons HS, Kalb SR, Cotter RJ, Raetz CR. Role of Mg2+ and pH in the modification of Salmonella lipid A after endocytosis by macrophage tumour cells. Mol Microbiol. 2005;55:425–440. doi: 10.1111/j.1365-2958.2004.04409.x. [DOI] [PubMed] [Google Scholar]
- Gunn JS. Bacterial modification of LPS and resistance to antimicrobial peptides. J Endotoxin Res. 2001;7:57–62. [PubMed] [Google Scholar]
- Gunn JS, Lim KB, Krueger J, Kim K, Guo L, Hackett M, Miller SI. PmrA-PmrB-regulated genes necessary for 4-aminoarabinose lipid A modification and polymyxin resistance. Mol Microbiol. 1998;27:1171–1182. doi: 10.1046/j.1365-2958.1998.00757.x. [DOI] [PubMed] [Google Scholar]
- Gunn JS, Ernst RK, McCoy AJ, Miller SI. Constitutive mutations of the Salmonella enterica serovar Typhimurium transcriptional virulence regulator phoP. Infect Immun. 2000;68:3758–3762. doi: 10.1128/iai.68.6.3758-3762.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Lim KB, Gunn JS, Bainbridge B, Darveau RP, Hackett M, Miller SI. Regulation of lipid A modifications by Salmonella typhimurium virulence genes phoP-phoQ. Science. 1997;276:250–253. doi: 10.1126/science.276.5310.250. [DOI] [PubMed] [Google Scholar]
- van Heijenoort J. Formation of the glycan chains in the synthesis of bacterial peptidoglycan. Glycobiology. 2001a;11:25R–36R. doi: 10.1093/glycob/11.3.25r. [DOI] [PubMed] [Google Scholar]
- van Heijenoort J. Recent advances in the formation of the bacterial peptidoglycan monomer unit. Nat Prod Rep. 2001b;18:503–519. doi: 10.1039/a804532a. [DOI] [PubMed] [Google Scholar]
- Icho T, Raetz CR. Multiple genes for membrane-bound phosphatases in Escherichia coli and their action on phospholipid precursors. J Bacteriol. 1983;153:722–730. doi: 10.1128/jb.153.2.722-730.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JG, Wilson DB. Role of a sugar-lipid intermediate in colanic acid synthesis by Escherichia coli. J Bacteriol. 1977;129:225–236. doi: 10.1128/jb.129.1.225-236.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanjilal-Kolar S, Raetz CR. Dodecaprenyl phosphate-galacturonic acid as a donor substrate for lipopolysaccharide core glycosylation in Rhizobium leguminosarum. J Biol Chem. 2006;281:12879–12887. doi: 10.1074/jbc.M513865200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato J, Fujisaki S, Nakajima K, Nishimura Y, Sato M, Nakano A. The Escherichia coli homologue of yeast RER2, a key enzyme of dolichol synthesis, is essential for carrier lipid formation in bacterial cell wall synthesis. J Bacteriol. 1999;181:2733–2738. doi: 10.1128/jb.181.9.2733-2738.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Jia W, Parreira VR, Bishop RE, Gyles CL. Phosphoethanolamine substitution in the lipid A of Escherichia coli O157: H7 and its association with PmrC. Microbiology. 2006;152:657–666. doi: 10.1099/mic.0.28692-0. [DOI] [PubMed] [Google Scholar]
- Meredith TC, Aggarwal P, Mamat U, Lindner B, Woodard RW. Redefining the requisite lipopolysaccharide structure in Escherichia coli. ACS Chem Biol. 2006;1:33–42. doi: 10.1021/cb0500015. [DOI] [PubMed] [Google Scholar]
- Miller JR. Experiments in Molecular Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- Nanninga N. Morphogenesis of Escherichia coli. Microbiol Mol Biol Rev. 1998;62:110–129. doi: 10.1128/mmbr.62.1.110-129.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol Mol Biol Rev. 2003;67:593–656. doi: 10.1128/MMBR.67.4.593-656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz CR, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz CR, Reynolds CM, Trent MS, Bishop RE. Lipid a modification systems in gram-negative bacteria. Annu Rev Biochem. 2007;76:295–329. doi: 10.1146/annurev.biochem.76.010307.145803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CM, Ribeiro AA, McGrath SC, Cotter RJ, Raetz CR, Trent MS. An outer membrane enzyme encoded by Salmonella typhimurium lpxR that removes the 3′-acyloxyacyl moiety of lipid A. J Biol Chem. 2006;281:21974–21987. doi: 10.1074/jbc.M603527200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rick PD, Hubbard GL, Kitaoka M, Nagaki H, Kinoshita T, Dowd S, et al. Characterization of the lipid-carrier involved in the synthesis of enterobacterial common antigen (ECA) and identification of a novel phosphoglyceride in a mutant of Salmonella typhimurium defective in ECA synthesis. Glycobiology. 1998;8:557–567. doi: 10.1093/glycob/8.6.557. [DOI] [PubMed] [Google Scholar]
- Scher M, Lennarz WJ, Sweeley CC. The biosynthesis of mannosyl-1-phosphoryl-polyisoprenol in Micrococcus lysodeikticus and its role in mannan synthesis. Proc Natl Acad Sci USA. 1968;59:1313–1320. doi: 10.1073/pnas.59.4.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleifer KH, Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972;36:407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Sonnhammer EL, von Heijne G, Krogh A. A hidden Markov model for predicting transmembrane helices in protein sequences. Proc Int Conf Intell Syst Mol Biol. 1998;6:175–182. [PubMed] [Google Scholar]
- Storm DR, Strominger JL. Complex formation between bacitracin peptides and isoprenyl pyrophosphates. The specificity of lipid–peptide interactions. J Biol Chem. 1973;248:3940–3945. [PubMed] [Google Scholar]
- Stukey J, Carman GM. Identification of a novel phosphatase sequence motif. Protein Sci. 1997;6:469–472. doi: 10.1002/pro.5560060226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar LD, Marolda CL, Polischuk AN, van Leeuwen D, Valvano MA. An Escherichia coli undecaprenyl-pyrophosphate phosphatase implicated in undecaprenyl phosphate recycling. Microbiology. 2007;153:2518–2529. doi: 10.1099/mic.0.2007/006312-0. [DOI] [PubMed] [Google Scholar]
- Tran AX, Karbarz MJ, Wang X, Raetz CR, McGrath SC, Cotter RJ, Trent MS. Periplasmic cleavage and modification of the 1-phosphate group of Helicobacter pylori lipid A. J Biol Chem. 2004;279:55780–55791. doi: 10.1074/jbc.M406480200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trent MS, Ribeiro AA, Lin S, Cotter RJ, Raetz CR. An inner membrane enzyme in Salmonella and Escherichia coli that transfers 4-amino-4-deoxy-L-arabinose to lipid A: induction on polymyxin-resistant mutants and role of a novel lipid-linked donor. J Biol Chem. 2001a;276:43122–43131. doi: 10.1074/jbc.M106961200. [DOI] [PubMed] [Google Scholar]
- Trent MS, Ribeiro AA, Doerrler WT, Lin S, Cotter RJ, Raetz CR. Accumulation of a polyisoprene-linked amino sugar in polymyxin-resistant Salmonella typhimurium and Escherichia coli: structural characterization and transfer to lipid A in the periplasm. J Biol Chem. 2001b;276:43132–43144. doi: 10.1074/jbc.M106962200. [DOI] [PubMed] [Google Scholar]
- Trent MS, Pabich W, Raetz CR, Miller SI. A PhoP/PhoQ-induced Lipase (PagL) that catalyzes 3-O-deacylation of lipid A precursors in membranes of Salmonella typhimurium. J Biol Chem. 2001c;276:9083–9092. doi: 10.1074/jbc.M010730200. [DOI] [PubMed] [Google Scholar]
- Trent MS, Stead CM, Tran AX, Hankins JV. Diversity of endotoxin and its impact on pathogenesis. J Endotoxin Res. 2006;12:205–223. doi: 10.1179/096805106X118825. [DOI] [PubMed] [Google Scholar]
- van Velkinburgh JC, Gunn JS. PhoP-PhoQ-regulated loci are required for enhanced bile resistance in Salmonella spp. Infect Immun. 1999;67:1614–1622. doi: 10.1128/iai.67.4.1614-1622.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, McGrath SC, Cotter RJ, Raetz CR. Expression cloning and periplasmic orientation of the Francisella novicida lipid A 4′-phosphatase LpxF. J Biol Chem. 2006a;281:9321–9330. doi: 10.1074/jbc.M600435200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ribeiro AA, Guan Z, McGrath SC, Cotter RJ, Raetz CR. Structure and biosynthesis of free lipid A molecules that replace lipopolysaccharide in Francisella tularensis subsp. novicida. Biochemistry. 2006b;45:14427–14440. doi: 10.1021/bi061767s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkinson RJ, Hussey H, Baddiley J. Shared lipid phosphate carrier in the biosynthesis of teichoic acid and peptidoglycan. Nat New Biol. 1971;229:57–59. doi: 10.1038/newbio229057a0. [DOI] [PubMed] [Google Scholar]
- Wheeler DL, Church DM, Federhen S, Lash AE, Madden TL, Pontius JU, et al. Database resources of the National Center for Biotechnology. Nucleic Acids Res. 2003;31:28–33. doi: 10.1093/nar/gkg033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield C. Biosynthesis of lipopolysaccharide O antigens. Trends Microbiol. 1995;3:178–185. doi: 10.1016/s0966-842x(00)88917-9. [DOI] [PubMed] [Google Scholar]
- Wright A, Dankert M, Fennessey P, Robbins PW. Characterization of a polyisoprenoid compound functional in O-antigen biosynthesis. Proc Natl Acad Sci USA. 1967;57:1798–1803. doi: 10.1073/pnas.57.6.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WI, Liu Y, Riedel B, Wissing JB, Fischl AS, Carman GM. Purification and characterization of diacylglycerol pyrophosphate phosphatase from Saccharomyces cerevisiae. J Biol Chem. 1996;271:1868–1876. doi: 10.1074/jbc.271.4.1868. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Lin S, Cotter RJ, Raetz CR. Lipid A modifications characteristic of Salmonella typhimurium are induced by NH4VO3 in Escherichia coli K12. Detection of 4-amino-4-deoxy-L-arabinose, phosphoethanolamine and palmitate. J Biol Chem. 1999;274:18503–18514. doi: 10.1074/jbc.274.26.18503. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Ribeiro AA, Lin S, Cotter RJ, Miller SI, Raetz CR. Lipid A modifications in polymyxin-resistant Salmonella typhimurium: PMRA-dependent 4-amino-4-deoxy-L-arabinose, and phosphoethanolamine incorporation. J Biol Chem. 2001;276:43111–43121. doi: 10.1074/jbc.M106960200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


